Abstract
Background
Arsenic (As) methylation capacity in epidemiologic studies is typically indicated by the proportions of inorganic As (%InAs), monomethylarsonic acid (%MMA), and dimethylarsinic acid (%DMA) in urine as a fraction of total urinary As. The relationship between renal function and indicators of As methylation capacity has not been thoroughly investigated.
Objectives
Our two aims were to examine (1) associations between estimated glomerular filtration rate (eGFR) and %As metabolites in blood and urine, and (2) whether renal function modifies the relationship of blood %As metabolites with respective urinary %As metabolites. Methods: In a cross-sectional study of 375 As-exposed Bangladeshi adults, we measured blood and urinary As metabolites, and calculated eGFR from plasma cystatin C.
Results
In covariate-adjusted linear models, a one ml/min/1.73 m2 increase in eGFR was associated with a 0.39% increase in urinary %InAs (p<0.0001) and a mean decrease in urinary %DMA of 0.07 (p=0.0005). In the 292 participants with measurable blood As metabolites, the associations of eGFR with increased blood %InAs and decreased blood %DMA did not reach statistical significance. eGFR was not associated with urinary or blood %MMA in covariate-adjusted models. For a given increase in blood %InAs, the increase in urinary %InAs was smaller in those with reduced eGFR, compared to those with normal eGFR (p=0.06); this effect modification was not observed for %MMA or %DMA.
Conclusions
Urinary excretion of InAs may be impaired in individuals with reduced renal function. Alternatively, increased As methylation capacity (as indicated by decreased urinary %InAs) may be detrimental to renal function.
Keywords: Arsenic, Bangladesh, kidney, glomerular filtration rate, methylation
1. Introduction
Exposure to inorganic arsenic (As) in drinking water is a public health problem estimated to impact over 140 million people globally (World Health Organization, 2008). Arsenic exposure has been associated with skin, lung, and bladder cancers, cardiovascular disease, nonmalignant respiratory illness, and neurologic deficits (National Research Council, 2013). Arsenic metabolism is thought to play an important role in the toxicity of As (Hughes, 2002).
The metabolism of inorganic As (InAs) occurs in a series of oxidative methylation and reduction reactions which promote As excretion in urine. Arsenite (AsIII) undergoes oxidative methylation to form monomethylarsonic acid (MMAV). MMAV is reduced to MMAIII, which then undergoes oxidative methylation to form dimethylarsinic acid (DMAV) (Challenger, 1951). The methylation reactions are catalyzed by arsenic methyltransferase (AS3MT) (Lin et al., 2002). The toxicity of As metabolites varies by methylation and valence state, with MMAIII being most toxic, and DMAV being least toxic (Styblo et al., 2002).
Methylation of As is crucial for As elimination through urine; AS3MT knockout mice retain greater amounts of As in their tissues (primarily InAs) and excrete less As in urine than wild-type mice (Drobna et al., 2009; Hughes et al., 2010). The reduced ability of the AS3MT knockout mice to excrete As is likely due to impaired synthesis of DMA, which is rapidly excreted in urine (Buchet et al., 1981). DMA is the most prevalent As species in human urine (approximately 60–80% of total urine As on average), while MMA (10–20%) and InAs (10–30%) comprise a much smaller proportion of total urine As (Vahter, 2000).
The proportions of InAs, MMA, and DMA in urine are often used as indicators of As methylation capacity; these proportions, particularly the percentage of MMA in urine (u%MMA), have been associated with risk for various diseases including atherosclerosis, bladder cancer, lung cancer, skin cancer, and skin lesions (Chen et al., 2013; Steinmaus et al., 2010). An important yet overlooked factor in using these indicators is the potential relationship between renal function and the urinary excretion of As metabolites. Other than studies on the reabsorption of InAsV in dogs (Ginsburg and Lotspeich, 1963; Tsukamoto et al., 1983) and the effect of nephrectomy on InAsV methylation in rabbits (De Kimpe et al., 1999), very little is known regarding the renal handling of As species and how this might be influenced by reductions in glomerular filtration rate (GFR) or by proximal tubule injury. The dearth of knowledge regarding renal filtration, secretion, and reabsorption of As species, and the influence of these factors on urine composition of As metabolites, was highlighted in a review by Carter, Aposhian, and Gandolfi in 2003 (Carter et al., 2003), yet research in this area is still severely lacking. In a previous report of two cross-sectional samples of As-exposed Bangladeshi adults, we observed that the estimated GFR (eGFR) was associated with increased %InAs in urine (u%InAs) in both samples, and decreased u%DMA in one of the samples, while there was no association of eGFR with u%MMA (Peters et al., 2014). We hypothesized that these associations could be due to renal function influencing the urinary excretion of As metabolites, or that As metabolites are differentially detrimental to renal function.
The current analysis builds upon a rich dataset from the Folate and Oxidative Stress (FOX) study (Hall et al., 2013), a cross-sectional study designed to examine the dose-response relationship between As exposure and biomarkers of oxidative stress. Two advantages of the FOX study are: 1) by design, participants had a wide range of As exposure, and 2) As metabolites were measured in both blood and urine. In this study, we examined the association of eGFR with As metabolite proportions in both blood and urine, and tested whether eGFR modifies the relationship between blood and urinary As metabolite proportions. The latter analysis may elucidate whether reduced renal function impedes the excretion of some As species from blood to urine.
