Abstract
Background and objectives
Ultrasonographic detection of subclinical atheromatosis is a noninvasive method predicting cardiovascular events. Risk factors predicting atheromatosis progression in CKD are unknown. Predictors of atheromatosis progression were evaluated in patients with CKD.
Design, setting, participants, & measurements
Our multicenter, prospective, observational study included 1553 patients with CKD (2009–2011). Carotid and femoral ultrasounds were performed at baseline and after 24 months. A subgroup of 476 patients with CKD was also randomized to undergo ultrasound examination at 12 months. Progression of atheromatosis was defined as an increase in the number of plaque territories analyzed by multivariate logistic regression.
Results
Prevalence of atheromatosis was 68.7% and progressed in 59.8% of patients after 24 months. CKD progression was associated with atheromatosis progression, suggesting a close association between pathologies. Variables significantly predicting atheromatosis progression, independent from CKD stages, were diabetes and two interactions of age with ferritin and plaque at baseline. Given that multiple interactions were found between CKD stage and age, phosphate, smoking, dyslipidemia, body mass index, systolic BP (SBP), carotid intima-media thickness, plaque at baseline, uric acid, cholesterol, 25-hydroxy vitamin D (25OH vitamin D), and antiplatelet and phosphate binders use, the analysis was stratified by CKD stages. In stage 3, two interactions (age with phosphate and plaque at baseline) were found, and smoking, diabetes, SBP, low levels of 25OH vitamin D, and no treatment with phosphate binders were positively associated with atheromatosis progression. In stages 4 and 5, three interactions (age with ferritin and plaque and plaque with smoking) were found, and SBP was positively associated with atheromatosis progression. In dialysis, an interaction between body mass index and 25OH vitamin D was found, and age, dyslipidemia, carotid intima-media thickness, low cholesterol, ferritin, and uric acid were positively associated with atheromatosis progression.
Conclusions
Atheromatosis progression affects more than one half of patients with CKD, and predictive factors differ depending on CKD stage.
Keywords: atherosclerosis; chronic kidney disease; cardiovascular disease; blood pressure; carotid intima-media thickness; follow-up studies; humans; renal insufficiency, chronic; smoking; vitamin D
Introduction
Cardiovascular disease is the main cause of death in patients with CKD, in which cardiovascular death is a more likely outcome than progression to ESRD (1). Classic risk prediction equations underestimate cardiovascular disease risk in adults with CKD (2,3), in which most events occur in patients with low-moderate risk (4,5). Therefore, new tools for risk prediction in renal patients are needed. Arterial ultrasonography assessment of subclinical atheromatosis is a noninvasive imaging technique that could predict incidence of cardiovascular events better than traditional models (6–9). It has been shown that increases in plaque burden have a strong predictive value assessing cardiovascular events in patients on dialysis (10) and the general population (7). However, data on patients in early stages of CKD are scarce (11).
Although numerous studies show an association between decreased kidney function and cardiovascular disease and mortality, mechanisms underlying this association are incompletely understood. This could be related to differences in cardiovascular events types and the associated factors amid the progression of CKD. Thus, in early stages, there is a high mortality rate due to atherothrombotic ischemic events (12–14), but in dialysis mortality is mainly caused by heart failure and sudden cardiac death (15). Determining risk factors predicting atherosclerosis progression in every CKD stage could help to establish effective preventive measures to decrease cardiovascular disease in patients with CKD.
Recently, baseline data from the National Observatory of Atherosclerosis in Nephrology (NEFRONA) (16) Study have shown that patients in early CKD stages already have a higher prevalence of atheromatous plaques than those without CKD (17,18), and as previously shown, this prevalence is even higher in advanced stages of CKD (19–21). However, it is uncertain what the velocity of atheromatosis progression in CKD is (22,23), and little is known about the predictors of plaque progression in patients with CKD. Only two studies have addressed this question: one with patients on dialysis (10) and one with a limited number of patients with CKD stage 3 or 4 with a short interval ultrasound control (22).
We present here the first longitudinal results from the NEFRONA Study regarding the risk factors predicting progression of atheromatosis in a large CKD patient population. Furthermore, we assessed whether atheromatosis progression is associated with CKD progression.
Materials and Methods
Design and Study Population
The protocol of the study was approved by the ethics committees of each hospital, and all patients were included after signing informed consent. This research followed the principles of the Declaration of Helsinki. The design and objectives of the NEFRONA Study have been published in detail (24,25). Briefly, 2445 patients with CKD (937 in CKD stage 3, 820 in CKD stage 4 or 5, and 688 on dialysis) who were 18–75 years of age were enrolled from 81 Spanish hospitals between October of 2009 and June of 2011, with a scheduled follow-up visit after 24 months. Additionally, 476 patients with CKD were randomly selected to undergo ultrasound examination at 12 months. Patients from the NEFRONA Study who had stenotic carotid plaque, had ankle-brachial index (ABI) <0.7 at baseline, had a cardiovascular event, received a renal allograft, or died after the first ultrasound exploration were excluded from the follow-up visit.
Clinical Data and Laboratory Examinations
Current health status, medical history, former cardiovascular risk factors, and drug use information were obtained at baseline. A physical examination was performed, consisting of anthropometric measures, standard vital tests, and ABI measurements as previously described (16). A pathologic ABI was described as ≤0.9 or ≥1.4. Biochemical data were obtained from a routine fasting blood test within 3 months from the vascular study. GFR was estimated using the Modification of Diet in Renal Disease Study formula. Parathyroid hormone (PTH) levels in patients on dialysis were corrected using a well established method to avoid intermethod variability between different centers (26).
Ultrasound Imaging
B-mode ultrasound of the carotid and femoral arteries was performed using the Vivid BT09 apparatus (GE Healthcare, Waukesha, WI) equipped with a 6- to 13-MHz broadband linear array probe as previously described (27). An atheromatous plaque presence analysis was performed in 10 territories (internal, bulb, and common carotids and both common and superficial femoral arteries) by a single reader in blinded fashion using semiautomatic software (EchoPAC Dimension; GE Healthcare). To assess intraobserver reliability, a sample of 20 individuals was measured three to five times on different days. A κ-coefficient of one was obtained for plaque assessment, indicating excellent intraobserver reliability. The reader was unaware of patients’ clinical histories.
