Abstract
Technologic advances, such as continuous RRT, provide lifesaving therapy for many patients. AKI in the critically ill patient, a fatal diagnosis in the past, is now often a survivable condition. Dialysis decision making for the critically ill patient with AKI is complex. What was once a question solely of survival now is nuanced by an individual’s definition of quality of life, personal values, and short– and long–term prognoses. Clinical evaluation of AKI in the critically ill is multifaceted. Treatment decision making requires consideration of the natural evolution of the patient’s AKI within the context of the global prognosis. Situations are often marked by prognostic uncertainty and clinical unknowns. In the face of these uncertainties, establishment of patient-directed therapies is imperative. A time–limited trial of continuous RRT in this setting is often appropriate but difficult to execute. Using patient preferences as a clinical guide, a proper time–limited trial requires assessment of prognosis, elicitation of patient values, strong communication skills, clear documentation, and often, appropriate integration of palliative care services. A well conducted time–limited trial can avoid interprofessional conflict and provide support for the patient, family, and staff.
Keywords: intensive care unit, communication, time-limited trials, acute kidney injury, decision making, humans, palliative care, quality of life, renal dialysis, renal replacement therapy
Introduction
Dr. A was a 56-year-old nonpracticing physician with alcoholic cirrhosis who was transferred to the intensive care unit (ICU) for management of a myocardial infarction, bacteremia, and cellulitis. On arrival, he had a creatinine of 1.1 mg/dl (GFR>60 ml/min per 1.73 m2) with a significant transaminitis (aspartate aminotransferase=488 units/L; alanine aminotransferase=94 units/L), elevated bilirubin (total =2.4 mg/dl; direct =2.0 mg/dl), and ultrasound evidence of hepatic cirrhosis and splenomegaly. He lived with his wife and two teenage children, and he was actively drinking alcohol before his hospitalization and thus, not a candidate for liver transplantation. He presented with anasarca, ascites, and erythema below the left knee. His persistent bacteremia eventually caused septic shock requiring vasopressor support. Two days after transfer, the nephrology service was consulted for oliguric AKI with a creatinine of 7.0 mg/dl and severe electrolyte abnormalities (potassium =6.0 mmol/L and bicarbonate =15 mmol/L). Urine microscopy and fractional excretion of sodium were consistent with acute tubular necrosis. Dr. A was often somnolent and delirious but when lucid, stated his goal was to leave the hospital to be able to return to his family. Because of his lack of consistent decision–making abilities, his wife was appointed health–care surrogate. She described Dr. A as a highly functional man who would want to pursue all treatments if there was a chance for him to go home to be with his family. The ICU team had told her that his kidney failure was impeding his overall recovery and that, without dialysis, he would die from renal failure. She was told that the nephrology service would be consulted for initiation of dialysis.
On consultation, the nephrology service felt uncertain about Dr. A’s prognosis. Mrs. A told the nephrologist that she heard that dialysis would correct the metabolic abnormalities that were inhibiting his improvement. She also heard that some of his altered mental status could be caused by toxin buildup secondary to his kidney failure. The nephrologist explained to Mrs. A that, although his kidney function might recover, his overall prognosis was poor because of his liver failure, and he likely would not survive this hospitalization. Furthermore, there was also a chance that his kidneys would never recover and that he could be permanently dialysis dependent. Mrs. A stated that, if there was any chance for recovery, she would want to try dialysis. The primary team agreed. After discussing Dr. A’s uncertain renal and global prognosis with the ICU team, the nephrologist initiated continuous RRT (CRRT) as a time–limited trial (TLT) to support Dr. A while he was receiving full disease–directed therapy by the ICU. It was discussed with the ICU team that the trial’s timeframe was shaped by milestones marking global clinical improvement, such as decreased vasopressor requirements, recovery of mental status, and recovery of kidney function. If Dr. A clinically worsened to the point where he would not be able to leave the ICU and interact with his family, then CRRT would be withdrawn. The nephrologist explained these limitations to the family and said that there would be daily re-evaluations and that they should know if he was responding in a matter of days. This was documented in Dr. A’s chart. Mrs. A expressed understanding and remained hopeful.
In the next 48 hours, Dr. A was diagnosed with endocarditis with increased hemodynamic instability requiring escalation of vasopressors. He failed to show signs of renal recovery. Nephrology discussed the lack of perceived clinical improvement in his overall condition with the ICU team, stressing their concern that CRRT was not adding meaningful benefit and suggested a broader conversation about goals of care. The ICU team wanted to continue all therapy but was worried enough to call a palliative care (PC) consultation. PC facilitated an interdisciplinary family meeting (IDFM), where the initial goals of the TLT of CRRT were reviewed with expressed worry that these were unachievable because of multiorgan failure. Mrs. A described Dr. A’s definition of quality of life (QOL) as she knew it and believed that he would not want to live on machines. She did feel conflicted given that some providers had told her that there was a chance of recovery. It was agreed to continue CRRT for another 24 hours. PC stated they would meet with her again and described how the transition to comfort-directed care would occur if needed. The next day, Dr. A worsened, and Mrs. A stated that she did not want him to suffer anymore. She agreed to withdraw all life–sustaining interventions, including CRRT. PC aggressively managed his symptoms, and Dr. A died peacefully 4 days later. The family expressed appreciation to the nephrologist that CRRT was tried in accordance with his wishes.