2. Materials and Methods
2.1 Study population and design
The cross-sectional Folate and Oxidative stress (FOX) study included 379 adults from Araihazar, Bangladesh who were selected based on the As concentration of their wells [Group A: 0 – 10 μg/L (n=76), Group B: 10 – 100 μg/L (n=104), Group C: 100 – 200 μg/L (n=86), Group D: 200 – 300 μg/L (n=67), Group E: > 300 μg/L (n=45)]. The study has been described in detail previously (Hall et al., 2013). Briefly, we recruited individuals between the ages of 30 and 65 who had been drinking from their current well for at least 3 months. Exclusion criteria were as follows: (1) women who were pregnant, (2) individuals taking nutritional supplements, and (3) individuals having known diabetes, cardiovascular disease, renal disease, chronic obstructive pulmonary disease, or cancer. For the current analysis, participants missing data on water As, plasma folate, specific gravity, or BMI were excluded, leaving a final sample size of N=375. Blood As (bAs) metabolites were only measured in participants with total bAs≥ 5 μg/L, because one or more As metabolites would likely fall below detection limits in those with total bAs< 5 μg/L, leaving a subset of N=292 for analyses with bAs metabolites.
Oral informed consent was obtained by our Bangladeshi field staff physicians, who read an approved assent form to the study participants. This study was approved by the Bangladesh Medical Research Council and the institutional review board of Columbia University Medical Center.
2.2 Water arsenic
Water samples were analyzed for total As by high-resolution inductively coupled plasma mass spectrometry, as previously described (Cheng et al., 2004). The detection limit of the method is < 0.2 μg/L. A standard with an As concentration of 51 µg/L was run multiple times in each batch. The intra- and inter-assay coefficients of variation (CVs) for this standard were 6.01% and 3.76%, respectively.
2.3 Total blood arsenic
As described previously (Hall et al., 2006), total blood As concentrations were measured using a Perkin-Elmer Elan DRC II ICP-MS equipped with an AS 93+ autosampler. The intra- and inter-assay CVs were 2.1% and 4.9%, respectively.
2.4 Blood and urine arsenic metabolites
The As metabolites [arsenite (AIII), arsenate (AsV), monomethylarsonous acid plus monomethylarsonic acid (MMAIII+V), and dimethylarsinous acid plus dimethylarsinic acid (DMAIII+V)] were measured in blood and urine by coupling HPLC to dynamic reaction cell inductively coupled plasma MS (Reuter et al., 2003). The percentages of InAsIII+V (%InAs), MMAIII+V (%MMA), and DMAIII+V (%DMA) were calculated using the sum of the inorganic and methylated metabolites as the denominator. The intra-assay CVs for urinary AsIII, AsV, MMA, and DMA were 3.6%, 4.5%, 1.5%, and 0.6%, respectively; those for blood were 0.9%, 11.5%, 3.6%, and 2.6%, respectively. The inter-assay CVs for urinary metabolites were 9.7%, 10.6%, 3.5%, and 2.8%, respectively, whereas those for blood were 3.7%, 23.2%, 2.9%, and 3.5%, respectively. The total urinary As (uAs) and blood As (bAs) variables that are used in our analyses were calculated as the sum of AsIII, AsV, MMA, and DMA in urine and blood, respectively. Specific gravity (SG) was measured by refractometer, and total uAs was adjusted for SG using the following formula: [uAs*(overall mean SG-1)/(measured SG-1)] (Cone et al., 2009).
2.5 Plasma cystatin C and eGFR
GFR is typically estimated from plasma creatinine and/or cystatin C using population-based equations; we calculated eGFR from plasma cystatin C using the 2012 CKD-EPI Cystatin C equation (Inker et al., 2012). Plasma cystatin C was measured by ELISA according to the manufacturer’s protocol (R&D Systems Human Cystatin C Duoset Catalog# DY1196). We used a 6 point standard curve with a high standard of 3000 pg/ml. Samples were diluted 1:2000 in PBS with 10% fetal bovine serum (Sigma Aldrich F6178). Recovery of the IFCC certified reference material for serum cystatin C (ERM-DA 471/IFCC) was 104%. The intra- and inter-assay CVs were 7% and 10%, respectively.
2.6 Plasma nutrients
Plasma folate and B12 were measured by radio-protein binding assay (SimulTRAC-S, MP Biomedicals). The intra- and inter-assay CVs for folate were 9% and 14%, respectively, and those for B12 were 5% and 9%, respectively. Plasma total homocysteine (tHcys) was measured by HPLC with fluorescence detection (Pfeiffer et al., 1999). The intra- and inter-assay CVs for tHcy were 2% and 9%, respectively. LC-MS/MS was used to measure plasma concentrations of choline, betaine, and dimethylglycine (DMG) (Holm et al., 2003; Wang et al., 2008) with modifications based on the instrumentation in the laboratory of Dr. Marie Caudill at Cornell University (Yan et al., 2011). The intra-assay CVs were 3.4% for choline, 3.9% for betaine, and 4.7% for dimethylglycine. The inter-assay CVs were 9.0% for choline, 7.8% for betaine, and 8.1% for dimethylglycine.
2.7 Statistical analysis
To describe the sample characteristics, we calculated means and standard deviations for continuous variables, and frequencies for categorical variables, in the total sample and stratified by eGFR (reduced vs. normal). We considered those with eGFR<90 ml/min/1.73 m2 as having reduced renal function, based on the cut-point for mild GFR reduction from the Kidney Disease Outcomes Quality Initiative (National Kidney Foundation, 2002), and those with eGFR≥90 ml/min/1.73 m2 as having normal renal function. We used the Wilcoxon rank-sum test or the Chi-squared test to detect differences in continuous or categorical variables, respectively, between those with normal and reduced renal function.
