Abstract
Background and objectives
The generation of key uremic nephrovascular toxins, indoxyl sulfate (IS), and p-cresyl sulfate (PCS), is attributed to the dysbiotic gut microbiota in CKD. The aim of our study was to evaluate whether synbiotic (pre- and probiotic) therapy alters the gut microbiota and reduces serum concentrations of microbiome–generated uremic toxins, IS and PCS, in patients with CKD.
Design, setting, participants, & measurements
Predialysis adult participants with CKD (eGFR=10–30 ml/min per 1.73 m2) were recruited between January 5, 2013 and November 12, 2013 to a randomized, double–blind, placebo–controlled, crossover trial of synbiotic therapy over 6 weeks (4-week washout). The primary outcome was serum IS. Secondary outcomes included serum PCS, stool microbiota profile, eGFR, proteinuria-albuminuria, urinary kidney injury molecule-1, serum inflammatory biomarkers (IL-1β, IL-6, IL-10, and TNF-α), serum oxidative stress biomarkers (F2-isoprostanes and glutathione peroxidase), serum LPS, patient-reported health, Gastrointestinal Symptom Score, and dietary intake. A prespecified subgroup analysis explored the effect of antibiotic use on treatment effect.
Results
Of 37 individuals randomized (age =69±10 years old; 57% men; eGFR=24±8 ml/min per 1.73 m2), 31 completed the study. Synbiotic therapy did not significantly reduce serum IS (−2 μmol/L; 95% confidence interval [95% CI], −5 to 1 μmol/L) but did significantly reduce serum PCS (−14 μmol/L; 95% CI, −27 to −2 μmol/L). Decreases in both PCS and IS concentrations were more pronounced in patients who did not receive antibiotics during the study (n=21; serum PCS, −25 μmol/L; 95% CI, −38 to −12 μmol/L; serum IS, −5 μmol/L; 95% CI, −8 to −1 μmol/L). Synbiotics also altered the stool microbiome, particularly with enrichment of Bifidobacterium and depletion of Ruminococcaceae. Except for an increase in albuminuria of 38 mg/24 h (P=0.03) in the synbiotic arm, no changes were observed in the other secondary outcomes.
Conclusion
In patients with CKD, synbiotics did not significantly reduce serum IS but did decrease serum PCS and favorably modified the stool microbiome. Large–scale clinical trials are justified.
Keywords: chronic kidney disease; indoxyl sulphate; probiotics; synbiotics; uremic toxins; p-cresyl sulphate; glomerular filtration rate; humans; microbiota; renal insufficiency, chronic
Introduction
CKD is a major growing public health problem, with a prevalence estimated between 8% and 16% worldwide (1). Patients with CKD are at greatly increased risk of cardiovascular disease (CVD) and ≤20 times more likely to die prematurely than survive to the point of reaching ESRD (2).
The increased risk of CVD is only partially accounted for by traditional cardiac risk factors, leading many investigators to look for therapeutically modifiable nontraditional risk factors (3–5). Promising candidates in this regard are uremic toxins, particularly p-cresyl sulfate (PCS) and indoxyl sulfate (IS) (6,7). Both toxins are exclusively produced by the bacterial community resident within the large bowel (termed the gut microbiota), which is altered (dysbiotic) in the CKD population. Moreover, these toxins have been shown to promote and further aggravate kidney disease progression and CVD (8).
Although a number of therapeutic approaches have been proposed to mitigate the production and/or extraintestinal release of IS and PCS (9), none have been shown to exert proven clinical benefit, including oral adsorbents (10). More recently, administration of pre- and/or probiotics has emerged as a promising bowel–targeted therapeutic approach for modifying bacterial production of IS and PCS on the basis of a systematic review of clinical studies (11). Nonetheless, the conclusions that can be drawn from the available studies are appreciably limited by poor methodologic quality, clinical heterogeneity (including with respect to pre- and probiotic formulations), limited control for dietary intake, and a lack of exploration of effects on the gut microbiota (12,13).
The aim of this study was to ascertain the efficacy of synbiotics (coadministration of pre- and probiotics) as a potential treatment to decrease the microbial production of PCS and IS by alterations to the form and/or function of the gut microbiota. This is a proof-of-concept study to elucidate the clinical applicability of this novel therapy in the CKD setting.
Materials and Methods
The Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY) Study was a single–center, double–blind, placebo–controlled, randomized crossover trial investigating the effects of synbiotics on serum PCS and IS in patients with moderate to severe CKD. A detailed description of the SYNERGY Study protocol has been published elsewhere (12). Ethical approval was granted through the Metro South Human Research Ethics Committee and the University of Queensland Human Research Ethics Committee, and the study adhered to the Declaration of Helsinki. Informed consent was obtained before enrolment and participation. The SYNERGY Study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12613000493741).
Participants
Patients with CKD stage 4 or 5 nondialyzed (eGFR=10–30 ml/min per 1.73 m2) ages ≥18 years old were eligible for inclusion. The narrow GFR range was selected on the basis of this patient population’s high toxin levels. Patients were excluded if they met any of the following criteria: previous renal transplant; receiving or have received bowel radiation or had large bowel resection; consumed pre- or probiotics or had antibiotic therapy within 1 month of study commencement; medically diagnosed irritable bowel syndrome, Crohn disease, or ulcerative colitis; non-English speaking or unable to give informed consent; likely to receive a transplant or progress to dialysis within 6 months; severely malnourished (Subjective Global Assessment: C); or having had a clinically significant change to their immunosuppressant dose within 6 months (determined by the medical team).