Carotid intima-media thickness (cIMT) was only measured in common carotid regions without plaque and calculated as the average between left and right sides. The presence of atheromatous plaques was defined as a cIMT>1.5 mm protruding into the lumen according to the American Society of Echocardiography Consensus Statement (28) and the Mannheim cIMT Consensus (29).
Evaluation of Progression
Atheromatosis progression was defined as an increase in the number of territories showing a plaque with respect to the baseline visit as previously used in the Multi-Ethnic Study of Atherosclerosis (MESA) Study (30). Individuals doubling basal serum creatinine or starting RRT were considered CKD progressive. Thus, patients on dialysis at baseline were excluded from this last variable.
Statistical Analyses
Data are expressed as means and SDs for quantitative variables and relative frequencies for qualitative variables. In some quantitative variables, in which nonlinear effects were detected, tertiles were used.
The relationship between potential risk factors with plaque progression at 24 months was analyzed by univariate and multivariate logistic regression. Chi-squared or McNemar tests were used to compare qualitative variables between progression and nonprogression; the Mann–Whitney test was used for quantitative variables. Significant variables in univariate analyses and potential confounders were used to develop appropriate multivariate logistic regression models: age, sex, diabetes, smoking status, body mass index (BMI), systolic BP (SBP), triglycerides, ferritin, cholesterol, dyslipidemia, uric acid, CKD stage, 25-hydroxy vitamin D (25OH vitamin D), cIMT, plaque at baseline, statins treatment, and antiplatelet drugs. A forward step procedure was used to build the multivariate model, including the variable showing maximum contribution identifying those patients with 24-month atheromatosis progression according to the likelihood ratio test. Those variables without a statistically significant contribution but modifying in >10% the value of the coefficients (β) of any of the significant variables when removed from the model were considered confounders and included in the final model. The model with the contributing or confounding variables was assessed for interactions of first and second order in a backward procedure to optimize Akaike information criterion. The contribution of the interactions identified by the former procedure was assessed by the likelihood ratio test to discard those interactions without a statistically significant contribution to the model. In case an explanatory variable showed multiple interactions with other covariates in the model, the analysis was stratified by that variable to facilitate interpretation. A statistical significance level of 0.05 was used.
All analyses were made using a standard statistical package (SPSS 21.0; SPSS Inc., Chicago, IL) and R program.
Results
Baseline Characteristics
From the original NEFRONA Study cohort (2445 patients with CKD), 888 were excluded from the 24-month analysis, namely deaths (46), cardiovascular events (80), renal allografts during 2 years (359), and second visit nonattendees (403). Finally, 1553 (709 CKD stage 3, 578 CKD stage 4 or 5, and 266 on dialysis) patients were included in the plaque progression analysis at 24 months, because four presented plaque in all ten territories at baseline. Furthermore, there were 476 patients who were also assessed after 12 months.
The cohort consisted of 61.6% men, and mean age was 58.8 years old. Of the patients, 29% were diabetic, and 56% smokers. Baseline characteristics in participants according to CKD stages are detailed in Table 1. The percentage of men, smokers, and the average age were higher among patients with CKD stage 3. Patients on dialysis showed lower BMI, SBP, total cholesterol, LDL-cholesterol, corrected serum calcium, and 1,25-dihydroxy vitamin D, whereas serum PTH and high–sensitivity C–reactive protein were higher. Atheromatous plaque prevalence was 68.7% without significant differences between CKD stages. At baseline, 53% of patients had plaques in multiple territories, and 16% had a single plaque; 37.2% had plaque in carotids and femorals, whereas 18.4% had plaque only in carotids, and 13.1% had plaque exclusively in femorals. Prevalence of pathologic ABI was 23.3%. The percentage of patients with pathologic ABI was higher among patients on dialysis because of a high prevalence of ABI>1.4.
Table 1.
Main baseline characteristics in participants according to CKD stage (n=1553)
| Variables | CKD 3 | CKD 4–5 | CKD 5D | P Value |
|---|---|---|---|---|
| n | 709 (46) | 578 (37) | 266 (17) | |
| Men | 481 (67.8) | 328 (56.7) | 147 (55.3) | <0.001 |
| Age, yr | 63 (56, 69) | 61 (51, 68) | 55 (44, 66) | <0.001 |
| Race, white | 700 (98.7) | 562 (97.2) | 247 (92.9) | <0.001 |
| Medical history | ||||
| Smoker | 413 (58.3) | 317 (58.4) | 139 (52.2) | <0.001 |
| Diabetes | 186 (26.2) | 158 (27.3) | 40 (15) | <0.001 |
| Hypertension | 645 (91) | 550 (95.2) | 232 (87.2) | <0.001 |
| Dyslipidemia | 506 (71.4) | 409 (70.8) | 151 (56.8) | <0.001 |
