Abstract
Background and objectives
Despite the many studies showing an association between CKD and a high risk of ischemic events and mortality, the association of CKD with peripheral arterial disease (PAD) still has not been well described.
Design, setting, participants, & measurements
This large cohort study assessed the association of CKD, even in the earlier stages, with morbidity, short- and long-term outcome, and costs among patients with PAD.
Results
We identified 41,882 patients with PAD who had an index hospitalization between January 1, 2009, and December 31, 2011. Of these, 8470 (20.2%) also had CKD (CKD stage 2: n=2158 [26%]; stage 3: n=3941 [47%]; stage 4: n=935 [11%]; stage 5: n=1436 [17%]). The ratio of women to men was 1:1.2. Compared with patients without known CKD, those with CKD had higher frequencies of coronary artery disease (1.8-fold higher; P<0.001), chronic heart failure (3.3-fold higher; P<0.001), and Rutherford PAD categories 5 and 6 (1.8-fold higher; P<0.001); underwent significantly fewer revascularizations (0.9-fold fewer; P<0.001); had a nearly two-fold higher amputation rate (P<0.001); had higher frequencies of in-hospital infections (2.1-fold higher; P<0.001), acute renal failure (2.8-fold higher; P<0.001), and sepsis (1.9-fold higher; P<0.001); had a 2.5-fold higher frequency of myocardial infarction (P<0.001); and had a nearly three-fold higher in-hospital mortality rate (P<0.001). In an adjusted multivariable Cox regression model, CKD remained a significant predictor of long-term outcome of patients with PAD during follow-up for up to 4 years (until December 31, 2012; median, 775 days; 25th–75th percentiles, 469–1120 days); the hazard ratio was 2.59 (95% confidence interval, 2.21 to 2.78; P<0.001). The projected mortality rates after 4 years were 27% in patients without known CKD and 46%, 52%, 72%, and 78% in those with CKD stages 2, 3, 4, and 5, respectively. Lengths of hospital stay and reimbursement costs were on average nearly 1.4-fold higher (P<0.001) in patients who also had CKD.
Conclusions
This analysis illustrates the significant and important association of CKD with in-hospital and long-term mortality, morbidity, amputation rates, duration and costs of hospitalization, in-hospital treatment, and complications in patients with PAD.
Keywords: chronic kidney disease, peripheral arterial disease, outcome, acute kidney injury, amputation, cohort studies, hospital mortality, hospitalization, humans, length of stay
Introduction
Lower-extremity peripheral artery disease (PAD) is associated with a fatal outcome (1,2). CKD also predicts mortality, is related to substantially higher rates of vascular ischemic events (3), and seems to be associated with severe PAD requiring revascularization and higher amputation rates (2). Moreover, it is presents one of the fastest-growing global health and socioeconomic burdens (4–6).
Despite the many studies showing the association of CKD with a high risk of ischemic events and mortality, the association of CKD with PAD is not well described. In particular, to our knowledge no studies have sufficiently evaluated the clinical importance of the earlier stages of both diseases according to morbidity and outcome of patients with PAD. Therefore, physicians still lack robust data and guidelines on how to treat vascular diseases in patients with CKD. Evaluations of large-scale data on morbidity, treatment, complications, outcome, and costs in patients with PAD who also have CKD are essential for discussion of this topic.
The aim of this study was to evaluate the association of different stages of CKD with morbidity, in-hospital treatment and complications, mortality, and longterm outcome and costs in patients with PAD.
Materials and Methods
Explanation of the German Diagnosis-Related Group System
Since 2004, the diagnosis and procedure–related reimbursement system (German Diagnosis-Related Groups [G-DRG] system) has been used for hospital reimbursement in Germany. All hospitals are obligated to transfer all data on diagnoses, comorbidities, and complications to health insurance companies in the form of detailed and mandatory coding guidelines according to the International Classification of Diseases (ICD). As is done for the ICD, all diagnostic and therapeutic procedures have to be coded according to the German procedure classification (Operations Procedure Codes [OPS]). If this is not done, payment for a hospital treatment is not reimbursed.
The G-DRG system requires one code for the main diagnosis, which must be carefully chosen after discharge of the in-hospital patient. An unlimited number of additional codes representing secondary diagnoses can also be used to reflect the presence or occurrence of comorbidities and complications during the hospital stay. These additional codes can increase the patients’ complexity level and therefore affect reimbursement.