Design
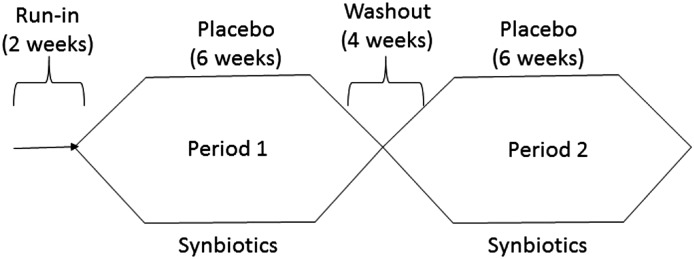
Participants underwent a 2-week run–in period followed by randomization in a 1:1 ratio to either synbiotic supplements or placebo for 6 weeks. Thereafter, participants underwent a further 4-week washout period followed by crossover to the alternative intervention (Figure 1). All participants underwent face-to-face dietary education and counseling with a qualified dietitian to achieve evidence–based guideline–recommended targets (14) during the first week of run-in. Throughout the intervention, patients were encouraged to maintain stable dietary intakes, with a focus on stabilizing protein and fiber intake. Participants were also provided with a standard evening meal preceding their overnight fast before each blood collection. This was a precautionary measure to minimize any potential residual influences of the macronutrient distribution of proximal meals on participants’ serum IS and PCS levels. A computer–generated blocked randomization list with blocks of size 2 was produced by an external statistical consultant and maintained on a secure server not accessible to those recruiting for the trial. Random allocation was performed by telephoning the list custodian, who reported the next numbered supplement kit in the list. Supplement packaging was done offsite.
Figure 1.
The Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY) Study schema.
Intervention
The prebiotic component of the synbiotic therapy consisted of a combination of high–molecular weight inulin (inulin high performance), fructo-oligosaccharides, and galacto-oligosaccharides (GOSs) and the probiotic component including nine different strains across the Lactobacillus, Bifidobacteria, and Streptococcus genera. The synbiotic therapy (prebiotic powder and probiotic capsule) and the identically matched placebo (maltodextrin powder and capsule) were manufactured by BioCeuticals. The rationale behind supplement selection and study design has been described elsewhere (11).
The study design included a dose escalation, where the supplements were commenced at a one-half dose for the first 3 weeks. This consisted of 7.5 g (one level scoop) of the placebo/prebiotic powder and one placebo/probiotic capsule containing 45 billion CFU taken in the morning with food. After the dose escalation, participants took an additional dose (7.5 g powder and one capsule) with their evening meal, equating to a daily dose of 15 g (two level scoops) powder and two capsules during both interventions.
Outcome Measures
The primary outcome measure was serum IS concentration at the end of the 6-week study period. The secondary outcomes included serum PCS, eGFR, proteinuria-albuminuria, urinary kidney injury molecule-1, serum inflammatory biomarkers (IL-1β, IL-6, IL-10, and TNF-α), serum oxidative stress biomarkers (F2-isoprostanes and glutathione peroxidase), serum LPS, patient-reported health, Gastrointestinal Symptom Score, dietary intake, and stool microbiota profile. Outcome measures were collected and analyzed using validated methods detailed in Supplemental Material.
Gut Microbiota Analyses
Stool samples were collected from consenting patients at baseline and the end of each intervention arm and stored at −80°C. The microbiome profiles of these samples were determined using 16S ribosomal ribonucleic acid gene sequencing approaches undertaken by the Australian Centre for Ecogenomics (ecogenomics.org). The DNA extraction and sequencing methods are described in detail in Supplemental Material, with QIIME (version 1.8.0) used to produce the taxonomic profiles and diversity measurements.
Study Compliance/Deviations
Adherence to therapy was measured by pill count and powder weight at the end of each intervention. Nonadherence was defined as >20% of prescribed pills/powder not taken by the participant.
Participants’ dietary intakes and adherence to a stable diet during the study were assessed using an open–ended, structured diet history method at baseline and the end of each intervention period. Dietary data were analyzed using Food Works 7 (Xyris Software; version 7.0.2915) Australian Food, Supplement and Nutrient Database 2007. In addition, dietary energy intakes were verified using body weight, and dietary protein intakes were verified according to the formula by Maroni et al. (15) using 24-hour urinary urea nitrogen and body weight measured at baseline and the end of each intervention arm.
Adverse Events
A serious adverse event (SAE) was defined as any event that suggested a significant hazard, contraindication, side effect, or precaution, including fatal or life-threatening events, permanent disability incidents, and experiences requiring in-patient hospitalization. All SAEs were documented and reported to the ethics committee for review.