Identification of the Ethical Questions at Hand
AKI occurs in 10%–56% of all ICU admissions and is associated with increased hospital mortality (adjusted odds ratio [OR], 2.89; 95% confidence interval [95% CI], 2.41 to 3.46) (1,2). When first described during World War II, AKI was an affliction with 100% mortality (3). In the 1940s, mortality was reduced with the invention of hemodialysis. CRRT was introduced in the 1970s, allowing nephrologists to dialyze the sickest patients with AKI: the critically ill. The decision to initiate RRT in a critically ill patient is complex, with consequences reaching beyond survival. Treatment plans affect QOL, the emotional experience of family and staff with the critical illness, and often, the actual experience of death (4). Described as the “technological imperative,” a nephrologist is often asked to view RRT as the correct moral treatment decision just because it exists as a technical option (5).
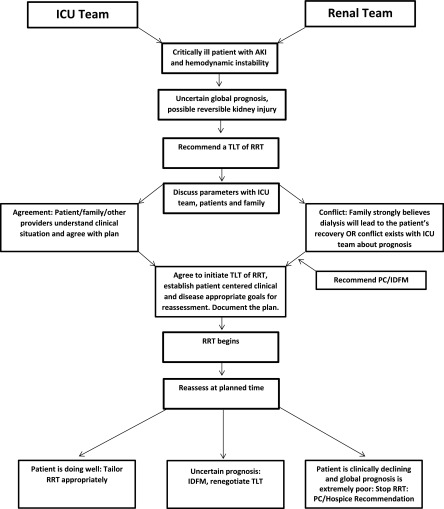
Because of prognostic uncertainty, decision making in the ICU is particularly challenging. Forgoing RRT may deny a patient lifesaving treatment, but its initiation could inflict indefinite suffering. The commencement of RRT as a trial with time that is limited by clear clinical milestones and QOL goals is often appropriate in the face of an unknown prognosis (6). Because of hemodynamic instability and comorbidity, most patients considered for CRRT have an uncertain overall prognosis. Nephrologists commonly find themselves the mediators of care with these patients, because RRT often represents a path of intensive care and thus, serves as a gateway for broader conversations about life-sustaining treatments. These situations are often marked by significant stress for families, interprofessional conflict, and strong emotional responses by staff. Although common, these patients can be challenging for nephrologists, and little literature exists for guidance. Our patient presents the following ethical questions. (1) Should a TLT of CRRT be offered? (2) How should treatment options be presented to Dr. A and his family? (3) How should treatment plans be discussed with other providers to ensure cohesive teamwork and comfort with medical decisions? This paper will serve as a guide for the implementation of a TLT of CRRT in a critically ill patient by addressing these three ethical questions. Figure 1 illustrates an approach to implementing a TLT of RRT and will be discussed throughout the text.
Figure 1.
An approach to implementing a time–limited trial (TLT) of RRT. ICU, intensive care unit; IDFM, interdisciplinary family meeting; PC, palliative care.
Should a TLT of CRRT Be Offered?
The Definition of a TLT of CRRT
A TLT is a patient–centered ethical process incorporating the best estimate of prognosis, QOL factors, and patient values. A TLT of CRRT is defined as a goal-directed trial of RRT limited by predetermined outcomes that are evaluated at planned intervals. TLTs allow the patient and family to assess the experience of dialysis while providing the nephrologist with time to evaluate clinical response (7). As illustrated in Figure 1, the time limits chosen are dependent on the etiology of AKI and the patient’s overall prognosis and should be flexible as the clinical scenario evolves. Each reassessment serves as a pause point for reflection of the burdens and benefits of dialysis within the developing clinical situation (8). Although TLTs for AKI can last weeks, it is appropriate to arrange for a planned reassessment within days of CRRT initiation given the dynamic nature of critical illness, subsequent changing goals of care, and involvement of multiple medical providers. If prognosis remains uncertain, then CRRT can be continued, and another planned reassessment can be negotiated. At pause points, clear communication of prognosis, long– and short–term goals, and plans for both successful and failed responses to therapy allows for smooth transitions of care and maximum support of the patient and family (6). Table 1 outlines suggested steps of a TLT of CRRT in the ICU.
Table 1.