We applied separate linear regression models using continuous eGFR to predict each %As metabolite, with and without adjustment for covariates. A priori we decided to adjust for age and sex in covariate-adjusted models, as well as a measure of As exposure (total bAs when the outcome was a bAs metabolite, and total uAs adjusted for SG when the outcome was a uAs metabolite). Other potential confounders relating to physiology (BMI, systolic blood pressure, diastolic blood pressure, urinary creatinine), nutrition (plasma folate, B12, betaine, choline, homocysteine), inflammation (plasma C-reactive protein, α-1 acid glycoprotein), substance abuse (smoking, betel nut chewing), and socio-economic status (television ownership, education) were considered based on their Spearman correlations with eGFR and with the %As metabolites, adjusting for age and sex. The control variables in the final models were those that were associated with eGFR and with any one %As metabolite (p<0.10), and resulted in an appreciable (>10%) change in the regression coefficient for the association between eGFR and a %As metabolite. Variables with skewed distributions (u%InAs, b%InAs, age, BMI, uAs [SG adjusted], bAs, plasma folate, plasma betaine) were natural log transformed for linear regression analyses. For the models using eGFR to predict %As metabolites in urine, we checked whether the results were similar when restricting on the sample of participants with total bAs ≥ 5 (i.e. participants in which blood As metabolites could be measured) (N=292).
We also examined whether these findings were consistent between men and women, as there are well documented sex differences in the metabolism and toxicity of As (reviewed in (National Research Council, 2013)). To do this, we repeated the linear regression analyses outlined above stratified by sex. We used the Wald test to detect between-gender differences in covariate-adjusted associations of eGFR with the %As metabolites.
To examine whether excretion of As metabolites from blood to urine may be impeded among those with reduced renal function, we conducted a linear regression analysis using %As metabolites in blood as predictors of respective urinary %As metabolite outcomes, stratifying by renal function (normal or reduced). Covariates for these models were chosen based on their bivariate Spearman correlations with %As metabolites in blood and urine at significance level α< 0.10, and their impact on the estimated regression coefficient of interest (change in estimated coefficient >10%). We used the Wald test to detect differences in the covariate-adjusted regression coefficient between those with normal and reduced renal function.
Statistical tests were two-sided with a significance value of 0.05. All analyses were performed using R version 3.0.3.
3. Results
Characteristics of the study population are presented in Table 1, in the total sample and stratified by renal function. The study enrolled approximately equal numbers of men and women, and participants ranged in age from 30 to 63 years old, on average had low BMI (20.4±3.5 kg/m2), and a low level of education (3.4±3.6 years). Additionally, there was a high proportion of smokers and betel-nut chewers. By design, there was a wide range of water As exposure in this study (0.4–700 μg/L), and mean ± SD water As exposure in the population was 137.8±123.8 μg/L. Participants with reduced renal function (eGFR<90 ml/min/1.73 m2) tended to be older, and were more likely to be male, smokers, and users of betelnut, than participants with normal renal function. Additionally, those with reduced renal function had lower plasma folate, and higher plasma betaine, choline, and homocysteine, than those with normal renal function.
Table 1.
Descriptive characteristics of participants in the FOX study.
| Total Sample (N=375) |
Normal eGFR (≥90 ml/min/1.73 m2) (N=222) |
Reduced eGFR (<90 ml/min/1.73 m2) (N=153) |
pa | |
|---|---|---|---|---|
|
| ||||
| Mean ± SD or % | Mean ± SD or % | Mean ± SD or % | ||
| Age | 43.2 ± 8.3 | 40.7 ± 7.5 | 46.8 ± 8.2 | <0.0001 |
| Male (%) | 48.5 | 34.7 | 68.6 | <0.0001 |
| BMI (kg/m2) | 20.4 ± 3.5 | 20.5 ± 3.4 | 20.3 ± 3.6 | 0.44 |
| Education (yrs) | 3.4 ± 3.6 | 3.3 ± 3.5 | 3.5 ± 3.8 | 0.98 |
| Smokers (ever) (%) | 36.3 | 23.0 | 55.6 | <0.0001 |
| Betel Nut Use (ever) (%) | 42.7 | 36.5 | 51.6 | 0.004 |
| Own TV (%) | 58.1 | 58.6 | 57.5 | 0.84 |
|
| ||||
| Water arsenic (μg/L) | 137.8 ± 123.8 | 135.9 ± 120.1 | 140.6 ± 129.4 | 0.77 |
| Urinary As (SG adjusted) | 231.1 ± 204.5 | 219.4 ± 196.4 | 248.1 ± 215.3 | 0.15 |
| Blood arsenic (μg/L)b | 14.8 ± 8.3 | 13.9 ± 6.9 | 16.0 ± 9.7 | 0.20 |
| Blood %InAsb | 29.6 ± 4.3 | 30.0 ± 3.8 | 29.2 ± 4.8 | 0.06 |
| Blood %MMAb | 39.0 ± 5.6 | 38.8 ± 5.4 | 39.4 ± 5.8 | 0.22 |
| Blood %DMAb | 31.3 ± 5.5 | 31.2 ± 5.5 | 31.5 ± 5.6 | 0.72 |
| Urinary %InAs | 17.7 ± 5.5 | 18.8 ± 6 | 16.3 ± 4.3 | <0.0001 |
| Urinary %MMA | 13.9 ± 5.0 | 13.2 ± 4.7 | 15.0 ± 5.2 | 0.002 |
| Urinary %DMA | 68.3 ± 7.9 | 68.0 ± 8.0 | 68.8 ± 7.7 | 0.60 |
|
| ||||
| Plasma folate (nmol/L) | 12.9 ± 7.2 | 13.5 ± 7.4 | 11.9 ± 6.7 | 0.002 |
| Plasma B12c (pmol/L) | 203.2 ± 112.6 | 204.7 ± 120.1 | 201.2 ± 101.4 | 0.82 |
| Plasma betaine (μmol/L) | 47.6 ± 19.8 | 45.5 ± 19.8 | 50.5 ± 19.6 | 0.007 |
| Plasma choline (μmol/L) | 11.6 ± 3.5 | 11.2 ± 3.3 | 12.2 ± 3.7 | 0.007 |
| Plasma tHcys (μmol/L) | 11.2 ± 13.0 | 9.8 ± 9.9 | 13.3 ± 16.4 | <0.0001 |
|
| ||||
| Plasma cystatin C (ng/mL) | 906.8 ± 257.2 | 736.4 ± 111.0 | 1154.2 ± 201.9 | <0.0001 |
| eGFR (ml/min/1.73m2) | 94.2 ± 24.4 | 111.6 ± 11.8 | 69.0 ± 13.6 | <0.0001 |
| Proteinuria (%)d | 1.6 | 1.4 | 2.0 | 0.63 |
| Systolic blood pressure (mm Hg)d | 108.2 ± 13.5 | 107.1 ± 13.2 | 109.9 ± 13.9 | 0.06 |
| Diastolic blood pressure (mm Hg)d | 72.2 ± 8.6 | 71.7 ± 8.7 | 72.9 ± 8.6 | 0.17 |
P-value for difference between participants with normal and reduced eGFR, from Wilcoxon rank-sum test for continuous variables and Chi-square test for categorical variables;
N=292;
N=369;
N=370.