DRGs are built via the Grouper software program, which is certified by the Institut für das Entgeltsystem im Krankenhaus in line with the diagnosis and procedure catalogs (German Modification of the ICD, 10th Revision [ICD-10-GM], and OPS). The inclusion and exclusion criteria of the individual ICD and OPS codes must be in line with the German coding rules Deutsche Kodierrichtlinie of the Institut für das Entgeltsystem im Krankenhaus. All of these codes are checked by the software; moreover, about 20% are controlled and, if needed, corrected by specialized physicians (Medizinischer Dienst der Krankenversicherung) independently of health insurance companies and hospitals.
All inhabitants of Germany must have private or public health insurance by law.
Study Population, Data Sources, and Inclusion Criteria
We evaluated anonymized data of a large public health insurance company in Germany (BARMER GEK) as described elsewhere in detail (7). With around 8.7 million insured patients, it represents about 10% of the entire German population. Our cohort contains all patients with an index hospitalization between January 1, 2009, and December 31, 2011, who had lower-limb PAD (ICD-10 code, I70.2*) as a main diagnosis or codiagnosis.
Diagnoses, Operations Procedure Codes, and Reimbursement
All main or additional diagnoses were coded according to the ICD-10-GM. Annual adaptations of the ICD-10-GM did not affect any of the analyzed diagnoses in this study.
PAD was classified according to Rutherford categories: I70.24 was coded as Rutherford 6 (severe ischemic ulcers or frank gangrene); I70.23 as Rutherford 5 (ischemic ulceration not exceeding ulcer of the digits of the foot); I70.22 as Rutherford 4 (rest pain); and I70.20 or I70.21 as Rutherford 1 (asymptomatic), 2 (mild claudication), and 3 (severe claudication). CKD was classified as follows: N18.82 as CKD stage 2 (normal or mildly reduced renal function), N18.83 as CKD stage 3 (moderate renal insufficiency), N18.84 as CKD stage 4 (severe renal insufficiency), and N18.0 as CKD stage 5 (established kidney failure [GFR<15 ml/min per 1.73 m2], permanent RRT, or ESRD). Additionally, the OPS codes for diagnostic angiography (3–605.0, 3–607.0), endovascular treatment (8–836*, 8–84*), vascular surgery (5–380*, 5–381*, 5–383*, 5–386*, 5–388*, 5–393*, 5–395*), and amputations (5–864.*, 5–865.*) were evaluated. Amputations of the upper extremities or amputations due to reasons other than limb ischemia were not included.
All in-hospital costs are presented in this study. Costs resulting from outpatient care are not included in this analysis.
Follow-Up Data
All inpatient and outpatient cardiovascular diagnoses and procedure codes were recorded during the index hospitalization and for at least 24 months after discharge until December 31, 2012.
Statistical Analyses
Frequencies of the distinct ICD codes and OPS codes are given as absolute numbers (n) and percentages of the total numbers for each CKD subgroup. Statistical comparisons for these categorical variables were made by the chi-squared test. Continuous variables are presented as means±SDs and were compared by the ANOVA F-test. P values <0.05 were considered to represent statistically significant differences. Relative risks for death and amputation during follow-up, as well as the predictive value of baseline parameters concerning long-term outcomes, were analyzed in separate multivariable Cox regression model analyses using SPSS 22.0 (covariates: age, sex, hypertension, obesity, dyslipidemia, smoking, diabetes, PAD, coronary artery disease, chronic heart failure, and malignancies).
Results
The cohort contains 41,882 patients with PAD who met the inclusion criteria mentioned in the Materials and Methods section. Of these, 8470 (20.2%) also had CKD. Most patients had PAD in Rutherford categories 1–3 (n=20,491 [49.0%]), followed by category 6 (n=8964 [21.4%]). Of those with CKD, 26% (n=2158) had normal or mildly reduced renal function (CKD stage 2), 47% (n=3941) had moderate renal insufficiency (CKD stage 3), 11% (n=935) had severe renal insufficiency (CKD stage 4), and 17% (n=1436) had ESRD (CKD stage 5). The ratio of women to men was 1:1.2. Baseline characteristics and comorbidities of all patients in relation to CKD stages are presented in Table 1.