| Etiology of renal disease | ||||
| Vascular disease | 217 (37.2) | 94 (18.4) | 9 (11) | <0.001 |
| Diabetic nephropathy | 84 (14.4) | 90 (17.6) | 25 (11.5) | 0.12 |
| Others | 282 (48.4) | 326 (63.9) | 169 (77.5) | <0.001 |
| Body mass index, kg/m2 | 28.8 (26, 31.8) | 27.7 (24.9, 32) | 26.1 (23.1, 30) | <0.001 |
| Abdominal obesitya | 142.5 (52.6) | 294 (51.9) | 84 (45.4)a | 0.21 |
| Systolic BP, mmHg | 142.4 (19.5) | 143.9 (20.5) | 139.4 (23) | 0.03 |
| Diastolic BP, mmHg | 81.9 (10.07) | 82 (11.1) | 80.5 (13.5) | 0.33 |
| Pulse pressure, mmHg | 60.5 (15.8) | 61.9 (18) | 58.8 (18.6) | 0.10 |
| Time on dialysis, mo | 16.6 (7, 33.8) | NA | ||
| Total cholesterol, g/dl | 188.3 (36.9) | 177.9 (37.1) | 165.03 (45.2) | <0.001 |
| LDL-cholesterol, g/dl | 111 (32.5) | 101.1 (32.3) | 90.9 (34.7) | <0.001 |
| HDL-cholesterol, g/dl | 48 (40, 58) | 47 (37.8, 59) | 45 (36.4, 56) | <0.001 |
| Non–HDL-cholesterol, mg/dl | 138.2 (34.8) | 128.9 (35.1) | 118.8 (43.3) | <0.001 |
| Triglycerides, mg/dl | 122 (92, 174) | 125 (91, 171) | 121 (90, 170) | 0.95 |
| Hemoglobin A1c, %b | 6 (5.5, 6.7) | 5.9 (5.4, 6.8) | 5.1 (4.8, 5.8) | <0.001 |
| Albumin, g/dl | 4.2 (0.4) | 4.1 (0.4) | 3.8 (0.4) | <0.001 |
| Hematocrit, % | 41.6 (4.7) | 37.4 (4.2) | 35.8 (4.4) | <0.001 |
| Ferritin, mg/dl | 110.1 (60, 210) | 148 (74, 268) | 316 (159, 474) | <0.001 |
| Corrected calcium, mg/dl | 9.27 (0.5) | 9.2 (0.5) | 9.1 (0.7) | <0.001 |
| Phosphate, mg/dl | 3.3 (0.5) | 4 (0.7) | 4.8 (1.2) | <0.001 |
| PTH, pg/ml | 68.7 (49.2, 98) | 137 (91.6, 212) | 226 (145, 344) | <0.001 |
| Uric acid, g/dl | 6.79 (1.5) | 6.99 (1.6) | 6.02 (1.4) | <0.001 |
| 25-hydroxy vitamin D, pg/ml | 15.2 (11.8,20.1) | 15.7 (11.3, 19.9) | 13.6 (10, 18.9) | <0.001 |
| 1,25-hydroxy vitamin D, ng/ml | 19.3(14, 26.3) | 15.3 (10.1, 22.2) | 6.2 (4.1, 9.9) | <0.001 |
| High–sensitivity C–reactive protein, mg/L | 1.8 (0.9, 3.7) | 1.72 (0.84, 3.98) | 2.5 (0.99, 6.6) | 0.01 |
| UACR, mg/gc | 51.5 (7.6, 264) | 173.4 (30, 677) | NAc | <0.001 |
| CKD progression | 12 (1.7) | 146 (25.3) | NA | <0.001 |
| Atheromatosis progression | 418 (59) | 344 (59.5) | 168 (63.2) | 0.47 |
| Treatments | ||||
| Antihypertensive | 655 (92.4) | 560 (96.9) | 194 (72.9) | <0.001 |
| Statins | 425 (67.8) | 370 (64) | 136 (51.1) | <0.001 |
| Phosphate binders | 38 (5.4) | 186 (32.2) | 210 (78.9) | <0.001 |
| Vitamin D | 128 (18.1) | 281 (48.6) | 136 (51.1) | <0.001 |
| Antiplatelet drugs | 153 (21.6) | 134 (23.2) | 76 (28.6) | 0.07 |
| Plaque presence | 495 (69.8) | 385 (66.6) | 186 (69.9) | 0.41 |
| Multiple plaquesd | 363 (73.3) | 310 (80.5) | 143 (76.9) | 0.12 |
| No. of territories with plaqued | 3 (1, 4) | 3 (2, 4) | 3 (2, 4.3) | 0.88 |
| cIMT, mme | 0.74 (0.14) | 0.7 (0.1) | 0.7 (0.1) | <0.001 |
| Pathologic ankle-brachial index | 147 (20.7) | 126 (21.9) | 88 (33.2) | <0.001 |
| ≤0.9 | 109 (15.4) | 76 (13.2) | 30 (11.3) | 0.22 |
| ≥1.4 | 38 (5.4) | 50 (8.7) | 58 (21.9) | <0.001 |
Quantitative data are expressed as means and SDs or medians (percentile 25, percentile 75) depending on the normality of the distribution. Qualitative variables are expressed as N (%). NA, not applicable; PTH, parathyroid hormone; UACR, urine albumin-to-creatinine ratio; cIMT, common carotid artery intima media thickness.
Abdominal obesity: waist circumference ≥102 cm (men) or ≥88 cm (women; not measured in patients on peritoneal dialysis).
Hemoglobin A1c was measured only in patients with diabetes.
UACR and CKD progression were not measured in patients on dialysis.
In patients with plaque at baseline.
P values reflect differences between CKD stages.
Analysis of Progression at 24 months
The percentage of patients with plaque increased from 68.6% to 81.4% at 24 months. Atheromatosis progression occurred in 59.8% of patients. Mean number of territories with plaque increased from 3.16 (SD=1.97) to 4.48 (SD=2.34).
Baseline potential factors predicting atheromatosis progression in univariate analysis are detailed in Table 2. In a multivariate regression model assessing all significant predictors in the univariate analysis (Supplemental Table 1), only diabetes and two interactions of first order (age with ferritin and the presence of plaque at baseline) were independent predictors of atheromatosis progression, with a similar effect across all CKD stages. Thus, the association of ferritin and plaque with the progression of atherosclerosis decreased with age. Significant interactions were found between CKD stage and age, phosphate, smoking, dyslipidemia, BMI, SBP, cIMT, plaque at baseline, uric acid, cholesterol, 25OH vitamin D, and antiplatelet and phosphate binders use, three of them of second order. Therefore, to better understand risk factors for progression of atheromatosis, a multivariate model stratified by CKD stages was built. This decision was justified by the existence of multiple interactions of CKD stage with other clinical variables, and also, many of those parameters show a very wide range when individuals from different CKD stages are pooled together. For instance, PTH or phosphate levels stay relatively stable in early stages, increasing very steeply in dialysis.
Table 2.
