Abstract
While α1-adrenergic receptors (ARs) have been previously shown to limit ischemic cardiac damage, the mechanisms remain unclear. Most previous studies utilized low oxygen conditions in addition to ischemic buffers with glucose deficiencies but we discovered profound differences if the two conditions are separated. We assessed both mouse neonatal and adult myocytes and HL-1 cells in a series of assays assessing ischemic damage under hypoxic or low glucose conditions. We found that α1-AR stimulation protected against increased lactate dehydrogenase release or annexin V+ apoptosis under conditions that were due to low glucose concentration, not to hypoxia. The α1-AR antagonist prazosin or nonselective PKC inhibitors blocked the protective effect. α1-AR stimulation increased 3H-deoxyglucose uptake that was blocked with either an inhibitor to glucose transporter 1 or 4 (GLUT1 or GLUT4) or siRNA against PKCδ. GLUT1/4 inhibition also blocked α1-AR-mediated protection from apoptosis. The PKC inhibitor rottlerin or siRNA against PKCδ blocked α1-AR stimulated GLUT1 or GLUT4 plasma membrane translocation. α1-AR stimulation increased plasma membrane concentration of either GLUT1 or GLUT4 in a time-dependent fashion. Transgenic mice over-expressing the α1A-AR but not α1B-AR mice displayed increased glucose uptake and increased GLUT1 and GLUT4 plasma membrane translocation in the adult heart while α1A-AR but not α1B-AR knockout mice displayed lowered glucose uptake and GLUT translocation. Our results suggest that α1-AR activation is anti-apoptotic and protective during cardiac ischemia due to glucose deprivation and not hypoxia by enhancing glucose uptake into the heart via PKCδ-mediated GLUT translocation that may be specific to the α1A-AR subtype.
Key Terms: adrenergic receptor, cardiac, glucose transporter
Introduction
α1-ARs are G-protein coupled receptors that mediate the sympathetic nervous system, but are best characterized in regulating heart function, cardiac hypertrophy and blood pressure (1). Of the two α1-AR subtypes (α1A, α1B) expressed in the myocyte, the α1B-AR is hypothesized to be cardiac maladaptive (2–4) as it induces heart disease in transgenic mouse models. In contrast, α1A-AR expressing mice show functional protection from myocardial ischemia or pressure overload (5–8) and are inherently preconditioned (7), suggesting that it is cardiac adaptive. However, the detailed mechanism(s) by which activation of α1A-ARs are cardiac adaptive and those specific to ischemic protection remain unclear.
Previous studies that explored cardiac apoptosis attribute both pro- and anti-apoptotic effects of catecholamines through β1 and β2-ARs, respectively (9–10). While there are few reports of α1-AR mediated protection from apoptosis in myocytes (11–13), no mechanism was reported nor its role during ischemia addressed.
Myocardial ischemia leads to cardiac muscle damage due to blood restriction. One critical factor regulating the extent of ischemic cell injury is hypoxia, defined as low oxygen availability. While ischemia always leads to hypoxia, hypoxia can occur without ischemia and may have different mechanisms of action, particularly in the heart (14–15). Glucose becomes very important during ischemia as the high rate of metabolism in the heart starves the organ during energy-consuming stress (16). The mechanism of cell death becomes important during ischemia as apoptosis, necrosis and autophagy all contribute (17–20). Despite these findings, we found few studies that dissociate the contribution of glucose deficiency from hypoxic damage during cardiac ischemia (21–22). There are no studies that explored these variables in α1-AR mediated ischemic protection.
In this report, we show that α1-AR stimulation can protect mouse myocytes and adult HL-1 cells against ischemic damage and apoptosis mostly due to glucose deprivation and not hypoxia through a pathway involving PKCδ. We show that PKCδ mediates both GLUT1 and GLUT4 translocation to increase glucose uptake. The α1A-AR appears selective for this process as only CAM α1A-AR but not CAM α1B-AR mice increased glucose uptake in the heart and the plasma membrane translocation of both GLUT1 and GLUT4 while α1A-AR knockout (KO) mice displayed the opposite phenotype. Our novel results suggest that α1A-AR activation is anti-apoptotic and protective during cardiac ischemia because of its ability to increase glucose availability via PKCδ-mediated GLUT 1/4 translocation.
Materials and Methods
Animal use
Transgenic mouse models (B6CBA) that systemically over express the α1A-AR or α1B-AR subtypes have been characterized and previously described (7, 23). These mice express constitutively active mutants (CAM) of the receptors under the control of their native mouse promoter to increase subtype-selective signaling in tissues that naturally express that subtype. Equal numbers of both male and female mice were used in each experiment. The α1-AR KO mice were also previously characterized (24–25) and are maintained on a C57 background. Neonatal pups of 3 days of age or less are decapitated after using hypothermia and waiting until the cessation of movement as anesthesia negatively affects myocyte function. The hypothermia method was approved on November 10, 1998 by the National Institutes of Health Animal Research Advisory Committee if justified. The hearts from all other mice are harvested after injection of pentobarbitol (i.p. 60mg/kg body weight). Mice were housed and provided veterinary care in an AAALAC-accredited animal care facility. The experimental protocols employed in this study conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by the Animal Care and Use Committee at our institutions (Protocol 0844).
Neonatal Myocytes
Myocytes were isolated from the hearts of neonatal pups from 1 to 3 days old according to the instructions of the Neomyt Cardiomyocte Isolation Kit (nc-6031) from Cellutron Life Technologies (Baltimore, MD). All of the cells were plated onto 96 well plates in NS Media (Cellultron Life Technologies) and placed in a 37°C incubator with 5% CO2.
Adult Myocytes
The isolation was performed using the adult rat cardiomyocyte isolation kit (ac-7031) from Cellutron Life Technologies (Baltimore, MD). A simplified Langendorff system was used. The system was sterilized using 70% ethanol for 5 minutes and was then rinsed with autoclaved water thoroughly. An adult mouse was injected with 500 U/kg heparin (Sigma, St. Louis, MO) i.p. and then anesthetized using 150 mg/kg sodium pentobarbital (Nembutal, Oak Pharmaceuticals, Inc., Lake Forest, IL). The heart was removed and placed in ice cold WB (from kit). The aorta was cannulated using a 23G blunt needle and then tied with sterile 6-0 silk suture. The heart was then attached to the perfusion system under a laminar flow hood and perfused according to the manufacturer. The cell suspension was passed through sterile 300 μm Nylon mesh and then centrifuged at 1200 rpm for 3 minutes and suspended in AS media (Cellutron). The cells (1 ml/well) were pipetted into a 48-well non-tissue culture treated plated coated with 10 ug/ml mouse laminin (Invitrogen, Grand Island, NY) for several hours at 37°C. The cells were incubated at 37°C overnight with 5% CO2. The experiment was performed the following day by removing the media and washing once with buffer before addition of the test reagents.
HL-1 cells are maintained in Claycomb Media (Sigma-Aldrich, MO)(Claycomb et al., 1998) supplemented with 10% fetal bovine serum and 4mM L-glutamine at 37°C and 5% CO2. The media was changed every 24 hours. At confluence, the cells are passage 1:3 after splitting with trypsin.