Table 1.
Baseline characteristics and comorbidities per CKD stages
| Characteristic | No CKD | CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | CKD Stage 5 | All | P Value |
|---|---|---|---|---|---|---|---|
| Patients | 33,412 (79.8) | 2158 (5.2) | 3941 (9.4) | 935 (2.2) | 1436 (3.4) | 41,882 (100.0) | |
| Mean age±SD, yr | 70.3±11.5 | 75.1±10.2 | 77.1±9.5 | 78.2±10.1 | 72.2±10.4 | 71.4±11.4 | <0.001 |
| Women | 14,905 (44.6) | 910 (42.2) | 1819 (46.2) | 491 (52.5) | 466 (32.5) | 18,591 (44.4) | <0.001 |
| Hypertension | 22,261 (66.6) | 1623 (75.2) | 2962 (75.2) | 656 (70.2) | 983 (68.5) | 28,485 (68.0) | <0.001 |
| Obesity | 2346 (7.0) | 211 (9.8) | 362 (9.2) | 70 (7.5) | 84 (5.8) | 3073 (7.3) | <0.001 |
| Dyslipidemia | 10,209 (30.6) | 779 (36.1) | 1354 (34.4) | 245 (26.2) | 367 (25.6) | 12,954 (30.9) | <0.001 |
| Smoking | 4310 (12.9) | 134 (6.2) | 194 (4.9) | 27 (2.9) | 40 (2.8) | 4705 (11.2) | <0.001 |
| Diabetes | 9438 (28.2) | 949 (44.0) | 1866 (47.3) | 498 (53.3) | 810 (56.4) | 13,561 (32.4) | <0.001 |
| Coronary artery disease | 7233 (21.6) | 781 (36.2) | 1516 (38.5) | 319 (34.1) | 616 (42.9) | 10,465 (25.0) | <0.001 |
| Chronic heart failure | 2269 (6.8) | 389 (18.0) | 903 (22.9) | 266 (28.4) | 296 (20.6) | 4123 (9.8) | <0.001 |
| PAD Rutherford stage | 17,846 (53.5) | 812 (37.6) | 1394 (35.4) | 203 (21.7) | 236 (16.5) | 20,491 (49.0) | <0.001 |
| 1–3 | |||||||
| 4 | 4559 (13.7) | 312 (14.5) | 557 (14.1) | 110 (11.8) | 131 (9.2) | 5669 (13.5) | |
| 5 | 4795 (14.4) | 434 (20.1) | 903 (22.9) | 238 (25.5) | 353 (24.7) | 6723 (16.1) | |
| 6 | 6188 (18.5) | 600 (27.8) | 1083 (27.5) | 383 (41.0) | 710 (49.7) | 8964 (21.4) | |
| Malignancies | 578 (1.7) | 55 (2.5) | 88 (2.2) | 19 (2.0) | 34 (2.4) | 774 (1.8) | 0.01 |
Unless otherwise noted, values are the number (percentage) of patients. PAD, peripheral arterial disease.
Compared with patients without known CKD, patients with CKD were older; more often had hypertension, diabetes, obesity, coronary artery disease, and chronic heart failure; and showed more severe PAD (Table 1). Fewer patients with classic cardiovascular risk factors, such as hypertension, obesity, dyslipidemia, and smoking, and more patients with diabetes, coronary artery disease, and chronic heart failure were associated with higher CKD stages (P<0.001). Patients in the most severe stage of CKD had the highest prevalence of severe PAD (tissue ulceration and gangrene [Rutherford stages 5 and 6]).
In-Hospital Treatment, In-Hospital Complications
Patients with PAD and CKD received significantly fewer revascularizations procedures (surgical bypasses, thromboendarterectomy, and endovascular procedures) than patients with PAD who did not have CKD. Moreover, the frequency of revascularization procedures was inversely related to the CKD stage (i.e., patients with higher CKD stage and more severe PAD stage received fewer revascularization procedures than those with lower CKD stages and lower Rutherford categories).
A total of 1353 (16.0%) patients with CKD had amputation during their index hospitalization. Thus, the amputation frequency was nearly two-fold higher in patients with known CKD than in those without and was associated with higher CKD stage. Details of in-hospital treatment during the index hospitalization are shown in Table 2.
