Abstract
Objectives:
To examine direct and indirect associations of sleep duration and quality with insulin resistance, considering body mass index (BMI) as a potential mediator in newly diagnosed type 2 diabetes mellitus patients.
Methods:
Cross-sectional data from patients enrolled in the Early Activity in Diabetes study. We studied 522 newly diagnosed type 2 diabetes mellitus patients, 65.9% male, mean age 63.5 ± 10.1 years. Of the total sample 53% had a BMI of ⩾30 kg/m2. Participants completed a 7-day sleep diary and sleep questionnaire. Average sleep duration (minutes), average nap duration (minutes) and average number of night awakenings were derived. Objective measures of height and body weight were obtained for the BMI calculation (kg/m2). Insulin resistance was obtained using the homeostatic model assessment – insulin resistance (HOMA2-IR) standardized technique.
Results:
Average number of night awakenings was positively correlated with BMI (r= 0.22, p < 0.001) and negatively associated with logged HOMA2-IR (r= -0.16, p = 0.04). Path analysis demonstrated night awakenings were directly associated with BMI and indirectly associated with insulin resistance, whilst considering BMI as a potential mediator (p < 0.05). Sleep duration was not associated with BMI or insulin resistance (p > 0.05).
Conclusions:
Sleep quality, not sleep duration, plays an important role in insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMI may mediate the relationship between indicators of sleep quality and insulin resistance. There is a need to examine the impact of improving sleep quality on obesity and insulin resistance in patients with type 2 diabetes mellitus.
Keywords: body mass index, insulin resistance, sleep quality
Introduction
The prevalence of obesity and type 2 diabetes mellitus is rapidly increasing worldwide. Sleep duration as well as sleep quality have emerged as potential contributors to metabolic dysfunction, diabetes and obesity [Buxton and Marcelli, 2010; Arora et al. 2011; Buxton et al. 2012; Hung et al. 2013; Reutrakul et al. 2013; Wan Mahmood et al. 2013].
Although sleep duration may play a significant role in the development of obesity and/or diabetes, sleep quality and napping may be equally important [Lam et al. 2010]. Previous studies have examined overall sleep quality using the Pittsburg Sleep Quality Index (PSQI) in those with pre-existing type 2 diabetes mellitus and found that insufficient sleep duration and reduced sleep quality were associated with poorer glucose control, assessed by glycated hemoglobin (HbA1c) levels [Knutson et al. 2006]. Similarly, napping frequency and duration, both potential indicators of sleep quality, has been associated with diabetes both cross-sectionally and longitudinally [Lam et al. 2010; Xu et al. 2010]. A recent study of 3570 Japanese workers without diabetes, followed annually, showed that those with poorer overall sleep quality were almost four times more likely to develop diabetes. Night awakenings were also a significant and positive risk factor for developing diabetes [Kita et al. 2012]. The number of night awakenings and nap duration in those with diabetes, however, have not yet been explored but may be crucial for a better understanding of how individual indicators of sleep quality, rather than overall sleep quality, may be related to chronic conditions such as diabetes mellitus and obesity.
Type 2 diabetes mellitus is closely associated with adiposity, with many diabetes patients having increased BMI scores compared to healthy counterparts. Both conditions are also associated with sleep duration and other aspects of disordered sleep. It is therefore possible that sleep parameters may contribute to insulin resistance through the mediated effects of BMI, although this has not yet been investigated. We therefore hypothesized that sleep duration, frequency of night awakenings and nap duration would be directly associated with BMI and indirectly related to insulin resistance through the effects of BMI.