Ischemic Conditions and Cellular Treatments
After myocytes were prepared and equilibrated for 24 hours at atmospheric O2 levels and with normal non-ischemic media (DMEM which contains 22.5mM glucose), the media was drawn off and replaced with 200 μl Ischemia Media (118mM NaCl, 16mM KCl, 1.2mM MgCl2, 1mM NaH2PO4, 2 mM NaHCO3, 2.5mM CaCl2, 20mM sodium lactate and 1.375 mM glucose, pH 6.2). pH was monitored before and after ischemia and no changes in pH was noted. Control cells received 0.5% DMSO to match any DMSO concentration in treated cells. Treated cells contained the α1-AR agonist 100μM phenylephrine HCl (Sigma-Aldrich, St. Louis, MO, P-6126) in the presence of 1 μM propranolol and 0.1μM rauwolscine to block β-and α2-ARs respectively, with or without freshly prepared prazosin (1μM; Sigma-Aldrich, P-7791), a non-selective α1-AR antagonist. For hypoxia, neonatal or HL-1 cells were placed in a hypoxic chamber (C-chamber) from BioSpherix (Lacona, NY) with a ProCO2 carbon dioxide controller attached to a carbon dioxide tank set at 5% CO2 and a Pro-ox Model 110 oxygen controller attached to a nitrogen tank set at 1.0% oxygen for 24 hours. Adult myocytes were incubated for a shorter time period of 5 hours. Two 150mm petri dishes containing water were placed in the bottom of the C-chamber to provide humidity. The C-chamber was placed in a dry incubator set at 37 °C. For normoxic conditions, cells are placed in a regular 37 °C/5 CO2 incubator for 24 hours.
Lactate Dehydrogenase Assay
To measure the extent of cell damage due to ischemia, we assessed the amount of lactate dehydrogenase (LDH) released into the cell media (26). Neonatal myocytes were cultured onto 96-well plates coated with SureCoat while adult myocytes were cultured onto 48 well plates coated with mouse laminin and subjected to normoxic high glucose, normoxic low glucose or hypoxic low glucose conditions. 100 μl of media was removed for the LDH assay after plates were first centrifuged at 1200 rpm in a Sorvall RT6000B centrifuge for 3 minutes. The LDH Cytotoxicity Detection Kit (630117) from Clontech Laboratories, Inc. (Mountain View, CA) was used according to manufacturer’s directions. The absorbance at 490 nm was read in a SpectraMax Plus 384 plate reader from Molecular Devices Corporation (Sunnydale, CA). Triton X-100 was added for a final concentration of 1% to wells for high cytotoxicity positive controls.
Flow Cytometric Analysis of Apoptosis
Cells cultured under different conditions were washed twice with PBS, trypsinized for 5 min, centrifuged and resuspended in Annexin V binding buffer (10x stock: 0.1M HEPES, pH 7.4; 1.4M NaCl; 25mM CaCl2. 2 μl of FITC-Annexin V (BD Pharmingen, CA). 10 μl (50μg/ml) of propidium iodide (Sigma-Aldrich, MO) was added to 105 cells in 300 μl of the binding buffer and incubated for 15 min at room temperature in the dark. Stained cells in the binding buffer were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Ca) following manufacture’s instructions. Raw data were analyzed by CellQuest software (Becton Dickinson). Quadrant dot plot was introduced to identify living and necrotic cells and cells in early or late phase of apoptosis (27). Apoptotic cells were identified as positive for annexin V-FITC. Cells are expressed as percentage of the total number of stained cells counted.
Inhibitors
To first assess the signal transduction pathway mediated by α1-ARs that protect against ischemic damage, inhibitors are added to the media 1 hour before the start of hypoxia or low glucose conditions: 1.5μM Ro-31-8220 (PKCε inhibitor), 1.25μM rottlerin (PKCδ inhibitor), 25μM PD98059 (ERK inhibitor), 10μM SB203580 (p38 inhibitor), 10μM PP2 (Src inhibitor), 25μM AG490 (Jak2 inhibitor) and 1μM Glucose Transporter Inhibitor II (Calbiochem Cat #400035).
Cellular 2-Deoxyglucose Uptake
Cells were seeded at 60–80% confluence in 12-well plates in serum containing Claycomb medium for HL-1 cells, NS medium for neonatal myocytes or AS medium for adult myocytes. Cells were incubated overnight, then switch to serum free medium for 2–4 hr. Cells were treated with phenylephrine (100 μM) for 16hr then washed twice with PBS. [3H]-2-deoxyglucose (2DG) was added to each well with PBS (0.5 μCi/well) and incubated for 10 minutes. The cells were then washed three times in PBS, then lysed with 0.4 ml of 1% SDS. The cell lysate was transferred to a scintillation vial containing 4 ml liquid scintillation cocktail. 3H-2DG uptake was detected using Beckman scintillation counter.
Tissue 2-Deoxyglucose Uptake
Equal numbers of male and female mice were fasted for 6 hours then injected with [3H]-2DG (20uCi/mouse; ip). Blood samples were taken from the tail vein at 30, 60, 90 mins post-injection to determine blood glucose levels using a Nova Max Plus glucometer according to manufacturer’s instructions (Nova Biomedical Corporation, Waltham, MA) to determine [3H]-2DG specific activity. After the final collection of blood, mice were euthanized with pentobarbitol (i.p. 60mg/kg body weight) and the heart and brain removed, rinsed in PBS, diced and frozen in dry ice. Tissues were processed with perchloric acid, barium hydroxide/zinc sulfate and glucose uptake rate was calculated according to the method of Ferre’, et al (28).
siRNA transfection
All siRNAs are premade and verified from Qiagen, CA. siPKCε (Cat # SIO1388800), siMAPK1 (Cat # 1027321) and siPKCδ (Cat # SI02738197) each are a blend of 4 preselected target sequences to each gene. All of the above siRNAs were selected from a series of siRNAs from each target protein and verified to knockdown the target in neonatal myocytes (29). We also utilized the AllStars siNegative Control, which is the most thoroughly tested and validated negative control siRNA currently available. It has no homology to any mammalian gene and validated using Affymetrix GeneChip and other assays to minimize nonspecific effects. The samples were dissolved in RNAse free water to a concentration of 20 μM. HiPerfect Transfection Reagent (Qiagen, CA) (12 μl) was added to the diluted siRNA to a final concentration of 5nM following manufacturer’s instructions and mixed by vortexing. The samples were allowed to sit 5–10 minutes at room temperature to allow the formation of transfection complexes. The complexes were added dropwise onto the cells and gently swirled to ensure the uniform distribution of the transfection complexes and incubated overnight. After 2 days, cells were washed and serum-free Claycomb medium was added for 4 hr and then treated with phenylephrine for 16 hr. Cells were then processed for plasma membrane extraction and/or western analysis as described below.
Plasma Membrane Preparation and Western Analysis
Heart tissue or cells were isolated, washed in PBS and diced into small pieces. 20–30mg of tissue or cells were homogenized in a polytron or glass douncer in 0.5ml osmotic lysis buffer (25mM Tris HCl, pH 7.4, 5mM EDTA pH 8.0) containing a proteinase cocktail (0.5 mM 4-(2-aminoethyl) benzenesulfonylfluoride, 0.15μM aprotinin, 0.5mM EDTA, 1μM leupeptin), transferred to a 1.5ml microfuge tube and rotated for 20 min. The sample was centrifuged at 500g for 10 min and the pellet discarded. The sample was then centrifuged at 30000g for 30 min. The supernatant is the cytosol fraction while the pellet is the plasma membrane fraction. Samples were incubated in a SDS-based lysis buffer (50 mM Tris, 100 mM DTT, 2% SDS, 10% glycerol). Fresh solutions of proteinase inhibitors were added to the lysis buffer immediately before use. Equal amounts of protein were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was immunoblotted with primary antibodies (GLUT4: Cell Signaling 1:1000, Cat# 2213; GLUT1: Santa Cruz, 1:500, Cat# sc-7903) overnight at 4°C. After removal of blotting solution containing primary antibody, the blot was incubated with an HRP-conjugated secondary antibodies at room temperature for 1 h, and the signal was detected by chemiluminescence (Pierce). Total amounts of protein were normalized to GAPDH. Images were scanned and analyzed using Image J software.
Statistical Analysis
One Way Analysis of Variance and Newman-Keuls post-test were used to compare functional and signaling parameters in the different mouse models and experimental conditions. A probability value p< 0.05 was considered statistically significant. Prism software (GraphPad, San Diego, CA) was used for all data analyses.
