Abstract
Purpose
Up to 50% of patients diagnosed with stage I non-seminomatous germ cell tumors (NSGCT) harbor occult metastases. Patients are managed by surveillance with chemotherapy at relapse or adjuvant treatment up-front. Late toxicities from chemotherapy are increasingly recognised. Based on a potential biological role in germ cells/tumors and pilot data, our aim was to evaluate tumor expression of the chemokine CXCL12 alongside previously proposed markers as clinically useful biomarkers of relapse.
Experimental design
Immunohistochemistry for tumor expression of CXCL12 was assessed as a biomarker of relapse alongside vascular invasion, histology (percentage embryonal carcinoma) and MIB1 staining for proliferationin formalin fixed paraffin-embedded orchidectomy samples from patients enrolled in the Medical Research Council’s TE08/22 prospective trials of surveillance in stage I NSGCT.
Results
TE08/TE22 trial patients had a 76.4% 2-year relapse free rate (RFR) and both CXCL12 expression and percentage embryonal carcinoma provided prognostic value independently of vascular invasion (stratified log rank test p=0.006 for both). There was no additional prognostic value for MIB1 staining. A model using CXCL12, percentage embryonal carcinoma and VI defines 3 prognostic groups that were independantly validated.
Conclusions
CXCL12 and percentage embryonal carcinoma both stratify patients’ relapse risk over and above vascular invasion alone. This is anticipated to improve the stratification of patients and identify high-risk cases to be considered for adjuvant therapy.
Keywords: Testicular Germ Cell Tumors, Stage I Non-Seminomas, CXCL12, Adjuvant Chemotherapy, Surveillance
Introduction
Although overall rare cancers, Testicular Germ Cell Tumors (TGCT) are the most common solid malignancy to affect young adult Caucasian males. They are divided into seminomas that resemble primordial germ cells or non-seminomas (NSGCT) that exhibit embryonal or extra-embryonal patterns of differentiation (1). 60% of NSGCT present with stage I disease (confined to the testes) and this proportion is increasing (2). 15-50% of such patients may harbor micro-metastatic disease and will relapse without further treatment (3, 4).
Assuming protocols are closely adhered to (5), immediate adjuvant treatment (1-2 cycles of Bleomycin, Etoposide and Cisplatin – BEP (6)) or surveillance with chemotherapy as salvage both have excellent rates of cure. Chemotherapy may have significant long-term effects including cardiovascular disease (7, 8), second malignancies (9, 10), Reynauds syndrome, neuropathies, fertility and emotional disorders. For this reason the routine use of adjuvant chemotherapy has been criticised in some quarters. Adjuvant retroperitoneal lymph node dissection is an alternative adjuvant strategy but with associated potential complications and morbidities these patients would also benefit from improved risk-stratification (11-13).
Histological evidence of vascular invasion (VI) is the only validated histological prognostic factor currently used to define risk of relapse in clinical stage I NSGCT (3, 4), not-with-standing the distinct role that plasma tumor markers play in assessing disease state. Tumors with VI have relapse rates of up to 50% and patients may be offered adjuvant chemotherapy reducing subsequent relapses to approximately 2% (6). In the absence of VI, around 15% patients relapse and surveillance may be a more reasonable option. More accurate stratification of patients for likelihood of relapse would improve patient management, decreasing the risk of unneccessary treatment with associated side-effects and reducing risks and costs associated with over-treatment and excess imaging (14).
A systematic review (4) identified the percentage of Embryonal Carcinoma (%EC) within the primary tumor and the proliferation marker MIB1 as promising markers for relapse. Both are continuous variables and studies used differing cut-offs with variable levels of risk prediction. Univariate odds ratios for relapse range from 2.8 to 9 (4, 15-18). However %EC and VI were correlated and in multivariate analysis the prognostic effect of %EC diminished (4). In a subsequent study, 77% of metastasizing tumors showed MIB1 staining in >70% cells, equivalent to an odds ratio of 3.18 (95% CI: 1.51, 6.65). However, these data derive from a series of 195 patients after retroperitoneal lymph node dissections and not strictly a surveillance population (19).
Considering novel molecular markers, TGCT resemble primordial germ cells (PGC) (20) that physiologically utilize KITLG/KIT and CXCL12/CXCR4 for migration and survival during embryological development (21-23) and in the maintenance of the spermatogonial stem cell niche (24). Both signalling pathways are implicated in the malignant counterpart; KIT is strongly implicated in testicular tumorigenesis with both discrete amplification and activating mutations described (20) and TGCT express the receptor CXCR4 that can mediate invasive migration towards its ligand, CXCL12 (25, 26). Importantly, NSGCTs can express CXCL12 in an autocrine fashion and in a pilot study containing a high proportion of Embryonal Carcinoma cases (n=80) of TGCT this was associated with reduced risk of relapse (25). Here we investigate this feature further for clinical utility alongside VI, %EC and MIB1 staining and in addition to other clinicopathological characteristics.
Two recent clinical trials conducted by the Medical Research Council (MRC) investigated surveillance strategies in clinical stage I NSGCT and provide a unique large cohort of well-characterized stage I NSGCT patients managed on prospective protocols. TE08 (NCT00003420) (27) compared two frequencies of CT scanning during surveillance of stage I NSGCT. TE22 (NCT00045045) (28) investigated the ability of a baseline FDG PET scan to distinguish patients at lower risk of relapse who might safely be managed by surveillance. In total 501 patients were managed by surveillance, with a relapse rate of 21% (103 patients). 130/501 (26%) had VI, although in an unselected population this would be closer to 50%.
To refine treatment stratification in patients diagnosed with stage I NSGCT we set out to investigate and validate CXCL12, %EC and MIB1 as biomarkers prognostic for relapse. Additional evidence of a prognostic effect for CXCL12 over and above the previous pilot data (25) was first sought using a tissue microarray (TMA) comprising representative cores from 59 stage I NSGCT patients managed with surveillance. We then investigated the markers MIB1, CXCL12 and %EC in the samples from stage I NSGCT patients managed by surveillance in the MRC TE08/TE22 clinical trials. Finally, we validated a combined prognostic model using VI, CXCL12 and %EC in the previous cohort of samples (25).