Baseline factors associated with progression of atheromatosis in all patients (n=1553)
| Atheromatosis Progression | No Atheromatosis Progression | P Value | |
|---|---|---|---|
| N | 930 (59.8) | 623 (40.1) | |
| Men | 608 (67.8) | 348 (56.7) | <0.001 |
| Age, yr | 64 (56, 70) | 57 (43, 66) | <0.001 |
| Race, white | 914 (98.3) | 595 (95.5) | <0.001 |
| Medical history | |||
| Smoker | 561 (60.3) | 308 (49.4) | <0.001 |
| Diabetes | 877 (94.3) | 550 (88.3) | <0.001 |
| Hypertension | 278 (29.9) | 106 (17) | <0.001 |
| Dyslipidemia | 674 (72.5) | 392 (62.9) | <0.001 |
| Etiology of renal disease | |||
| Vascular disease | 210 (27.1) | 125 (23.3) | 0.12 |
| Diabetic nephropathy | 143 (18.5) | 56 (10.4) | <0.001 |
| Others | 422 (54.5) | 355 (66.2) | <0.001 |
| Body mass index, kg/m2 | 28.5 (25.4, 31.8) | 27.7 (24.5, 31.6) | <0.001 |
| Abdominal obesitya | 469 (53.8) | 287 (48.1) | 0.03 |
| Systolic BP, mmHg | 144.8 (21.2) | 138.9 (19) | <0.001 |
| Diastolic BP, mmHg | 81.57 (11.4) | 81.96 (10.7) | <0.001 |
| Pulse pressure, mmHg | 63.29 (18.0) | 57.01(15.2) | <0.001 |
| CKD stage | 0.48 | ||
| 3 | 418 (44.9) | 291 (46.7) | |
| 4 or 5 | 344 (37) | 234 (37.6) | |
| 5D | 168 (18.1) | 98 (15.7) | |
| Time on dialysis, mo | 17.5 (6.7, 33.4) | 16.3 (7.4, 34.2) | 0.82 |
| Total cholesterol, g/dl | 179.9 (39.8) | 181.8 (38.6) | 0.27 |
| LDL-cholesterol, g/dl | 102.9 (33.5) | 105.2 (33.7) | 0.26 |
| HDL-cholesterol, g/dl | 49 (15.2) | 50.1 (14.7) | 0.11 |
| Non–HDL-cholesterol, mg/dl | 131.1 (38.3) | 131.7 (35.4) | 0.49 |
| Triglycerides, mg/dl | 126 (94, 176.5) | 118 (86, 165) | <0.001 |
| Hemoglobin A1c, %b | 6 (5.4, 6.7) | 5.7 (5.2, 6.4) | <0.001 |
| Albumin, g/dl | 4.2 (0.4) | 4.1 (0.4) | 0.93 |
| Hematocrit, % | 39 (5) | 39.2 (5.1) | 0.51 |
| Ferritin, mg/dl | 160.1 (78, 313.2) | 136.2 (66.5, 242) | <0.001 |
| Corrected calcium, mg/dl | 9.2 (0.5) | 9.2 (0.5) | 0.10 |
| Phosphate, mg/dl | 3.9 (0.9) | 3.8 (0.9) | 0.42 |
| PTH, pg/ml | 114.8 (68.4, 193.8) | 101 (62.3, 186) | 0.08 |
| Uric acid, g/dl | 6.71 (1.6) | 6.78 (1.6) | 0.29 |
| 25-hydroxy vitamin D, pg/ml | 14.7 (11.2, 19.1) | 15.9 (11.9, 20.7) | <0.001 |
| 1,25-hydroxy vitamin D, ng/ml | 16.1 (10.3, 22.9) | 15.6 (10.1, 22.7) | 0.83 |
| High–sensitivity C–reactive protein, mg/L | 2 (0.96, 4.7) | 1.77 (0.8, 3.8) | <0.001 |
| UACR, mg/gc | 92.5 (12, 438.6) | 100.8 (13.7, 404) | 0.83 |
| Treatments | |||
| Antihypertensive | 857 (92.2) | 552 (88.6) | 0.02 |
| Hypolipemiant | 578 (69.5) | 353 (60.5) | <0.001 |
| Phosphate binders | 257 (27.6) | 177 (28.4) | 0.74 |
| Vitamin D | 334 (35.9) | 211 (33.9) | 0.41 |
| Antiplatelet drugs | 238 (25.6) | 125 (20.1) | 0.01 |
| Plaque at baseline | 733 (78.8) | 333 (53.5) | <0.001 |
| Multiple plaquesd | 562 (76.6) | 254 (76.2) | 0.89 |
| No. of territories with plaqued | 2 (1, 4) | 1 (0, 3) | 0.43 |
| cIMT, mm | 0.74 (0.14) | 0.67 (0.13) | <0.001 |
| Pathologic ankle-brachial index | 226 (24.3) | 135 (21.7) | 0.24 |
| ≤0.9 | 128 (13.8) | 87 (14) | 0.90 |
| ≥1.4 | 98 (10.5) | 48 (7.7) | 0.06 |
| CKD progression | 109 (14.3) | 49 (9.4) | <0.001 |
Quantitative data expressed as means and SDs or medians (percentile 25, percentile 75) depending on the normality of the distribution. Qualitative variables are expressed as N (%). PTH, parathyroid hormone; UACR, urine albumin-to-creatinine ratio; cIMT, common carotid artery intima media thickness.
Abdominal obesity: waist circumference ≥102 cm (men) or ≥88 cm (women; not measured in patients on peritoneal dialysis).
Hemoglobin A1c was measured only in patients with diabetes.
UACR and CKD progression were not measured in patients on dialysis.
In patients with plaque at baseline.
Atheromatosis Progression at 24 months Stratified by CKD Stage
Risk factors of atheromatosis progression were analyzed in each CKD stage (Table 3). In stage 3, age, SBP, smoking, phosphate, the lack of phosphate binder use, diabetes, plaque presence, and low 25OH vitamin D significantly predicted progression of atheromatosis. Two interactions of age with phosphate and plaque at baseline were also found. In CKD stages 4 and 5, age, SBP, smoking, plaque presence, and ferritin significantly predicted the formation of new plaque, with significant interactions of age with plaque and ferritin as well as smoking with plaque. In dialysis, factors associated with new plaque formation were age, basal cIMT, dyslipidemia, ferritin, and uric acid, whereas BMI, total cholesterol, and 25OH vitamin D were inversely associated. A significant interaction was found between vitamin D and BMI.
Table 3.