Steps of a time–limited trial of continuous RRT in the intensive care unit
| Steps |
|---|
| Preparation |
| Define the prognosis of the patient’s AKI by reviewing the medical facts; consider all medical information in the context of the patient’s overall prognosis |
| Discuss prognosis with other providers to reach a consensus (Table 4) |
| Identify relevant short– and long–term clinical milestones to mark improvement or deterioration |
| Assess decision–making capabilities of the patient, or appoint a surrogate if needed |
| Identify if a PC consultation would be helpful to aid in discussions or to provide an added layer of support for family and providers; discuss this suggestion with the primary team |
| Communication with the patient/family |
| Share prognosis with the patient and family and explore the patient’s values and definition of quality of life; use empathetic communication techniques (Table 3) |
| Suggest initiation of CRRT as a TLT if appropriate |
| Discuss the identified clinical milestones with the patient and family on the basis of the individual’s values and goals |
| Share the anticipated timeframe of the trial with all of those involved, although stress that it is variable, patient specific, and flexible |
| Document the goals of the trial as determined by the team and the family |
| After initiation of the TLT |
| Meet with the family and providers regularly to review the patient’s progress |
| Larger planned IDFMs can be held at specific time intervals with the help of PC; attempt to participate if available, and communicate with providers before and after |
| Be sure to review the prognosis and treatment plan regularly with all providers to communicate a unified message |
| If the patient improves, tailor RRT appropriately |
| Consider available choices if, at the predetermined end of the TLT, the patient has not met the goals: |
| Either a new TLT can be negotiated, or the TLT can be stopped and aggressive PC can be pursued |
| If appropriate, the patient can transition to hospice care |
PC, palliative care; CRRT, continuous RRT; TLT, time–limited trial; IDFM, interdisciplinary family meeting. Modified from reference 6.
Bioethical and Historical Precedent of TLTs
There are several ethical and clinical practice guidelines outlining the foundation of TLTs, and some are specific to nephrology. Precedent for TLTs was first established in the 1983 President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research publication entitled Deciding to Forego Life-Sustaining Treatment (9). The Commission writes, “A physician and patient can agree to a TLT of a particular intervention, with the understanding that unless the intervention achieves certain goals it should be stopped” (9). The Commission argues that a treatment’s effect is unknown unless tried and failed, that lack of clinical benefit justifies stopping the intervention, and that greater moral justification should be given to withdrawing an intervention compared with withholding, despite ethical equivalence (9). A trial of treatment implementation is, thus, a concept laying the groundwork for TLTs.
In 1991, the Institute of Medicine (IOM) published the report Kidney Failure and the Federal Government, which described TLTs of dialysis (in reference to patients on chronic dialysis) as an approach appropriate when benefit is unclear, but the patient or family wants treatment (10). This allows the patient to understand the effect of dialysis and facilitates informed decision making. The IOM recommended that, before treatment initiation, clear parameters delineating outcomes justifying dialysis continuation should be determined (10). The Renal Physicians Association (RPA) and American Society of Nephrology clinical practice guideline Clinical Practice of Nephrology: Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis, published in 2000, included a rationale for TLTs (11). Nephrologists who were aware of the guideline reported more comfort with end-of-life decision making and higher use of TLTs (12). The second edition of the guideline (2010) endorsed TLTs not only for situations of clinical uncertainty but also, when a consensus concerning dialysis could not be reached (7), thus providing a framework for managing commonly faced dialysis–related interprofessional conflicts.
Most recently, the 2013 The Hastings Center Guidelines for Decisions on Life-Sustaining Treatment and Care near the End of Life states that, if a patient’s goals are consistent with a TLT that has a potential for benefit, the clinician should offer this option if appropriate (4). This approach is described as ethically preferred.
In summary, the common themes resonating in each guideline or report support TLTs as the ethically favored route in the face of clinical uncertainty or conflict, with patient autonomy and informed consent driving the decision for treatment. Clear predefined patient and relevant clinical goals reflecting the balance of beneficence and nonmaleficence serve as the basis of the trial’s limitations.
Defining Prognosis: Review of the Clinical Facts
A key step in ethical decision making is review of the medical prognosis and consideration of interventions with the goals of prevention, cure, and care of illness (13). Although full analysis of Dr. A’s prognosis is beyond the scope of this paper, this step is a crucial part of TLTs, because it allows for risk stratification of patients and formulation of a prognostic estimate. However, it is important to recognize that exact patient prognostication is difficult and that uncertainty is the norm. Prognostication is particularly challenging for patients with AKI, because the heterogeneity of relevant clinical trials often makes the data nongeneralizable. Overall, severe AKI in the ICU has a mortality of >50%, and those requiring CRRT or those with a higher severity of organ failure have equal or increased mortality depending on the studies reviewed (14–18). Dr. A also had septic shock. The mortality for septic shock has been decreasing because of improved supportive therapies but remains at 20%–30% (19,20) and up to 75% in the setting of acute tubular necrosis with septic shock (14–20). This highlights Dr. A’s uncertain but presumably poor prognosis and the appropriateness of a TLT given his goals and his family’s goals. General prognostic scores for critically ill patients or those with liver failure that incorporate renal function, such as the Model for End Stage Liver Disease, Sequential Organ Failure Assessment, and Acute Physiology Age Chronic Health Evaluation II scores, may also be useful and can serve as discussion points for providers. Table 2 shows additional scenarios where a TLT may be considered.
Table 2.