Materials and Methods
Patients and tumor samples
This study has national research ethics committee approval (09/MRE00/30) and complies with the REMARK guidelines for biomarker studies (29). Samples from stage I NSGCT patients managed by surveillance were collected from the Medical Research Council (MRC) trials TE08 (27, 28). Specifically these were patients diagnosed with stage I NSGCT (negative tumour markers and CT scan confirming stage I) and enrolled post-operatively into a randomised study of two alternate imaging surveillance protocols (TE08) or in the case of TE22, undergoing FDG-PET imaging followed by surveillance if negative, to assess the negative predictive value of this scan. Formalin fixed paraffin embedded (FFPE) tumor blocks were available for 200/501 (40%) cases; 139 from TE08 and 61 from TE22.10 of the 61 TE22 patients were not eligible for this study as 7 were PET positive and received adjuvant chemotherapy and 1 was PET negative but received adjuvant chemotherapy at the patient’s request. 2 cases were lost to follow up. The final trial samples consisted of material from 190 patients (Table 1). Importantly, this cohort was representative of the overall trial sample set with a relapse-free rate of ~78% at 2 years after orchidectomy (Supplementary Material Figure S1). Complete tumor cases were retrieved from each patient, and a full set of haematoxylin and eosin (H&E) sections from tumor for each case were examined by a board certified histopathologist. Representative tumour material, to include all significant areas of pathology, was selected from each case. VI was assessed as previously described (16). Additionally, sections from a TMA containing 0.6mm diameter cores were available from Princess Margaret Hospital, Toronto (JS) representing primary tumors from 59 patients with stage I NSGCT managed by surveillance and a minimum follow-up of 2 years (Supplementary Material Table S1). Finally, TMAs comprising material from 80 patients with stage I non-seminomatous germ cell tumors managed with surveillance at the Royal Marsden Hospital (RMH, previously described in (25)) were re-scored as per the below by a pathologist (DB) blinded to outcomes.
Table 1.
Histological subtype, % Embryonal Carcinoma, tumour marker status and immunohistochemistry for CXCL12 and MIB1 for 190 stage I NSGCT primary tumours from patients treated in the TE08/TE22 clinical trials. EC - embryonal carcinoma; CC - choriocarcinoma; AFP - alpha fetoprotein; HCG - human chorionic gonadotrophin.
| Vascular invasion | Total | ||||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| N | % | N | % | N | % | ||
| Histology | Pure EC | 23 | 19% | 18 | 27% | 41 | 22% |
| Mixed NSGCT | 79 | 65% | 39 | 57% | 118 | 62% | |
| Yolk sac | 8 | 7% | 3 | 4% | 11 | 6% | |
| Differentiated Teratoma | 9 | 7% | 1 | 2% | 10 | 5% | |
| Other type | 3 | 3% | 7 | 10% | 10 | 5% | |
| EC presence | EC absent | 29 | 26% | 9 | 14% | 38 | 22% |
| EC present | 83 | 74% | 56 | 86% | 139 | 79% | |
| Not known | 10 | 3 | 13 | ||||
| % EC Optimal categories | <=25% | 60 | 54% | 20 | 31% | 80 | 45% |
| 26-99% | 29 | 26% | 27 | 42% | 56 | 32% | |
| 100% | 23 | 21% | 18 | 28% | 41 | 23% | |
| Not known | 10 | 3 | 13 | ||||
| AFP pre-orchidectomy | normal | 53 | 43% | 25 | 37% | 78 | 41% |
| raised | 69 | 57% | 43 | 63% | 112 | 59% | |
| HCG pre-orchidectomy | normal | 65 | 53% | 29 | 43% | 94 | 50% |
| raised | 57 | 47% | 39 | 57% | 96 | 50% | |
| CXCL12 (≤1%=weak/absent) | Insufficient tumor | 7 | 6% | 1 | 2% | 8 | 4% |
| absent/weak | 26 | 21% | 11 | 16% | 37 | 20% | |
| moderate/strong | 89 | 73% | 56 | 82% | 145 | 76% | |
| MIB1 staining | Weak | 37 | 33% | 8 | 12% | 45 | 25% |
| High | 77 | 68% | 57 | 88% | 134 | 75% | |
| Not assessable | 8 | 3 | 11 | ||||
| Total | 122 | 64% | 68 | 36% | 190 | 100% | |
Sectioning, histology review and staining
Sections were stained with H&E and assessed to ensure adequate tumor material. Sections were deparaffinized prior to staining for CXCL12 (Antibody 79018, 1:100, R&D Systems, Minneapolis MN, USA), including positive (tonsillar crypt) and negative controls as previously described (25), and MIB1 (Antibody M7240, 1:100, Dako). Antibodies were visualized using the Bond Polymer Refine Kit (Leica Biosystems, Newcastle, UK). Immunostaining was performed on a Bond max automated immunostainer.
Scoring and categorization
H&E slides were scored for %EC (by DB) as a continuous variable, and then additionally grouped as described in previous studies or a new data-derived grouping for subsequent analysis. Immunostaining was scored by two independent histopathologists (KT and IC) recording intensity of staining as 0-3 (absent, weak, medium and high intensity) and % cells staining positive. Samples where scores differed were reviewed and a consensus obtained. Scores were categorized as absent/weak CXCL12 if <1% cells across the whole tumor stained positive for CXCL12. A second exploratory analysis was also performed classifying <10% cells staining for CXCL12 as CXCL12 absent/weak. Analysis for MIB1 was performed separately using both intensity and % cells positive using cut-offs described in the previous studies i.e. ≥70% and ≥40% (15-18) as well as additional exploratory analyses.