Statistical Analyses
Summary statistics for patients’ characteristics were expressed as means±SDs for normally distributed continuous data, medians (interquartile ranges) for skewed continuous data, and frequencies (percentages) for categorical data. The primary analyses of uremic toxin concentrations and other normally distributed continuous outcome variables were undertaken using regression modeling, with the difference between serum toxins at the end of interventions A and B as the dependent variable and treatment sequence allocation as the independent variable (16). Because the use of antibiotics was likely to decrease the effect of synbiotic therapy because of alterations of the gut microbiota, a prespecified sensitivity analysis was undertaken to determine whether there was a significant interaction between antibiotic use and treatment effect. In addition, additional sensitivity analyses were undertaken, including adjusting for baseline toxin concentrations and assessing carryover effects as described by Jones and Kenward (16). Changes in potential confounders between treatment, including dietary intake (protein and fiber) and eGFR, were also assessed. Secondary analysis of the primary outcome included mixed modeling to account for missing at random data. Continuous outcome measures with non–normal difference distributions were analyzed using the Wilcoxon rank sum test. Categorical outcome variables were analyzed using the exact McNemar test.
Prospective sample size calculations indicated that the SYNERGY Study would have 90% power to detect a 30% reduction in IS levels with an error level of 0.05 if 24 participants completed the study. Allowing for a 20% dropout and using the adjustment factor 1/1(1 − υ)2, a total of 37 participants needed to be randomized. The null hypothesis was rejected at the 0.05 level. All of the statistical analyses were performed using Stata (version 12; StataCorp., College Station, TX).
Results
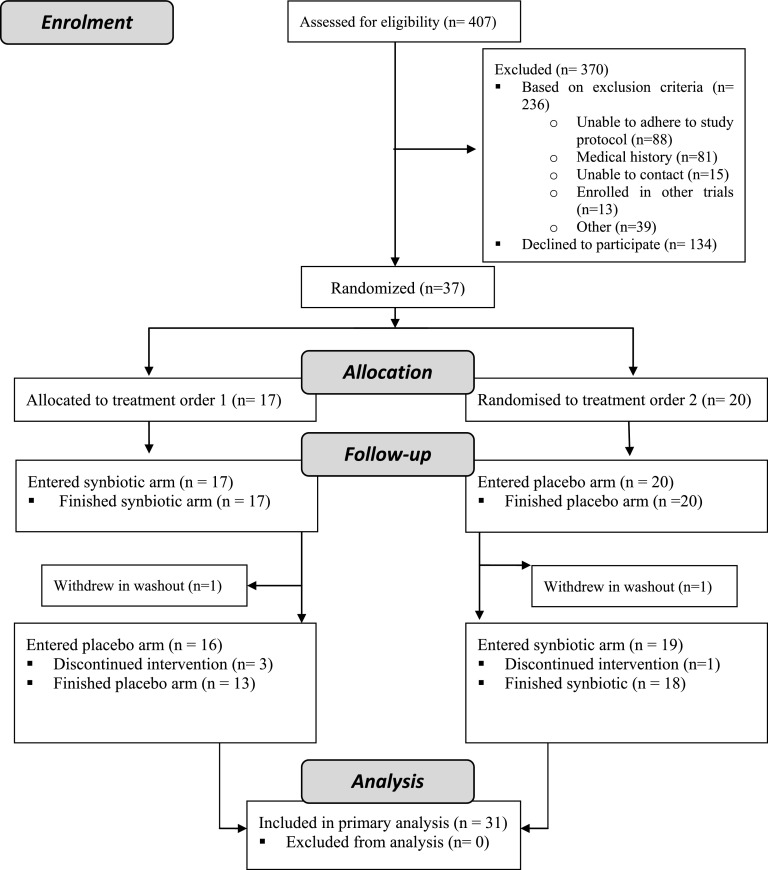
Thirty-seven participants were recruited between May 1, 2013 and December 11, 2013 from a single–center nephrology outpatient department and randomized to one of two treatment orders: synbiotic-placebo or placebo-synbiotic (baseline characteristics are outlined in Table 1). Thirty-one patients completed both arms of the trial (ending April 16, 2014, 18 weeks after the recruitment of the last participant) as detailed in the participant flow Consolidated Standards of Reporting Trials diagram (Figure 2). Compliance to study medications was achieved by 90% of patients.
Table 1.
