Abstract
Background
Disordered swallowing, or dysphagia, is almost always present to some degree in people with Parkinson’s disease (PD), either causing aspiration or greatly increasing the risk for aspiration during swallowing. This likely contributes to aspiration pneumonia, a leading cause of death in this patient population. Effective airway protection is dependent upon multiple behaviors, including cough and swallowing. Single voluntary cough function is disordered in people with PD and dysphagia. However, the appropriate response to aspirate material is more than one cough, or sequential cough. The goal of this study was to examine voluntary sequential coughing in people with PD, with and without dysphagia.
Methods
Forty adults diagnosed with idiopathic PD produced two trials of sequential voluntary cough. The cough airflows were obtained using pneumotachograph and facemask and subsequently digitized and recorded. All participants received a modified barium swallow study as part of their clinical care, and the worst penetration–aspiration score observed was used to determine whether the patient had dysphagia.
Results
There were significant differences in the compression phase duration, peak expiratory flow rates, and amount of air expired of the sequential cough produced by participants with and without dysphagia.
Conclusions
The presence of dysphagia in people with PD is associated with disordered cough function. Sequential cough, which is important in removing aspirate material from large- and smaller-diameter airways, is also impaired in people with PD and dysphagia compared with those without dysphagia. There may be common neuroanatomical substrates for cough and swallowing impairment in PD leading to the co-occurrence of these dysfunctions.
Keywords: Parkinson’s disease, Airway protection, Voluntary cough, Swallowing, Aspiration
Introduction
The ability to effectively protect the airway is dependent upon multiple behaviors, including both coughing and swallowing. Swallowing and coughing are sensorimotor behaviors that prevent material from entering the lower airway or eject material from the lower airway, respectively. Both airway-protective mechanisms can become disordered; however, the traditional focus of clinical evaluation and treatment has been swallowing (dysphagia). Research completed in the past decade provides strong evidence that cough function is degraded with neurologic and neurodegenerative diseases and that disordered cough is likely to co-occur with disordered swallowing [1-3].
The organization and effectiveness of a single voluntary cough can provide insight into swallowing dysfunction. Smith-Hammond and colleagues [1] measured cough airflow in stroke patients and found significant reductions in cough expiratory airflow; this finding corresponded to the severity of dysphagia. People who were categorized as “severe” aspirators had the lowest peak expiratory airflows during a single voluntary cough. Similar findings were reported for patients with Parkinson’s disease (PD). Pitts et al. [2] observed that people with PD who exhibit aspiration during a fluoroscopic swallowing evaluation have significantly reduced peak expiratory flow rates (PEFR) and increased time to achieve peak expiratory flow during single voluntary cough when compared to people with PD who do not aspirate.
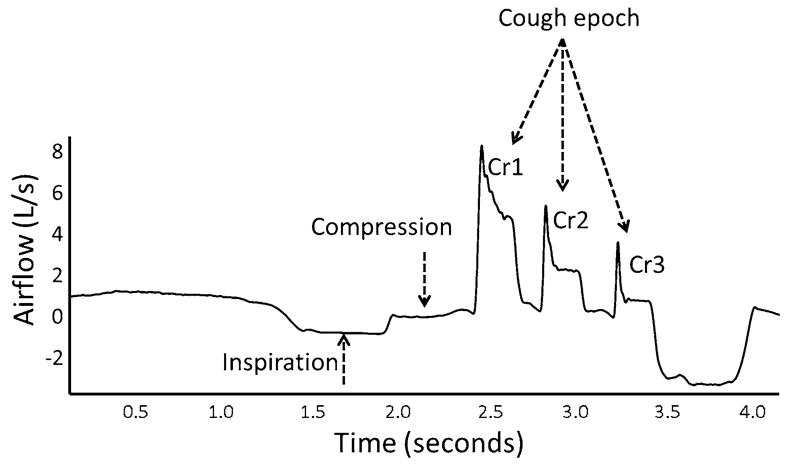
Cough, which is a reflexive mechanism that protects the airway from aspirate material, typically results in more than a single event. In fact, most reflex cough testing protocols call for at least two coughs to determine a cough reflex threshold [4]. Physiologically, this observation may be related to the ability of the cough to clear material from the lower airways. The dynamic compression that occurs during cough, which accelerates and aerosolizes aspirate particulates, typically begins in the larger airway passages (trachea, main stem bronchi) and moves distally as lung volume decreases [5]. Thus, theoretically, in order to clear material from the smaller airways, cough must be produced at progressively lower and lower lung volumes. The clearing of particulate material is accomplished by producing cough epochs that consist of multiple cough reaccelerations. A cough epoch is defined as a single inhalation followed by multiple cough reaccelerations (Fig. 1) [6, 7]. The lung volume at the initiation of each successive cough reacceleration in the epoch decreases [8].