We examined the effect of eGFR on %As metabolite outcomes in blood and urine using linear regression models (Table 2). For each outcome, we examined the covariate-unadjusted effect of eGFR (Model 1), and the covariate-adjusted effect of eGFR, either adjusting for variables selected a priori (Model 2), or adjusting for both a priori variables and additional variables that influenced the estimated coefficient of the eGFR variable (Model 3). In Model 3, a one ml/min/1.73 m2 increase in eGFR was associated with a 0.39% increase in u%InAs (p<0.0001) and a mean decrease in u%DMA of 0.07 (p=0.0005). However, in the N=292 participants with measurable blood As metabolites (total bAs ≥ 5 μg/L), the associations of eGFR with increased b%InAs and with decreased b%DMA, did not reach statistical significance at α=0.05. eGFR was not associated with either u%MMA or b%MMA in the covariate-adjusted models. Results were similar with and without adjustment for total blood or urinary As. Additionally, the results of the urinary %As metabolite models were similar when restricting the sample to those with blood As metabolite data available (data not shown).
Table 2.
Linear regression models using eGFR (ml/min/1.73 m2) as a predictor of %As metabolites in blood and urine.
| Blood metabolite outcomes (N=292) | Urine metabolite outcomes (N=375) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Model | Outcome | B (SE) | p | Δ R2 (%)a | Outcome | B (SE) | p | Δ R2 (%) |
| Model 1b | b%InAsc | 0.10 (0.03) |
0.003 | 3.04 | u%InAsc | 0.34 (0.06) |
<0.0001 | 8.02 |
| Model 2d | 0.07 (0.04) |
0.05 | 1.01 | 0.38 (0.07) |
<0.0001 | 6.61 | ||
| Model 3e | 0.06 (0.04) |
0.10 | 0.69 | 0.39 (0.07) |
<0.0001 | 6.43 | ||
|
| ||||||||
| Model 1 | b%MMA | −0.02 (0.01) |
0.18 | 0.61 | u%MMA | −0.04 (0.01) |
0.0002 | 3.62 |
| Model 2 | 0.00 (0.01) |
0.83 | 0.01 | −0.00 (0.01) |
0.74 | 0.02 | ||
| Model 3 | 0.01 (0.01) |
0.42 | 0.14 | −0.01 (0.01) |
0.52 | 0.08 | ||
|
| ||||||||
| Model 1 | b%DMA | −0.01 (0.01) |
0.47 | 0.18 | u%DMA | −0.03 (0.02) |
0.10 | 0.73 |
| Model 2 | −0.02 (0.02) |
0.21 | 0.49 | −0.07 (0.02) |
0.0003 | 2.99 | ||
| Model 3 | −0.02 (0.02) |
0.12 | 0.70 | −0.07 (0.02) |
0.0005 | 2.63 | ||
The change in R2 after adding eGFR to the model.
Model 1 is unadjusted (i.e. eGFR only).
Parameter reflects the percent change in the geometric mean of %InAs for 1 unit increase in eGFR.
Model 2 adjusted for ln(age), sex, and either ln(total bAs) (for blood metabolite models) or ln(total uAs adjusted for SG) (for urine metabolite models).
Model 3 adjusts for all covariates in Model 2, ever-smoking, ln(BMI), ln(plasma folate), and ln(plasma betaine).
To examine differences by gender, the fully adjusted linear regression models (Model 3) were repeated separately in men and women (Table 3). The covariate-adjusted associations of eGFR with the blood %As metabolite outcomes did not differ by sex. In contrast, the covariate-adjusted associations of eGFR with u%InAs and u%DMA did differ by sex. In women, a one ml/min/1.73 m2 increase in eGFR was associated with a 0.62% increase in u%InAs (p<0.0001) and a mean decrease in u%DMA of 0.14 (p<0.0001). In men, a one ml/min/1.73 m2 increase in eGFR was associated with a 0.20% increase in u%InAs (p=0.03), with no association between eGFR and u%DMA. Sex significantly modified the effect of eGFR on u%InAs (PWald=0.004) and u%DMA (PWald=0.0006).
Table 3.
Linear regression models using eGFR to predict %As metabolites in blood and urine, stratified by sex.