Baseline characteristics of participants in the Synbiotics Easing Renal Failure by Improving Gut Microbiology Study who were randomized by treatment order (n=37)
| Characteristic | All Patients Randomized (n=37) | Treatment Order 1 (n=17)a | Treatment Order 2 (n=20) |
|---|---|---|---|
| Age, yr | 69±10 | 68±10 | 69±10 |
| Range | 43–82 | 43–82 | 50–82 |
| Men | 21 (57) | 7 (41) | 14 (70) |
| White | 35 (95) | 16 (94) | 19 (95) |
| Cause of kidney disease | |||
| GN | 5 (14) | 2 (12) | 3 (15) |
| Hypertension/vascular | 7 (19) | 3 (18) | 4 (20) |
| Diabetic nephropathy | 14 (38) | 9 (53) | 5 (25) |
| BMI, kg/m2 | 29±6 | 30±8 | 28±4 |
| Comorbidities (treated) | |||
| Hypertension | 37 (100) | 17 (100) | 20 (100) |
| Hyperlipidemia | 29 (78) | 13 (76) | 16 (80) |
| No. of antihypertensive medications | 2.3±1.1 | 2.5±1.4 | 2.0±0.7 |
| Angiotensin–converting enzyme inhibitor | 8 (22) | 3 (18) | 5 (25) |
| Angiotensin receptor II blocker | 22 (59) | 12 (71) | 10 (50) |
| Diuretics | 13 (35) | 6(35) | 7 (35) |
| Smoking history | 20 (54) | 8 (47) | 12 (60) |
| EPI GFR, ml/min per 1.73 m2 | 24±8 | 24±9 | 25±7 |
| Proteinuria, mg/24 h | 318 (165–1600) | 523 (160–1700) | 263 (168–1100) |
| Albuminuria, mg/24 h | 107 (20–1100) | 275 (18–1200) | 97 (20–677) |
| Uremic toxins (μmol/L) | |||
| Total indoxyl sulfate | 18 (12–27) | 20 (16–27) | 15 (10–25) |
| Total p-cresyl sulfate | 110 (71–130) | 128 (88–174) | 100 (61–119) |
| Free indoxyl sulfate | 0.7 (0.4–1.0) | 0.8 (0.5–1.1) | 0.6 (0.3–0.9) |
| Free p-cresyl sulfate | 3.0 (2.0–3.9) | 3.3 (2.0–5.2) | 2.5 (1.7–3.3) |
| Indoxyl sulfate-to-p-cresyl sulfate ratio | 0.23±0.17 | 0.19±0.11 | 0.27±0.22 |
| Percentage free fraction | |||
| Indoxyl sulfate | 4.1±1.5 | 4.4±1.8 | 3.8±1.0 |
| P-cresyl sulfate | 2.9±1.0 | 3.1±1.2 | 2.7±0.8 |
Data are presented as means±SDs, medians (interquartile ranges), or numbers (%). BMI, body mass index; EPI, epidemiology collaboration.
The imbalance in numbers arose because of the spoiling of two randomized kits, which were replaced by the next available kits.
Figure 2.
Summary of patient flow through in the Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY) Study.
Uremic Toxins
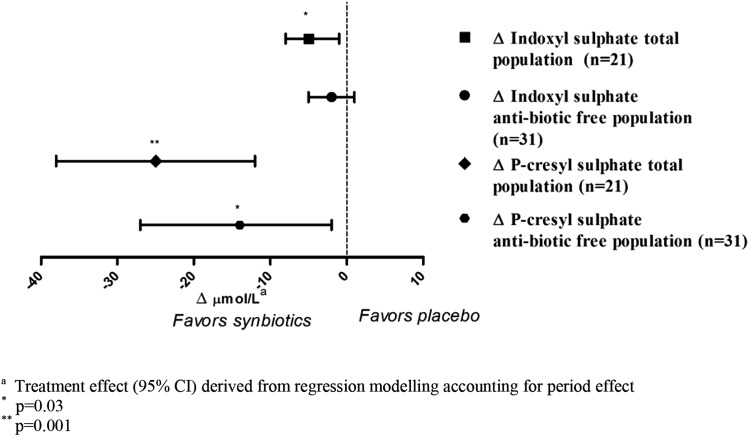
Compared with placebo, the synbiotic therapy resulted in a significant mean reduction in serum concentration of PCS of 14 μmol/L (95% confidence interval [95% CI], −27 to −2 μmol/L; 13% reduction), but the reduction in the primary outcome, IS, did not reach statistical significance (Figure 3, Table 2). However, when those participants who received antibiotics were excluded from the comparison, synbiotic therapy significantly reduced the free and total serum concentrations of both PCS and IS in the remaining 21 participants by 22%–28% (Figure 3, Table 2). Interestingly, there was a progressive reduction in serum concentrations of PCS (P=0.002) but not IS over the course of the study (Supplemental Figures 1 and 2).The effects of synbiotic administration on serum PCS and IS concentrations were independent of baseline toxin concentrations, changes in kidney function, changes in dietary fiber and protein intakes, changes in eGFR, and treatment order (Supplemental Table 2). There was no apparent carryover effect of the intervention on either toxin when analyzed for the order of synbiotic administration (both P>0.30), suggesting that reductions in serum PCS and IS concentration require sustained use of the synbiotic therapy. A linear mixed model sensitivity analysis yielded similar findings (Supplemental Table 2).
Figure 3.
Treatment effect of synbiotics on serum uremic toxins in all completing patients (n=31) and patients who were antibiotic free (n=21). aTreatment effect (95% confidence interval) derived from regression modeling accounting for period effect. *P=0.03; **P=0.001.
Table 2.