Materials and methods
This study used baseline data from the Early Activity in Diabetes (Early ACTID) randomized controlled trial [ISRCTN Registry: 92162869] [Andrews et al. 2011]. Briefly, participants aged 30–80 years with newly diagnosed type 2 diabetes mellitus were recruited and randomly allocated to receive either lifestyle intervention (dietary modification or dietary modification plus additional physical activity) or usual care. Study exclusion criteria included age >80 years, HbA1c concentration >10%, blood pressure >180/100 mm Hg, low-density lipoprotein (LDL) cholesterol concentration >4 mmol/l, body mass index (BMI) <25 kg/m2, body weight >180 kg, use of weight loss medication, taking sulphonylurea at maximum dose, unstable angina, a myocardial infarction 3 months prior to study, inability of increased physical activity, and pregnancy or intention of planning pregnancy during the study. A total of 593 participants were recruited and underwent a detailed assessment, including medical history, and a physical examination. A 7-day sleep diary along with a sleep questionnaire, previously employed in a well-established sleep cohort study [Taheri et al. 2004], were completed prior to the commencement of the trial. The study received approval from the Bath Hospital Research Ethics Committee and all participants provided written informed consent.
Sleep parameters
A 7-day sleep diary was administered to all participants. Diary sleep duration was calculated according to the difference between ‘time you think you fell to sleep’ and ‘time woken up’ for each day/night the diary was completed. Average sleep duration from the sleep diary (minutes) was calculated by totaling sleep duration for all completed days/nights and dividing by the same number.
The sleep questionnaire asked participants to report their average nap duration (minutes). Those who did not nap (n = 147) were excluded from further path analysis since we were only interested in nap duration in the individuals who napped. Information on the average estimated number of night awakenings from the sleep questionnaire was also used in subsequent analyses.
Information concerning daytime sleepiness was acquired from the Epworth Sleepiness Scale (ESS), previously validated against polysomnography [Johns, 1991]. The ESS is comprised of 8 items and a total score (0–24) was derived (a higher score indicates a higher level of perceived daytime sleepiness).
Anthropometric measures and insulin resistance
Measurements of height (cm) and weight (kg) were recorded and used for subsequent BMI calculation. The levels of serum glucose and insulin were assessed using standard techniques from a venous blood sample collected in the morning following an overnight fast. Homeostatic model assessment of insulin resistance (HOMA2-IR) was calculated to investigate insulin resistance [Levy et al. 1998].
Other measures
Self-reported information on age, gender, ethnicity (Caucasian, other) smoking (yes, no), average weekly alcohol consumption (units) per week and sleep problems (none/diagnosed obstructive sleep apnea/snoring/other) were obtained. The number of diabetes mellitus medications taken was obtained from medical records. Physical activity levels were assessed across a 7-day period where participants wore an accelerometer (GT1M; ActiGraph LLC, Pensacola, FL, USA). Participants wore the accelerometer on awakening and then removed the device at bedtime. Data were downloaded and average counts per minute (cpm) was derived from all days that the pedometer was worn [Cooper et al. 2012].
Statistical analysis
Data analyses were performed using IBM Statistical Package for the Social Sciences (SPSS, version 19.0 Chicago, IL, USA) and SPSS AMOS. Continuous data were checked for normal distribution. HOMA2-IR values were log transformed due to non-normal distribution of raw data. Descriptive statistics were calculated for the study sample and BMI was dichotomized (<30 and ⩾30) to compare obese with non-obese. Independent t-tests, Mann–Whitney U tests and chi-square analysis was performed to examine differences between study characteristics according to BMI. Due to a non-normal distribution of ESS (daytime sleepiness), Spearman’s rho bivariate correlation was conducted to assess the relationships between ESS and the three sleep parameters (sleep duration, night awakenings and nap duration). A series of further bivariate correlations were then performed to examine the relationships between sleep duration and the number of night awakenings, BMI and logged HOMA2-IR.
Path analysis was then performed in Stata version 13 (Texas, USA) to assess the direct, indirect and total effects where the three sleep parameters were predictors, BMI was considered as a mediator and logged HOMA2-IR was entered as the outcome. The path analysis was conducted in a sample of 375 participants as we included nap duration in the model and adjusted for potential confounders including age, gender, number of diabetes medications, physical activity level and self-reported sleep problems. This allowed us to perform bootstrapping (n = 200), a resampling technique used to obtain estimates of summary statistics. We analyzed the model using standard goodness of fit statistics: chi-square, Comparative Fit Index (CFI) (>0.95), and mean square error of approximation (RMSEA) (<0.05), with a p value for the test of closeness of fit >0.50.