All models were adjusted for ln(age), smoking, ln(BMI), ln(plasma folate), ln(plasma betaine), and either ln(total bAs) (for blood metabolite models) or ln(total uAs adjusted for SG) (for urine metabolite models).
| Blood metabolite outcomes | Urine metabolite outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Men (N=146) | Women (N=146) | Men (N=182) | Women (N=193) | ||||||||||
|
| |||||||||||||
| Outcome | B (SE) | p | Δ R2 (%)a | B (SE) | p | Δ R2 (%) | Outcome | B (SE) | p | Δ R2 (%) | B (SE) | p | Δ R2 (%) |
| b%InAsb | 0.05 (0.05) |
0.33 | 0.49 | 0.08 (0.05) |
0.15 | 1.05 | u%InAsb,c | 0.20 (0.09) |
0.03 | 2.32 | 0.62 (0.11) |
<0.0001 | 12.55 |
| b%MMA | 0.00 (0.02) |
0.98 | 0.00 | 0.03 (0.02) |
0.16 | 0.94 | u%MMA | −0.02 (0.02) |
0.12 | 1.01 | 0.02 (0.02) |
0.24 | 0.65 |
| b%DMA | −0.01 (0.02) |
0.53 | 0.23 | −0.05 (0.03) |
0.07 | 2.17 | u%DMAd | −0.01 (0.02) |
0.59 | 0.12 | −0.14 (0.03) |
<0.0001 | 10.08 |
The change in R2 after adding eGFR to the model.
Parameter reflects the percent change in %InAs for 1 unit increase in eGFR.
Coefficient for eGFR different between men and women by the Wald test, p=0.004.
Coefficient for eGFR different between men and women by the Wald test, p=0.0006.
The %As metabolites in blood are correlated with their respective %As metabolites in urine (Spearman correlations: %InAs r=0.41, %MMA r=0.46, %DMA r=0.67, p<0.0001 for all). Using linear models with blood %As metabolites as predictors of respective urinary %As metabolites controlling for water As and gender, we observed that, for a given increase in b%InAs, the increase in u%InAs was smaller in those with reduced renal function (B=0.90, 95% CI: 0.64, 1.15), compared to those with normal renal function (B=1.29, 95% CI: 0.97, 1.62) (Table 4; Figure 1). The effect modification by renal function was marginally significant (p=0.06). The pattern of a larger effect of b%InAs on u%InAs in those with normal renal function, compared to those with reduced renal function, was observed in both men and women, however effect modification by renal function was not significant within either gender (Table 4). In contrast, renal function did not modify the relationship between blood and urinary %MMA, or blood and urinary %DMA, in the total sample or in gender-stratified analyses.
Table 4.
Estimated regression coefficient (95% CI) of blood %As metabolites as predictors of respective urinary %As metabolites in linear models, stratified by renal function.
| Normal eGFR (≥90 ml/min/1.73 m2) |
Reduced eGFR (<90 ml/min/1.73 m2) |
pa | |
|---|---|---|---|
| Total sampleb | N=166 | N=126 | |
|
| |||
| %InAsc | 1.29 (0.97, 1.62) | 0.90 (0.64, 1.15) | 0.06 |
| %MMA | 0.32 (0.19, 0.45) | 0.33 (0.17, 0.48) | 0.96 |
| %DMA | 0.88 (0.69, 1.07) | 0.82 (0.64, 0.99) | 0.62 |
|
| |||
| Mend | N=58 | N=88 | |
|
| |||
| %InAsc | 1.10 (0.62, 1.57) | 0.89 (0.58, 1.20) | 0.47 |
| %MMA | 0.24 (−0.04, 0.52) | 0.39 (0.18, 0.60) | 0.40 |
| %DMA | 1.14 (0.82, 1.45) | 0.92 (0.69, 1.15) | 0.27 |
|
| |||
| Womend | N=108 | N=38 | |
|
| |||
| %InAsc | 1.37 (0.93, 1.81) | 0.83 (0.28, 1.37) | 0.12 |
| %MMA | 0.34 (0.19, 0.50) | 0.21 (−0.01, 0.44) | 0.33 |
| %DMA | 0.80 (0.56, 1.04) | 0.61 (0.30, 0.93) | 0.34 |
p-value from Wald test for difference in regression coefficient of interest between participants with normal and reduced renal function.
Models adjusted for ln(water As) and sex.
Blood and urinary %InAs are natural log transformed.
Models adjusted for ln(water As).
Figure 1.
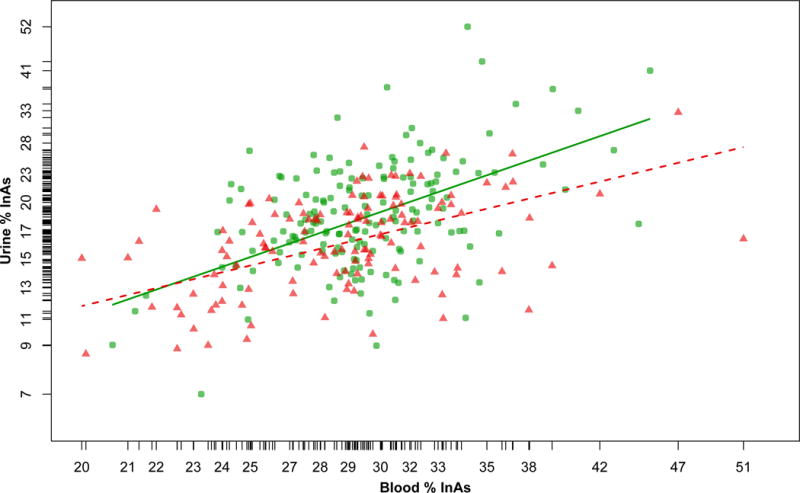
Scatter plot of the relationship between blood %InAs and urinary %InAs in those with normal (green circles; eGFR≥90 ml/min/1.73 m2) or reduced (red triangles; eGFR<90 ml/min/1.73 m2) renal function. The lines are regression lines from the adjusted models in the total sample in Table 4 (green solid line, normal renal function; red dotted line, reduced renal function).