Multivariate logistic regression to model plaque progression at 24 months stratified by CKD stages 3, 4–5, and 5D
| CKD Stage 3 | CKD Stages 4 and 5 | CKD Stage 5D | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95 %CI) | P Value | OR (95% CI) | P Value | |
| Age, decades | 1.65 (1.24 to 2.25)a | <0.001a | 1.95 (1.43 to 2.75)a | <0.001a | 1.48 (1.07 to 2.06)a | 0.02a |
| Smoking, current and former versus no | 1.77 (1.24 to 2.55)a | <0.001a | 3.95 (1.91 to 8.50)a | <0.001a | 1.32 (0.64 to 2.74) | 0.45 |
| Diabetes, yes versus no | 1.59 (1.06 to 2.41)a | 0.03a | 1.45 (0.92 to 2.31) | 0.11 | 2.37 (0.66 to 10.74) | 0.22 |
| Systolic BP ≥150 mmHg | 1.46 (1.01 to 2.12)a | 0.04a | 2.84 (1.86 to 4.39)a | <0.001a | 0.64 (0.29 to 1.40) | 0.26 |
| Phosphate >3.6 mg/dl | 1.62 (1.09 to 2.43)a | 0.02a | 0.84 (0.54 to 1.30) | 0.44 | 0.80 (0.28 to 2.17) | 0.66 |
| Phosphate binders use, yes versus no | 0.42 (0.19 to 0.88)a | 0.02a | 0.78 (0.51 to 1.19) | 0.25 | 1.38 (0.56 to 3.34) | 0.48 |
| 25-hydroxy vitamin D, pg/ml | 0.62 (0.43 to 0.89)a | 0.01a | 1.34 (0.82 to 2.20) | 0.24 | 0.08 (0.01 to 0.47)a | 0.01a |
| Plaque at baseline, yes versus no | 1.33 (0.85 to 2.06) | 0.21 | 3.16 (1.68 to 6.02)a | <0.001a | 1.20 (0.50 to 2.82) | 0.67 |
| Age × phosphate | 1.70 (1.16 to 2.54)a | 0.01a | 0.95 (0.63 to 1.42) | 0.22 | 1.02 (0.44 to 2.09) | 0.95 |
| Age × plaque at baseline | 0.66 (0.45 to 0.96)a | 0.03a | 0.62 (0.41 to 0.93)a | 0.02a | 0.58 (0.29 to 1.11) | 0.11 |
| Ferritin >220 ng/ml | 1.33 (0.82 to 2.18) | 0.25 | 1.37 (0.89 to 2.14) | 0.16 | 2.54 (1.20 to 5.52)a | 0.02a |
| Age × ferritin >220 ng/ml | 0.91 (0.58 to 1.49) | 0.69 | 0.66 (0.45 to 0.97)a | 0.04a | 1.22 (0.70 to 2.11) | 0.95 |
| Smoking × plaque at baseline | 0.45 (0.19 to 1.02) | 0.06 | 0.32 (0.13 to 0.78)a | 0.01a | 2.75 (0.60 to 13.12) | 0.20 |
| BMI, kg/m2 | 0.99 (0.96 to 1.03) | 0.76 | 1.01 (0.97 to 1.05) | 0.67 | 0.79 (0.66 to 0.93)a | 0.01a |
| Dyslipidemia, yes versus no | 1.27 (0.86 to 1.85) | 0.23 | 1.39 (0.89 to 2.15) | 0.14 | 5.14 (2.34 to 12.01)a | <0.001a |
| Cholesterol >180 mg/dl | 1.15 (0.81 to 1.63) | 0.45 | 1.06 (0.71 to 1.6) | 0.77 | 0.31 (0.13 to 0.70)a | 0.01a |
| Uric acid, mg/dl | 1.08 (0.96 to 1.21) | 0.22 | 1.02 (0.91 to 1.16) | 0.72 | 1.38 (1.06 to 1.84)a | 0.02a |
| cIMT, mm | 1.07 (0.93 to 1.23) | 0.37 | 1.12 (0.95 to 1.33) | 0.18 | 1.66 (1.22 to 2.32)a | <0.001a |
| Ln 25-hydroxy vitamin D × BMI | 1.01 (0.94 to 1.09) | 0.82 | 0.95 (0.87 to 1.03) | 0.22 | 1.16 (1.02 to 1.33)a | 0.02a |
Results are expressed as odds ratios (ORs; exponential-β for independent variables with interactions) and 95% confidence intervals (95% CIs). The following variables were introduced to build multivariate models by CKD stages, because they were significant on bivariate testing or potential confounders: sex, CKD stage, age (decades), diabetes, smoking, dyslipidemia, systolic BP ≥150 mmHg (highest tertile in CKD stage 3), pulse pressure, body mass index (BMI), basal plaque, common carotid artery intima media thickness (cIMT), ferritin >220 mg/dl (highest tertile in CKD stages 4 and 5), uric acid, C-reactive protein, total cholesterol >180 mg/dl (the level of 180 was selected on the basis of clinical criteria), LDL-cholesterol, hematocrit, statins, antiplatelet drugs, triglycerides, 25-hydroxy vitamin D (ln 25-hydroxy vitamin D for interactions and CKD stages 4–5 and 5D and 25-hydroxy vitamin D ≥18.1; vitamin D highest tertile in CKD stage 3), phosphate (highest tertile in CKD stage 3 >3.6 mg/dl), and parathyroid hormone. Only significant variables in multivariate analysis in each group of CKD were included in the final model. The exponential-β and P values of the rest of the variables are the values obtained if added to the final model. CKD stage 3: Hosmer Lemeshow = 0.46; AUC=0.72; CKD stages 4 and 5: Hosmer Lemeshow = 0.82; AUC=0.74; and CKD stage 5D: Hosmer Lemeshow = 0.16; AUC=0.83. AUC, area under the curve.
Significant variables included in each model.