Results
Of the 593 participants, information was available on 522 (88%) for all variables of interest, excluding 147 non-napping participants, where there were 375 cases. Study sample characteristics are shown in Table 1 according to obesity status. Briefly, gender, age, physical activity (measured by cpm), weekly alcohol consumption, logged HOMA2-IR, self-reported sleep problems and number of night awakenings were all significantly associated with obesity status.
Table 1.
Study sample characteristics in 522 volunteers with type 2 diabetes mellitus according to obesity status.
| Characteristic | Non-obese (n = 246) | Obese (n = 276) | p value |
|---|---|---|---|
| Gender | 0.012 | ||
| Men | 176 (71.5) | 168 (60.9) | |
| Women | 70 (28.5) | 108 (39.1) | |
| Age (years) | 63 (56–68) | 58 (51–65) | <0.001 |
| Ethnicity | 0.261 | ||
| Caucasian | 234 (95.1) | 268 (97.1) | |
| Other | 12 (4.9) | 8 (2.9) | |
| Smoker | 0.436 | ||
| Yes | 222 (90.2) | 255 (92.4) | |
| No | 24 (9.8) | 21 (7.6) | |
| Physical activity* | 300 (237–380) | 267 (206–352) | <0.001 |
| Alcohol units per week | 6 (1–15) | 3 (0–10) | 0.001 |
| Logged HOMA-IR | 1.37 ± 0.53 | 1.85 ± 0.68 | <0.001 |
| Sleep duration (minutes) | 471 ± 61 | 467 ± 67 | 0.468 |
| Night awakenings | 1 (1–2) | 2 (1–3) | 0.001 |
| ESS score | 7 (5–9) | 7 (4–10) | 0.423 |
| Nap duration (minutes)$ | 30 (20–60) | 30 (20–60) | 0.697 |
| Number of DM medications | 0 (0–1) | 0 (0–1) | 0.412 |
| Self-reported sleep problems | <0.001 | ||
| Diagnosed OSA | 1 (0.4) | 20 (7.3) | |
| None | 238 (96.8) | 245 (88.8) | |
| Snoring | 4 (1.6) | 3 (1.0) | |
| Other | 3 (1.2) | 8 (2.9) |
Data are presented as mean ± standard deviation, median (IQR) or n (%).
p values were calculated using the independent t-test, Mann–Whitney U test or chi square, as appropriate.
Counts per minute.
n = 375.
DM, diabetes mellitus; ESS, Epworth Sleepiness Scale; HOMA-IR, homeostatic model assessment – insulin resistance; OSA, obstructive sleep apnea.
We observed no significant associations between perceived daytime sleepiness and sleep duration (r= -0.07, p = 0.19), night awakenings (r= 0.10, p = 0.07) or nap duration (r= 0.07, p = 0.16). Within the total sample, significant positive correlations were observed between logged HOMA2-IR and BMI (r= 0.46, p < 0.001) as well as night awakenings and BMI (r= 0.22, p < 0.001) (Table 2). Our stratified analysis demonstrated a significant positive relationship between logged HOMA2-IR and BMI (r= 0.34, p < 0.001) and a negative relationship between frequency of night awakenings and logged HOMA2-IR (r= -0.16, p = 0.04) in non-obese (Table 3). A similar association was observed in obese individuals between BMI and HOMA2-IR (r= 0.33, p < 0.001). A positive association was found between night awakenings and BMI in the obese group (r= 0.22, p = 0.002) (Table 4).
Table 2.