Fig. 1.
Sequential cough airflow waveform and the cough epoch. Cr cough reacceleration
Harris and Lawson [9] described the organization of sequential voluntary cough epochs in healthy adults. Across a 3-cough epoch, slightly over 50 % of the total air expired occurred during the first cough, followed by 28 and 19 % for the second and third cough, respectively. Hegland et al. [6] observed that equivalent expired air distributions across reflexive cough epochs produced by healthy young adults occurred in response to capsaicin. There was also a concomitant decrease in peak expiratory airflow from the first to the second and finally even a greater reduction with the third cough.
PD affects the coordination, strength, and precision of movement, and there are measurable deficits in swallowing and single voluntary cough function [2, 10, 11]. The goal of this study was to identify whether similar deficits exist in sequential voluntary cough function. We hypothesized that PD participants with penetration or aspiration of material to the airway (PD-PA) would have significantly reduced voluntary sequential cough airflow parameters and changes to the distribution of cough-expired airflow compared to a group of PD participants who did not have penetration or aspiration of material into the airway (PD-noPA).
Methods
Forty patients provided informed consent and were enrolled in an Institutional Review Board (IRB)-approved DBS database (INFORM-PD). For all participants, the diagnosis of idiopathic PD was determined by a University of Florida Neurologist fellow trained in movement disorders using UK Brain Bank Criteria [12]. Participant demographic information is included in Table 1. Participants were excluded from analysis if they had a history of other neurologic diseases, deep brain stimulation surgery, chronic respiratory disease, head or neck cancer, or dysphagia stemming from another disorder other than PD. All participants were able to follow the instructions for both the cough and swallow evaluations.
Table 1.
Study participants’ demographic information
| Participant | Sex | Age (years) | H&Y score | PAS | Years since onset |
|---|---|---|---|---|---|
| PD-noPA | |||||
| 1 | F | 62 | 2 | 1 | 6 |
| 2 | M | 67 | 2 | 2 | 6 |
| 3 | M | 68 | 2 | 1 | 3 |
| 4 | M | 62 | 2 | 1 | 4 |
| 5 | M | 71 | 2 | 1 | 4 |
| 6 | F | 69 | 3 | 1 | 4 |
| 7 | M | 70 | 2 | 1 | 1 |
| 8 | M | 63 | 2 | 1 | 7 |
| 9 | F | 61 | 3 | 1 | 4 |
| 10 | M | 67 | 2 | 1 | 1 |
| 11 | F | 63 | 4 | 1 | 13 |
| 12 | F | 60 | 3 | 2 | 6 |
| 13 | M | 58 | 2 | 1 | 7 |
| 14 | M | 69 | 1.5 | 1 | 3 |
| 15 | M | 77 | 2 | 2 | 2 |
| 16 | F | 56 | 3 | 1 | 8 |
| 17 | M | 63 | 2.5 | 2 | 12 |
| 18 | M | 55 | 2 | 2 | 3 |
| Total | 6 F; 12 M | 64.5 (mean) | 2 (median) | 1 (median) | 5.22 (mean) |
| PD-PA | |||||
| 19 | M | 79 | 3 | 4 | 12 |
| 20 | F | 76 | 3 | 5 | 17 |
| 21 | M | 60 | 4 | 5 | 12 |
| 22 | F | 81 | 2.5 | 8 | 6 |
| 23 | M | 81 | 2.5 | 8 | 12 |
| 24 | M | 87 | 3 | 6 | 11 |
| 25 | M | 68 | 2 | 7 | 10 |
| 26 | M | 78 | 4 | 4 | 1 |