Statistical methods
The primary outcome measure was relapse-free rate, measured from the date of orchidectomy to the date of relapse confirmation, with relapse-free patients censored on the date last known to be alive. Relapse free rates on Kaplan Meier survival curves were compared by the logrank test, with an initial assessment of the independence of %EC, CXCL12 and MIB1 over VI determined by logrank tests stratified for VI. Subsequently, a proportional hazards regression model was fitted to adjust for baseline clinical variables, VI, %EC, MIB1 and CXCL12 staining, using forward and backwards stepwise selection. Chi squared tests were used to investigate the association of %EC and CXCL12/MIB1 staining with clinico-pathological variables.
Results
TMA CXCL12 expression and outcome
To investigate CXCL12 as a marker for relapse prior to application to the clinical trial sample sets, we first studied the TMA representing 59 cases. 25/59 cases (42.4%) demonstrated moderate/strong expression of CXCL12 cells of which 3 had relapsed (RFR 88.0%). Of the 34 patients with absent/weak staining for CXCL12, 11 had relapsed (RFR 67.6%, log rank test p=0.68). Although not reaching statistical significance, the rates of CXCL12 expression and subsequent relapse were consistent with previous data [25] and analysis of CXCL12 expression was taken forward to TE08/TE22 samples.
TE08/22 – CXCL12 and relapse
Samples representing 182/190 samples from patients in the TE08 and TE22 trials were assessable for CXCL12 staining (Table 1; Figure 1A,B,C,D). Using <1% as the cut off, 37 (20.3%) tumors were classified as absent/weakwith the other 145 (79.7%) moderate/strong (as scored by two pathologists, κ=0.465, p<0.001). In an exploratory analysis, a <10% cut off was also assessed and produced a similar performance (Supplementary Table S2 and Figure S2). Therefore either cut-off may be used. There was no association between CXCL12 and VI, the presence of seminomatous elements or raised markers pre-orchidectomy. There was however a strong association with the presence of Embryonal Carcinoma which was more prevalent in those with absent/weak staining, (75.7% vs 30.3% of those with moderate/high staining, chi-square p < 0.001). The logrank test shows evidence of a prognostic impact, alone and stratified by VI (p=0.006, Table 2) for CXCL12 with reduced relapse-free rate in the absent/weak group (Figure 2A). In VI+ve patients, CXCL12 further stratified relapse rates; 56 patients with VI but moderate/strong staining had a 2-year RFR of 62.4% versus 11 patients with VI and absent/weak staining for CXCL12 where a RFR of 27.3% was observed (95% CI 1-53.6%).
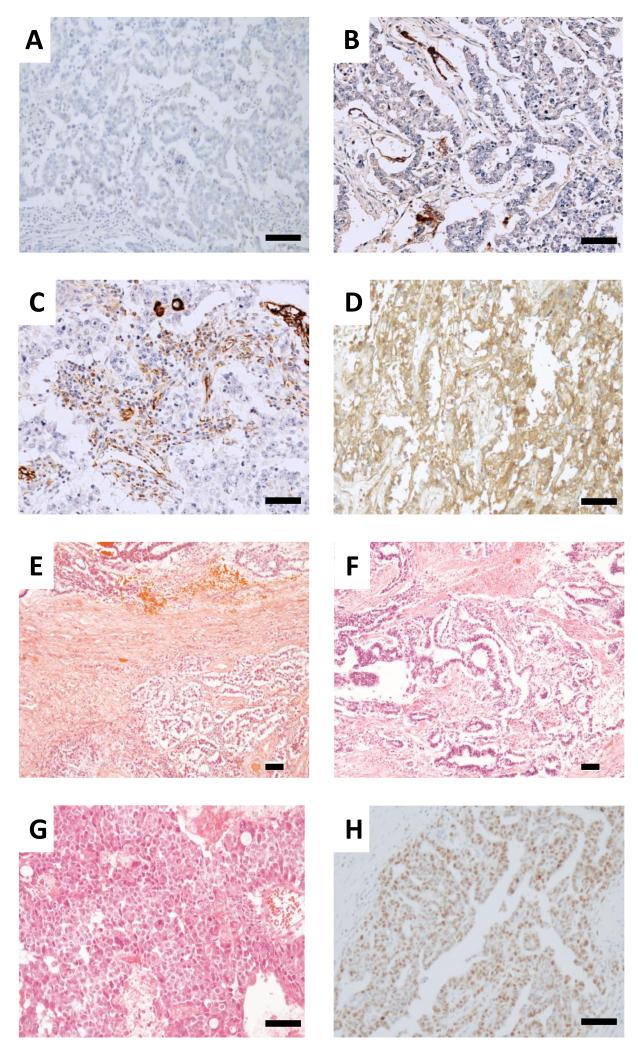
Figure 1. Representative staining of stage I non-seminomatous germ cell tumor samples.
Immunohistochemistry for CXCL12 staining A negative B <10% C ~30% and D 100% positive. E Haematoxylin and Eosin staining showing a combined seminomatous (bottom of photomicrograph) and non-seminomatous tumour (top) composed of less than 10% embryonal carcinoma. F Tumor composed of 25% embryonal carcinoma and 75% yolk sac tumor. The yolk sac and embryonal carcinoma are intermingled in a polyembryo matous fashion, mimicking the earliest stages of embryonic development. G Tumor entirely composed of embryonal carcinoma. H Embryonal carcinoma showing 75% positivity for MIB1 immunohistochemistry. (Scale bar, 100 microns).
Table 2.