Correlation coefficients between sleep duration, night awakenings, BMI and logged HOMA-IR in 375 volunteers with type 2 diabetes mellitus.
| BMI | Logged HOMA-IR | Nap duration | Night awakenings | Sleep duration | |
|---|---|---|---|---|---|
| BMI | 1.000 | ||||
| Logged HOMA-IR |
0.460
<0.001 |
1.00 | |||
| Nap duration |
0.064
0.218 |
0.064
0.218 |
1.00 | ||
| Night awakenings |
0.218
<0.001 |
0.045
0.387 |
−0.005
0.923 |
1.00 | |
| Sleep duration |
0.011
0.826 |
−0.012
0.822 |
−0.022
0.676 |
0.045
0.382 |
1.00 |
Correlation coefficients are presented in italics with p values detailed below.
BMI, body mass index; HOMA-IR, homeostatic model assessment – insulin resistance. As noted in the table, the p values are provided underneath the r values and highlighted in bold when significant.
Table 3.
Correlation coefficients between sleep duration, night awakenings, BMI and logged HOMA-IR in 182 non-obese volunteers with type 2 diabetes mellitus.
| BMI | Logged HOMA-IR | Nap duration | Night awakenings | Sleep duration | |
|---|---|---|---|---|---|
| BMI | 1.000 | ||||
| Logged HOMA-IR |
0.341
<0.001 |
1.00 | |||
| Nap duration |
0.071
0.339 |
0.030
0.684 |
1.00 | ||
| Night awakenings |
−0.041
0.580 |
–0.155
0.037 |
−0.033
0.655 |
1.00 | |
| Sleep duration |
0.066
0.364 |
−0.015
0.832 |
−0.037
0.616 |
0.032
0.661 |
1.00 |
Correlation coefficients are presented in italics with p values detailed below.
BMI, body mass index; HOMA-IR, homeostatic model assessment – insulin resistance. As noted in the table, the p values are provided underneath the r values and highlighted in bold when significant.
Table 4.
Correlation coefficients between sleep duration, night awakenings, BMI and logged HOMA-IR in 193 obese volunteers with type 2 diabetes mellitus.
| BMI | Logged HOMA-IR | Nap duration | Night awakenings | Sleep duration | |
|---|---|---|---|---|---|
| BMI | 1.000 | ||||
| Logged HOMA-IR |
0.326
<0.001 |
1.00 | |||
| Nap duration |
0.118
0.103 |
0.095
0.189 |
1.00 | ||
| Night awakenings |
0.224
0.002 |
0.101
0.163 |
0.033
0.684 |
1.00 | |
| Sleep duration |
0.066
0.364 |
−0.015
0.832 |
−0.012
0.864 |
0.032
0.661 |
1.00 |
Correlation coefficients are presented in italics with p values detailed below.
BMI, body mass index; HOMA-IR, homeostatic model assessment – insulin resistance.As noted in the table, the p values are provided underneath the r values and highlighted in bold when significant.
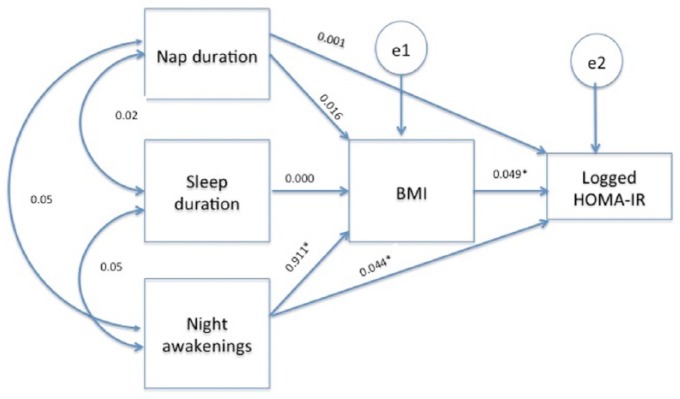
Our proposed theoretical path model is shown in Figure 1. The results from the path analysis showed that the model had an excellent fit to the data [χ2 (1, n = 375) = 0.08, p = 0.77, CFI = 1.00, RMSEA = 0.00]. The direct, indirect and total effects for the model are presented in Table 5. The path model was theory driven, although no significant correlations were observed between the three sleep parameters. Significant direct effects were found for night awakenings on BMI but not for sleep duration. BMI was significantly and positively related to logged HOMA2-IR, as expected. Significant indirect effects were observed for night awakenings on logged HOMA2-IR through BMI. The greatest significant direct effect was found for night awakenings on BMI and indirect effect on logged HOMA2-IR through the mediating effects of BMI.