Situations in the intensive care unit appropriate for a time–limited trial of RRT
| Example | Rationale for a TLT | Benefits of a TLT |
|---|---|---|
| ATN secondary to septic shock | AKI with an uncertain prognosis that is possibly reversible | Allows the nephrologist time to assess reversibility of the kidney injury, evaluate the patient’s response to RRT, and assess the often changing prognosis |
| ATN secondary to septic shock with multiorgan failure in a patient with cardiac disease and no advance directives or health–care proxy; the family is hopeful for complete recovery and insists on doing everything leading to improvement; they have never discussed goals of care with the patient | AKI where dialysis is feasible, prognosis is uncertain but seems to be poor, and the patient lacks decision–making capacity | Allows family to come to terms with the poor prognosis and trust the nephrologist, avoiding a sense of abandonment; allows for the possibility that the patient will gain decision–making capacity and express his/her wishes |
| Decompensated end stage heart failure with AKI from cardiorenal syndrome; not a candidate for heart transplant or LVAD and dependent on inotropes and vasopressors; cardiology informs the patient and family that CRRT with fluid removal is needed to help with volume status and unload the heart; the primary team does not want a PC consult called | Patient is able to tolerate CRRT, and conflict exists between providers | Allows time for the primary team to assess patient response and global prognosis and address interprofessional conflict; allows the primary team to feel as if their plan is being honored, while setting limits on care |
TLT, time–limited trial; ATN, acute tubular necrosis; LVAD, left ventricular assist device; CRRT, continuous RRT; PC, palliative care.
Patient Preferences and QOL: Prognostic Outcomes of Consideration beyond Survival
Medical ethicists divide decision making into four categories: medical indications, patient preferences, QOL, and contextual features (13). In the case of a vulnerable critically ill patient, preferences and QOL goals may take on a larger role, particularly when setting treatment limitations (21). Few studies have examined the effects of CRRT on QOL, and most are observational in nature. Some have shown a trend toward an acceptable health–related QOL for AKI survivors who require hemodialysis, although scores are lower than in the global general population (22). In a study of 153 survivors of AKI requiring RRT, 48% reported problems ambulating, 42% reported problems with performing usual activities, 56% described moderate to severe pain, and 30% suffered from anxiety or depression (23); 25% of 60-day survivors of the Veterans Affairs/National Institutes of Health (VA/NIH) Renal Trial Network Study reported a QOL comparable with death (24). However, even with known decreased QOL, survivors of AKI requiring RRT report that they would still undergo dialysis (25). Given the effect on QOL, RRT initiation provides a focal point for larger discussions of personal values that can help guide consideration of other intensive procedures, such as mechanical ventilation, tracheostomy, or feeding tubes. Initiation of RRT as a TLT allows this conversation to happen without depriving the patient of life-sustaining treatment.
Although the initial goals of TLTs are typically short term in the setting of acute illness, for patients who stabilize, discussions can start to focus on long-term prognosis; ≤25% of survivors of AKI in the ICU requiring RRT remain dialysis dependent 90 days after initiation (26). In the VA/NIH Renal Trial Network Study, by day 60, only 16% of patients had returned home dialysis independent (15). Questions of long-term prognosis are often asked of nephrologists for patients with AKI. This dialogue is relevant to ensure that patients’ wishes are clearly followed in the future. Data from patients with prolonged ICU stays (2 weeks) from the landmark Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatments showed that only 38% of patients had discussions with their physicians about their prognosis and that 47% of those who preferred a palliative approach believed that they were receiving contrary treatment (27). Patient–desired comfort–oriented care was two times more likely if individual preferences had been discussed with physicians (27). Despite the frequency with which nephrologists face these serious conversations, graduating fellows feel unprepared for this task (28).
How Should Treatment Options Be Presented to the Patient and Family?
The Robert Wood Johnson Foundation Critical Care End-of-Life Workgroup identified adequate communication between teams and with families as well as patient–centered decision making as specific quality domains of ICU PC (29). Separately rounding ICU consultants provide highly technical organ–specific expertise but often contribute to fragmented care and present conflicting messages to patients and families (30). A well planned communication strategy that considers early involvement of PC may facilitate effective patient–centered shared decision making and care consistent with patient values. For the incapacitated patient, such as Dr. A, care teams rely on a surrogate or proxy for decision making. Three principles guide decision making for the incapacitated patient: (1) autonomy and respect for the patient’s previously expressed wishes, (2) substituted judgment, where families are asked to infer what the patient would have wanted on the basis of the patient’s known values, or (3) best interests, when little is known about the patient, and decision makers do what is considered the best (31). Decision aids that consider the role of dialysis exist and can facilitate communication with surrogates (http://decisionaid.ohri.ca/docs/das/Critically_Ill_Decision_Support.pdf) (32).
It is not uncommon for providers to confront conflict when working with a surrogate decision maker. Given the vulnerability of an incapacitated patient, all efforts must be made to ensure clear communication and decisions consistent with the patient’s values. In a recent multisociety–endorsed policy statement, regular ICU IDFMs with early involvement of expert consultants, such as PC, are recognized as a method for management of conflicts with surrogates (33). A useful approach to the ideal IDFM discussing a TLT of CRRT is the setting, perception, invitation, knowledge, emotions, summarize, and strategize protocol (Table 3) (34). However, real world experiences are rarely so ideal: family meetings tend to occur later in the ICU stay and often lack mention of patient preferences and values (31). For nephrologists, clinical responsibilities and schedules may prevent attendance at all meetings. Given the importance of RRT in decision making, if the renal team is not able to attend, it is imperative to convey prognosis and recommendations to the ICU team before these meetings and follow-up on outcomes with both the family and other providers.
Table 3.