Presence of Plaque at Baseline, CKD Progression, and Atheromatosis Progression
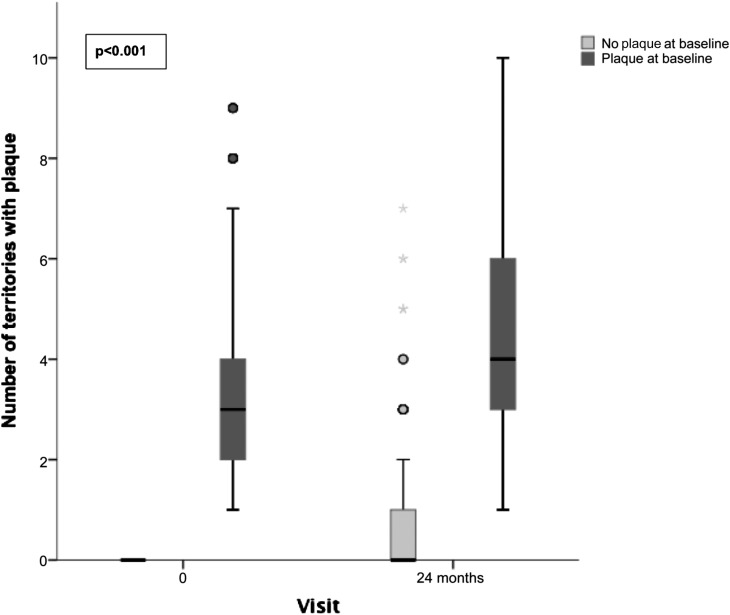
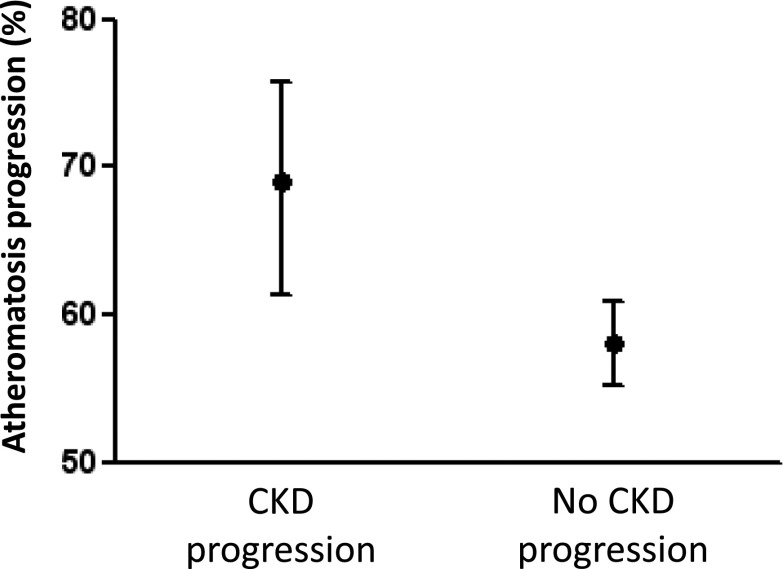
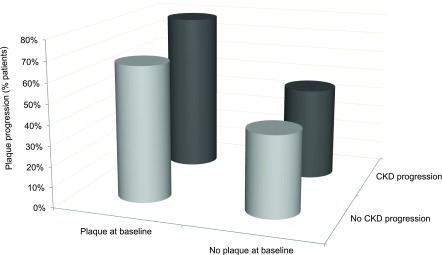
In contrast to patients with plaque at baseline, atheromatosis progression in patients free from disease at baseline was very low (Figure 1). Furthermore, bivariate analysis shows that the percentage of patients with atheromatosis progression was higher in the group with CKD progression (Table 2). Figure 2 shows that progression of atheromatosis was significantly more frequent in patients with CKD progression. Figure 3 shows the percentage of patients with atheromatosis progression according to CKD progression and the presence of plaques at baseline. Almost 80% of patients in whom CKD progressed and had plaque at baseline experienced aggravation of atheromatosis. At the other end, around 60% of patients in whom CKD did not progress and who were free of plaques at baseline did not suffer an increase in atheromatous burden. Spearman correlation between progressors in both CKD and atheromatosis was positive and statistically significant (r=0.10; P=0.004).
Figure 1.
Atheromatous disease progress more in patients with plaque at baseline than in patients without plaque at baseline. Boxplot showing the number of territories with plaque at baseline and after 24 months of follow-up according to the presence of plaque at baseline. The highest whisker indicates the last value that is less than or equal to the result of the standard formula percentile 75+1.5× interquartile range (IQR). Circles and stars represent readings that are outliers or far outliers, respectively (defined as higher than percentile 75+1.5×IQR or percentile 75+3×IQR, respectively). P<0.001 between progression in patients with plaque at baseline versus progression in patients without plaque at baseline.
Figure 2.
Percentages and 95% confidence intervals of patients who showed atheromatosis progression according to CKD progression after 24 months. P<0.001.
Figure 3.
Percentage of patients with progression of atheromatosis over 24 months according to basal plaque and CKD progression in CKD stages 3 and 4–5. P<0.001 between progression in patients with neither CKD progression nor plaque at baseline and progression in patients with CKD progression and plaque at baseline.
Atheromatosis Progression at 12 months
In one patient subgroup, progression of atheromatosis was also evaluated after 12 months from the first visit, and plaque progression was observed in 47% of the patients. Of those, 54% of them progressed even more at 24 months. Of the nonprogressors at 12 months, 38% progressed at 24 months (Supplemental Figure 1).
Discussion
In this work, we analyze atheromatosis progression and the factors predicting it in a subpopulation of the NEFRONA Study who did not suffer a cardiovascular event during the 24 months of follow-up. We had four main findings. (1) In CKD, there is a high prevalence of patients with atheromatous plaque in carotid and/or femoral territories, but about 30% are free from atheromatosis. Moreover, in about 40% of patients, atheromatosis progression is absent after 2 years. (2) The only variables with a homogeneous association with the progression of plaque across CKD stages were the presence of diabetes and two interactions of age with ferritin and the presence of plaque at baseline. (3) Apart from those variables, risk factors predicting atheromatosis progression are different depending on the CKD stage. (4) The progression of atheromatosis is associated with the progression of CKD.
The data also show that, although this is a population with high cardiovascular risk, a significant proportion of patients is free from atheromatosis. Interestingly, in these particular patients without plaque at baseline, atheromatosis progression is slow or nonexistent, suggesting a phenotype of individuals protected from the disease. Identifying the phenotypic characteristics in these patients is of great interest to the study of the pathogenesis of atheromatosis.
The progression of atheromatosis was assessed by quantifying the number of carotid and femoral territories with plaque as previously reported in other studies (30). Quantifying did not require special software (for a more sophisticated analysis, such as plaque area or volume) and also predicts cardiovascular events more accurately than cIMT (9). Benedetto et al. (10) showed that progression of atheromatosis is a better predictor of cardiovascular events than the baseline number of plaques in a cohort of patients with ESRD, indicating that monitoring the evolution of plaque burden adds independent prognostic information. One novelty included in our study is the analysis of femoral arteries, which are not routinely explored. Our results indicate that, if only carotid territories had been explored, the presence of plaque would have been underdiagnosed in around 13% of patients. Although exploration of the femoral territories may cause discomfort to the patient, it does not increase the cost of the exploration and provides valuable information for a significant proportion of patients.
Interestingly, it was found that CKD progression is associated with atheromatosis progression. Although both occur concurrently and we cannot use one as a predictor of the other, the results show that both pathologies are closely related and that patients who progress in one are more likely to progress in the other, independent of other factors.