Uremic toxins serum concentrations (micromoles per liter) at the end of synbiotic and placebo intervention
| Uremic toxin | All Completers (n=31) | Antibiotic-Free Completers (n=21) | ||||||
|---|---|---|---|---|---|---|---|---|
| Synbiotics | Placebo | Treatment Effecta (95% CI) | P Value | Synbiotics | Placebo | Treatment Effecta (95% CI) | P Value | |
| Total IS | 15 (10–26) | 16 (12–27) | −2 (−5 to 1) | 0.12 | 15 (11–26) | 20 (12–32) | −5 (−8 to −1) | 0.03 |
| Total PCS | 75 (36–101) | 93 (54–136) | −14 (−27 to −2) | 0.03 | 68 (34–97) | 91 (54–130) | −25 (−38 to −12) | 0.001 |
| Free IS | 0.6 (0.4–0.8) | 0.5 (0.4–1.0) | −0.08 (−0.20 to 0.04) | 0.20 | 0.5 (0.4–0.7) | 0.5 (0.4–1.0) | −0.18 (−0.32 to −0.04) | 0.02 |
| Free PCS | 2.2 (0.7–2.8) | 2.4 (1.1–3.4) | −0.23 (−0.70 to 0.25) | 0.34 | 2.0 (0.6–2.4) | 2.4 (1.1–3.1) | −0.70 (−1.10 to −0.30) | 0.01 |
Data are presented as medians (interquartile ranges). 95% CI, 95% confidence interval; IS, indoxyl sulfate; PCS, p-cresyl sulfate.
Treatment effect derived from regression modeling accounting for period effect.
Clinical Outcomes
Synbiotic therapy significantly increased albuminuria by 38 mg/24 h (95% CI, 1 to 295 mg/24 h) but did not affect proteinuria (Table 3). No significant changes were observed in eGFR, urinary kidney injury molecule-1 concentration, serum inflammatory biomarker concentrations (IL-1β, IL-6, IL-10, and TNF-α), serum oxidative stress biomarkers (F2-isoprostanes and glutathione peroxidase), serum endotoxin (LPS) concentration, patient-reported health short form-36), Gastrointestinal Symptom Rating Scale, or dietary intakes of energy, protein, or fiber (Table 3).
Table 3.
Secondary outcomes at the end of synbiotic and placebo intervention
| Variable | All Completers (n=31) | Antibiotic-Free Completers (n=21) | ||||
|---|---|---|---|---|---|---|
| Synbiotics | Placebo | P Valuea | Synbiotics | Placebo | P Valuea | |
| Kidney related | ||||||
| EPI GFR, ml/min per 1.73 m2 | 24±8 | 24±8 | 0.67 | 23±7 | 23±7 | 0.46 |
| Serum creatinine, μmol/L | 231±75 | 233±74 | 0.94 | 236±72 | 241±74 | 0.59 |
| Kidney injury molecule-1 (n=27), ng/ml | 1.1 (0.4–2.7) | 1.1 (0.4–2.1) | 0.12 | 1.0 (0.3–1.7) | 1.1 (0.3–1.7) | 0.54 |
| Proteinuria (n=28), mg/24 h | 369 (162–1550) | 323 (169–1150) | 0.20 | 803 (175–1550) | 466 (224–1250) | 0.13 |
| Albuminuria (n=30), mg/24 h | 112 (16–758) | 111 (12–594) | 0.03b | 393 (47–970) | 240 (102–715) | 0.19 |
| Inflammatory | ||||||
| IL-1β, pg/ml | 0.8±0.7 | 0.8±0.6 | 0.98 | 0.8±0.6 | 0.8±0.7 | 0.36 |
| IL-6, pg/ml | 2.2±0.9 | 2.0±0.8 | 0.40 | 2.0±0.8 | 2.0±0.9 | 0.86 |
| IL-10, pg/ml | 3.6±2.0 | 3.6±2.1 | 0.84 | 3.4±1.7 | 3.7±2.1 | 0.32 |
| TNF-α, pg/ml | 2.2±0.8 | 2.0±0.7 | 0.09 | 2.1±0.7 | 2.1±0.7 | 0.58 |
| Oxidative stress | ||||||
| F2-isoprostanes, pg/ml | 141±76 | 167±112 | 0.16 | 127±63 | 139±97 | 0.68 |
| Glutathione peroxidase, U/L | 157±6 | 155±7 | 0.19 | 157±7 | 155±7 | 0.37 |
| Exploratory | ||||||
| Endotoxins, EU/ml | 0.29±0.13 | 0.27±0.13 | 0.16 | 0.29±0.13 | 0.27±0.12 | 0.18 |
| Patient reported health (n=27) | ||||||
| Physical composite score | 35±11 | 37±10 | 0.23 | 34±10 | 37±10 | 0.48 |
| Mental composite score | 51±10 | 52±9 | 0.75 | 50±10 | 52±9 | 0.67 |
| Gastrointestinal Symptom Score (n=30) | ||||||
| Mean score | 1.6±0.7 | 1.5±0.8 | 0.72 | 1.7±0.7 | 1.5±0.8 | 0.49 |
| Score >3, n (%) | ||||||
| Reflux | 0 (0) | 1 (3) | >0.99b | 0 (0) | 1 (5) | >0.99b |
| Abdominal pain | 0 (0) | 0 (0) | >0.99b | 0 (0) | 0 (0) | >0.99b |
| Indigestion | 4 (13) | 2 (7) | 0.69b | 4 (19) | 1 (5) | 0.38b |
| Constipation | 2 (7) | 5 (17) | 0.25b | 1 (5) | 3 (14) | 0.50b |
| Diarrhea | 3 (10) | 1 (3) | 0.50b | 3 (14) | 1 (5) | 0.50b |
| Dietary intake | ||||||
| Energy | ||||||
| Diet history, MJ | 7.6±2.5 | 7.4±2.5 | 0.19 | 7.9±2.7 | 7.7±2.7 | 0.08 |
| Body weight, kg | 80±18 | 80±18 | 0.47 | 80±21 | 80±21 | 0.43 |
| Protein | ||||||
| Diet history, g | 79±24 | 80±24 | 0.75 | 80±26 | 82±27 | 0.46 |
| Estimated from 24-h urinary urea nitrogen, g | 67±20 | 70±19 | 0.38 | 68±23 | 72±20 | 0.24 |
| Fiberd, g | 23±9 | 23±9 | 0.38 | 24±9 | 24±10 | 0.15 |
Data presented as means±SDs or medians (interquartile ranges). Score >3 indicates moderate discomfort. EPI, epidemiology collaboration; MJ, megajoules.