Approach to delivering bad news in a family meeting: setting, perception, invitation, knowledge, emotions, summarize, and strategize
| Step | Action | Benefits |
|---|---|---|
| Setting | Arrange for a private, quite room with all pagers and cell phones on silent. Tissues should be available. Each participant should have a place to sit and be able to make eye contact with the patient and family. The leader of the meeting should have all participants introduce themselves and explain their relation to the patient, including family members | A comfortable and nonchaotic setting allows for a connection to be made between providers and patients. Allows for patient/family to focus on the information as much as possible |
| Perception | Begin by asking the patient or family what their understanding is of the medical situation. Example: “What is your understanding of your medical situation thus far?” | Allows for providers to assess where the patient or family is in their comprehension of the medical events. Allows for assessment of any coping mechanisms, such as denial |
| Invitation | Ask the patient or family for invitations about sharing medical information. Example: “How would you like to hear information? Are you the type of person who likes to know all of the details or just an overview with more emphasis on our next steps?”; “Would it be ok if I shared an update on his kidney function?” | Allows the patient to have control over the meeting and may lessen anxiety |
| Knowledge | Share medical information in short chunks without use of medical jargon or many numbers. Provide warning shots before delivering bad news. Use silence. Check in with the patient or family frequently to ensure comprehension. Example: “Unfortunately, your (or your loved one’s) kidneys have not recovered the way we would have hoped, and it’s quite serious now. [pause] At this time, your kidneys have stopped working all together. If it’s ok with you, I would like to share some of the options we have to support you going forward.” Check-in statement: “I know this is a lot of information and often, doctors speak quite quickly; does everything make sense so far?” | Allows for the patient or family to absorb as much information as possible in a tempo controlled by them |
| Emotions | Respond to patient’s or family’s emotions with empathic support statements. Explore the emotion and validate its presence. Example: “I can see how upsetting this is to you.” “Would you be able to share what your biggest worry is at this time?” “I can assure you, many patients experience what you are going through.” | Aligns you with the patient or family and allows for pause points during the meetings to respond to emotions |
| Summarize and strategize | Provide a short summary statement at the end that describes the content of the meeting and presents the next steps. Example: “To summarize, we have just gone over the events related to your dad’s kidney function, and at this time, we have decided to initiate a TLT of CRRT on the basis of the goals we have discussed. We expect that we will have a good idea of response to therapy in about a week. We will update you daily and together, look for the clinical markers we spoke about. We will arrange for another meeting with as many of your providers as possible in a few days.” | Allows for a short summary of the meeting and increases the ability for the patient or family to absorb the decisions given the emotional nature of the events |
TLT, time–limited trial; CRRT, continuous RRT.
How Should Treatment Plans Be Discussed with Other Providers to Ensure Cohesive Teamwork?
Interprofessional Conflict
Conflict among staff in the ICU is common and seen with 78% of patients in a study of 102 ICU admissions, with 48% being between staff (35). Areas of disagreement included communication of prognosis to the family, pain control, and treatment decisions (35). Dialysis initiation in the ICU itself is associated with physician discomfort. At least one provider in 56% of 657 critically ill patients receiving dialysis reported discomfort (36). Clinician unease was also more likely generated for patients receiving dialysis (OR, 2.53; 95% CI, 1.73 to 3.71) or those who had dialysis withheld (OR, 2.04; 95% CI, 1.58 to 2.62) (36). Providers cited treatment plans felt to be too aggressive, patient’s and family’s overestimation of survival, and prolongation of the dying process as reasons for discomfort (36). Providers’ unexamined emotional responses can lead to poor patient care, professional loneliness, loss of professional sense of purpose, cynicism, frustration, and burnout (37). PC physicians are trained to lead reflection on these emotional responses and may be helpful in these situations (38). Conflict between clinicians can be handled with consistent and open dialogue between providers utilizing respectful curiosity while discussing differing opinions (38). A short premeeting with providers before a larger IDFM can be helpful in the discussion of conflicting clinical assessments and proposed treatment plans. This process maintains professionalism by showing respect for opposing opinions. Table 4 outlines some of the barriers influencing TLTs, focusing on interprofessional factors. These issues are common and not easily resolved. Early involvement of PC to navigate interprofessional differences can facilitate collaborative clinical decision making and cohesive patient care. As consultants, nephrologists often join the primary team at a time when others have already decided with the family to provide dialysis without clearly identified short– and long–term goals that would define a TLT. This creates conflict for the nephrologist before evaluating the patient if the nephrologist is uncertain about the benefits of RRT. In such patients, ongoing attempts to educate providers and families of expected outcomes by identifying and documenting achievable goals consistent with the patient’s narrative and QOL standards may facilitate conflict resolution and establish appropriate care. The common encounter of the family and primary team together insisting on doing everything highlights the importance of upstream communication about the TLT of RRT and its predefined limitations with all of those involved. It is also important to understand the hopes and worries of the other clinical providers while sharing those of the renal team. Although limited literature exists regarding interprofessional conflict, RPA Guideline Recommendation No. 8 provides a detailed approach to conflict resolution surrounding differences of opinion regarding dialysis initiation (7).
Table 4.