Results
α1-AR stimulation protected both neonatal and adult myocytes against glucose deprivation damage and not hypoxia
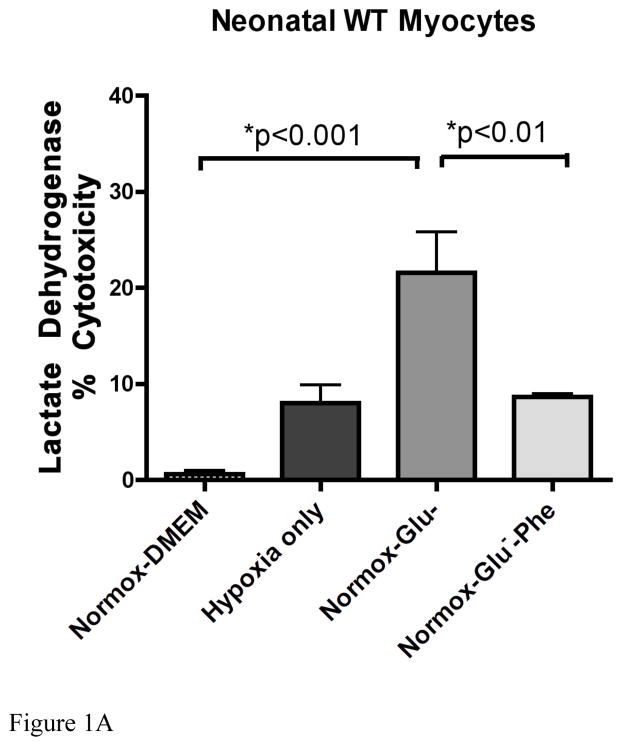
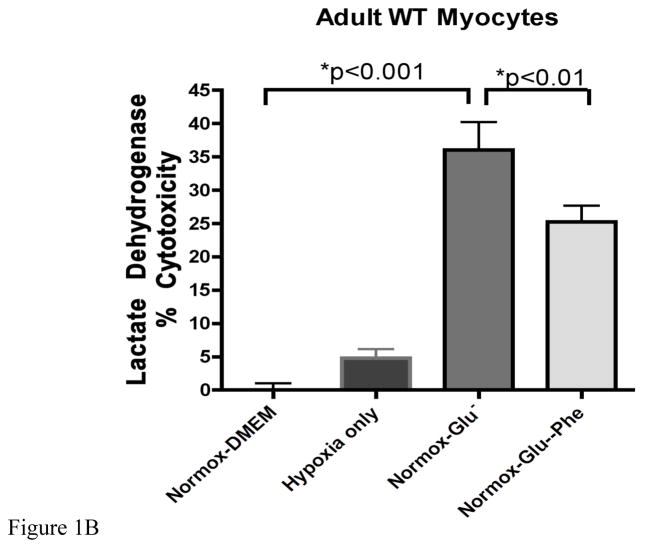
We assessed ischemic cell damage using a LDH assay that measured the amount of the enzyme released into the cell media. Using both normal neonatal (Fig 1A) and adult (Fig 1B) myocytes, we found that LDH released increased significantly under normoxic conditions only with a buffer containing a low glucose concentration (1.375 mM). Hypoxic conditions (1% O2) alone produced much lower amounts of LDH release. We also observed similar levels of LDH release when the oxygen levels were reduced to 0.5% (data not shown). α1-AR stimulation with 100μM phenylephrine significantly protected against low glucose damage in both cell types. Adult myocytes did display greater sensitivity to glucose deprivation but less protection conferred by phenylephrine than neonatal myocytes.
Figure 1. α1-AR stimulation reduced lactate dehydrogenase release in wild-type neonatal myocytes undergoing glucose but not oxygen deprivation.
A. Neonatal myocytes were subjected to normoxic (normox; atmospheric O2) or hypoxia (1% O2 for 24 hr) as described in materials and methods with either normal glucose (DMEM, 22.5mM) or low glucose concentrations (Glu−, 1.375 mM) with or without α1-AR stimulation (Phe; 100μM). B. Adult myocytes were subjected to same conditions as above except incubation time was 5 hours. The amount of LDH released (% cytotoxicity) into the media was measured using the LDH Cytotoxicity Detection Kit (Clontech Laboratories, Inc., Mountain View, CA). *Statistically significant. N = 4–6 independent experiments were performed in triplicate.
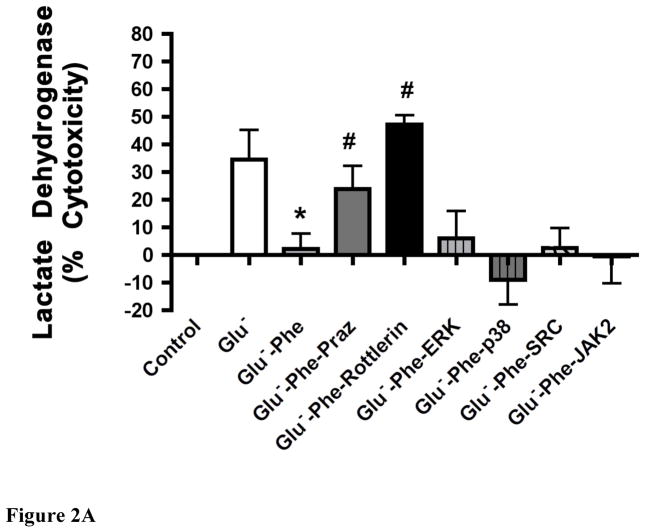
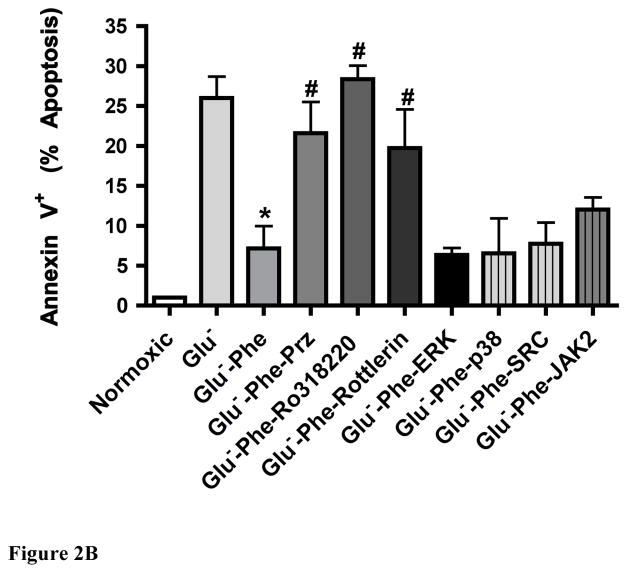
α1-AR stimulation protected myocytes against glucose deprivation apoptotic damage through PKC signaling
The α1-AR protective effect from LDH release (Fig 2A) or annexin V + apoptosis (Fig 2B) could be blocked by 1μM prazosin, a selective α1-AR antagonist or by PKC inhibitors for PKCε (Ro-31-8220) or PKCδ (rotterlin) but was not blocked when inhibitors for ERK, p38, SRC or JAK2 were applied. We also verified α1-AR mediated protection against low glucose-induced ischemic cell damage through the Calcein AM toxicity assay (data not shown). Cells that are positive for annexin V but negative for 7-AAD or PI are considered to be in early stage apoptosis (27). We also confirmed α1-AR mediated protection from apoptotic cell death using the Hoechst dye assay that detects compacted chromatin (data not shown).
Figure 2. α1-AR stimulation reduced lactate dehydrogenase release (A) or annexin V+ apoptosis (B) in WT neonatal myocytes undergoing glucose deprivation through a PKC-mediated pathway.