4. Discussion
In this cross-sectional study of Bangladeshi adults exposed to a wide range of As through contaminated drinking water, eGFR was a strong predictor of As metabolite proportions in urine, explaining ~6% of the variation in u%InAs, and ~3% of the variation in u%DMA. Our findings here are consistent with our previous study, in which eGFR was a positive predictor of u%InAs in two cross-sectional samples, and a negative predictor of u%DMA in one of the cross-sectional samples (Peters et al., 2014). Given that the present study has replicated these findings, it is unlikely that they are due to chance. Here we were additionally able to examine the associations between eGFR and As metabolite proportions in blood. The associations of eGFR with b%InAs and b%DMA were in the same direction as the associations of eGFR with u%InAs and u%DMA, respectively, although they did not reach statistical significance at p<0.05. Finally, for a given increase in b%InAs, u%InAs increased less in those with reduced renal function, as compared to those with normal renal function, possibly indicating that those with reduced renal function may have impaired excretion of InAs from blood to urine.
The mechanism by which GFR may be associated with As metabolite proportions is unknown. We previously reported two hypothesized mechanisms: either renal function influences the urinary excretion of As metabolites, or As metabolites are differentially detrimental to renal function (Peters et al., 2014). A third possibility that cannot be ruled out is that renal function indirectly influences AS3MT activity (i.e. through effects of uremic substances). Our finding that eGFR was associated with increased %InAs in urine, and that eGFR modified the magnitude of the association between blood and urinary %InAs, may support a hypothesis that individuals with better renal function are more efficient at excreting InAs. The negative association between eGFR and u%DMA may be a consequence of this, since as one As metabolite proportion increases, at least one other must decrease.
Few other studies, aside from the current study and our previous cross-sectional study in Bangladesh, have examined the relationship between renal function and indicators of As methylation capacity in humans. In a case-control study in Taiwan, urinary %As metabolites were not associated with risk for chronic kidney disease (CKD) (Hsueh et al., 2009). In a cross-sectional study of American Indians in the Unites States, increased u%MMA was associated with decreased prevalence of albuminuria, which is a marker of renal dysfunction (Zheng et al., 2013). These studies differ from the present study on the level of As exposure in the population; average uAs (μg/g creatinine) in both the Taiwan study and the American Indian study were substantially lower than the average uAs (μg/g creatinine) in the current study. A study in patients with acute promyelocytic leukemia (APL) undergoing multiple-dose As trioxide chemotherapy over a 4-week period supports the notion that renal function influences urinary InAs excretion (Sweeney et al., 2010). In this APL study, the percentage of the As dose excreted in urine, and the percentage of total As excreted as AsIII (i.e. u%InAs), were reduced in patients with mild to severe renal impairment, as compared to patients with normal renal function. Serum MMAV and DMAV were increased in the patients with mild to severe renal impairment, indicating impaired excretion of these metabolites as well, although we did not observe that eGFR modified the relationship between blood and urine %MMA, or blood and urine %DMA. Interestingly, renal insufficiency induced by partial nephrectomy (3/6 or 5/6 kidney removed) in rabbits resulted in decreased methylation efficiency of an intraperitoneally administered arsenate dose (1.2 μg), characterized by decreased DMA in tissues, plasma, and urine (De Kimpe et al., 1999). This result is in contrast to ours, where decreased renal function appears to be associated with an increased As methylation capacity; however, the extent of renal dysfunction in the current study is likely much less severe than that induced by nephrectomy.
Our contention that InAs excretion is impaired among those with reduced renal function is also supported by recent cross-sectional and prospective analyses of a large American Indian cohort with substantially lower As exposure than our study population (median total uAs 9.7 μg/L vs. 125 μg/L in our study) (Zheng et al., 2015). In the cross-sectional analysis of that cohort, urinary InAs concentrations were inversely associated with CKD prevalence (i.e. positively associated with eGFR), while urinary MMA and DMA concentrations were positively associated with CKD prevalence only after adjustment for urinary InAs. In a prospective analysis of the same cohort, urinary InAs concentration was not associated with incident CKD, while urinary MMA and DMA concentration were positively associated with incident CKD. This study did not examine the relationship between As metabolite proportions and prevalent or incident CKD. The authors concluded that these data suggest that CKD influences the excretion of InAs.
The marginal associations between eGFR and the blood As metabolite proportions are difficult to interpret. While As in urine is the net result of kidney processing (i.e. filtration, secretion, and reabsorption), As in blood has many sources and sinks, including intestinal absorption, flux in and out of tissues, renal excretion, and biliary excretion. We might expect that increased flux of InAs through the kidney in response to improved GFR would result in increased u%InAs, with an accompanying decrease in b%InAs, however, because of the complex sources and sinks of blood As this is not necessarily the case. Accordingly, in the APL study, serum AsIII only became elevated in patients with severe renal impairment, and not in mild-moderate renal impairment (Sweeney et al., 2010). It is possible that participants with better renal function, having a higher capacity to excrete InAs from blood, may have increased flux of InAs from tissues to blood; this scenario may result in the marginal positive association we observe between eGFR and b%InAs.
Alternatively, since this is a cross-sectional study, we must also consider reverse causation: perhaps a certain As methylation profile, involving decreased %InAs (and increased %DMA in women), may be detrimental to renal function. In this scenario, we would expect that the %As metabolites in blood would be associated with eGFR in the same direction as the %As metabolites in urine, which is what we observe. Our observations are in contrast with epidemiologic studies of other health outcomes which have shown that As methylation profiles of increased u%MMA and decreased u%DMA are associated with adverse outcomes, including cardiovascular disease, skin lesions, skin cancer, and bladder cancer (Chen et al., 2013; Steinmaus et al., 2010).