| 27 | F | 59 | 3 | 5 | 6 |
| 28 | F | 65 | 2.5 | 5 | 4 |
| 29 | M | 71 | 2 | 5 | 8 |
| 30 | M | 64 | 2 | 7 | 3 |
| 31 | F | 80 | 2 | 5 | 5 |
| 32 | M | 66 | 1.5 | 5 | 3 |
| 33 | M | 80 | 2 | 5 | 5 |
| 34 | M | 84 | 3 | 8 | 3 |
| 35 | M | 82 | 4 | 5 | 3 |
| 36 | M | 76 | 2 | 5 | 6 |
| 37 | M | 62 | 2.5 | 8 | 12 |
| 39 | F | 64 | 2.5 | 3 | 10 |
| 39 | M | 64 | 2.5 | 3 | 4 |
| 40 | F | 74 | 2 | 3 | 6 |
| Total | 7 F; 15 M | 72.7 (mean) | 2.5 (median) | 5 (median) | 7.22 (mean) |
Equipment
Voluntary sequential cough airflow data were recorded via face mask in line with an antibacterial filter attached to a pneumotachograph (MLT 1000; ADInstruments, Dunedin, New Zealand). The pneumotachograph input differential pressure change to the digital spirometer (ADInstruments), where it was then digitized (PowerLab, ADInstruments) and recorded (LabChart 7, ADInstruments) for analysis. A 3-L syringe was used to calibrate airflow and volume.
The videofluoroscopic swallowing evaluation was completed in the lateral view using our standard clinical protocol consisting of multiple bolus consistencies and volumes. Bolus types included thin liquid, pudding-thick, and a cookie coated with pudding-thick barium. All consistencies were 40 % w/v ratio of barium sulfate concentration (Varibar®, E-Z-EM, Inc., Melville, NY, USA).
Procedures
Evaluation of voluntary sequential cough function was completed in our clinical research space. Participants were seated upright with a face mask placed securely over the nose and mouth, with careful attention paid to ensure a complete seal of the mask to prevent air leaks. Participants completed 30 s of tidal breathing in order to become comfortable with the mask in place. They were then given the instruction to “cough as if something went down the wrong pipe.” In our clinical experience, this instruction elicits multiple sequential coughs. In cases where multiple coughs were not produced, the behavior was modeled by the clinician. Two trials of the sequential cough task were produced by each participant. Participants rested 10–30 s between the two trials. We chose to have the participants perform two trials based on time restraints related to other speech and swallow testing that were completed during the same clinical visit.
Modified barium swallowing evaluations were completed as part of our comprehensive clinical protocol by a clinically certified speech-language pathologist with expertise in the evaluation of patients with movement disorders. Participants were seated upright and positioned in the lateral viewing plane using a properly collimated Siemens radiographic/fluoroscopic unit. The images were recorded at 30 frames per second. Our standard protocol for bolus delivery is two small thin-liquid boluses by spoon (~5 mL; clinician-delivered), one large cup sip (~20 mL; self-delivered), two sips in a row by cup (self-delivered), pudding-thick bolus by spoon (clinician-delivered), and cookie coated with barium (clinician-delivered).