Neonatal myocytes seeded onto 96-well plates were subjected to normoxic conditions (control) or with low glucose concentrations (Glu−; 1.375mM) with or without α1-AR stimulation (Phe; 100μM). α1-AR stimulated cells were incubated with a series of inhibitors: prazosin, Praz (1μM); protein kinase C, PKC (1.5μM Ro-31-8220 or 1.25μM rottlerin), ERK (25μM PD98059), p38 (10μM SB203580), SRC (10μM PP2) and JAK2 (25μM AG490). *Statistically significant p≤0.05 from Glu−. #Statistically significant p≤0.05 from Glu−-Phe. N = 5 independent experiments performed in triplicate.
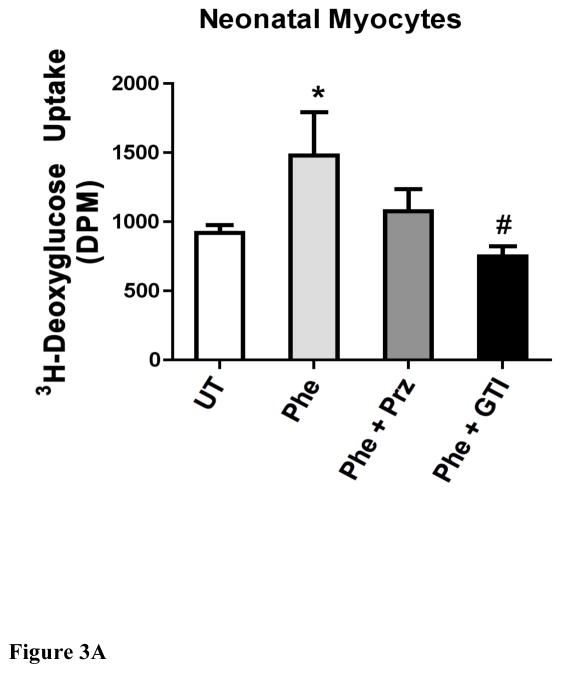
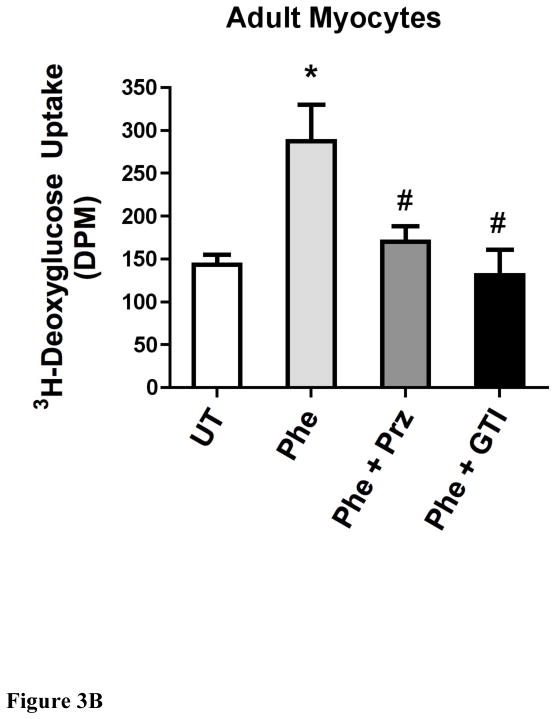
α1-AR stimulation increased deoxyglucose uptake in either neonatal or adult myocytes that was blocked with a glucose transport inhibitor to GLUT1 and GLUT4
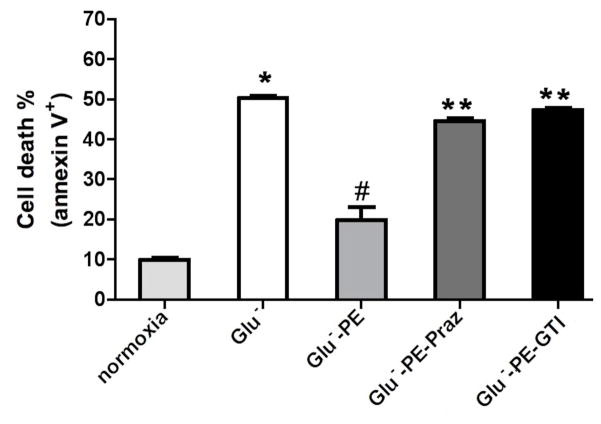
As α1-ARs protected against low glucose damage, we next determined if α1-AR stimulation increased glucose uptake utilizing 2-deoxyglucose (2DG). 2DG is readily incorporated into cells and is converted by cellular metabolism to 2-deoxyglucose-6-phosphate, which becomes trapped inside the cell. α1-AR stimulation increased deoxyglucose uptake in either neonatal (Fig 3A) or adult (Fig 3B) myocytes that was blocked by either prazosin or the glucose transporter inhibitor II that inhibits both GLUT1 and GLUT 4. Neonatal myocytes did incorporate more glucose into the cells compared with adult cells. We also confirmed that glucose uptake was responsible for ischemic protection as glucose transporter II could also block α1-AR protection from low glucose-induced apoptosis (Fig 4).
Figure 3. α1-AR stimulation increased deoxyglucose uptake in either neonatal (A) or adult (B) myocytes.
Cells were seeded at 60–80% confluence, incubated overnight, then switch to serum free medium for 2–4 hr. Cells were treated with or without (untreated, UT) phenylephrine (PE, 100 μM) for 16hr and either prazosin (Praz, 1 μM) or the glucose transporter inhibitor II (GTI, 1μM). [3H]-2-deoxyglucose (2DG) was added to each well (0.5 μCi/well) and incubated for 10 minutes. The cells were washed, lysed and transferred to a scintillation vial where 3H-2DG uptake was detected using Beckman scintillation counter. *Statistically significant p≤0.05 from untreated control. #Statistically significant p≤0.05 from phenylephrine stimulation. N = 5 independent experiments performed in triplicate.
Figure 4. α1-AR mediated glucose uptake protected against annexin V+ apoptosis in HL-1 cells.
Cells (105) were subjected to normoxic conditions (control) or with low glucose concentrations (Glu−; 1.375mM) with or without α1-AR stimulation (PE; 100μM). α1-AR stimulated cells were incubated with prazosin, Praz (1μM) or the glucose transporter inhibitor II (GTI, 1μM). Cells were suspended in Annexin V binding buffer (10x stock: 0.1M HEPES, pH 7.4; 1.4M NaCl; 25mM CaCl2. 2 μl of FITC-Annexin V (BD Pharmingen, CA), propidium iodide and incubated for 15 min in the dark. Cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Ca) following manufacture’s instructions. Raw data were analyzed by CellQuest software (Becton Dickinson). Cells are expressed as percentage of the total number of stained cells counted. *Statistically significant p≤0.05 from control. #Statistically significant p≤0.05 from Glu−. **Statistically significant p≤0.05 from Glu−-PE. N = 7 independent experiments.
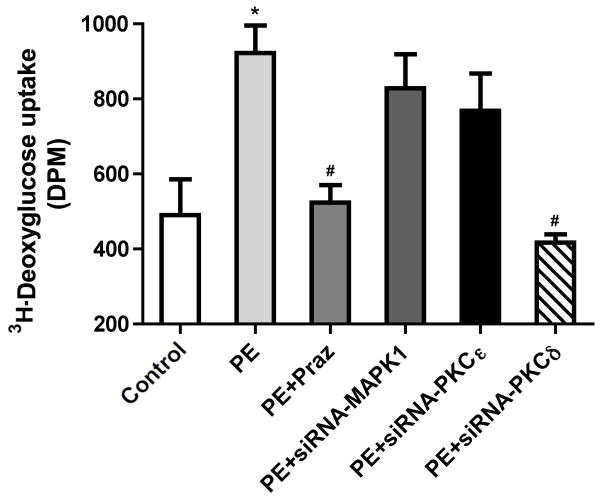
α1-AR stimulated glucose uptake is mediated through PKCδ in HL-1 cells
We also tested whether α1-ARs regulated glucose uptake in HL-1 cells, an adult cardiac muscle cell line derived from mouse atria. HL-1 maintains the ability to contract with myocyte morphological, biochemical and electrophysiological properties (31) and are more readily transfected with siRNA for mechanistic studies. As α1-AR-mediated protection against low glucose damage was blocked by PKC inhibitors, we next determined if PKC and what isoform was involved in mediating glucose uptake. Using a series of siRNAs, we found that only siRNA against PKCδ but not PKCε or ERK blocked α1-AR stimulated glucose uptake in HL-1 cells (Fig 5).