Figure 1.
Theoretical path model.
Data are presented as unstandardized coefficients and adjusted for age, gender, diabetes medication, physical activity level and sleep problems.
*p < 0.05.
BMI, body mass index; HOMA-IR, homeostatic model assessment – insulin resistance; e=error.
Table 5.
Unstandardized and standardized coefficients for the direct, indirect and total effects for the theoretical path model.
| Causal variable | BMI |
Logged HOMA-IR |
||
|---|---|---|---|---|
| Unstd. (SE) | Std. | Unstd. (SE) | Std. | |
| Sleep duration | ||||
| Direct effect | 0.000 (0.004) | 0.005 | 0.000 (0.000) | 0.000 |
| Indirect effect | 0.000 (0.000) | 0.002 | ||
| Total effect | 0.000 (0.000) | 0.002 | ||
| Night awakenings | ||||
| Direct effect | 0.911* (0.267) | 0.249 | −0.025 (0.023) | −0.064 |
| Indirect effect | 0.044* (0.012) | 0.114 | ||
| Total effect | 0.020 (0.027) | 0.050 | ||
| Nap duration | ||||
| Direct effect | 0.016 (0.009) | 0.105 | 0.001 (0.001) | 0.040 |
| Indirect effect | 0.001 (0.000) | 0.048 | ||
| Total effect | 0.001 (0.000) | 0.088 | ||
| BMI | ||||
| Direct effect | 0.049* (0.007) | 0.458 | ||
Adjusted for age, gender, number of diabetes mellitus medications, physical activity level, and self-reported sleep problems.
BMI, body mass index; HOMA-IR, homeostatic model assessment – insulin resistance; SE, standard error; Std., standardized coefficient; Unstd., unstandardized coefficient.
p < 0.05.
Discussion
In our sample of 522 participants with newly diagnosed type 2 diabetes mellitus, we observed significant relationships between BMI with gender, age, physical activity, logged HOMA2-IR and night awakenings. We also found a significant positive relationship between night awakenings and BMI in obese individuals, but a negative relationship between night awakenings and logged HOMA2-IR in non-obese. BMI was also positively associated with logged HOMA2-IR, as expected. Our theoretical path model demonstrated that night awakenings were directly associated with BMI but sleep duration was not. BMI was shown to mediate the effects of night awakenings on logged HOMA2-IR.
Previous evidence has suggested relationships between sleep duration and/or overall sleep quality and diabetes as well as obesity development [Spiegel et al. 2005; Buxton and Marcelli, 2010; Hung et al. 2013; Wan Mahmood et al. 2013]. Cross-sectional studies have shown associations between short sleep duration (⩽6 hours) and BMI [Taheri et al. 2004] as well as diabetes [Gottlieb et al. 2005; Chaput et al. 2007]. Prospective data are consistent with cross-sectional studies showing that those with ⩽5 hours of sleep were more likely to have developed diabetes at 8–10 year follow up [Gangwisch et al. 2007]. A similar observation was found for short sleepers (⩽5 hours) with weight gain after 16 years follow up in women [Patel et al. 2006]. Furthermore, experimental studies deploying chronic partial sleep restriction (4 hours per night for 6 consecutive nights) in healthy young men have demonstrated that glucose tolerance and glucose effectiveness are reduced by 40% and 30%, respectively [Spiegel et al. 1999].