Data Analysis
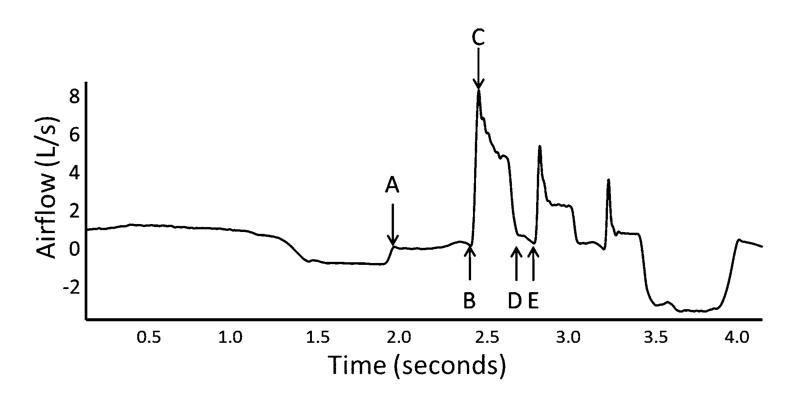
The airflow data were measured by research assistants who were blinded to the participant’s PA-group status. From the voluntary sequential cough waveform, a cough (Cr1) was defined as an inspiration followed by expiratory effort against a closed glottis (compression phase) and high expiratory airflow. Cough reaccelerations (CrN) followed the initial cough (Cr1) and included the compression phase, glottal opening, and high expiratory airflow, but were not preceded by an inspiration. A cough epoch was defined as a Cr1 and all subsequent CrN associated with the same inspiration (Fig. 1). The first three cough reaccelerations (Cr1, Cr2, and Cr3) produced for the two trials were selected for further analysis. The following parameters were measured from each of the airflow waveforms and are illustrated in Fig. 2:
Compression phase duration (CPD; seconds [s])
Peak expiratory flow rate (PEFR; L/s)
Cough volume acceleration (CVA; PEFR/peak expiratory flow rise time; L/s/s)
% of cough-expired airflow volume (%CEV; the percent of total expired in the epoch for each Cr)
Fig. 2.
Variables measured from the sequential cough airflow wave-form. A–B, compression phase duration (CPD; seconds); C, peak expiratory flow rate (PEFR; L/s); C/(B–C duration; seconds), cough volume acceleration (CVA; L/s/s); B–E, cough-expired air for that cough reacceleration (liters of air, expressed as a percent of total air expired for all reaccelerations in the epoch); D–E, subsequent CPD. Each variable then repeats for the subsequent cough reaccelerations in the epoch
Group membership (PD-PA and PD-noPA) was determined based on the worst penetration-aspiration score [13] observed by the evaluating clinician during the swallow study. We identified a PA score of 3 or worse as “PD-PA” and 1 or 2 as “PD-noPA.”
Statistical Analysis
Descriptive statistics were used to summarize the data. A two-way multivariate analysis of variance (MANOVA) was used to test the hypothesis that significant differences would exist for CPD, PEFR, CVA, and %CEV between the PD-PA and PD-noPA groups, according to the sequential cough number (CrNum) in the epoch.
Results
Participants in the PD-noPA group were about 8 years younger than those in the PD-PA group (65.4 and 72.7 years, respectively), and had PD for about 2 years less than the PD-PA group (Table 1). All participants produced more than one cough when given the instructions to “cough as if something has gone down the wrong pipe,” with a range of 3–8 coughs in the PD-noPA group (median = 4), and 2–9 in the PD-PA group (median = 4). Means and standard error for the dependent variables according to group (PD-PA/PD-noPA) and CrNum are given in Table 2.
Table 2.
Summary of dependent variables according to group and cough number
| Group | Cough No. |
CPD (s) |
PEFR (L/ s) |
CVA (L/s/ s) |
%CEV |
|---|---|---|---|---|---|
| PD- noPA |
1 | 0.45a | 5.51b | 102.42b | 49a |
| 2 | 0.08 | 3.96b | 93.18b | 24 | |
| 3 | 0.08 | 2.67 | 77.59 | 15a | |
| PD-PA | 1 | 0.22a | 4.19b | 80.72b | 42a |
| 2 | 0.09 | 3.26b | 81.10b | 25 | |
| 3 | 0.10 | 2.74 | 73.03 | 19a |
Indicates significant interaction effect
Indicates significant main effect for group
Results of multivariate testing revealed a significant Group × CrNum interaction for the overall model (F = 6.576, df = 10, 414, p < .001). Between subjects, tests showed significant interaction effects for CPD (F = 8.091, df = 2, p < .001), PEFR (F = 4.448, df = 2, p = .013), and %CEV (F = 3.430, df = 2, p = .034). There was no significant interaction effect on CVA; however, there was a significant main effect for Group (F = 6.540, df = 1, p = .011). In general, the PD-noPA group had higher PEFR and CVA values than the PD-noPA group, with the exception of those for Cr3, where the PEFR was equivalent for both groups. CPD was longest for the first cough (Cr1) for both groups and was longer for the PD-noPA group than for the PD-PA group for Cr1 only. The PD-noPA group had a higher %CEV for Cr1 than the PD-PA group but a lower %CEV in Cr3 than the PD-PA group. The %CEV for Cr2 was the same for both groups.