Figure 5. siRNA to PKCδ blocked α1-AR stimulated deoxyglucose uptake into HL-1 cells.
HL-1 cells were seeded at 60–80% confluence in 12-well plates and incubated overnight. Cells were washed and switched into serum-free medium for 2–4 hr and treated with or without (untreated, UT) phenylephrine (PE, 100 μM) for 16hr and either prazosin (Praz, 1 μM) or with different siRNAs according to Materials and Methods. [3H]-2DG was added to each well (0.5 μCi/well) and incubated for 10 minutes. The cells were washed, lysed and transferred to a scintillation vial where 3H-2DG uptake was detected using Beckman scintillation counter. *Statistically significant p≤0.05 from untreated control. #Statistically significant p≤0.05 from phenylephrine stimulation. N = 6 independent experiments.
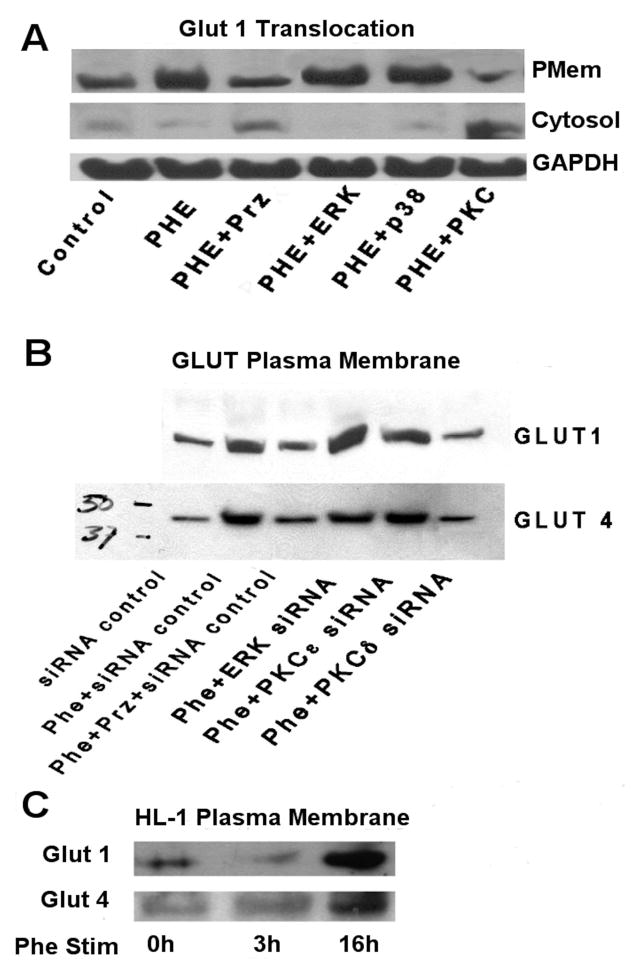
α1-AR stimulation increased GLUT1 or GLUT 4 plasma membrane translocation through PKCδ
As glucose is actively transported into cells via GLUT 1 and GLUT 4 in myocytes, we next determined if α1-ARs regulated the plasma membrane translocation of GLUTs which is required for their activation. GLUT4 is the major insulin-regulated isoform in the heart (32), while GLUT1 is responsible for basal glucose uptake (33). α1-AR stimulated the plasma membrane translocation of GLUT 1 in HL-1 cells that was blocked with either the α1-AR antagonist prazosin or the PKC inhibitor rottlerin (Fig 6A) but not with inhibitors against PKCε or ERK. α1-AR stimulation increased both GLUT 1 and GLUT 4 plasma membrane translocation that was blocked with either prazosin or siRNA against PKCδ but not PKCε or ERK (Fig 6B). α1-AR stimulation increased both GLUT 1 and GLUT 4 plasma membrane translocation in HL-1 cells in a time-dependent fashion (Fig 6C).
Figure 6. α1-AR stimulation translocated GLUT 1 and GLUT 4 to the plasma membrane and is blocked by siRNA against PKCδ.
A. HL-1 cells were incubated with or without (control) phenylephrine (Phe; 100μM) for 16 hours with a series of inhibitors: prazosin, Prz (1μM); ERK (25μM PD98059), p38 (10μM SB203580); PKC (1.25 μM rottlerin). B. HL-1 cells were incubated with or without (control) phenylephrine (Phe; 100μM) for 16 hours with a series of different siRNAs against ERK, PKCε and PKCδ in addition to a control siRNA. C. HL-1 cells were incubated with or without (0h) phenylephrine (Phe; 100μM) for 3 or 16 hours. Plasma membrane protein and cytosolic proteins were prepared according to Materials and Methods. Equal amounts of protein were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was immunoblotted with primary antibodies (GLUT4: Cell Signaling 1:1000, Cat# 2213; GLUT1: Santa Cruz, 1:500, Cat# sc-7903) overnight at 4°C. After removal of blotting solution containing primary antibody, the blot was incubated with an HRP-conjugated secondary antibodies at room temperature for 1 h and the signal was detected by chemiluminescence (Pierce). Total amounts of protein were normalized to GAPDH. Images were scanned and analyzed using Image J software. N = 6 independent experiments.
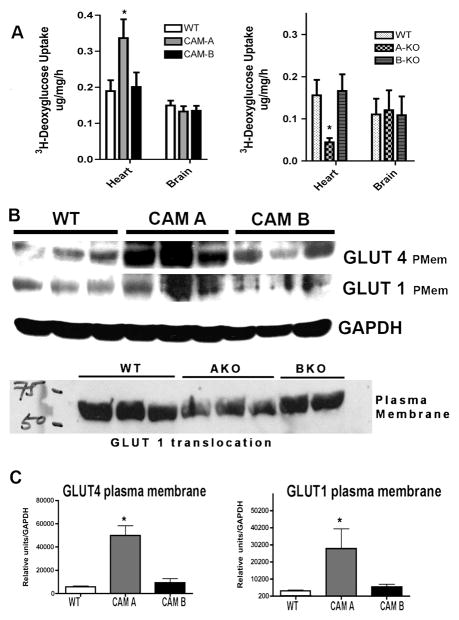
CAM α1A-AR but not CAM α1B-AR mice increased 2-deoxyglucose uptake selectively into adult heart tissue while α1A-AR KO mice decreased glucose uptake
We next determined what α1-AR subtype was regulating glucose uptake in the myocyte. We utilized CAM α1A-AR and CAM α1B-AR mice and analyzed two high glucose-utilizing tissues in the body: the adult heart and brain. We found that CAM α1A-AR but not CAM α1B-AR mice enhanced 2DG uptake and this was significant only in heart tissue. In confirmation, we also found that only the α1A-AR but not α1B-AR KO mice displayed decreased glucose uptake in the heart (Fig 7A).
Figure 7. CAM α1A-AR mice enhanced while α1A-AR KO decreased the rate of 2-deoxyglucose uptake into the heart and the cardiac plasma membrane translocation of GLUT1 and GLUT4.