Arsenic metabolism has been considered both a detoxification and bioactivation process: DMAV is rapidly excreted in urine, however trivalent intermediates of As metabolism are significantly more cytotoxic than pentavalent forms. Among the forms of trivalent As, MMAIII is the most cytotoxic, followed by DMAIII and AsIII (Styblo et al., 2002). Because we observe that DMA is the most prevalent As metabolite in urine, but not in blood, it is logical to surmise that the urinary space (i.e. from the proximal tubules of nephrons to the bladder) may be exposed to a greater concentration of DMA, and a lower concentration of InAs and MMA, than other tissues. It is not entirely known why DMA is more readily excreted than InAs or MMA; some possibilities are (a) greater binding strength of InAsIII and MMAIII with dithiols in proteins than of DMAIII with monothiols (Carter et al., 2003), (b) the glomerular filter may be more permeable to DMA than InAs or MMA, (c) secretion of DMA into the proximal tubule may be greater than that for InAs or MMA, or (d) reabsorption of DMA from the proximal tubule may be less than that for InAs or MMA. Whatever the reason, the kidney could have increased susceptibility to harm from a “better” methylation capacity, which could possibly explain our findings. However, increased u%DMA was protective against bladder cancer in a prospective cohort study (Huang et al., 2008), which does not lend support to this hypothesis.
We unexpectedly observed sex differences in the associations of eGFR with the urinary As metabolite proportions: the positive association of eGFR with u%InAs was stronger in women than in men, and the negative association of eGFR with u%DMA was observed in women only. We are unaware of a mechanism by which these differences may exist, however they may relate to differences in renal function or As methylation capacity between the men and women of this study population. The women in this study had significantly higher eGFR than the men. There is no established sex difference in measured GFR (Delanaye et al., 2012), and both measured GFR (Jafar et al., 2011) and eGFR (Shah et al., 2015) have been reported not to differ by sex in South Asian populations, although another study in Bangladesh also observed higher eGFR in women than men (Huda et al., 2012). The GFR estimating equation we have used was shown to be similar in accuracy and bias between men and women (Inker et al., 2012), however it has not yet been validated in a South Asian population against measured GFR. It is therefore unclear whether the sex difference in eGFR we have observed is real, or the result of the equation insufficiently accounting for sex differences in cystatin C unrelated to GFR in this particular population. The women in this study also had a significantly higher As methylation capacity (lower %MMA and higher %DMA in urine and blood) than men; this sex difference in As methylation is well documented (National Research Council, 2013), and may be attributed to lower homocysteine concentrations (Jacques et al., 1999) or potentially a greater availability of choline (a source of methyl groups) in women (Zeisel, 2011). Thus, the sex differences in the ‘eGFR – As metabolite proportion’ associations may be a result of women having higher eGFR or enhanced As methylation capacity, both of which could make these associations more apparent in women.
Our results may have implications for epidemiologic studies which use As methylation capacity to predict risk for disease, given that renal function is adversely affected by a variety of disease states, such as diabetes, hypertension, and infections (Atkins, 2005; Barsoum, 2006) and that CKD is associated with increased risk for CVD (Gansevoort et al., 2013). Depending on the nature of the true relationship between GFR and As metabolite proportions, and the nature of the relationship between GFR and the disease of interest, several scenarios arise in which adjustment for eGFR may or may not be appropriate. For example, if GFR differentially influences the urinary excretion of the As metabolites, and reduced renal function increases risk for the disease (e.g. cardiovascular disease), GFR could be a confounder of the exposure/disease relationship, and should be adjusted for. Alternatively, if the As metabolites are differentially detrimental to GFR, and the disease (e.g. diabetes) causes a deterioration of GFR, adjustment for GFR (a collider) could create a biased exposure/disease relationship.
The strengths of this study include the large sample size, the measurement of a sensitive biomarker of GFR, and the speciation of As metabolites in both blood and urine. Additionally, we were able to account for many factors that may influence As metabolism and/or renal function, such as age, nutrition, homocysteine, inflammation, blood pressure, urinary creatinine, total As exposure, and socioeconomic indices; this makes it less likely that our results are due to unmeasured confounding. However, our study has several limitations. First, because our study is cross-sectional and therefore lacks temporality, we cannot determine the causality or temporal direction of the associations between eGFR and the As metabolite proportions. Second, our sample size of participants with moderately to severely reduced eGFR (<60 ml/min/1.73 m2) was very small. Lastly, we were not able to measure biomarkers of proximal tubule injury, due to instability of these biomarkers in urine samples that have undergone multiple freeze-thaw cycles. Measurement of additional markers of renal function and injury might aid in the interpretation of the current findings.
5. Conclusions
In conclusion, we have observed that eGFR is associated with the proportions of As metabolites in urine, and that excretion of InAs from blood to urine may be modified by renal function. These findings may indicate that InAs excretion is impaired in those with reduced renal function, and/or there may be an effect of As methylation capacity on renal function. This study presents several avenues for further investigation. Experiments in animal models can elucidate the effect of renal injury on the distribution of As metabolites in blood and urine. Studies in humans should examine whether As metabolites proportions are similarly associated with eGFR estimated from serum creatinine, to help rule out the possibility that As metabolite proportions may be associated with cystatin C independently of actual GFR. Finally, prospective cohort studies examining the association of As methylation with the incidence of CKD may provide evidence that As methylation capacity plays a role in the nephrotoxicity of As.
Highlights.
We examined the association between renal function and As methylation capacity.
Blood and urine As metabolites and eGFR were analyzed in 375 As-exposed adults.
eGFR was significantly associated with increased %InAs and decreased %DMA in urine.
The relationship between %InAs in blood vs. in urine was weaker in low eGFR strata.
Urinary excretion of InAs may be impaired in those with reduced eGFR.
Acknowledgments
Sources of funding support
This work was supported by funding from NIH grants R01CA133595, P42ES10349, P30ES09089, R01ES017875, and T32ES007322.
Abbreviations
- As
arsenic
- AS3MT
arsenic methyltransferase
- bAs
blood arsenic
- CKD
chronic kidney disease
- DMA
dimethylarsinic acid
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- InAs
inorganic arsenic
- MMA
monomethylarsonic acid
- SG
specific gravity
- uAs
urinary arsenic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests Statement
The authors declare they have no actual or potential competing financial interests.