Derived from regression modeling with normally distributed data and Wilcoxon Mann–Whitney with non-normal data, with both methods accounting for period effect.
Treatment effect of 38 mg/24 h.
Exact McNemar significance probability.
From diet only and not including synbiotic supplement.
There were six SAEs in total: one occurred during the synbiotic intervention, and the other five occurred in placebo (n=2) or washout (n=3). Initial hospitalization accounted for all SAEs.
Stool Microbiota
Of 30 patients consenting to the fecal collection substudy, nine had incomplete samples, and one sample was contaminated. Therefore, 20 patients with complete fecal samples at visits three and six were included in the microbial analysis. The coverage of the microbiota diversity for all 40 samples was very high (in total, 7,166,577 reads were produced; mean =174,794 reads per sample; range =57,631–437,450; with Good coverage values in excess of 90%), and overall, 39 bacterial and archaebacterial families were identified (Supplemental Figure 3). Members of the Bacteroidaceae, Lachnospiraceae, and Ruminococcaceae comprised the majority of the communities recovered from all samples, with the majority of these microbes assigned to 74 different genera. Many of these genera showed some small changes in relative abundance between the placebo and synbiotic arms of the study (approximately 1%), and the mean Simpson coefficient of the fecal microbiota profiles was not significantly changed between interventions (P=0.42). However, although the Simpson coefficient is a good measure of bacterial diversity, it is weighted toward the most abundant members of a community (17), and our results do show that there were differences observed in the relative abundances of some key but less abundant taxa in response to synbiotic therapy. The most pronounced effect of synbiotic therapy was the increased relative abundance of Bifidobacterium spp. (3.2%; P=0.003), which represents a fivefold increase relative to placebo (Supplemental Figure 4). The relative increases in Bifidobacterium spp. were not attributable to the baseline Bidifobacterium abundance within individual subjects (P=0.13). There was a significant inverse correlation observed between changes in the relative abundance of Bifidobacterium spp. and free serum concentrations of both PCS and IS (r=−0.55, P=0.01 and r=−0.59, P=0.01, respectively). The analyses also showed that the relative abundances of Bifidobacterium spp. were only sustained during the period of synbiotic therapy, with no apparent crossover effect (P=0.89). There was also a small increase in the relative abundance of Lactobacillus spp. identified in the fecal samples on synbiotic therapy, but this was not statistically significant (0.7%; P=0.36).
The relative abundance of sequences assigned to unclassified members of the Lachnospiraceae family also showed an increase (2.1%; P=0.01). Interestingly, the relative abundance of Faecalibacterium spp. was also found to be significantly greater during symbiotic administration but only in those patients not treated with antibiotics during the interventions (n=15; 1.1%; P=0.04) (Supplemental Figure 4). There were concordant decreases in the relative abundances of bacteria assigned to the Clostridiales and more specifically, the Ruminococcaceae (4.3%; P=0.01).
Discussion
In this study, synbiotic therapy did not significantly reduce the serum concentration of IS (the primary outcome measure) but did result in significant alteration of several key secondary outcome measures, including a mean 14-μmol/L reduction in serum concentration of the nephrovascular uremic toxin, PCS, and an appreciable shift in the stool microbiome (particularly with enrichment of Bifidobacterium and depletion of Ruminococcaceae). When only patients who did not receive antibiotics during the study period were analyzed as part of a prespecified sensitivity analysis, synbiotic therapy resulted in statistically significant and potentially clinically important 22%–28% reductions in the serum concentrations of both IS and PCS. Moreover, the treatment was well tolerated, achieved excellent compliance, and did not adversely affect patient-reported health in contrast to other bowel therapies.
The SYNERGY Study findings support those of a recent meta-analysis, which reported a significant reduction of serum IS in dialysis and urinary PCS in the healthy population after supplementation (12). However, this meta-analysis was appreciably limited by suboptimal study quality, a lack of randomized, controlled trials, and clinical heterogeneity (including with respect to pre- and probiotic formulations). In addition, few studies reported how antibiotic use was managed during the intervention.
To date, there have been two other synbiotic intervention studies: one controlled trial in predialysis patients (18) and one uncontrolled trial in patients on hemodialysis (19), both of which measured p-cresol (PC; a surrogate marker of PCS [20]). Although no details concerning antibiotic use were provided, both studies reported greater reductions in PC after 30 days (21 μmol/L) and 14 days (26.8 μmol/L), respectively, compared with the SYNERGY Study’s primary analysis (14 μmol/L). Nonetheless, these changes were of similar magnitude to the subanalysis including only patients who remained antibiotic free (25 μmol/L; n=21).