Despite longitudinal evidence demonstrating an association between short sleep duration and weight gain in women [Patel et al. 2006] and experimental studies showing sleep restriction is related to insulin resistance [Spiegel et al. 1999, 2005], it is still unclear if these relationships are bidirectional. For example, a recent study showed that Japanese workers without diabetes who were followed annually were more likely to develop the condition if they had reduced sleep quality or short sleep duration [Kita et al. 2012]. As increased BMI usually precedes type 2 diabetes mellitus and the sleep–obesity evidence is significant, it is possible that BMI mediates the effects of sleep parameters on insulin resistance.
Our study is the first to examine BMI as a potential mediator of multiple sleep parameters on insulin resistance in a large sample of participants with diabetes. Our findings support the hypothesis that indicators of sleep quality (night awakenings) are significantly and directly associated with BMI. Average calculated sleep duration from the 7-day sleep diary was not directly associated with BMI. Diabetes patients are prone to increased levels of daytime sleepiness and may therefore nap more during the day. We only considered nocturnal sleep duration and not total sleep duration, which may explain the absence of the sleep–obesity association. It may be possible that short sleep duration precedes weight gain [Patel et al. 2006], but then sleep duration is extended with diabetes development. Interestingly, napping has been shown to be more frequent in those with diabetes[Xu et al. 2010], but it is not known if BMI mediates this relationship. Our findings show that nap duration is not independently associated with insulin resistance but is directly related to BMI. Similar, but stronger, effects were observed for night awakenings with BMI-mediated effects. The two indicators of sleep quality assessed in our study both showed direct effects on BMI but not insulin resistance. However, night awakenings (a sleep quality parameter) was mediated by BMI with indirect effects on logged HOMA2-IR.
Our results demonstrate the importance of BMI for sleep quality parameters and levels of insulin resistance in those with diabetes. It is possible that obstructive sleep apnoea (OSA) may be partly responsible for the observed relationships. Risk factors for OSA include increased BMI and type 2 diabetes. OSA is also associated with poorer sleep quality through sleep interruptions following apnoeas and decreased levels of restorative sleep, namely slow wave sleep (SWS). If OSA were responsible for the findings, however, it would be expected that sleep duration, which is affected by OSA, would show significant associations within our theoretical model, but this was not present. Furthermore, we observed no significant correlations between perceived daytime sleepiness and the three sleep parameters assessed in our study (data not shown). Nevertheless, OSA is an important and underinvestigated condition that is common amongst patients with type 2 diabetes [Foster et al. 2009; Fredheim et al. 2011] and especially those at risk of cardiovascular disease [Leong et al. 2013].
Our findings have several clinical implications that need to be investigated through future studies that should employ objective measures of sleep quality. If napping is a contributor to metabolic dysfunction and obesity, reducing napping (frequency and/or duration) could have beneficial metabolic effects. Whilst a recent study demonstrated a dose-dependent relationship between nap duration and prevalence of type 2 diabetes mellitus as well as impaired fasting glucose [Fang et al. 2013], there is limited information concerning what a ‘healthy’ nap duration is or indeed if napping has any beneficial effects in these patient populations [Lam et al. 2010], Similarly, more comprehensive work needs to investigate sleep quality, although emerging evidence from the US National Health and Nutrition Examination Survey showed a significant relationship between this sleep parameter and increased risk of pre diabetes [Engeda et al. 2013]. There is currently a need for greater attention to identifying potential sleep problems, including OSA, which could have a significant impact on diabetes. Deficiencies in sleep quality could not only influence body weight with downstream effects on insulin sensitivity, but could also have a significant effect on mental health and quality of life – two significant aspects of type 2 diabetes.