Discussion
This study examined voluntary sequential cough function in people with PD, with and without penetration or aspiration of material into the airway during a modified barium swallow evaluation. The results revealed significant differences in cough airflow measures, and overall cough organization, between the two groups (Table 2, Fig. 2). The findings provide strong support of our central hypothesis that multiple behaviors responsible for protecting the airway are simultaneously disordered with PD. In this cohort of PD participants, those with observed aspiration or penetration of bolus material to the airway exhibited changes to their cough function that affected not only the first cough in an epoch but in some cases the second and third as well.
Similar to swallowing, coughing is a sensorimotor behavior that requires precise coordination of upper airway structures, as well as coordination with the respiratory system [14-16]. Our a priori hypothesis was that people with both PD and observed penetration or aspiration would exhibit differences in their cough function compared with those who did not exhibit airway compromise. Multiple aspects of sequential cough airflow were significantly different between the two PD groups (Fig. 3), including CPD, PEFR, CVA, and percent cough-expired volume (%CEV). Each of these parameters is important to overall cough effectiveness. The compression phase relates to the ability to develop tracheal pressure necessary to quickly achieve the high peak expiratory airflow at the onset of the expiratory phase [16]. Cough-expired volume relates to the ability to sustain airflow in the post-peak portion of the cough, highly important for removal of material from the airway [9, 17]. Previous studies [6, 8] have shown strong relationships between these variables in sequential coughing that were not maintained in this cohort of persons with PD and penetration-aspiration.
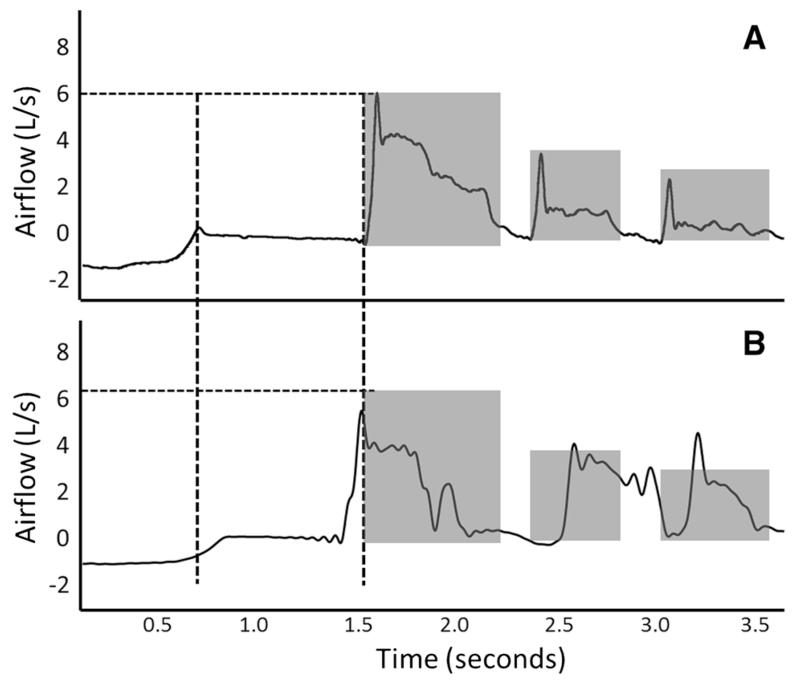
Fig. 3.
