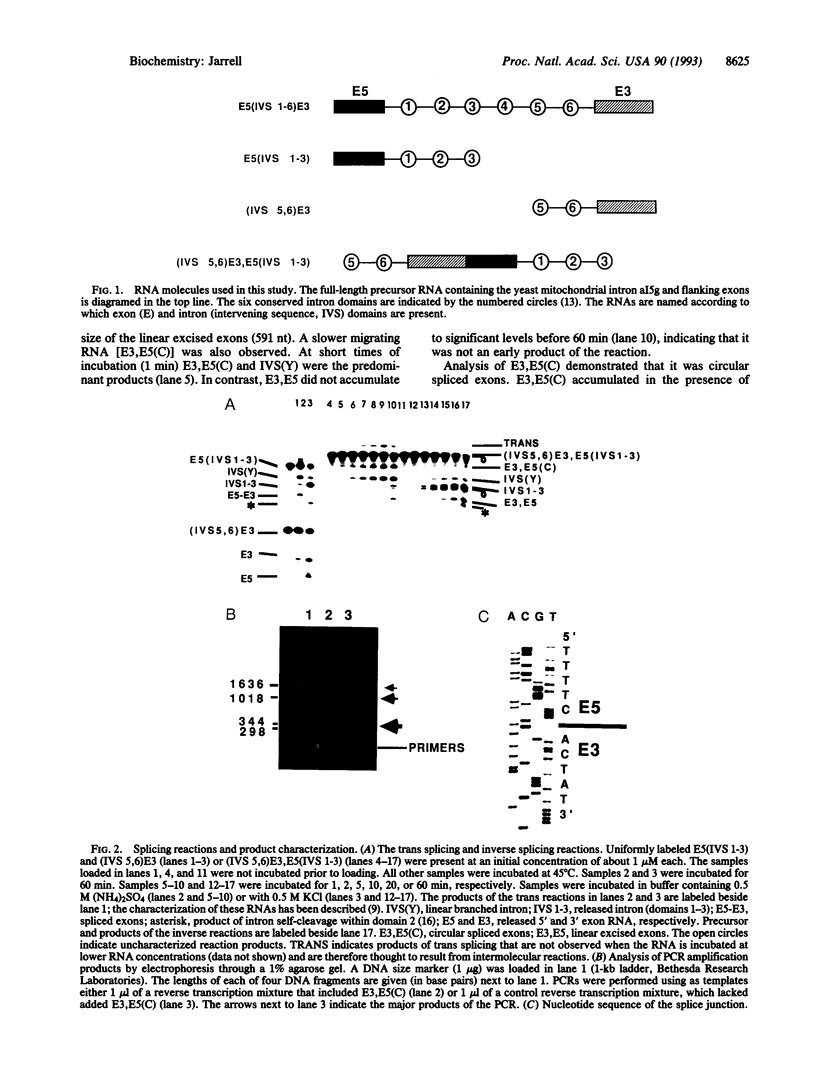
Abstract
I describe the self-splicing of an RNA that consists of exon sequences flanked by group II intron sequences. I find that this RNA undergoes accurate splicing in vitro, yielding an excised exon circle. This splicing reaction involves the joining of the 5' splice site at the end of an exon to the 3' splice site at the beginning of the same exon; thus, I term it inverse splicing. Inverse splicing provides a potential mechanism for exon scrambling, for exon deletion in alternative splicing pathways, and for exon shuffling in gene evolution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapdelaine Y., Bonen L. The wheat mitochondrial gene for subunit I of the NADH dehydrogenase complex: a trans-splicing model for this gene-in-pieces. Cell. 1991 May 3;65(3):465–472. doi: 10.1016/0092-8674(91)90464-a. [DOI] [PubMed] [Google Scholar]
- Cocquerelle C., Daubersies P., Majérus M. A., Kerckaert J. P., Bailleul B. Splicing with inverted order of exons occurs proximal to large introns. EMBO J. 1992 Mar;11(3):1095–1098. doi: 10.1002/j.1460-2075.1992.tb05148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Choquet Y., Girard-Bascou J., Michel F., Schirmer-Rahire M., Rochaix J. D. A small chloroplast RNA may be required for trans-splicing in Chlamydomonas reinhardtii. Cell. 1991 Apr 5;65(1):135–143. doi: 10.1016/0092-8674(91)90415-u. [DOI] [PubMed] [Google Scholar]
- Grabowski P. J., Zaug A. J., Cech T. R. The intervening sequence of the ribosomal RNA precursor is converted to a circular RNA in isolated nuclei of Tetrahymena. Cell. 1981 Feb;23(2):467–476. doi: 10.1016/0092-8674(81)90142-2. [DOI] [PubMed] [Google Scholar]
- Jarrell K. A., Dietrich R. C., Perlman P. S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol. 1988 Jun;8(6):2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. A., Peebles C. L., Dietrich R. C., Romiti S. L., Perlman P. S. Group II intron self-splicing. Alternative reaction conditions yield novel products. J Biol Chem. 1988 Mar 5;263(7):3432–3439. [PubMed] [Google Scholar]
- Jorcyk C. L., Watson D. K., Mavrothalassitis G. J., Papas T. S. The human ETS1 gene: genomic structure, promoter characterization and alternative splicing. Oncogene. 1991 Apr;6(4):523–532. [PubMed] [Google Scholar]
- Madhani H. D., Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992 Nov 27;71(5):803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Newman A. J., Norman C. U5 snRNA interacts with exon sequences at 5' and 3' splice sites. Cell. 1992 Feb 21;68(4):743–754. doi: 10.1016/0092-8674(92)90149-7. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Cho K. R., Fearon E. R., Kern S. E., Ruppert J. M., Oliner J. D., Kinzler K. W., Vogelstein B. Scrambled exons. Cell. 1991 Feb 8;64(3):607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- Parker R., Siliciano P. G., Guthrie C. Recognition of the TACTAAC box during mRNA splicing in yeast involves base pairing to the U2-like snRNA. Cell. 1987 Apr 24;49(2):229–239. doi: 10.1016/0092-8674(87)90564-2. [DOI] [PubMed] [Google Scholar]
- Peebles C. L., Perlman P. S., Mecklenburg K. L., Petrillo M. L., Tabor J. H., Jarrell K. A., Cheng H. L. A self-splicing RNA excises an intron lariat. Cell. 1986 Jan 31;44(2):213–223. doi: 10.1016/0092-8674(86)90755-5. [DOI] [PubMed] [Google Scholar]
- Puttaraju M., Been M. D. Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res. 1992 Oct 25;20(20):5357–5364. doi: 10.1093/nar/20.20.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissinger B., Schuster W., Brennicke A. Trans splicing in Oenothera mitochondria: nad1 mRNAs are edited in exon and trans-splicing group II intron sequences. Cell. 1991 May 3;65(3):473–482. doi: 10.1016/0092-8674(91)90465-b. [DOI] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., van der Horst G., Bonen L., Tabak H. F., Grivell L. A. Excised group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell. 1986 Jan 31;44(2):225–234. doi: 10.1016/0092-8674(86)90756-7. [DOI] [PubMed] [Google Scholar]