Barriers to time–limited trials and suggested strategies
| Barrier | Strategy |
|---|---|
| Lack of consensus between treatment teams | Reach out to individual practitioners to understand their concerns, hopes, and prognosis. Attempt to negotiate a plan that represents a middle ground. Explain how a TLT does this |
| Family has already been told that dialysis will be offered. They hope and believe that, with the initiation of dialysis, their loved one will recover | Meet with the family in a quiet setting and explore their perception of the current illness. Using communication tools, such as warning shots, explain that the patient is seriously ill and that there is worry about the overall clinical outcome. Support the primary team in their suggestion of dialysis but express concern about the risks and benefits of the procedure. Realign the family’s expectations with realistic outcomes |
| Critically ill with an uncertain global prognosis | Review relevant prognostic literature related to outcomes of AKI in patients with the demographics of the consulted patient. Relate the AKI prognosis to the patient’s global clinical state. Acknowledge that global prognosis is uncertain and what recovery or lack of clinical response to therapy may look like |
| Uneasiness communicating prognosis | If the primary team is in agreement, involve PC consultation teams early to help with the complex communication needs. Continue consistent dialogue with the patient and family to address any changing clinical status |
| Changing teams and providers over time | Establish and document clear and realistic goals at the start of the TLT. Re-evaluate these goals daily, and update any new clinical teams that are rounding on the patient |
| Discomfort with use of the surrogate decision maker as proxy | Recognize the patient and family as a unit. Use effective communication strategies to elicit patient’s values and goals. Examples include, “Given what you know of the situation, what would your loved one say if he/she was able to be part of this conversation?” |
| Family does not seem to understand the prognosis | Reach out to PC colleagues for guidance concerning communication if they are not officially consulted. Speak with family in a controlled setting, such as a family meeting room (if the patient cannot participate), to avoid the distractions of the ICU. Use the ask-tell-ask method to ensure understanding. Deliver medical information in small amounts without medical jargon |
| Family does not want to transition care at the end of the TLT and insists on continuing to do everything | Recognize that the family is being faced with a very difficult situation and express empathy. Explore with the family what everything means to them. Check that the family understands the situation (e.g., “Can you share with me your impression of your loved one’s hospital course so far?”). Revisit the initial goals established, especially those surrounding QOL. Renegotiate the TLT if needed |
| Family and primary team are in agreement to not transition care at the end of the TLT, and both want to continue to do everything | Explore the hopes and concerns of the primary team. Revisit the initial goals of the TLT, and ask how the primary team is interpreting the patient’s current clinical status. Express the renal team’s concern about prognosis and the achievement of the patient’s goals. Renegotiate and document the TLT if needed. Reference the RPA guideline for interprofessional conflict. Support the family as above |
| Transition of care at the end of the TLT | Reinforce nonabandonment if the patient does not recover. Present aggressive PC early on as intense medical management for the goal of comfort so that the transition is expected. Remain involved through the patient’s end of life, even after CRRT is stopped, to help manage uremic symptoms and continuity of care |
| Sense of physician failure if the patient does not recover | Institute educational initiatives in nephrology training programs to address care of dying critically ill patients. Include communication training. Continue to have interdisciplinary meetings for all providers to debrief about challenging patients, even after the patient’s death |
TLT, time–limited trial; PC, palliative care; ICU, intensive care unit; QOL, quality of life; RPA, Renal Physicians Association; CRRT, continuous RRT.
The Role of PC in a Critically Ill Patient Undergoing a TLT of CRRT
Families’ emotional responses to the stress of critical illness often serve as barriers to effective substituted decision making. Common responses are anxiety (70%), depression (35%), stress (33%), and difficulty understanding medical information (50%) (39). Involvement of PC specialists has been shown to increase patient understanding of prognosis, goals of care, and coping with emotional and spiritual distress (40). The effect of PC consultations specifically on patients on CRRT is unknown. One single–center retrospective study showed a trend in delayed referral to PC for patients undergoing CRRT (41). In this cohort, a PC consult was called a median of 9 days after initiation of CRRT and longer (10 versus 4 days) in those who died (41). These results suggest that a more protracted hospital course and slower clinical decline rather than severity of renal injury trigger PC consults. Of note, a PC consult was not associated with increased mortality, a common worry of clinicians (41). Current research examining ICU triggers for PC consultation focuses on patients who have a high risk of ICU or hospital death (42). This cohort should include those with AKI requiring dialysis who have a hospital mortality of about 50% or higher depending on the cause of the AKI and the patient’s underlying condition. Early involvement of PC provides an extra layer of support for the patient and family throughout the illness as well as easy transitions of care to comfort care if needed (43). If the patient worsens, this timely exposure also allows for more time for closure. If the patient recovers, PC is available to manage symptoms; support patients, families, and staff; address advance care planning when appropriate; and assist in disposition on the basis of the patient’s goals as needed.
As consultants, nephrologists are not in the position to call another consult and often, are providing care without the assistance of PC. Given that nephrologists commonly face situations requiring expert communication skills, it would be beneficial to expand our knowledge base in this field. Nephrology societies recognize the importance of communication and promote the integration of PC skills into standard practice (7,44). Graduating nephrology fellows cited a required PC rotation as a means of obtaining these skills (28). Educational tools and courses promoting the learning of such expertise specifically targeting nephrologists are increasingly available (45,46).