Illustration of sequential cough epoch organization between the PD-noPA (a) group and PD-PA (b) groups. a Dotted lines represent the compression phase duration (vertical) and peak expiratory flow rate (horizontal) of the first cough in the epoch (horizontal). Shaded areas represent cough-expired volume for each cough reacceleration. b The compression phase duration, peak expiratory flow rate, and cough-expired volume for PD-noPA group superimposed on PD-PA group
In general, the CPD in the PD-PA group was 50 % that of the PD-no PA group, and the peak airflows for the PD-PA group was 1 L/s slower. Participants in the PD-noPA group had significantly more expired air in Cr1 and significantly less expired air in Cr3 compared to those in the PD-PA group, although the changes were relatively small (49 vs. 42 % and 15 vs. 19 % for coughs 1 and 3, respectively). Interestingly, CPD, PEFR, CVA, and %CEV measures in the PD-noPA group were similar to those previously reported for induced sequential cough in healthy young adults [6]. This indicates that multiple factors that determine overall cough effectiveness are affected in those with PD-PA, resulting in an uncoordinated sequential cough pattern (Fig. 3). In the presence of airway compromise during swallowing, the ability to remove aspirate material from the airway is imperative. Assuming the ability to detect that a stimulus is intact, as it seems to be at least for moderate-stage PD [3, 18], the motor response must generate the airflow and dynamic compression necessary to aerosolize and remove aspirate particulates from the airway if effective coughing is to be achieved. These results strongly suggest that in those with both PD and airway compromise during swallowing, the effectiveness of the cough motor response is reduced.
The cough in response to aspirate material is reflexive in nature, a consideration that must be addressed given that the current study included voluntary sequential coughs. In terms of the cough motor output, the measurable differences between the two tasks are thought to be minimal, although few studies have directly compared their airflow measures in a patient population. Lasserson et al. [19] showed differences in the recruitment of expiratory and accessory muscle activation between voluntary and induced cough; however, the characteristic three-phase organization of coughing was maintained despite these differences. A recent study compared the peak expiratory airflow generated for voluntary and induced cough in patients who had suffered a traumatic brain injury. Results revealed slightly higher voluntary cough flow rates than that for induced cough, with a strong positive correlation between the two measures [20]. Ward et al. [21] studied voluntary and induced cough in stroke patients and found reduced PEFR, CVA, and CPD values in both types of cough compared with those in age-matched control participants. As in the TBI study, the voluntary cough peak flow was slightly higher than the induced cough peak flow [21]. These previous findings suggest that the current data may underestimate the degree to which reflexive cough airflows are reduced in people with PD-PA. Empirical testing is underway in our laboratory to directly assess this question.
Of particular interest is how the presence of penetration-aspiration relates to these changes. PD is known to affect the respiratory system [22], and because cough is an overlaid respiratory function, it is not surprising to find changes to the coordination of a sequential cough task in this cohort of PD participants. Recent reports by Pitts et al. [23] and Bolser et al. [24] assert that cough and swallowing central pattern generators are intricately connected within brainstem centers. Davenport et al. [25] hypothesized that brainstem respiratory centers are actually reconfigured to produce cough and swallow behaviors. This emerging evidence suggests common pathologic pathways that underlie degeneration of both behaviors at the level of the brainstem. It may be that additional supramedullary neural structures participating in the generation of each behavior, for example, the anterior cingulate, insular cortex, and primary and premotor areas [26, 27], also mediate these concomitant changes in function. These are important questions that should be addressed in future studies.
Study Limitations
Risk of airway compromise in this study was determined based on penetration-aspiration [13]. It was felt that this functional metric of airway protection was fitting for this particular population given that aspiration pneumonia is the leading cause of death in PD. It is entirely possible for a patient to be dysphagic without penetration or aspiration observed on instrumental examination. However, for this particular population where swallowing changes are known to occur with disease progression, the PA scale allows for quantification of the functional consequences of physiologic changes to the swallowing mechanism. Therefore, even in the PD-noPA group, in most cases the swallow would not be termed “normal”; instead, the physiologic changes occurring are not yet leading to the entrance of bolus material into the airway. Certainly, a more in-depth analysis of the specific physiologic and bolus flow characteristics, such as that obtained by using the MBSImp® (Modified Barium Swallow Impairment Profile; Northern Speech Services, Gaylord, MI, USA), would potentially yield more specific results.
In this study all participants were recruited clinically and had PD. While they arrive at our clinic “on” medication, we did not control the precise timing for this study. Also, it is important to study these parameters in a cohort of age-matched control participants in order to better understand which changes are part of healthy aging and which are specifically related to the presence of PD. Lastly, information on history of pneumonia should be included in future studies, as Yamanda et al. [28] have identified differences in urge-to-cough sensation in persons with a history of pneumonia. It remains unclear whether similar differences would exist for voluntary cough airflows according to pneumonia history.