(A) CAM α1A, CAM α1B, α1A-AR KO, α1B-AR KO or WT normal control mice were fasted for 6 hours then injected with [3H]-2-deoxyglucose (2DG)(20 uCi/mouse; ip). Blood samples were taken from the tail vein at 30, 60, 90 mins post-injection to determine blood glucose and [3H]-2-deoxyglucose specific activity. After the final collection of blood, mice were euthanized and the heart and brain removed, rinsed in PBS, diced and frozen in dry ice. Tissues were processed as outlined in procedures. *Statistically significant from WT control, p≤0.05. (B) Hearts from CAM α1A, CAM α1B, α1A-AR KO, α1B-AR KO or WT normal control mice were homogenized and processed for plasma membrane and cytosolic fractions according to procedures. Each fraction was separated by SDS-PAGE electrophoreses and subjected to western analysis using GLUT 1, GLUT 4 or GAPH antibodies. (C) Blots were scanned with Image J software and normalized to GAPDH levels. *Statistically significant from WT control, p≤0.05. Four hearts from each sex (8 hearts total) was used.
Adult hearts from CAM α1A-AR but not CAM α1B-AR mice increased plasma membrane translocation of GLUT 1/4 while α1A-AR KO mice decreased GLUT translocation
We determined the level of activated GLUTs in the hearts of CAM, KO and WT controls. We found that only the CAM α1A-AR but not CAM α1B-AR hearts had increased levels of plasma membrane GLUT 1/4 (Fig 7BC). In confirmation, we also found that only the α1A-AR but not α1B-AR KO mice displayed decreased GLUT1 in the heart (Fig 7B).
Discussion
Our results indicate that the α1-ARs conferred ischemic protection by regulating the influx of glucose into myocytes via PKCδ activation of glucose transporters 1/4. This is the first report of glucose transport being involved in α1-AR ischemic protection. As neonatal myocytes metabolize more glucose to fulfill their energy requirements than adult myocytes that utilize more fatty acid oxidation (30), it is possible that neonatal myocytes are more sensitized to glucose deprivation damage and the protection afforded through α1-AR glucose influx. While neonatal myocytes did have almost a 10-fold increase (in total DPMs) in deoxyglucose uptake than adult myocytes, we found that α1-ARs did increase glucose uptake (Fig 3AB) and protected against low-glucose induced cytotoxicity or apoptosis in both adult and neonatal myocytes (Fig 1AB, Fig 2, Fig 4), although not to the same degree. We believe that the α1A-AR subtype is the mediator of this cardioprotection as only CAM α1A-AR but not CAM α1B-AR hearts increased deoxyglucose uptake in vivo (Fig 7A) and GLUT 1/4 translocation (Fig 7B) while α1A-AR KO hearts were impaired.
The role of α1-ARs in cardiac ischemic protection is well established (34–38) with transgenic mouse models suggesting that the α1A-AR but not the α1B-AR subtype mediating cardioprotection (7–8, 13). This α1-AR protective mechanism was previously postulated to involve PKC, downstream mitoK (ATP), or cardiac sarcolemmal K(ATP) channels (36, 39–40). We previously suggested utilizing CAM α1A-AR mice that the kinase responsible for cardiac protection was staurosporine-sensitive (7), a broad spectrum PKC inhibitor. Several previous studies suggested a role of PKCε in ischemic protection with the cardioprotective targets residing at the mitochondria (41). Another theory is that PKCε and PKCδ have opposite targets with PKCε conferring ischemic preconditioning while PKCδ inhibition protects against reperfusion-induced damage (41–44). However, a large clinical trial using PKCδ inhibition in primary percutaneous coronary intervention failed to reduce any biomarker of ischemic injury (45). However, there is previous evidence that PKCδ activation may be involved in α1-AR ischemic preconditioning as chemical inhibitors to PKCδ but not PKCε blocked cardioprotection with the target postulated to be the cardiac sarcolemmal K(ATP) channel (40). PKCδ-stimulation also mediates cardioprotection through opioid receptor activation (46). Our results using siRNA suggests that PKCδ isoform blocked both glucose uptake (Fig 5) and Glut 1/4 translocation/activation (Fig 6) to conferred ischemic protection through α1-AR activation. PKCδ KO mice show a shift from glucose to lipid metabolism in murine hearts (47) and impaired preconditioning (48) consistent with our results.
Most previous studies in ischemic protection utilized both hypoxic as well as low glucose conditions and therefore, the role of glucose in ischemic protection is not known in these studies. Since ischemic damage is both oxygen and nutrient dependent, we initially explored the role of each of these conditions and how they may influence α1-AR ischemic protection. There are few studies that dissociate damage due to glucose deficiency versus hypoxia in cardiac ischemia (21–22) and there are no studies that explored these two variables in GPCR-mediated protection during ischemia. Previous studies showing specific α1-AR ischemic protection utilized both low oxygen as well as low glucose conditions (34–38). We report here for the first time that a major part of the mechanism on how the α1-AR limits ischemic damage involves protection from glucose deficiency and not hypoxia and/or reperfusion damage (Fig 1).
It has been known in clinical studies that intravenous glucose-insulin-potassium treatment metabolically protects the myocardium against ischemic injury and slows the rate of cell death during cardiac surgery (49). Under ischemic conditions, metabolism is shifted from an aerobic to anaerobic state and promotes glycolysis (50–51). During reperfusion, while ATP-producing fatty acid oxidation resumes, oxidative free radicals are harmful (50). Therefore, therapies that shift substrate utilization from fatty acid to glucose may offer better functional recovery from ischemia (52–53) and our results suggests that α1A-AR activation may be efficient at promoting glucose utilization in the heart by selectively increasing uptake and GLUT 1/4 plasma membrane translocation (Fig 7).
Several studies have suggested a role of enhanced glucose metabolism (54) in mediating cardiac protection (39, 55–57). The transport of glucose across the cell membrane is the rate-limiting step of glucose utilization. Increased GLUT translocation has been shown to be cardioprotective in ischemia (58–59). Our study is the first to demonstrate that glucose entry and GLUT 1 and GLUT4 translocation in the myocyte is α1-AR mediated and α1A-AR specific (Fig 7AB). As GLUT 1 is expressed in most cell types and responsible for low-level basal glucose uptake, α1-ARs may be responsible for glucose uptake in a variety of different cell types.
In addition to replenishing metabolic pools, glucose-insulin-potassium treatment reduced reactive oxygen species and promotes insulin-mediated anti-apoptotic protection (60). We also show that glucose deprivation caused increased cell death due to apoptosis (Fig 2B) that is prevented through α1-AR activation of PKCδ and GLUTs. As different cellular stressors can induce various responses such as apoptosis, necrosis or autophagy (61), our results are important for its potential treatment against ischemic damage. Deprivation of serum and glucose have been shown to induce the mitochondrial apoptotic pathway in myocytes (62–63) as well as some necrosis with the degree of apoptosis dependent proportionally upon the concentration of glucose present (64). In most of the literature, α1-ARs have been shown to be anti-apoptotic in cardiac cells depending upon the stressor. In rat neonatal myocytes, phenylephrine inhibited 8-Br-cAMP-induced apoptosis in opposition to β-AR mediated apoptosis (65). α1-ARs protect against okadaic acid-induced apoptosis in rat neonatal myocytes involving PKC and PKA signaling (12). In rabbit heart, α1-AR stimulation increased the bclx/bax ratio and inhibited TUNEL-positive apoptosis (11). The α1A-AR but not the α1B-AR protected against doxorubicin or hydrogen peroxide-induced cell death in adult myocytes through ERK signaling (13). In contrast, Gq overexpression promoted apoptosis in myocytes and in myocardium (66) suggesting that for our studies, α1-ARs mediate apoptotic protection against ischemia through a non-Gq coupled PKC pathway.
CONCLUSIONS
We conclude that α1A-ARs confer cardiac ischemic protection by limiting glucose deprivation damage and apoptosis through a PKCδ-mediated pathway. α1A-ARs conferred this protection by increasing glucose substrate availability in the starved myocyte through the translocation of GLUT 1 and GLUT 4. Metabolic-targeted drugs are emerging as a novel therapeutic treatment in heart disease because it is highly amenable to intervention (51, 67). The ability of α1A-ARs to confer cardioprotection may be due, in part, to its regulation of glucose metabolism.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. This work was supported by The Heart Lung Blood Institute from The National Institutes of Health [RO1HL098279] and an American Heart Association Grant in Aid (Great Rivers Affiliate) to DMP.