Univariate and stratified logrank test results for factors of interest in 177 cases with complete data.
| No. Pts | 2 yr RFR |
95% CI | Log rank p-value |
Stratified (by VI) log rank p- value |
|
|---|---|---|---|---|---|
|
| |||||
| Vascular invasion | |||||
|
| |||||
| absent | 112 | 88.3 | (82.2, 94.4) | <0.001 | n/a |
| present | 65 | 58.3 | (46.0, 70.6) | ||
|
| |||||
| CXCL12 | |||||
|
| |||||
| mod/high, no VI | 87 | 90.7 | (84.6, 96.8) | 0.078 | 0.009 |
| mod/high, VI | 55 | 63.7 | (50.6, 76.8) | ||
|
| |||||
| Sub-total mod/high | 142 | 80.4 | (73.7, 87.1) | ||
|
| |||||
| absent /weak, no VI | 25 | 80.0 | (64.3, 95.7) | ||
| absent /weak, VI | 10 | 30.0 | (1.6, 58.4) | ||
|
| |||||
| Sub-total absent | 35 | 65.7 | (50.0, 81.4) | ||
|
| |||||
| MIB1 | |||||
|
| |||||
| Weak, no VI | 35 | 91.4 | (82.2, 99.9) | 0.007 | 0.045 |
| Weak, VI | 7 | 100.0 | (39.8, 99.9) | ||
|
| |||||
| Sub-total weak | 42 | 92.8 | (85.0, 99.9) | ||
|
| |||||
| High, no VI | 76 | 86.7 | (79.1, 94.3) | ||
| High, VI | 56 | 53.7 | (40.4, 67.0) | ||
|
| |||||
| Sub-total high | 132 | 73.0 | (65.4, 80.6) | ||
|
| |||||
| EC (Present/absent) | |||||
|
| |||||
| Absent, no VI | 29 | 96.3 | (89.2, 99.9) | 0.096 | 0.243 |
| Absent, VI | 9 | 66.7 | (35.9, 97.5) | ||
|
| |||||
| Sub-total absent | 38 | 89.2 | (79.2, 99.2) | ||
|
| |||||
| Present, no VI | 83 | 85.4 | (77.8, 93.0) | ||
| Present, VI | 56 | 56.8 | (43.5, 70.1) | ||
|
| |||||
| Sub-total present | 139 | 74.3 | (66.9, 81.7) | ||
|
| |||||
| EC (optimal categories) | |||||
|
| |||||
| ≤25%EC, no VI | 60 | 94.9 | (89.2, 99.9) | <0.001 | 0.006 |
| ≤25%EC, VI | 20 | 68.1 | (46.9, 89.3) | ||
|
| |||||
| Sub-total ≤25% | 80 | 88.4 | (81.3, 95.5) | ||
|
| |||||
| 26-99%EC, no VI | 29 | 93.1 | (83.9, 99.9) | ||
| 26-99%EC, VI | 27 | 57.3 | (38.1, 76.5) | ||
|
| |||||
| Sub-total 26-99% | 56 | 76.4 | (65.2, 87.6) | ||
|
| |||||
| 100% EC, no VI | 23 | 63.6 | (43.4, 83.8) | ||
| 100%EC, VI | 18 | 50.0 | (26.9, 73.1) | ||
|
| |||||
| Sub-total 100% | 41 | 57.5 | (42.2, 72.8) | ||
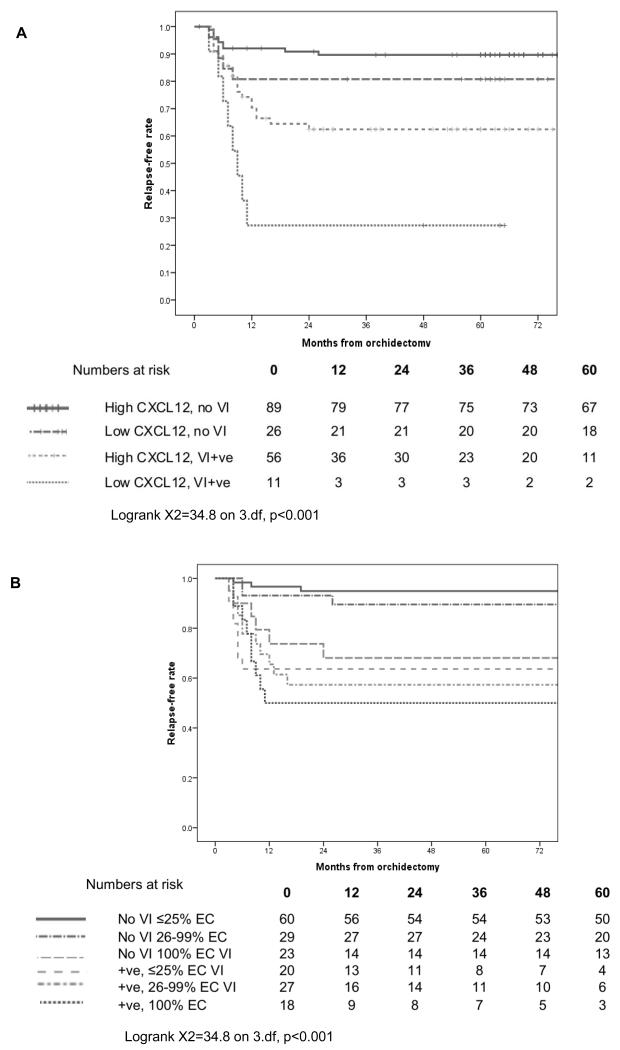
Figure 2. Relapse-free survival data for.
A182 patients with stage I NSGCT managed by surveillance with the MRC TE08 and TE22 clinical trials, stratified by vascular invasion (VI) and immunohistochemistry for CXCL12 in combination with the presence/absence of histological VI (stratified log rank test p=0.006). B 177 patients with stage I NSGCT managed with surveillance stratified by %EC and VI, illustrated using optimal cut-offs for %EC.
TE08/22 - %EC and relapse
177/190 patients were assessable for %EC (Table 1; Figure 1E,F,G). This showed a bimodal distribution, with clusters at 0% and 100% and a relatively even spread of the remaining values between these levels. %EC was significantly higher in patients with VI (median 70% vs 20%, Mann Whitney test p=0.013), and also in those with absent/weak CXCL12 intensity (medians 100% vs 20% for moderate/strong, p<0.001) and with presence of MIB1 staining (medians 50% vs 10%, p=0.012).