Human subjects research approval
This study was approved by the Bangladesh Medical Research Council and the institutional review board of Columbia University Medical Center.
References
- Atkins RC. The epidemiology of chronic kidney disease. Kidney Int Suppl. 2005:S14–8. doi: 10.1111/j.1523-1755.2005.09403.x. [DOI] [PubMed] [Google Scholar]
- Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–9. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- Buchet JP, et al. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1981;48:71–9. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- Carter DE, et al. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol. 2003;193:309–34. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Challenger F. Biological methylation. Adv Enzymol Relat Subj Biochem. 1951;12:429–91. doi: 10.1002/9780470122570.ch8. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect. 2013;121:832–8. doi: 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, et al. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–8. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- Cone EJ, et al. Normalization of urinary drug concentrations with specific gravity and creatinine. J Anal Toxicol. 2009;33:1–7. doi: 10.1093/jat/33.1.1. [DOI] [PubMed] [Google Scholar]
- De Kimpe J, et al. 74As-arsenate metabolism in Flemish Giant rabbits with renal insufficiency. J Trace Elem Med Biol. 1999;13:7–14. doi: 10.1016/S0946-672X(99)80017-0. [DOI] [PubMed] [Google Scholar]
- Delanaye P, et al. Normal reference values for glomerular filtration rate: what do we really know? Nephrol Dial Transplant. 2012;27:2664–72. doi: 10.1093/ndt/gfs265. [DOI] [PubMed] [Google Scholar]
- Drobna Z, et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol. 2009;22:1713–20. doi: 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansevoort RT, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–52. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- Ginsburg JM, Lotspeich WD. Interrelations of arsenate and phosphate transport in the dog kidney. Am J Physiol. 1963;205:707–14. doi: 10.1152/ajplegacy.1963.205.4.707. [DOI] [PubMed] [Google Scholar]
- Hall M, et al. Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology. 2006;225:225–33. doi: 10.1016/j.tox.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hall MN, et al. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect. 2013;121:1068–74. doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm PI, et al. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem. 2003;49:286–94. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- Hsueh YM, et al. Urinary arsenic species and CKD in a Taiwanese population: a case-control study. Am J Kidney Dis. 2009;54:859–70. doi: 10.1053/j.ajkd.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Huang YK, et al. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control. 2008;19:829–39. doi: 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- Huda MN, et al. Prevalence of chronic kidney disease and its association with risk factors in disadvantageous population. Int J Nephrol. 2012;2012:267329. doi: 10.1155/2012/267329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Hughes MF, et al. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol Appl Pharmacol. 2010;249:217–23. doi: 10.1016/j.taap.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Inker LA, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques PF, et al. Serum total homocysteine concentrations in adolescent and adult Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 1999;69:482–9. doi: 10.1093/ajcn/69.3.482. [DOI] [PubMed] [Google Scholar]
- Jafar TH, et al. Level and determinants of kidney function in a South Asian population in Pakistan. Am J Kidney Dis. 2011;58:764–72. doi: 10.1053/j.ajkd.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, et al. A NovelS-Adenosyl-l-methionine:Arsenic(III) Methyltransferase from Rat Liver Cytosol. Journal of Biological Chemistry. 2002;277:10795–10803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- National Research Council. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic. The National Academies Press; Washington, DC: 2013. [Google Scholar]
- Peters BA, et al. Creatinine, arsenic metabolism, and renal function in an arsenicexposed population in bangladesh. PLoS One. 2014;9:e113760. doi: 10.1371/journal.pone.0113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CM, et al. Rapid and Accurate HPLC Assay for Plasma Total Homocysteine and Cysteine in a Clinical Laboratory Setting. Clinical Chemistry. 1999;45:290–292. [PubMed] [Google Scholar]
- Reuter W, et al. Speciation of Five Arsenic Compounds in Urine by HPLC/ICP-MS. PerkinElmer, Inc; Shelton, CT: 2003. [Google Scholar]
- Shah AD, et al. The association between body composition and cystatin C in South Asians: results from the MASALA study. Obes Res Clin Pract. 2015;9:180–3. doi: 10.1016/j.orcp.2014.10.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, et al. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol. 2010;247:138–45. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, et al. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect. 2002;110(Suppl 5):767–71. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney CJ, et al. A pharmacokinetic and safety study of intravenous arsenic trioxide in adult cancer patients with renal impairment. Cancer Chemother Pharmacol. 2010;66:345–56. doi: 10.1007/s00280-009-1169-4. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, et al. Metabolism and renal handling of sodium arsenate in dogs. Am J Vet Res. 1983;44:2331–5. [PubMed] [Google Scholar]
- Vahter M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett. 2000;112–113:209–17. doi: 10.1016/s0378-4274(99)00271-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Analysis of acetylcholine, choline and butyrobetaine in human liver tissues by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2008;47:870–5. doi: 10.1016/j.jpba.2008.02.022. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Third edition, incorporating first and second addenda. WHO Press; Geneva: 2008. Guidelines for drinking-water quality - Volume 1: Recommendations. [PubMed] [Google Scholar]
- Yan J, et al. MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am J Clin Nutr. 2011;93:348–55. doi: 10.3945/ajcn.110.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional genomics: defining the dietary requirement and effects of choline. J Nutr. 2011;141:531–4. doi: 10.3945/jn.110.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LY, et al. Urine arsenic and prevalent albuminuria: evidence from a population-based study. Am J Kidney Dis. 2013;61:385–94. doi: 10.1053/j.ajkd.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LY, et al. The Association of Urine Arsenic with Prevalent and Incident Chronic Kidney Disease: Evidence from the Strong Heart Study. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]