Our study has several strengths and limitations. Firstly, our study benefitted from a relatively large sample of participants with newly diagnosed type 2 diabetes mellitus. Secondly, we applied a novel approach to examining the complex causal pathways between three sleep parameters, BMI and insulin resistance, analysis that has not been previously performed in any population, including patients with early diagnosis of type 2 diabetes mellitus. Limitations include the use of self-reported sleep data, although our group has previously shown that sleep duration from sleep diary estimates has a good level of agreement with objective measures of sleep using wrist-worn actigraphy [Arora et al. 2013]. Whilst we obtained self-reported information on sleep problems, we did not perform objective screening for the presence of OSA and therefore undiagnosed cases of this sleep disorder (and others) could potentially confound our observations. We did, however, stratify our analysis according to obesity status (a well-known proxy for OSA) but no significant differences were observed for daytime sleepiness (measured using the ESS), sleep duration or nap duration, which are mostly associated with OSA. Future work should, however, repeat the analysis in those with and without an OSA diagnosis to assess if OSA is responsible for the observed associations. Furthermore, we did not routinely ascertain information about psychological illness in our study, such as depression. Depression has been previously associated with obesity and diabetes mellitus, and may have confounded the relationships investigated. Finally, we also acknowledge that our findings report on the cross-sectional relationships and do not therefore determine sequential associations. Reverse causality remains problematic with the possibility of diabetes related outcomes resulting in sleep alterations.
Increased BMI is problematic both for the development of diabetes mellitus and for sleep quality. In patients with diabetes, we have shown that BMI is positively and significantly associated with nap duration and night awakenings, although only the latter was indirectly related to insulin resistance through the effects of BMI. Diabetes mellitus and obesity are major public health concerns and future studies should investigate and establish successful approaches for reducing BMI in those who are overweight or obese which may, in turn, improve sleep quality and insulin resistance. Improving sleep quality, in turn, could aid weight loss and its maintenance.
Acknowledgments
We thank the members of the steering committee: Jonathan Levy, Susan Jebb, Edwin Gale and Chris Riddoch. We also thank Lisa Treeby, Koba Koplatadze, Mimi Chen, Isy Douek, Nicola McLintock and Sarah Sainsbury for their help with the study. S.T., O.M.O and T.A. are funded by the Biomedical Research Program (BMRP) at Weill Cornell Medicine in Qatar, supported by the Qatar Foundation. A.R.C. is supported by the National Institute for Health Research (NIHR) Bristol Nutrition Biomedical Research Unit based at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Funding: This study was funded by Diabetes UK and the UK Department of Health. The funding bodies did not contribute to the design/conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript or the decision to submit the manuscript for publication.
Conflict of interest statement: All authors have no conflicts of interest to declare in preparing this article.
Contributor Information
Teresa Arora, Weill Cornell Medicine in Qatar and New York, USA.
Mimi Z. Chen, School of Clinical Sciences, University of Bristol, UK National Institute for Health Research, Bristol Biomedical Research Unit in Nutrition, Diet and Lifestyle, University of Bristol, UK.
Omar M. Omar, Weill Cornell Medicine in Qatar and New York, USA
Ashley R. Cooper, National Institute for Health Research, Bristol Biomedical Research Unit in Nutrition, Diet and Lifestyle Centre for Exercise, Nutrition and Health Sciences, School for Policy Studies, University of Bristol, UK
Rob C. Andrews, School of Clinical Sciences, University of Bristol, UK National Institute for Health Research, Bristol Biomedical Research Unit in Nutrition, Diet and Lifestyle, University of Bristol, UK.
Shahrad Taheri, Weill Cornell Medicine in Qatar, Qatar Foundation, Education City, PO Box 24144, Doha, Qatar.