%EC was assessed as in previous reports as a continuous variable, a binary variable (presence/absence) and applying previously reported cut-offs, (<45% 46-70%, >70%; above or below 50%) (4). In addition, to better reflect the unusual distribution of %EC, a data-derived categorization was investigated, formed by dividing the data initially into approximate quintiles (0%, 1-25%, 26-75%, 76-99%, 100%), collapsing groups with similar relapse free rates to create an “optimal” categorisation (≤25%, 26-99%, 100%). With the exception of presence/absence of EC, higher %EC was associated with higher relapse rates (Figure 2B, Table 2), independent of VI, for all categorizations.
TE08/TE22 - MIB1 and relapse
179 cases were assessable for MIB1 staining; 45 (25.1%) were MIB1 weak on both intensity and proportion of cells staining (Table 1; Figure 1H). There was a significant association of both MIB1 intensity and proportion of cells staining for MIB1 with decreasing likelihood of the tumor containing seminomatous elements (Mann Whitney test p<0.001) and increasing likelihood of VI (Mann-Whitney test p=0.004 for intensity and p<0.001 for % cells staining).
There was no evidence of prognostic value for MIB1 staining intensity (logrank test for trend p=0.26) nor for the proportion of cells staining positive for MIB1, either when analysed as per previous reports (≥70% and ≥40%), as quartiles or using a log rank test for trend. In contrast to previous studies, only 5/179 patients (3%) had MIB1 staining in >70% of cells. The main distinction observed was between the 45 samples with weak vs any staining for MIB1 (Table 2). Analyzing the samples in this binary fashion (MIB1 positive or negative) has a prognostic effect (univariate analysis), which was reduced after stratification for VI (Table 2).
TE08/TE22 multivariate analyses
Multivariate analyses were performed on the 177 patients with complete data to assess the additional prognostic value of these factors over and above the presence/absence of VI and clinical variables, specifically VI (yes/no), histology type, seminomatous elements present/absent, age (continuous variable), alpha feto protein (AFP) raised pre-orchidectomy (yes/no), human chorionic gonadotrophin (HCG) raised pre-orchidectomy (yes/no), CXCL12 expression, MIB1 staining (high/weak), %EC (as a continuous variable), EC present/absent, and %EC categorised according to previous studies, (<45, 46-79, >80%; <50% vs ≥50% (4)) and %EC categorised according to the optimal cut offs for this dataset (<25%, 26-99% and 100%).
Both forward and backwards stepwise model selection procedures were used which all led to a model (Supplementary Table S3) including only VI and %EC (continuous variable). Dropping EC as a continuous variable but keeping all the other variations, the model includes only VI and %EC, using the “optimal” categorization (Figure 2B). However, if %EC is used as previously reported (4), then VI and CXCL12, but not %EC, are retained as independent variables (Figure 2A).
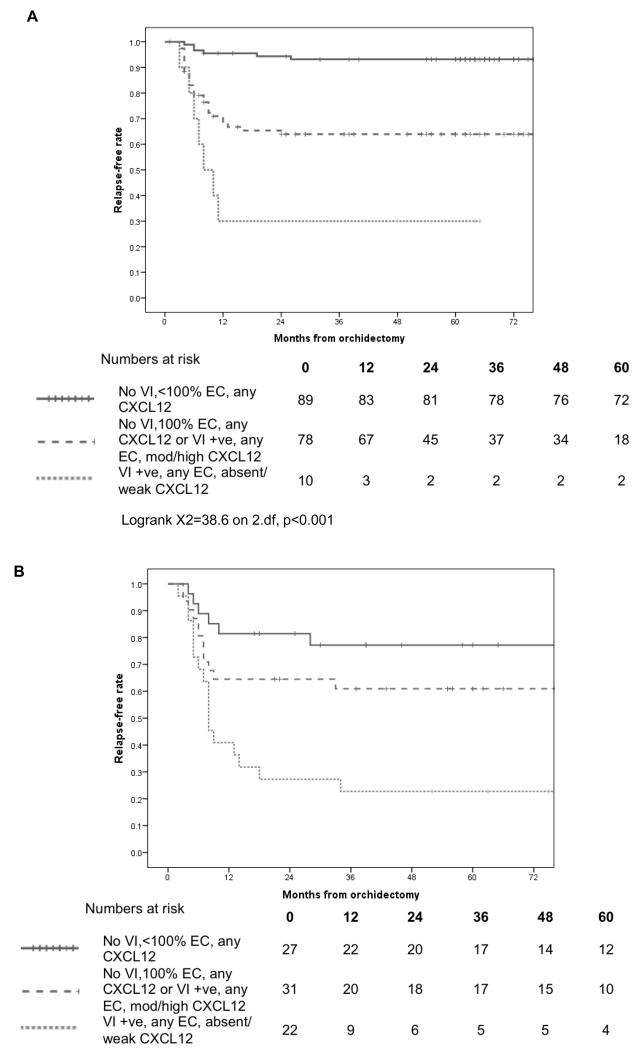
Components of both groupings have potential clinical utility in defining subsequent relapse risk, and as such we also present a combined model (Figure 3A) using 3 prognostic groups. Specfically these are a) VI negative, <100% EC and any CXCL12, b) VI negative, 100% EC any CXCL12 or VI positive, any EC and mod/high CXCL12 and c) VI +ve, any EC, absent/weak CXCL12 with 2 year RFRs of 94.3% (95% CI 89.4%, 99.2%), 63.9% (52.9%, 74.9%) and 30% (1.6%, 58.4%) respectively (Logrank X2=38.6 on 2.df, p<0.001).
Figure 3. Relapse free survival data for.
A 177 patients with stage I NSGCT managed with surveillance according to data-derived risk groups. B An independent cohort of 80 patients with stage I NSGCT managed with surveillance stratified by the risk grouping derived in A.