References
- 1.Perez DM, Doze VA. Cardiac and Neuroprotection Regulated by α1-Adrenergic Receptor Subtypes. J Recept Signal Transduct Res. 2011;31:98–110. doi: 10.3109/10799893.2010.550008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupp IL, Lorenz JN, Walsh RA, Boivin GP, Rindt H. Overexpression of α1B-adrenergic receptor induces left ventricular dysfunction in the absence of hypertrophy. Am J Physiol. 1998;44:H1338–H1350. doi: 10.1152/ajpheart.1998.275.4.H1338. [DOI] [PubMed] [Google Scholar]
- 3.Wang BH, Du XJ, Autelitano DJ, Milano CA, Woodcock EA. Adverse effects of constitutively active α1B-adrenergic receptors after pressure overload in mouse hearts. Am J Physiol Heart Circ Physiol. 2000;279:H1079–H1086. doi: 10.1152/ajpheart.2000.279.3.H1079. [DOI] [PubMed] [Google Scholar]
- 4.Zuscik MJ, Chalothorn D, Hellard D, Deighan C, McGee A, Daly CJ, Waugh DJ, Ross SA, Gaivin RJ, Morehead AJ, Thomas JD, Plow EF, McGrath JC, Piascik MT, Perez DM. Hypotension, autonomic failure, and cardiac hypertrophy in transgenic mice overexpressing the α1B-adrenergic receptor. J Biol Chem. 2001;276:13738–13743. doi: 10.1074/jbc.M008693200. [DOI] [PubMed] [Google Scholar]
- 5.Lin G, Owens WA, Chen SH, Stevens ME, Kesteven S, Arthur JF, Woodcock EA, Feneley MP, Graham RM. Targeted α1A-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. 2001;89:343–350. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- 6.Du XJ, Fang L, Gao XM, Kiriazis H, Feng X, Hotchin E, Finch AM, Chaulet H, Graham RM. Genetic enhancement of ventricular contractility protects against pressure-overload-induced cardiac dysfunction. J Mol Cell Cardiol. 2004;37:979–987. doi: 10.1016/j.yjmcc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, Perez DM. α1A- but not α1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovasc Res. 2005;65:436–445. doi: 10.1016/j.cardiores.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Du X-J, Gao X-M, Kiriazis H, Moore XL, Ming Z, Su Y, Finch AM, Hannan RA, Dart AM, Graham RM. Transgenic α1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res. 2006;71:735–743. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shizukuda Y, Buttrick PM. Subtype specific roles of β-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol. 2002;34:823–831. doi: 10.1006/jmcc.2002.2020. [DOI] [PubMed] [Google Scholar]
- 11.Baghelai K, Graham LJ, Wechsler AS, Jakoi ER. Delayed myocardial preconditioning by α1-adrenoceptors involves inhibition of apoptosis. J Thorac Cardiovasc Surg. 1999;117:980–986. doi: 10.1016/s0022-5223(99)70379-x. [DOI] [PubMed] [Google Scholar]
- 12.Singh K, Communal C, Colucci WS. Inhibition of protein phosphatase 1 induces apoptosis in neonatal rat cardiac myocytes: role of adrenergic receptor stimulation. Basic Res Cardiol. 2000;95:389–96. doi: 10.1007/s003950070038. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O’Connell TD. An α1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 14.Wyman RM, Farhi ER, Bing OH, Johnson RG, Weintraub RM, Grossman W. Comparative effects of hypoxia and ischemia in the isolated, blood-perfused dog heart: evaluation of left ventricular diastolic chamber distensibility and wall thickness. Circ Res. 1989;64:121–128. doi: 10.1161/01.res.64.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Rocha-Singh KJ, Honbo NY, Karliner JS. Hypoxia and glucose independently regulate the beta-adrenergic receptor-adenylate cyclase system in cardiac myocytes. J Clin Invest. 1991;88:204–213. doi: 10.1172/JCI115279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 17.Ohno M, Takemura G, Ohno A, Misao J, Hayakawa Y, Minatoguchi S, Fujiwara T, Fujiwara H. “Apoptotic” myocytes in infarct area in rabbit hearts may be oncotic myocytes with DNA fragmentation: analysis by immunogold electron microscopy combined with in situ nick end labeling. Circulation. 1998;98:1422–1430. doi: 10.1161/01.cir.98.14.1422. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka R, Ogawa K, Yaoita H, Naganuma W, Maehara K, Maruyama Y. Characteristics of death of neonatal rat cardiomyocytes following hypoxia or hypoxia-reoxygenation: the association of apoptosis and cell membrane disintegrity. Heart Vessels. 2002;16:241–248. doi: 10.1007/s003800200031. [DOI] [PubMed] [Google Scholar]
- 19.Tong DL, Zhang DX, Xiang F, Teng M, Jiang XP, Hou JM, Zhang Q, Huang YS. Nicotinamide Pretreatment Protects Cardiomyocytes against Hypoxia-Induced Cell Death by Improving Mitochondrial Stress. Pharmacology. 2012;90:11–8. doi: 10.1159/000338628. [DOI] [PubMed] [Google Scholar]
- 20.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 21.Owen P, Dennis S, Opie LH. Glucose flux rate regulates onset of ischemic contracture in globally underperfused rat hearts. Circ Res. 1990;66:344–354. doi: 10.1161/01.res.66.2.344. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra R, Brosius FC. Glucose uptake and glycolysis reduce hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes. J Biol Chem. 1999;274:12567–12575. doi: 10.1074/jbc.274.18.12567. [DOI] [PubMed] [Google Scholar]
- 23.Zuscik MJ, Sand S, Ross SA, Waugh DJJ, Gaivin RJ, Morilak D, Perez DM. Overexpression of the α1b-Adrenergic receptor causes apoptotic neurodegeneration: A multiple system atrophy. Nature Medicine. 2000;6:1388–1394. doi: 10.1038/82207. [DOI] [PubMed] [Google Scholar]
- 24.O’Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. The α1A/C- and α1B-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, Simpson PC. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King JA. A routine method for estimation of lactate dehydrogenase activity. J Med Lab Technol. 1959;16:265–272. [PubMed] [Google Scholar]
- 27.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phospatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 28.Ferré P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985;228:103–10. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi T, Papay RS, Perez DM. α1A-AR differentially regulates STAT3 phosphorylation through PKCε and PKCδ in myocytes. J Receptors Signal Transduction. 2012;32:76–86. doi: 10.3109/10799893.2011.647353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calmettes G, John SA, Weiss JN, Ribalet B. Hexokinase-mitochondrial interactions regulate glucose metabolism differentially in adult and neonatal cardiac myocytes. J Gen Physiol. 2013;142:425–36. doi: 10.1085/jgp.201310968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calderhead DM, Kitagawa K, Lienhard GE, Gould GW. Translocation of the brain-type glucose transporter largely accounts for insulin stimulation of glucose transport in BC3H-1 myocytes. Biochem J. 1990;269:597–601. doi: 10.1042/bj2690597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraegen EW, Sowden JA, Halstead MB, Clark PW, Rodnick KJ, Chisholm DJ, James DE. Glucose transporters and in vivo glucose uptake in skeletal and cardiac muscle: fasting, insulin stimulation and immunoisolation studies of GLUT1 and GLUT4. Biochem J. 1993;295:287–293. doi: 10.1042/bj2950287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee A, Locke-Winter C, Rogers KB, Mitchell MB, Brew EC, Cairns CB, Bensard DD, Harken AH. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an α-adrenergic mechanism. Circ Res. 1993;73:656–670. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita N, Nishida M, Hoshida S, Igarashi J, Hori M, Kuzuya T, Tada M. α1-adrenergic stimulation induces cardiac tolerance to hypoxia via induction and activation of Mn-SOD. Am J Physiol. 1996;271:H1356–H1362. doi: 10.1152/ajpheart.1996.271.4.H1356. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Ashraf M. Activation of α1-adrenergic receptor during Ca2+ pre-conditioning elicits strong protection against Ca2+ overload injury via protein kinase C signaling pathway. J Mol Cell Cardiol. 1998;30:2423–2435. doi: 10.1006/jmcc.1998.0802. [DOI] [PubMed] [Google Scholar]