The multivariate logistic regression model to predict progression of atheromatosis in the whole population showed traditional risk factors similar to those in the results of the MESA Study (30), the Rotterdam Study (31), and the TromsØ Study (32), although we found multiple interactions between CKD stages and the other clinical variables. However, being diabetic predicted atheromatosis progression independent of the CKD stage. Furthermore, having plaque at baseline and high levels of ferritin also predicted the progression of atheromatosis independent of the degree of renal function, although this effect is modified by age, being lower in older patients.
The stratified analysis showed that, in CKD stage 3, SBP, smoking, and diabetes predicted progression of atheromatosis. Therefore, in early stages of CKD, we can use classic cardiovascular risk factors to predict plaque progression. Additionally, high phosphate levels and low 25OH vitamin D levels were also predictors of atheromatosis progression. Indeed, a potential role for phosphate and 25OH vitamin D levels has been previously suggested in the progression of atherosclerosis (33–37). Furthermore, a significant interaction between phosphate levels and age was found, showing that, when phosphate levels are >3.6 mg/dl, the association with age is much stronger. This is consistent with previous results only showing a phosphate binder benefit in older patients (38). However, in our study, patients treated with phosphate binders seemed to have lower odds of atheromatosis progression, independent of their age. However, the association with age is minor in patients with plaque already at baseline, because this in itself is a high-risk condition. In CKD stages 4 and 5, new risk factors appear, like the ferritin levels. Thus, it seems that inflammation starts to play a role in atheromatous plaque progression in predialysis stages. In patients on dialysis, risk factors for atheromatosis progression are completely different. A paradoxical association was observed with cholesterol levels. However, history of dyslipidemia still persists as a risk factor, suggesting that actual low cholesterol levels could be a reflection of a state of malnutrition, which is reflected by a better prognosis in patients with a higher BMI. Uric acid seems to be a predictor of atheromatosis progression, agreeing with previous results (39,40). Inflammation, estimated by high ferritin levels, persists as an important risk factor for atheromatosis. Low vitamin D levels are also predictors of plaque progression, confirming previous experimental studies showing the effect of a vitamin D deficit on atheromatosis (41–43). A significant interaction between vitamin D and BMI was also found. Only patients with low to moderate BMI showed lower odds of progression with high vitamin D levels. The results also confirm that higher cIMT emerges as a predictor of atheromatosis progression in this group of patients.
The main strength of this study is the large number of patients with longitudinal observations, which allows us to make predictive associations between multiple factors and atheromatosis progression and adjust for multiple confounders. Another strength is that the vascular exploration was performed by the same team and evaluated by a single reader.
This study also has several limitations. First, the large number of dropouts lowers the statistical power of the study. Given that only patients without cardiovascular events were evaluated at baseline and follow-up and that most of the dropouts were either patients receiving a transplant or patients who died, a subpopulation with intermediate health status was probably being analyzed. Second, plaque volume and density were not measured. Third, fibroblast growht factor 23 levels were lacking, which could have been mediating the effects of high phosphate in atheromatosis, and only a small portion of patients had levels of proteinuria.
In summary, data presented here show the validity of the determination of atheroma plaque presence by arterial ultrasound as a powerful tool to predict atheromatosis progression in patients with CKD. The factors predicting atheromatosis progression in each CKD stage are different. Furthermore, CKD progression and atheromatosis progression are closely associated.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the NEFRONA team (Eva Castro, Virtudes Maria, Teresa Molí, and Teresa Vidal) and the Biobank of RedInRen for valuable help.
The work presented here was funded by a research grant from Abbvie, the Spanish Government, and Fondos Europeos de Desarrollo European Funds for Regional Development (FEDER) funds Thematic Networks for Collaborative Research (RETIC) grant RD12/0021/0026 and Fund for Sanitary Research (FIS) grant PS10/00946.
Supplemental Material lists the NEFRONA investigators.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01240215/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Chang A, Kramer H: Should eGFR and albuminuria be added to the Framingham risk score? Chronic kidney disease and cardiovascular disease risk prediction. Nephron Clin Pract 119: c171–c177, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Coll B, Betriu A, Martínez-Alonso M, Borràs M, Craver L, Amoedo ML, Marco MP, Sarró F, Junyent M, Valdivielso JM, Fernández E: Cardiovascular risk factors underestimate atherosclerotic burden in chronic kidney disease: Usefulness of non-invasive tests in cardiovascular assessment. Nephrol Dial Transplant 25: 3017–3025, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Lamprea-Montealegre JA, Astor BC, McClelland RL, de Boer IH, Burke GL, Sibley CT, O’Leary D, Sharrett AR, Szklo M: CKD, plasma lipids, and common carotid intima-media thickness: Results from the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol 7: 1777–1785, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner DE, Tighiouart H, Griffith JL, Elsayed E, Levey AS, Salem DN, Sarnak MJ: Kidney disease, Framingham risk scores, and cardiac and mortality outcomes. Am J Med 120: 552.e1–552.e8, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB, Sr.: Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med 365: 213–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman MG, Harkness JR, Blankstein R, Budoff MJ, Agatston AS, Carr JJ, Lima JA, Blumenthal RS, Nasir K, Blaha MJ: Baseline subclinical atherosclerosis burden and distribution are associated with frequency and mode of future coronary revascularization: Multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging 7: 476–486, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katakami N, Kaneto H, Shimomura I: Carotid ultrasonography: A potent tool for better clinical practice in diagnosis of atherosclerosis in diabetic patients. J Diabetes Investig 5: 3–13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba Y, Chen JA, Bergmann SR: Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis 220: 128–133, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Benedetto FA, Tripepi G, Mallamaci F, Zoccali C: Rate of atherosclerotic plaque formation predicts cardiovascular events in ESRD. J Am Soc Nephrol 19: 757–763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JK, Song YR, Kim MG, Kim HJ, Kim SG: Clinical significance of subclinical carotid atherosclerosis and its relationship with echocardiographic parameters in non-diabetic chronic kidney disease patients. BMC Cardiovasc Disord 13: 96, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, Tsuruya K, Iida M, Kiyohara Y, Sueishi K: Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: The Hisayama study. Am J Kidney Dis 55: 21–30, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Betriu A, Martinez-Alonso M, Arcidiacono MV, Cannata-Andia J, Pascual J, Valdivielso JM, Fernández E, Investigators from the NEFRONA Study : Prevalence of subclinical atheromatosis and associated risk factors in chronic kidney disease: The NEFRONA study. Nephrol Dial Transplant 29: 1415–1422, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR: Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol 28: 354–360, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Herzog CA: Cardiac arrest in dialysis patients: Approaches to alter an abysmal outcome. Kidney Int Suppl 63: S197–S200, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Arroyo D, Betriu A, Martinez-Alonso M, Vidal T, Valdivielso JM, Fernández E, investigators from the NEFRONA study : Observational multicenter study to evaluate the prevalence and prognosis of subclinical atheromatosis in a Spanish chronic kidney disease cohort: Baseline data from the NEFRONA study. BMC Nephrol 15: 168, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leoncini G, Viazzi F, Parodi D, Ratto E, Vettoretti S, Vaccaro V, Ravera M, Deferrari G, Pontremoli R: Mild renal dysfunction and cardiovascular risk in hypertensive patients. J Am Soc Nephrol 15[Suppl 1]: S88–S90, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kokubo Y: Carotid atherosclerosis in kidney disease. Contrib Nephrol 179: 35–41, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Olechnowicz-Tietz S, Gluba A, Paradowska A, Banach M, Rysz J: The risk of atherosclerosis in patients with chronic kidney disease. Int Urol Nephrol 45: 1605–1612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leskinen Y, Lehtimäki T, Loimaala A, Lautamatti V, Kallio T, Huhtala H, Salenius JP, Saha H: Carotid atherosclerosis in chronic renal failure-the central role of increased plaque burden. Atherosclerosis 171: 295–302, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Arbel Y, Halkin A, Finkelstein A, Revivo M, Berliner S, Herz I, Keren G, Banai S: Impact of estimated glomerular filtration rate on vascular disease extent and adverse cardiovascular events in patients without chronic kidney disease. Can J Cardiol 29: 1374–1381, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Rigatto C, Levin A, House AA, Barrett B, Carlisle E, Fine A: Atheroma progression in chronic kidney disease. Clin J Am Soc Nephrol 4: 291–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desbien AM, Chonchol M, Gnahn H, Sander D: Kidney function and progression of carotid intima-media thickness in a community study. Am J Kidney Dis 51: 584–593, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Junyent M, Martínez M, Borràs M, Coll B, Valdivielso JM, Vidal T, Sarró F, Roig J, Craver L, Fernández E: Predicting cardiovascular disease morbidity and mortality in chronic kidney disease in Spain. The rationale and design of NEFRONA: A prospective, multicenter, observational cohort study. BMC Nephrol 11: 14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Junyent M, Martínez M, Borrás M, Bertriu A, Coll B, Craver L, Marco MP, Sarró F, Valdivielso JM, Fernández E: Usefulness of imaging techniques and novel biomarkers in the prediction of cardiovascular risk in patients with chronic kidney disease in Spain: The NEFRONA project. Nefrologia 30: 119–126, 2010 [DOI] [PubMed] [Google Scholar]
- 26.de La Piedra C, Fernández E, González Casaus ML, González Parra E: Different biological functions in PTH molecules. What are we measuring? Nefrologia 28: 123–128, 2008 [PubMed] [Google Scholar]
- 27.Coll B, Betriu A, Martínez-Alonso M, Amoedo ML, Arcidiacono MV, Borras M, Valdivielso JM, Fernández E: Large artery calcification on dialysis patients is located in the intima and related to atherosclerosis. Clin J Am Soc Nephrol 6: 303–310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS, American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine : Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. J Am Soc Echocardiogr 21: 93–111, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez R, Kownator S, Prati P, Rundek T, Taylor A, Bornstein N, Csiba L, Vicaut E, Woo KS, Zannad F, Advisory Board of the 3rd Watching the Risk Symposium 2004, 13th European Stroke Conference : Mannheim intima-media thickness consensus. Cerebrovasc Dis 18: 346–349, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Astor BC, Sheppard L, Kronmal RA, Stein JH: Predictors of carotid thickness and plaque progression during a decade: The Multi-Ethnic Study of Atherosclerosis. Stroke 45: 3257–3262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Meer IM, Iglesias del Sol A, Hak AE, Bots ML, Hofman A, Witteman JCM: Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: The Rotterdam Study. Stroke 34: 2374–2379, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Herder M, Johnsen SH, Arntzen KA, Mathiesen EB: Risk factors for progression of carotid intima-media thickness and total plaque area: A 13-year follow-up study: The Tromsø Study. Stroke 43: 1818–1823, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Valdivielso JM, Coll B, Fernandez E: Vitamin D and the vasculature: Can we teach an old drug new tricks? Expert Opin Ther Targets 13: 29–38, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Bozic M, Panizo S, Sevilla MA, Riera M, Soler MJ, Pascual J, Lopez I, Freixenet M, Fernandez E, Valdivielso JM: High phosphate diet increases arterial blood pressure via a parathyroid hormone mediated increase of renin. J Hypertens 32: 1822–1832, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Ellam TJ, Chico TJA: Phosphate: The new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 220: 310–318, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Martín M, Valls J, Betriu A, Fernández E, Valdivielso JM: Association of serum phosphorus with subclinical atherosclerosis in chronic kidney disease. Sex makes a difference. Atherosclerosis 241: 264–270, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Bozic M, Álvarez Á, de Pablo C, Sanchez-Niño MD, Ortiz A, Dolcet X, Encinas M, Fernandez E, Valdivielso JM: Impaired Vitamin D Signaling in Endothelial Cell Leads to an Enhanced Leukocyte-Endothelium Interplay: Implications for Atherosclerosis Development. PLoS One 10: e0136863, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suki WN, Dialysis Clinical Outcomes Revisited Investigators : Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients: Results of a randomized clinical trial. J Ren Nutr 18: 91–98, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Xia X, He F, Wu X, Peng F, Huang F, Yu X: Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am J Kidney Dis 64: 257–264, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J: Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5: 1388–1393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yadav AK, Banerjee D, Lal A, Jha V: Vitamin D deficiency, CD4+CD28null cells and accelerated atherosclerosis in chronic kidney disease. Nephrology (Carlton) 17: 575–581, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Szeto FL, Reardon CA, Yoon D, Wang Y, Wong KE, Chen Y, Kong J, Liu SQ, Thadhani R, Getz GS, Li YC: Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol Endocrinol 26: 1091–1101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valcheva P, Cardus A, Panizo S, Parisi E, Bozic M, Lopez Novoa JM, Dusso A, Fernández E, Valdivielso JM: Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis 235: 247–255, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.