Validation of model combining VI, CXCL12 and %EC on RMH cohort
Staining characterisics of the cohort of 80 RMH patients managed with surveillance mirror those of the TE08/TE22 patients and are detailed in Table 3. Overall this cohort experienced a 2 year relapse-free rate (RFR) of 60% (95% CI 59%-71%). For patients with VI this was 47% (31-63%) as opposed to 69% without (54-83%). CXCL12 expression was prognostic as previously described with tumors absent/weak for CXCL12 having a 2 year RFR a relapse rate of 34% (18-50%) whereas tumors with moderate/high statining for CXCL12 having an RFR of 80% (68-92%), HR 0.24 (95% CI 0.11-0.49) p<0.001. CXCL12 expression retained additional prognostic value over and above VI (stratifed log rank p<0.001). Finally %EC was also prognostic in this cohort, with the 2 year RFR ranging from 79% (61-87) for patients with <=25%EC, through 64% (47-82%) with 26-99% EC and 48% (31-66%) in cases that were 100%EC (p=0.04). Using the combined model developed above, the three risk groupings have 2 RFR of 81.5% (66.8-96.2) for patients that were VI negative and %EC<100, 64.5% (47.6-81.4) for those that were VI negative and 100 %EC or VI positive and moderate/high expression of CXCL12 and a 2 year RFR of just 27.3% (8.7-45.9) for those that had evidence of VI but absent/weak expression of CXCL12 (Figure 3B, log rank Mantel-Cox <0.001).
Table 3.
Tumor characteristics (VI, CXCL12 and %EC) for 80 stage I NSGCT RMH patients managed by surveillance
|
No Vascular Invasion |
Vascular invasion + |
Total | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| EC% | <=25% | 14 | 33.3% | 5 | 13.9% | 19 | 24.4% |
| 26-99% | 13 | 31.0% | 15 | 41.7% | 28 | 35.9% | |
| 100% | 15 | 35.7% | 16 | 44.4% | 31 | 39.7% | |
|
CXCL12 Intensity |
0 | 11 | 26.2% | 19 | 50.0% | 30 | 37.5% |
| 1 | 2 | 4.8% | 3 | 7.9% | 5 | 6.3% | |
| 2 | 9 | 21.4% | 5 | 13.2% | 14 | 17.5% | |
| 3 | 20 | 47.6% | 11 | 28.9% | 31 | 38.8% | |
| CXCL12 | Absent/low | 13 | 31.0% | 22 | 57.9% | 35 | 43.8% |
| Moderate/high | 29 | 69.0% | 16 | 42.1% | 45 | 56.3% | |
|
Proposed grouping |
No VI, %EC <100 | 27 | 64.3% | 0 | 0.0% | 27 | 33.8% |
| No VI and 100% EC or VI and mod/high CXCL12 |
15 | 35.7% | 16 | 42.1% | 31 | 38.8% | |
| VI and absent/low CXCL12 |
0 | 0.0% | 22 | 57.9% | 22 | 27.5% | |
| TOTALS | 42 | 100% | 38 | 100% | 80 | 100% | |
4. Discussion
Using samples from stage I NSGCT patients managed by surveillance on prospective clinical trials, we have demonstrated that CXCL12 expression and %EC in the primary tumor are both predictors of relapse independently of VI. Importantly, samples obtained for this study were representative of the total trial populations (~78% relapse free rate at 2 years, by which point most relapses have usually occurred) and hence represent patients with stage I NSGCT in general. This provides prognostic information of potential clinical relevance over and above the presence/absence of histological evidence of VI being able to identify patients at low, moderate or very high risk of relapse (Tables 2, S2 and 3). However, %EC and CXCL12 are strongly inversely correlated and in multivariate analyses one or the other – but not both - are selected in addition to VI, depending on how %EC is analyzed. This gives two alternative prognostic models, as illustrated in figure 2A and B. While the multivariate analysis does not support a model containing VI with both %EC and CXCL12, there are unique characteristics of each combination that an ideal model would combine, specifically identification of a very high risk group (VI+, CXCL12 absent) and identification of VI−ve patients who have a prognosis closer to that of VI+ve (VI−ve, 100% EC). We therefore derived an exploratory model that combines these elements into 3 prognostic groups (Figure 3A). Using a cohort of cases previously characterised for CXCL12 (25), through the additional inclusion of %EC scores and VI we demonstrate the validity of this model (Figure 3B).
As shown previously (4), %EC is a subjective analysis. CXCL12 expression (<1% or <10% of cells staining positive as thresholds to classify as CXCL12 absent) together with VI is a potentially more reproducible approach to determining prognosis, and one that identifies a small group of patients with distinctly poorer prognosis.
Adjuvant chemotherapy is highly effective treatment and even 1 cycle, currently being tested in a UK phase II single arm study (BEP 111), can reduce the risk of recurrence to <4% (11, 13, 30, 31). This use of adjuvant BEP has been criticized for exposing a high proportion of patients who would not go on to relapse to intensive chemotherapy. Refining the current risk model (utlizing lymphovascular invasion alone) would therefore be of clinical utility, allowing adjuvant therapy to be focused on those at highest risk. Our analysis in this cohort supports our previous study in showing that CXCL12 immunohistochemistry can add valuable additional prognostic information to the model based on VI, particularly in identifying a small cohort of patients with a very high risk of relapse. It also in identifies groups for whom surveillance, potentially using reduced intensity follow-up (at least to the reduced frequency arm used in TE08 (27)) may be most appropriate, and those suitable for either surveillance or adjuvant therapy depending on personal preferences. Identifying high-risk patients for minimal effective adjuvant therapy will ultimately reduce long-term side-effects for the stage I NSGCT population as a whole.
The prognostic value of MIB1 was analysed in a number of ways looking at intensity and percentage of cells both separately and combined and including previously used categorizations (15-18, 32).. Although patients with weak staining have a better prognosis than those with high MIB1 staining, MIB1 staining was associated with VI and in multivariate analysis does not add clear independent prognostic value.