Although causal relationships between PCS and clinical outcomes are yet to be established, a 3-year longitudinal study in 74 predialysis patients showed that each 5-μmol/L increase in serum PCS was associated with a 12% (95% CI, 1% to 21%) increased risk of a cardiovascular event and a 17% (95% CI, 5% to 30%) increased risk of progressing to dialysis after controlling for eGFR and age (21). These results were supported in a larger cohort (n=499), which showed that free serum PCS was associated with cardiovascular events independent of both eGFR and Framingham risk factors (hazard ratio, 1.39; 95% CI, 1.02 to 1.89) (22).
In oral adsorbent studies, 45% and 30% reductions in serum IS occurred alongside significant reductions in cardiovascular risk markers (including endothelial dysfunction [measured by flow-mediated dilation]) (23) and reduction in kidney failure progression (1/creatinine slope) (24), respectively. The plausibility of these clinical benefits are supported by mechanisms of toxicity reported in vitro, including concentration-dependent damage to human renal proximal tubule epithelial cells (25) and vascular endothelial cells (26).
The significant increase in albuminuria observed in the synbiotic arm should be considered, although the magnitude of increase was not regarded clinically significant and was not related to changes in toxin levels. Nevertheless, although it is possible that this result was a chance finding, additional exploration is warranted.
Serum IS and PCS concentrations are widely accepted to originate from dissimilatory schemes of protein metabolism by gut microbes. In this study, the measurable improvements in PCS levels during synbiotic therapy were linked principally with an increased relative abundance of Bifidobacterium spp. This supports the hypothesis that Bifidobacterium spp. regulate the growth of bacterial species with high enzymatic capacities to produce IS and PCS (27), such as bacteria from the Clostridales order and Ruminococcaceae family, which both decreased after synbiotics (28,29). The findings support those of a recent study that showed that consumption of Bifidobacterium-fermented milk and GOS reduced serum phenols in healthy adult women (30). The study findings also provide a mechanistic explanation for the observed PC level decreases in two other synbiotic studies that used combinations of Lactobacillus and Bifidobacterium spp. with GOS (19) and Lactobacillus, Bifidobacterium and Streptococcus spp. with inulin (18). Furthermore, Hida et al. (31) have previously reported that a probiotic–containing Bifidobacterium infantis, Lactobacillus acidophilus, and Enterococcus faecalis decreased serum IS after an increase in Bifidobacteriaceae and a decrease in the Enterobacteriaceae. Consequently, the ability of synbiotic formulations to reduce PCS and IS levels seems dependent on their Bifidogenic effects.
There were also modest alterations in the relative abundances of some but not all taxa implicated in affecting IS and PCS levels observed in this study. The increase in the relative abundance of Faecalibacterium in patients receiving synbiotics may have had several potentially beneficial effects, including production of anti-inflammatory factors (32) and reduction of the pool of tryptophan available for IS production (33).
The strengths of the SYNERGY Study include its randomized, double–blind, placebo–controlled, crossover trial design. Interindividual variations in gut microbiota profiles and toxin concentrations were eliminated by using patients as their own controls. Potential dietary confounders were controlled for by using multimodal validated dietary assessment methods applied by a qualified dietitian who was blinded, along with the participants, to the intervention. The measurement of both free and total serum IS and PCS concentrations provided the most comprehensive overview to date of how microbial-modulating therapies may affect uremic toxin production. To the best of our knowledge, the SYNERGY Study is the first study to have undertaken a nonculture–dependent microbial analysis exploring the effect of synbiotic therapy in CKD.
Balanced against these strengths, the SYNERGY Study was limited by a relatively small sample size and study duration (limiting statistical power for detection of changes in the primary outcome, serum IS, and secondary clinical outcomes; kidney function; and cardiovascular risk markers), use of surrogate outcome measures (IS and PCS), and single-center design (limited generalizability of the study findings). Although a 4-week washout period occurred between the two interventions, a carryover effect from the first intervention was possible, although not apparent in either the toxin or Bifidobacterium levels. In addition, antibiotics were used in >25% of the study sample and seemed to attenuate the effect of the synbiotic intervention, possibly as a result of relatively greater antimicrobial action exerted against probiotics than IS-producing bacteria. Furthermore, detailed exploration of the effect of antibiotic use on the gut microbiota was not possible because of the small sample size and the variety of antibiotics (Supplemental Table 3) prescribed to patients during the course of the study. The exclusion of patients who received antibiotics in the prespecified subgroup analysis, although clinically appropriate in light of the effect of antibiotics on the microbiota, could have potentially introduced selection bias. It is also important to note in the primary analysis that there were no changes in the free concentrations of either toxin, which may have been more pathophysiologically important than total concentrations. Lastly, the analysis of the stool microbiome may not have reflected alterations in the mucosal-associated microbiomes within the large and small bowel. Furthermore, despite recent studies inferring functional attributes of these microbial communities by comparison with reference microbial genomes (29), such an approach cannot readily account for variations in gene expression, which are influenced by the colonic environment (e.g., pH and carbohydrate availability) (28).