- 37.Meng X, Shames BD, Pulido EJ, Meldrum DR, Ao L, Joo KS, Harken AH, Banerjee A. Adrenergic induction of bimodal myocardial protection: signal transduction and cardiac gene reprogramming. Am J Physiol. 1999;276:R1525–R1533. doi: 10.1152/ajpregu.1999.276.5.R1525. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchida A, Liu Y, Liu GS, Chosen MV, Downey GM. α1-adrenergic receptor agonist preconditioning rabbit heart independent of adenosine by direct activation of protein kinase c. Circ Res. 1994;74:576–585. doi: 10.1161/01.res.75.3.576. [DOI] [PubMed] [Google Scholar]
- 39.Loubani M, Galiñanes M. Pharmacological and ischemic preconditioning of the human myocardium: mitoK(ATP) channels are upstream and p38MAPK is downstream of PKC. BMC Physiol. 2002;2:10. doi: 10.1186/1472-6793-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turrell HE, Rodrigo GC, Norman RI, Dickens M, Standen NB. Phenylephrine preconditioning involves modulation of cardiac sarcolemmal K(ATP) current by PKCδ, AMPK and p38 MAPK. J Mol Cell Cardiol. 2011;51(3):370–80. doi: 10.1016/j.yjmcc.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Cepsilon (PKCepsilon): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007a;35(Pt 5):1052–4. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98(20):11114–9. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inagaki K, Hahn HS, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108(7):869–75. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 44.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007b;55(6):523–36. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Lincoff AM, Roe M, Aylward P, Galla J, Rynkiewicz A, Guetta V, Zelizko M, Kleiman N, White H, McErlean E, Erlinge D, Laine M, Dos Santos Ferreira JM, Goodman S, Mehta S, Atar D, Suryapranata H, Jensen SE, Forster T, Fernandez-Ortiz A, Schoors D, Radke P, Belli G, Brennan D, Bell G, Krucoff M for the PROTECTION AMI Investigators. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI Randomized Controlled Trial. Eur Heart J. 2014;35:2516–2523. doi: 10.1093/eurheartj/ehu177. [DOI] [PubMed] [Google Scholar]
- 46.Fryer RM, Wang Y, Hsu AK, Gross GJ. Essential activation of PKC-δ in opioid-initiated cardioprotection. Am J Physiol Heart Circ Physiol. 2001;280:H1346–H1353. doi: 10.1152/ajpheart.2001.280.3.H1346. [DOI] [PubMed] [Google Scholar]
- 47.Mayr M, Chung YL, Mayr U, McGregor E, Troy H, Baier G, Leitges M, Dunn MJ, Griffiths JR, Xu Q. Loss of PKC-delta alters cardiac metabolism. Am J Physiol Heart Circ Physiol. 2004;287(2):H937–45. doi: 10.1152/ajpheart.00877.2003. [DOI] [PubMed] [Google Scholar]
- 48.Mayr M, Metzler B, Chung YL, McGregor E, Mayr U, Troy H, Hu Y, Leitges M, Pachinger O, Griffiths JR, Dunn MJ, Xu Q. Ischemic preconditioning exaggerates cardiac damage in PKC-delta null mice. Am J Physiol Heart Circ Physiol. 2004;287(2):H946–56. doi: 10.1152/ajpheart.00878.2003. [DOI] [PubMed] [Google Scholar]
- 49.Lazar HL. Enhanced preservation of acutely ischemic myocardium and improved clinical outcomes using glucose-insulin-potassium (GIK) solutions. Am J Cardiol. 1997;80:90A–93A. doi: 10.1016/s0002-9149(97)00462-1. [DOI] [PubMed] [Google Scholar]
- 50.Stanley WC, Sabbah HN. Metabolic therapy for ischemic heart disease: The rationale for inhibition of fatty acid oxidation. Heart Fail Rev. 2005;10:275–279. doi: 10.1007/s10741-005-7542-4. [DOI] [PubMed] [Google Scholar]
- 51.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation-a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Lopaschuk GD. Optimizing cardiac energy metabolism: How can fatty acid and carbohydrate metabolism be manipulated? Coron Artery Dis. 2001;12:S8–S11. [PubMed] [Google Scholar]
- 53.Belardinelli R, Purcaro A. Effects of trimetazidine on the contractile response of chronically dysfunctional myocardium to low-dose dobutamine in ischaemic cardiomyopathy. Eur Heart J. 2001;22:2164–2170. doi: 10.1053/euhj.2001.2653. [DOI] [PubMed] [Google Scholar]
- 54.Patterson B, Fields AV, Shannon RP. New insights into myocardial glucose metabolism: surviving under stress. Curr Opin Clin Nutr Metab Care. 2009;12:424–430. doi: 10.1097/MCO.0b013e32832c4167. [DOI] [PubMed] [Google Scholar]
- 55.Bugge E, Ytrehus K. Ischemic preconditioning is protein kinase C dependent but not through stimulation of alpha adrenergic or adenosine receptors in the isolated heart. Cardiovasc Res. 1995;29:401–406. [PubMed] [Google Scholar]
- 56.Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A. Preconditioning of isolated rat heart is mediated by protein Kinase C. Circ Res. 1995;76:75–81. doi: 10.1161/01.res.76.1.73. [DOI] [PubMed] [Google Scholar]
- 57.Weinbrenner C, Nelles M, Herzog N, Sarvary L, Strasser RH. Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: A newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res. 2002;55:590–601. doi: 10.1016/s0008-6363(02)00446-7. [DOI] [PubMed] [Google Scholar]
- 58.Koneru S, Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Han Z, Maulik G, Das DK, Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–H2072. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 59.Lekli I, Szabo G, Juhasz B, Das S, Das M, Varga E, Szendrei L, Gesztelyi R, Varadi J, Bak I, Das DK, Tosaki A. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol. 2008;294:H859–H866. doi: 10.1152/ajpheart.01048.2007. [DOI] [PubMed] [Google Scholar]
- 60.Suranadi IW, Demaison L, Chaté V, Peltier S, Richardson M, Leverve X. An increase in the redox state during reperfusion contributes to the cardioprotective effect of GIK solution. J Appl Physiol. 2012;113:775–784. doi: 10.1152/japplphysiol.01153.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bialik S, Cryns VL, Drincic A, Miyata S, Wollowick AL, Srinivasan A, Kitsis RN. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ Res. 1999;85:403–414. doi: 10.1161/01.res.85.5.403. [DOI] [PubMed] [Google Scholar]
- 63.Moley KH, Mueckler MM. Glucose transport and apoptosis. Apoptosis. 2000;5:99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- 64.Tatsumi T, Shiraishi J, Keira N, Akashi K, Mano A, Yamanaka S, Matoba S, Fushiki S, Fliss H, Nakagawa M. Intracellular ATP is required for mitochondrial apoptotic pathways in isolated hypoxic rat cardiac myocytes. Cardiovasc Res. 2003;59:428–440. doi: 10.1016/s0008-6363(03)00391-2. [DOI] [PubMed] [Google Scholar]
- 65.Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. α- and β-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999;100:305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- 66.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., II Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ventura-Clapier R, Garnier A, Veksler V, Joubert F. Bioenergetics of the failing heart. Biochim Biophys Acta. 2011;1813:1360–1372. doi: 10.1016/j.bbamcr.2010.09.006. [DOI] [PubMed] [Google Scholar]