Conclusions
CRRT can be lifesaving for patients who have reversible acute illness. A TLT of CRRT provides an organized plan of care that honors this potential but sets clear patient–defined boundaries that limit burdensome treatments (6). A successful TLT, whether the patient recovers or not, is dependent on clear communication and collaboration with ICU teams. Prognosis and decision making should consider other life–sustaining interventions as well as RRT. The complex nature of this decision in addition to the high mortality rate of AKI suggest that early involvement of PC services can benefit all of those involved (41). The evidence for TLTs is limited, and expert recommendations are few (7). The ever–growing highly technical environment of medical care calls for more research and guidelines in this field. Evidence does suggest that nephrologists can benefit from formal training about the role of TLTs and communication strategies needed for its successful implementation.
Patient Outcome in Context of Ethical Issues
Dr. A was clear about his wish to be home. When his prognosis worsened, his family honored those wishes through substituted judgment. With the help of PC, an open dialogue was created between the clinical team, the patient, his family, and providers. The upstream introduction of limits of CRRT with plans for multiple outcomes helped to avoid conflict. A smooth TLT was conducted with seamless transitions of care and maximal support of all involved. Although there was initial conflict between the nephrology and ICU teams, the initiation of CRRT as a TLT allowed for clarification of prognosis for both the family and providers. The PC team facilitated communication and sequential decision making that was patient and family centered. Although critically ill patients with AKI may present challenges in medical decision making and barriers to TLTs may include interprofessional and patient-surrogate issues, TLTs can be used (Figure 1) successfully to promote patient–centered decision making and care.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Case J, Khan S, Khalid R, Khan A: Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013: 479730, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liborio AB, Leite TT, Neves FM, Teles F, Bezerra CT: AKI complications in critically ill patients: Association with mortality rates and RRT. Clin J Am Soc Nephrol 10: 21–28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Himmelfarb J: Continuous renal replacement therapy in the treatment of acute renal failure: Critical assessment is required. Clin J Am Soc Nephrol 2: 385–389, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Berlinger N, Jennings B, Wolf SM: The Hastings Center Guidelines for Decisions on Life-Sustaining Treatment and Care near the End of Life, New York, Oxford University Press, 2013 [Google Scholar]
- 5.Barger-Lux MJ, Heaney RP: For better and worse: The technological imperative in health care. Soc Sci Med 22: 1313–1320, 1986 [DOI] [PubMed] [Google Scholar]
- 6.Quill TE, Holloway R: Time-limited trials near the end of life. JAMA 306: 1483–1484, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Renal Physicians Association: Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal From Dialysis, 2nd Ed., Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 8.Schell JO, Cohen RA: A communication framework for dialysis decision-making for frail elderly patients. Clin J Am Soc Nephrol 9: 2014–2021, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US President’s Commission: Deciding to Forgo Life-Sustaining Treatment, Washington, DC, The Commission, 1983 [Google Scholar]
- 10.Institute of Medicine Committee for the Study of the Medicare End-Stage Renal Disease P: Kidney Failure and the Federal Government, edited by Rettig RA, Levinsky NG, Washington, DC, National Academies Press, 1991 [PubMed] [Google Scholar]
- 11.Renal Physicians Association and American Society of Nephrology: Clinical Practice of Nephrology. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis, Washington, DC, Renal Physicians Association and American Society of Nephrology, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Davison SN, Jhangri GS, Holley JL, Moss AH: Nephrologists’ reported preparedness for end-of-life decision-making. Clin J Am Soc Nephrol 1: 1256–1262, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Albert R, Jonsen MS, William J: Winslade: Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine, 7th Ed., New York, McGraw-Hill Companies, Inc., 2010 [Google Scholar]
- 14.Kes P, Basic Jukic N: Acute kidney injury in the intensive care unit. Bosn J Basic Med Sci 10[Suppl 1]: S8–S12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R, Ronco C, Genyk Y, Arroyo V: Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 60: 702–709, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, Maliakkal B, Thacker LR, Bajaj JS; North American Consortium for Study of End-Stage Liver Disease: New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology 145: 1280–1288.e1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allegretti AS, Steele DJ, David-Kasdan JA, Bajwa E, Niles JL, Bhan I: Continuous renal replacement therapy outcomes in acute kidney injury and end-stage renal disease: A cohort study. Crit Care 17: R109, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angus DC, van der Poll T: Severe sepsis and septic shock. N Engl J Med 369: 840–851, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R: Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 311: 1308–1316, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Swidler M: Considerations in starting a patient with advanced frailty on dialysis: Complex biology meets challenging ethics. Clin J Am Soc Nephrol 8: 1421–1428, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagshaw SM: The long-term outcome after acute renal failure. Curr Opin Crit Care 12: 