The prognostic value of CXCL12 expression demonstrated here is consistent with a growing body of evidence for the CXCL12/CXCR4 axis supporting the male germ cell niche and the metastatic spread of cancers, including this possibility in germ cell tumors (20, 22, 24, 25, 26, 33). CXCR4 expression is associated with invasion and metastases in a range of tumor types where lower levels of CXCL12 in tumors predict a reduced risk of metastatic dissemination (34, 35). Furthermore in breast cancer, lower levels of plasma CXCL12 appear to be associated with increased risk of metastatic relapse (36). That autocrine expression of CXCL12 consistently reduces the subsequent risk of relapse in stage I NSGCT (independent from histological VI) suggests the abrogation of a chemokine gradient (towards CXCR4) might prevent extravasation into the vascular compartment and/or invasion at metastatic sites. To this end, assessment of stromal and/or plasma CXCL12 might provide additional prognostic information. The apparent association with histological subtypes of TGCT requires further work, aligned with a better understanding of how these tumours develop from the in situ counterpart. Further investigations will inform on these and other potential mechanisms of dissemination and relapse (37) in NSGCT patients.
As a paradigm of protecting future quality of life in an essentially curable diseasea prospective study is recommended (potentially also investigating novel imaging strategies in an effort to minimize radiation exposure e.g. MRI) to further validate the prognostic value of CXCL12 and/or %EC expression in addition to the presence/absence of histological VI. This is anticipated to lead to the ability to identify patients diagnosed with stage I NSGCT at high risk of relapse to be considered for adjuvant therapy whilst others are safely surveilled.
Supplementary Material
Translational Relevance.
Patients diagnosed with stage I non-seminomatous germ cell tumors face a choice between surveillance with treatment at relapse or up-front adjuvant therapy. Whilst adjuvant therapy is effective, it may be unneccessary and long-term effects of chemotherapy are increasingly recognized. Histological evidence of vascular invasion is currently used to target patients for adjuvant therapy, but improved markers for risk of relapse are required. Embryologically, primordial germ cells use CXCR12/CXCR4 and KITLG/KIT signaling to migrate to the developing gonads. Previously we showed that CXCL12 stimulates migration of germ cell tumor cells in a CXCR4-dependent manner and that tumor cell expression of CXCL12 was associated with reduced risk of metastatic relapse. Here we validate this finding in a large series of samples from patients that underwent surveillance within prospective clinical trials and propose CXCL12 expression and percentage of embryonal carcinoma as clinically useful biomarkers to assist in stratifying patients for adjuvant therapy.
Acknowledgments
This study was supported by the National Cancer Research Institute Testis Cancer Clinical Studies Group. The authors would like to acknowledge the help of Philippa Jones in performing the immunohistochemistry, Brenda Summersgill and Ewa Aladowicz for organizing samples and the pathology departments across the UK for their assistance in retrieving tumor blocks.
Grant Support: This study was funded by the Medical Research Council (MRC) Biomarkers Grant G0801477 and Dr Daniel Berney is supported by the Orchid charity. This work was also partially funded by NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest: The authors indicated no potential conflicts of interest.
References
- 1.Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–765. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- 2.Huddart RA. Survival from testicular cancer in England and Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S83–85. doi: 10.1038/sj.bjc.6604598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Read G, Stenning SP, Cullen MH, Parkinson MC, Horwich A, Kaye SB, et al. Medical Research Council prospective study of surveillance for stage I testicular teratoma. Medical Research Council Testicular Tumors Working Party. J Clin Oncol. 1992;10:1762–8. doi: 10.1200/JCO.1992.10.11.1762. [DOI] [PubMed] [Google Scholar]
- 4.Vergouwe Y, Steyerberg EW, Eijkemans MJ, Albers P, Habbema JD. Predictors of occult metastasis in clinical stage I nonseminoma: a systematic review. J Clin Oncol. 2003;21:4092–9. doi: 10.1200/JCO.2003.01.094. [DOI] [PubMed] [Google Scholar]
- 5.van As NJ, Gilbert DC, Money-Kyrle J, Bloomfield D, Beesley S, Dearnaley DP, et al. Evidence-based pragmatic guidelines for the follow-up of testicular cancer: optimising the detection of relapse. Br J Cancer. 2008;98:1894–902. doi: 10.1038/sj.bjc.6604280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen MH, Stenning SP, Parkinson MC, Fossa SD, Kaye SB, Horwich A, et al. Short-course adjuvant chemotherapy in high-risk stage I nonseminomatous germ cell tumors of the testis: a Medical Research Council report. J Clin Oncol. 1996;14:1106–13. doi: 10.1200/JCO.1996.14.4.1106. [DOI] [PubMed] [Google Scholar]
- 7.Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513–23. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 8.van den Belt-Dusebout AW, Nuver J, de Wit R, Gietema JA, ten BokkelHuinink WW, Rodrigus PT, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2006;24:467–75. doi: 10.1200/JCO.2005.02.7193. [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. Journal of the National Cancer Institute. 2005;97:1354–65. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 10.van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–8. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 11.Albers P, Siener R, Krege S, Schmelz HU, Dieckmann KP, Heidenreich A, et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I Nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966–72. doi: 10.1200/JCO.2007.12.0899. [DOI] [PubMed] [Google Scholar]
- 12.Nichols CR, Kollmannsberger C. Vox populi: Using community-based studies to determine best management of early-stage nonseminoma. J Clin Oncol. 2009;27:2114–6. doi: 10.1200/JCO.2008.21.1524. [DOI] [PubMed] [Google Scholar]