Conclusions and Future Directions
This study adds to a growing body of literature indicating that multiple motor aspects of cough function are affected in people who have both PD and airway compromise during swallow. There are likely several contributing factors that are interrelated, indicating several potential intervention approaches for improving cough function. A recent study by Ruddy et al. [29] reported an increased CPD and PEFR in three patients with glottal insufficiency who underwent Radiesse™ Voice injections (Merz Aesthetics, San Mateo, CA, USA). Pitts et al. [23] found that expiratory muscle strength training (EMST) improved maximum expiratory pressure and cough peak expiratory airflow in ten patients with PD. Inzelberg et al. [30] found increased inspiratory strength (measured as inspiratory pressure generated at the mouth) and endurance in a cohort of 20 participants with mid- to moderate-stage PD following inspiratory muscle training. These researchers did not measure cough function; however, these changes theoretically could lead to changes in lung volume at cough initiation, with subsequent effects on cough airflow measures. Given the coordinated nature of sequential cough epochs and the importance of sequential cough for clearing the lower airways, it is likely that any treatment for cough would require some focus on producing more than one cough at a time in a coordinated fashion. That is, the strength and coordination of coughs should be addressed along with task-specific behavioral training. Work in our laboratory is ongoing to develop multimodal treatments that improve not only strength but also coordination of cough function. We continue to work to identify underlying mechanisms of disordered cough in PD in order to understand the interrelationships between central control, laryngeal, and respiratory subsystems in the development of cough disorders, and ultimately aspiration pneumonia which is a leading cause of death in PD.
Acknowledgments
Dr. Hegland’s work is supported in part by the American Heart Association and BAE defense systems. Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >36 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME activities on movement disorders in the last 36 months sponsored by PeerView, Prime, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Dr. Troche’s work is supported in part by an NIH (NCATS) CTSA through the University of Florida (UL1TR000064 and KL2TR000065).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Karen Wheeler Hegland, Department of Speech, Language and Hearing Sciences, University of Florida, 336 Dauer Hall, Gainesville, FL 32611, USA; Center for Movement Disorders and Neurorestoration, University of Florida, Gainesville, FL, USA.
Michael S. Okun, Center for Movement Disorders and Neurorestoration, University of Florida, Gainesville, FL, USA; Department of Neurology, University of Florida, Gainesville, FL, USA
Michelle S. Troche, Department of Speech, Language and Hearing Sciences, University of Florida, 336 Dauer Hall, Gainesville, FL 32611, USA; Center for Movement Disorders and Neurorestoration, University of Florida, Gainesville, FL, USA
References
- 1.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 2.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23:297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, Sasaki H. Impaired efficacy of cough in patients with Parkinson disease. Chest. 2003;124:1009–1015. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 4.Dicpinigaitis PV, Alva RV. Safety of capsaicin cough challenge testing. Chest. 2005;128(1):196–202. doi: 10.1378/chest.128.1.196. [DOI] [PubMed] [Google Scholar]
- 5.Knudson RJ, Mead J, Knudson DE. Contribution of airway collapse to supramaximal expiratory flows. J Appl Physiol. 1974;36(6):653–667. doi: 10.1152/jappl.1974.36.6.653. [DOI] [PubMed] [Google Scholar]