561–566, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ahlstrom A, Tallgren M, Peltonen S, Rasanen P, Pettila V: Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31: 1222–1228, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM; VA/NIH Acute Renal Failure Trial Network: Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol 5: 1366–1372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maynard SE, Whittle J, Chelluri L, Arnold R: Quality of life and dialysis decisions in critically ill patients with acute renal failure. Intensive Care Med 29: 1589–1593, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Wald R, McArthur E, Adhikari NK, Bagshaw SM, Burns KE, Garg AX, Harel Z, Kitchlu A, Mazer CD, Nash DM, Scales DC, Silver SA, Ray JG, Friedrich JO: Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: A population-based cohort study. Am J Kidney Dis 65: 870–877, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Teno JM, Fisher E, Hamel MB, Wu AW, Murphy DJ, Wenger NS, Lynn J, Harrell FE, Jr.: Decision-making and outcomes of prolonged ICU stays in seriously ill patients. J Am Geriatr Soc 48[5 Suppl]: S70–S74, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Combs SA, Culp S, Matlock DD, Kutner JS, Holley JL, Moss AH: Update on end-of-life care training during nephrology fellowship: A cross-sectional national survey of fellows. Am J Kidney Dis 65: 233–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser M, Deliens L, Houttekier D: Physician-related barriers to communication and patient and family-centred decision-making towards the end of life in intensive care: A systematic review. Crit Care 18: 604, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fins JJ: A Palliative Ethic of Care: Clinical Wisdome at Life’s End, Sudbury, MA, Jones and Bartlett Publishers International, 2006 [Google Scholar]
- 31.Scheunemann LP, Cunningham TV, Arnold RM, Buddadhumaruk P, White DB: How clinicians discuss critically ill patients’ preferences and values with surrogates: An empirical analysis. Crit Care Med 43: 757–764, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottowa Patient Decision Aids: Understanding the Options, Planning for Care Critically Ill Patients in the Intensive Care Unit. Available at: http://decisionaid.ohri.ca/decaids.html. Accessed June 22, 2015
- 33.Bosslet GT, Pope TM, Rubenfeld GD, Lo B, Truog RD, Rushton CH, Curtis JR, Ford DW, Osborne M, Misak C, Au DH, Azoulay E, Brody B, Fahy BG, Hall JB, Kesecioglu J, Kon AA, Lindell KO, White DB: An official ATS/AACN/ACCP/ESICM/SCCM policy statement: Responding to requests for potentially inappropriate treatments in intensive care units. Am J Respir Crit Care Med 191: 1318–1330, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Judelk AP: SPIKES-A-Six-Step protocol for delivering bad news: Application to the patient with cancer. Oncologist 5: 302–311, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Breen CM, Abernethy AP, Abbott KH, Tulsky JA: Conflict associated with decisions to limit life-sustaining treatment in intensive care units. J Gen Intern Med 16: 283–289, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffith L, Cook D, Hanna S, Rocker G, Sjokvist P, Dodek P, Marshall J, Levy M, Varon J, Finfer S, Jaeschke R, Buckingham L, Guyatt G: Level of Care Investigators; Canadian Critical Care Trials Group: Clinician discomfort with life support plans for mechanically ventilated patients. Intensive Care Med 30: 1783–1790, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Meier DE, Back AL, Morrison RS: The inner life of physicians and care of the seriously ill. JAMA 286: 3007–3014, 2001 [DOI] [PubMed] [Google Scholar]
- 38.O’Neill LB, Back AL: What are the key elements to having a conversation about setting goals and communicating serious news. In: Evidence-Based Practzice of Palliative Medicine, edited by Goldstein NE, Morrison RS, Philadelphia, Elsevier Saunders, 2013, pp 244–250 [Google Scholar]
- 39.Azoulay E, Chaize M, Kentish-Barnes N: Involvement of ICU families in decisions: Fine-tuning the partnership. Ann Intensive Care 4: 37, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machare Delgado E, Callahan A, Paganelli G, Reville B, Parks SM, Marik PE: Multidisciplinary family meetings in the ICU facilitate end-of-life decision making. Am J Hosp Palliat Care 26: 295–302, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Okon TR, Vats HS, Dart RA: Palliative medicine referral in patients undergoing continuous renal replacement therapy for acute kidney injury. Ren Fail 33: 707–717, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Nelson JE, Curtis JR, Mulkerin C, Campbell M, Lustbader DR, Mosenthal AC, Puntillo K, Ray DE, Bassett R, Boss RD, Brasel KJ, Frontera JA, Hays RM, Weissman DE: Improving Palliative Care in the ICU (IPAL-ICU) Project Advisory Board: Choosing and using screening criteria for palliative care consultation in the ICU: A report from the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board. Crit Care Med 41: 2318–2327, 2013 [DOI] [PubMed] [Google Scholar]
- 43.O’Mahony S, McHenry J, Blank AE, Snow D, Eti Karakas S, Santoro G, Selwyn P, Kvetan V: Preliminary report of the integration of a palliative care team into an intensive care unit. Palliat Med 24: 154–165, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, Michael B, O’Hare AM, Schaefer HM, Shaffer RN, Trachtman H, Weiner DE, Falk AR: American Society of Nephrology Quality and Patient Safety Task Force: Critical and honest conversations: The evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Schell JO, Arnold RM: NephroTalk: Communication tools to enhance patient-centered care. Semin Dial 25: 611–616, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Schell JO, Green JA, Tulsky JA, Arnold RM: Communication skills training for dialysis decision-making and end-of-life care in nephrology. Clin J Am Soc Nephrol 8: 675–680, 2013 [DOI] [PubMed] [Google Scholar]