References
- Andrews R., Cooper A., Montgomery A., Norcross A., Peters T., Sharp D., et al. (2011) Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the early ACTID randomised controlled trial. Lancet 378: 129–139. [DOI] [PubMed] [Google Scholar]
- Arora T., Broglia E., Pushpakumar D., Lodhi T., Taheri S. (2013) An investigation into the strength of the association and agreement levels between subjective and objective sleep duration in adolescents. PLoS One 8: e72406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora T., Jiang C., Thomas G., Lam K., Zhang W., Cheng K., et al. (2011) Self-reported long total sleep duration is associated with metabolic syndrome: the Guangzhou Biobank Cohort Study. Diabetes Care 34: 2317–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton O., Cain S., O’Connor S., Porter J., Duffy J., Wang W., et al. (2012) Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4: 129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton O., Marcelli E. (2010) Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med 71: 1027–1036. [DOI] [PubMed] [Google Scholar]
- Chaput J., Despres J., Bouchard C., Tremblay A. (2007) Association of Sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia 50: 2298–2304. [DOI] [PubMed] [Google Scholar]
- Cooper A., Sebire S., Montgomery A., Peters T., Sharp D., Jackson N., et al. (2012) Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia 55: 589–599. [DOI] [PubMed] [Google Scholar]
- Engeda J., Mezuk B., Ratliff S., Ning Y. (2013) Association between duration and quality of sleep and the risk of pre-diabetes: evidence from NHANES. Diabet Med 30: 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., Li Z., Wu L., Cao Z., Liang Y., Yang H., et al. (2013) Longer Habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng-Tongji cohort of retired workers. Sleep Med 14: 950–954. [DOI] [PubMed] [Google Scholar]
- Foster G., Sanders M., Millman R., Zammit G., Borradaile K., Newman A., et al. (2009) Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 32: 1017–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredheim J., Rollheim J., Omland T., Hofso D., Roislien J., Vegsgaard K., et al. (2011) Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross-sectional study. Cardiovasc Diabetol 10: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch J., Heymsfield S., Boden-Albala B., Buijs R., Kreier F., Pickering T., et al. (2007) Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 30: 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D., Punjabi N., Newman A., Resnick H., Redline S., Baldwin C., et al. (2005) Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 165: 863–867. [DOI] [PubMed] [Google Scholar]
- Hung H., Yang Y., Ou H., Wu J., Lu F., Chang C. (2013) The Association between self-reported sleep quality and overweight in a Chinese population. Obesity 21: 486–492. [DOI] [PubMed] [Google Scholar]
- Johns M. (1991) A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 14: 540–545. [DOI] [PubMed] [Google Scholar]
- Kita T., Yoshioka E., Satoh H., Saijo Y., Kawaharada M., Okada E., et al. (2012) Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care 35: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson K., Ryden A., Mander B., Van Cauter E. (2006) Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 166: 1768–1774. [DOI] [PubMed] [Google Scholar]
- Lam K., Jiang C., Thomas G., Arora T., Zhang W., Taheri S., et al. (2010) Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep 33: 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong W., Arora T., Jenkinson D., Thomas A., Punamiya V., Banerjee D., et al. (2013) The Prevalence and severity of obstructive sleep apnea in severe obesity: the impact of ethnicity. J Clin Sleep Med 9: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Matthews D., Hermans M. (1998) Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21: 2191–2192. [DOI] [PubMed] [Google Scholar]
- Patel S., Malhotra A., White D., Gottlieb D., Hu F. (2006) Association between reduced sleep and weight gain in women. Am J Epidemiol 164: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S., Hood M., Crowley S., Morgan M., Teodori M., Knutson K., et al. (2013) Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care 36: 2523–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K., Knutson K., Leproult R., Tasali E., Van Cauter E. (2005) Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 99: 2008–2019. [DOI] [PubMed] [Google Scholar]
- Spiegel K., Leproult R., Van Cauter E. (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439. [DOI] [PubMed] [Google Scholar]
- Taheri S., Lin L., Austin D., Young T., Mignot E. (2004) Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Mahmood W., Draman Yusoff M., Behan L., Di Perna A., Kyaw Tun T., Mcdermott J., et al. (2013) Association between sleep disruption and levels of lipids in Caucasians with type 2 diabetes. Int J Endocrinol 2013: 341506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Song Y., Hollenbeck A., Blair A., Schatzkin A., Chen H. (2010) Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care 33: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]