In conclusion, synbiotic therapy effectively lowered serum concentrations of PCS and to a lesser extent, IS in patients with moderate to severe CKD, particularly when such patients were not prescribed antibiotics. The medication was well tolerated, and patient adherence was high. Larger randomized, controlled trials evaluating the effect of synbiotic therapy on patient-level outcomes in CKD are warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Rachel Hale for assistance as the study nurse, Ms. Alicia Kang for contributions to the fecal microbiota analyses, Dr. Stuart Denman for efforts to support the bioinformatics analyses of the microbiome data, David Briskey for the inflammatory and oxidative stress measurements, Amelia Fotheringham for technical expertise in kidney injury molecule-1 measurement, and BioCeuticals for providing the synbiotic and placebo supplements.
This study was funded through a project grant from the Princess Alexandra Private Practice Trust Fund (PPTF). M.R. is a recipient of the Princess Alexandra PPTF Postgraduate Scholarship. D.W.J. is supported by a Queensland Government Health and Medical Research (HMR) Health Research Fellowship. K.L.C. is supported by a Queensland Government HMR Health Research Fellowship and a Lions Senior Medical Research Fellowship.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Effect of Synbiotic Therapy on Gut–Derived Uremic Toxins and the Intestinal Microbiome in Patients with CKD,” on pages 199–201.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05240515/-/DCSupplemental.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Isbel NM, Haluska B, Johnson DW, Beller E, Hawley C, Marwick TH: Increased targeting of cardiovascular risk factors in patients with chronic kidney disease does not improve atheroma burden or cardiovascular function. Am Heart J 151: 745–753, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH: Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 8: 1494–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schepers E, Glorieux G, Vanholder R: The gut: The forgotten organ in uremia? Blood Purif 29: 130–136, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Meijers BKI, Evenepoel P: The gut-kidney axis: Indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant 26: 759–761, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Aronov PA, Luo FJ-G, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi M, Campbell KL, Johnson DW: Indoxyl sulphate and p-cresyl sulphate: Therapeutically modifiable nephrovascular toxins. OA Nephrol 1: 13–21, 2013 [Google Scholar]
- 10.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M: Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol 26: 1732–1746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi M, Klein K, Johnson DW, Campbell KL: Pre-, pro-, and synbiotics: Do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol 2012: 673631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi M, Johnson DW, Morrison M, Pascoe E, Coombes JS, Forbes JM, McWhinney BC, Ungerer JP, Dimeski G, Campbell KL: SYNbiotics Easing Renal failure by improving Gut microbiologY (SYNERGY): A protocol of placebo-controlled randomised cross-over trial. BMC Nephrol 15: 106, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanholder R, Glorieux G: The intestine and the kidneys: A bad marriage can be hazardous. Clin Kidney J 8: 168–179, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ash S, Campbell K, MacLaughlin H, McCoy E, Chan M, Anderson K, Corke K, Dumont R, Lloyd L, Meade A, Montgomery-Johnson R, Tasker T, Thrift P, Trotter B: Evidence based practice guidelines for the nutritional management of chronic kidney disease. Nutr Diet 63: S33–S45, 2006 [Google Scholar]
- 15.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Jones B, Kenward G: Design and Analysis of Cross-Over Trials, London, Chapman & Hall, 2003 [Google Scholar]
- 17.Magurran A: Measuring Biological Diversity, Oxford, United Kingdom, Blackwell Sciences, 2004 [Google Scholar]
- 18.Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, Barbato F, Cataldi M: Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr Metab Cardiovasc Dis 24: 1043–1049, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M: Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol Dial Transplant 26: 1094–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Vanholder R, Bammens B, de Loor H, Glorieux G, Meijers B, Schepers E, Massy Z, Evenepoel P: Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol Dial Transplant 26: 1464–1467, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Lin CJ, Pan CF, Chuang CK, Sun FJ, Wang DJ, Chen HH, Liu HL, Wu CJ: P-cresyl sulfate is a valuable predictor of clinical outcomes in pre-ESRD patients. BioMed Res Int 2014: 526932, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P: p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5: 1182–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu M, Kim YJ, Kang DH: Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol 6: 30–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owada A, Nakao M, Koike J, Ujiie K, Tomita K, Shiigai T: Effects of oral adsorbent AST-120 on the progression of chronic renal failure: A randomized controlled study. Kidney Int Suppl 63: S188–S190, 1997 [PubMed] [Google Scholar]
- 25.Poveda J, Sanchez-Niño MD, Glorieux G, Sanz AB, Egido J, Vanholder R, Ortiz A: p-cresyl sulphate has pro-inflammatory and cytotoxic actions on human proximal tubular epithelial cells. Nephrol Dial Transplant 29: 56–64, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P: The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442–451, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Gibson GR, Wang X: Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol 77: 412–420, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, Anderson SE, Flint HJ: Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res 57: 523–535, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND: Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39: 230–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki K, Masuoka N, Kano M, Iizuka R: Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef Microbes 5: 121–128, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y: Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74: 349–355, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P: Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105: 16731–16736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinken A, Khan MT, Paglia G, Rodionov DA, Harmsen HJM, Thiele I: Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J Bacteriol 196: 3289–3302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.