- 13.Tandstad T, Dahl O, Cohn-Cedermark G, Cavallin-Stahl E, Stierner U, Solberg A, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol. 2009;27:2122–8. doi: 10.1200/JCO.2008.18.8953. [DOI] [PubMed] [Google Scholar]
- 14.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 15.Albers P, Bierhoff E, Neu D, Fimmers R, Wernert N, Muller SC. MIB-1 immunohistochemistry in clinical stage I nonseminomatous testicular germ cell tumors predicts patients at low risk for metastasis. Cancer. 1997;79:1710–6. [PubMed] [Google Scholar]
- 16.Albers P, Orazi A, Ulbright TM, Miller GA, Haidar JH, Donohue JP, et al. Prognostic significance of immunohistochemical proliferation markers (Ki-67/MIB-1 and proliferation-associated nuclear antigen), p53 protein accumulation, and neovascularization in clinical stage A nonseminomatous testicular germ cell tumors. Mod Pathol. 1995;8:492–7. [PubMed] [Google Scholar]
- 17.Albers P, Siener R, Hartmann M, Weinknecht S, Schulze H, Rebmann U, et al. Risk factors for relapse in stage I non-seminomatous germ-cell tumors: preliminary results of the German Multicenter Trial. German Testicular Cancer Study Group. International journal of cancer. 1999;83:828–30. doi: 10.1002/(sici)1097-0215(19991210)83:6<828::aid-ijc23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich A, Sesterhenn IA, Mostofi FK, Moul JW. Prognostic risk factors that identify patients with clinical stage I nonseminomatous germ cell tumors at low risk and high risk for metastasis. Cancer. 1998;83:1002–11. [PubMed] [Google Scholar]
- 19.Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, et al. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol. 2003;21:1505–12. doi: 10.1200/JCO.2003.07.169. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert D, Rapley E, Shipley J. Testicular germ cell tumours: predisposition genes and the male germ cell niche. Nat Rev Cancer. 2011;11:278–88. doi: 10.1038/nrc3021. [DOI] [PubMed] [Google Scholar]
- 21.De Miguel MP, Cheng L, Holland EC, Federspiel MJ, Donovan PJ. Dissection of the c-Kit signaling pathway in mouse primordial germ cells by retroviral-mediated gene transfer. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10458–63. doi: 10.1073/pnas.122249399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–59. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 23.Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development (Cambridge, England) 2006;133:4861–9. doi: 10.1242/dev.02688. [DOI] [PubMed] [Google Scholar]
- 24.Kanatsu-Shinohara M, Inoue K, Takashima S, Takehashi M, Ogonuki N, Morimoto H, et al. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell stem cell. 2012;11:567–78. doi: 10.1016/j.stem.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert DC, Chandler I, McIntyre A, Goddard NC, Gabe R, Huddart RA, et al. Clinical and biological significance of CXCL12 and CXCR4 expression in adult testes and germ cell tumours of adults and adolescents. The Journal of pathology. 2009;217:94–102. doi: 10.1002/path.2436. [DOI] [PubMed] [Google Scholar]
- 26.McIver SC, Loveland KL, Roman SD, Nixon B, Kitazawa R, McLaughlin EA. The chemokine CXCL12 and its receptor CXCR4 are implicated in human seminoma metastasis. Andrology. 2013;1:517–29. doi: 10.1111/j.2047-2927.2013.00081.x. [DOI] [PubMed] [Google Scholar]
- 27.Rustin GJ, Mead GM, Stenning SP, Vasey PA, Aass N, Huddart RA, et al. Randomized trial of two or five computed tomography scans in the surveillance of patients with stage I nonseminomatous germ cell tumors of the testis: Medical Research Council Trial TE08, ISRCTN56475197--the National Cancer Research Institute Testis Cancer Clinical Studies Group. J Clin Oncol. 2007;25:1310–5. doi: 10.1200/JCO.2006.08.4889. [DOI] [PubMed] [Google Scholar]
- 28.Huddart RA, O'Doherty MJ, Padhani A, Rustin GJ, Mead GM, Joffe JK, et al. 18fluorodeoxyglucose positron emission tomography in the prediction of relapse in patients with high-risk, clinical stage I nonseminomatous germ cell tumors: preliminary report of MRC Trial TE22--the NCRI Testis Tumour Clinical Study Group. J Clin Oncol. 2007;25:3090–5. doi: 10.1200/JCO.2006.09.3831. [DOI] [PubMed] [Google Scholar]
- 29.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–72. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert DC, Norman AR, Nicholl J, Dearnaley DP, Horwich A, Huddart RA. Treating stage I nonseminomatous germ cell tumours with a single cycle of chemotherapy. BJU International. 2006;98:67–9. doi: 10.1111/j.1464-410X.2006.06188.x. [DOI] [PubMed] [Google Scholar]
- 31.Westermann DH, Schefer H, Thalmann GN, Karamitopoulou-Diamantis E, Fey MF, Studer UE. Long-term followup results of 1 cycle of adjuvant bleomycin, etoposide and cisplatin chemotherapy for high risk clinical stage I nonseminomatous germ cell tumors of the testis. The Journal of Urology. 2008;179:163–6. doi: 10.1016/j.juro.2007.08.172. [DOI] [PubMed] [Google Scholar]
- 32.Heidenreich A, Schenkmann NS, Sesterhenn IA, Mostofi FK, McCarthy WF, Heidenreich B, et al. Immunohistochemical expression of Ki-67 to predict lymph node involvement in clinical stage I nonseminomatous germ cell tumors. The Journal of urology. 1997;158:620–5. [PubMed] [Google Scholar]
- 33.Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–4. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 35.Hassan S, Baccarelli A, Salvucci O, Basik M. Plasma stromal cell-derived factor-1: host derived marker predictive of distant metastasis in breast cancer. Clin Cancer Res. 2008;14:446–54. doi: 10.1158/1078-0432.CCR-07-1189. [DOI] [PubMed] [Google Scholar]
- 36.Roy I, Zimmerman NP, Mackinnon AC, Tsai S, Evans DB, Dwinell MB. CXCL12 Chemokine Expression Suppresses Human Pancreatic Cancer Growth and Metastasis. PLoS ONE. 2014;9:e9040037. doi: 10.1371/journal.pone.0090400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert DC, McIntyre A, Summersgill B, Missiaglia E, Goddard NC, Chandler I, et al. Minimum Regions of Genomic Imbalance in Stage I Testicular Embryonal Carcinoma and Association of 22q Loss with Relapse. Genes Chromosomes Cancer. 2011;50:186–95. doi: 10.1002/gcc.20843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.