- 6.Hegland KW, Troche MS, Davenport PW. Cough expired volume and airflow rates during sequential induced cough. Front Physiol. 2013;4:167. doi: 10.3389/fphys.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vovk A, Bolser DC, Hey JA, Danzig M, Vickroy T, Berry R, Martin AD, Davenport PW. Capsaicin exposure elicits complex airway defensive motor patterns in normal humans in a concentration-dependent manner. Pulm Pharmacol Ther. 2007;20:423–432. doi: 10.1016/j.pupt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JA, Aliverti A, Quaranta M, McGuinness K, Kelsall A, Earis J, Calverley PM. Chest wall dynamics during voluntary and induced cough in healthy volunteers. J Physiol. 2012;590(Pt 3):563–574. doi: 10.1113/jphysiol.2011.213157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris RS, Lawson TV. The relative mechanical effectiveness and efficiency of successive voluntary coughs in healthy young adults. Clin Sci. 1968;34(3):569–577. [PubMed] [Google Scholar]
- 10.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18(3):231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 11.Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Curr Opin Otolaryngol Head Neck Surg. 2008;16(3):205–210. doi: 10.1097/MOO.0b013e3282febd3a. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 14.Gross RD, Steinhauer KM, Zajac DJ, Weissler MC. Direct measurement of subglottic air pressure while swallowing. Laryngoscope. 2006;116:753–761. doi: 10.1097/01.mlg.0000205168.39446.12. [DOI] [PubMed] [Google Scholar]
- 15.Macklem PT. Physiology of cough. Ann Otolaryngol. 1974;83:761–768. [Google Scholar]
- 16.Von Leden, Isshiki N. An analysis of cough at the level of the larynx. Arch Otolaryngol. 1965;81:616–625. doi: 10.1001/archotol.1965.00750050631016. [DOI] [PubMed] [Google Scholar]
- 17.Mahajan RP, Singh P, Murty GE, Aitkenhead AR. Relationship between expired lung volume, peak flow rate and peak velocity time during a voluntary cough manoeuvre. Br J Anaesth. 1994;72(3):298–301. doi: 10.1093/bja/72.3.298. [DOI] [PubMed] [Google Scholar]
- 18.Leow LP, Beckert L, Anderson T, Huckabee ML. Changes in chemosensitivity and mechanosensitivity in aging and Parkinson’s disease. Dysphagia. 2011;27(1):106–114. doi: 10.1007/s00455-011-9347-z. [DOI] [PubMed] [Google Scholar]
- 19.Lasserson D, Mills K, Arunachalam R, Polkey M, Moxham J, Kalra L. Differences in motor activation of voluntary and reflex cough in humans. Thorax. 2006;61(8):699–705. doi: 10.1136/thx.2005.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SC, Kang SW, Kim MT, Kim YK, Chang WH, Im SH. Correlation between voluntary cough and laryngeal cough reflex flows in patients with traumatic brain injury. Arch Phys Med Rehabil. 2013;94(8):1580–1583. doi: 10.1016/j.apmr.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Ward K, Seymour J, Steier J, Jolley C, Polkey M, Kalra L, Moxham J. Acute ischaemic hemispheric stroke is associated with impairment of reflex in addition to voluntary cough. Eur Respir J. 2010;36(6):1383–1390. doi: 10.1183/09031936.00010510. [DOI] [PubMed] [Google Scholar]
- 22.Hovestadt A, Bogaard JM, Meerwaldt JD, van der Meche FG, Stigt J. Pulmonary function in Parkinson’s disease. J Neurol Neurosurg Psychiatr. 1989;52(3):329–333. doi: 10.1136/jnnp.52.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitts T, Rose MJ, Mortensen AN, Poliacek I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol. 2013;189(3):543–551. doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngol Clin North Am. 2013;46(6):957–964. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davenport PW, Bolser DC, Morris KF. Swallow remodeling of respiratory neural networks. Head Neck. 2011;33(Suppl 1):S8–S13. doi: 10.1002/hed.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31(8):2948–2958. doi: 10.1523/JNEUROSCI.4597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–3226. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamanda S, Ebihara S, Ebihara T, Yamasaki M, Asamura T, Asada M, Une K, Arai H. Impaired urge-to-cough in elderly patients with aspiration pneumonia. Cough. 2008;4:11. doi: 10.1186/1745-9974-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruddy BH, Pitts TE, Lehman J, Spector B, Lewis V, Sapienza CM. Improved voluntary cough immediately following office based vocal fold medialization injections. Laryngoscope. 2013 doi: 10.1002/lary.24529. doi:10.1002/lary.24529. [DOI] [PubMed] [Google Scholar]
- 30.Inzelberg R, Peleg N, Nisipeanu P, Magadle R, Carasso RL, Weiner P. Inspiratory muscle training and the perception of dyspnea in Parkinson’s disease. Can J Neurol Sci. 2005;32(2):213–217. doi: 10.1017/s0317167100003991. [DOI] [PubMed] [Google Scholar]