Table 2.
Treatment, complications, and outcomes during index hospitalization
| Variable | No CKD | CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | CKD Stage 5 | All | P Value |
|---|---|---|---|---|---|---|---|
| Patients | 33,412 (79.8) | 2158 (5.2) | 3941 (9.4) | 935 (2.2) | 1436 (3.4) | 41,882 (100.0) | |
| Angiography | 18,302 (54.8) | 1245 (57.7) | 2200 (55.6) | 431 (46.1) | 888 (61.8) | 23,066 (55.1) | <0.001 |
| Endovascular revascularization | 15,067 (45.1) | 915 (42.4) | 1678 (42.6) | 297 (31.8) | 619 (43.1) | 18,576 (44.4) | <0.001 |
| Surgery | 8771 (26.3) | 545 (25.3) | 816 (20.7) | 193 (20.6) | 268 (18.7) | 10,593 (25.3) | <0.001 |
| Thromboendarterectomy | 4224 (12.6) | 222 (10.3) | 359 (9.1) | 77 (8.2) | 107 (7.5) | 4989 (11.9) | <0.001 |
| Bypass | 4256 (12.7) | 277 (12.8) | 415 (10.5) | 94 (10.1) | 168 (11.7) | 5210 (12.4) | <0.001 |
| Any revascularization procedure | 22,449 (67.2) | 1360 (63.0) | 2344 (59.5) | 449 (48.0) | 836 (58.2) | 27,438 (65.5) | <0.001 |
| Acute renal failure | 301 (0.9) | 43 (2.0) | 95 (2.4) | 59 (6.3) | 13 (0.9) | 511 (1.2) | <0.001 |
| Myocardial infarction | 190 (0.6) | 17 (0.8) | 62 (1.6) | 17 (1.8) | 31 (2.2) | 317 (0.8) | <0.001 |
| Ischemic stroke | 106 (0.3) | 11 (0.5) | 16 (0.4) | 5 (0.5) | 8 (0.6) | 146 (0.3) | 0.23 |
| In-hospital infections | 3817 (11.4) | 400 (18.5) | 866 (22.0) | 287 (30.7) | 379 (26.4) | 5749 (13.7) | <0.001 |
| Sepsis | 658 (2.0) | 59 (2.7) | 147 (3.7) | 37 (4.0) | 82 (5.7) | 983 (2.3) | <0.001 |
| Amputations | 3048 (9.1) | 287 (13.3) | 548 (13.9) | 175 (18.7) | 343 (23.9) | 4401 (10.5) | <0.001 |
| Death | 708 (2.1) | 85 (3.9) | 174 (4.4) | 96 (10.3) | 154 (10.7) | 1217 (2.9) | <0.001 |
| Median in-hospital stay (25th–75th percentiles), d | 7 (2–13) | 10 (4–19) | 10 (4–19) | 14 (7–23) | 12 (4–23) | 7 (2–14) | <0.001 |
| Median costs (25th–75th percentiles), € | 4065 (2174– 6442) | 4454 (2628–7230) | 4851 (2718–7363) | 5568 (2807–8471) | 6493 (3686–10,339) | 4199 (2368–6697) | <0.001 |
Unless otherwise noted, values are the number (percentage) of patients.
The leading complication was infectious disease (5749 patients [13.7%]), followed by acute renal failure (511 patients [1.2%]). Patients with known CKD had clearly higher frequencies of all observed in-hospital complications (Table 2). A total of 4401 patients (10.5%) had amputation during their index hospitalization. The frequency of amputations in patients with PAD and CKD (16.0%) was 1.8-fold higher than in patients with PAD who did not have known CKD (9.1%; P<0.001).
The frequency of myocardial infarction was 2.5-fold higher and that of stroke >1.5-fold higher in patients with known CKD.
Short- and Long-Term Outcome
The overall mortality during the index hospitalization in the entire cohort was 2.9% (1217 deaths). Patients with CKD had a nearly three-fold higher in-hospital mortality rate (6.0%) than those without known CKD (2.1%; P<0.001). Mortality was associated with higher CKD stage. A big step was observed between CKD stages 3 (4.4%) and 4 (10.3%), with more than a two-fold higher in-hospital mortality rate.
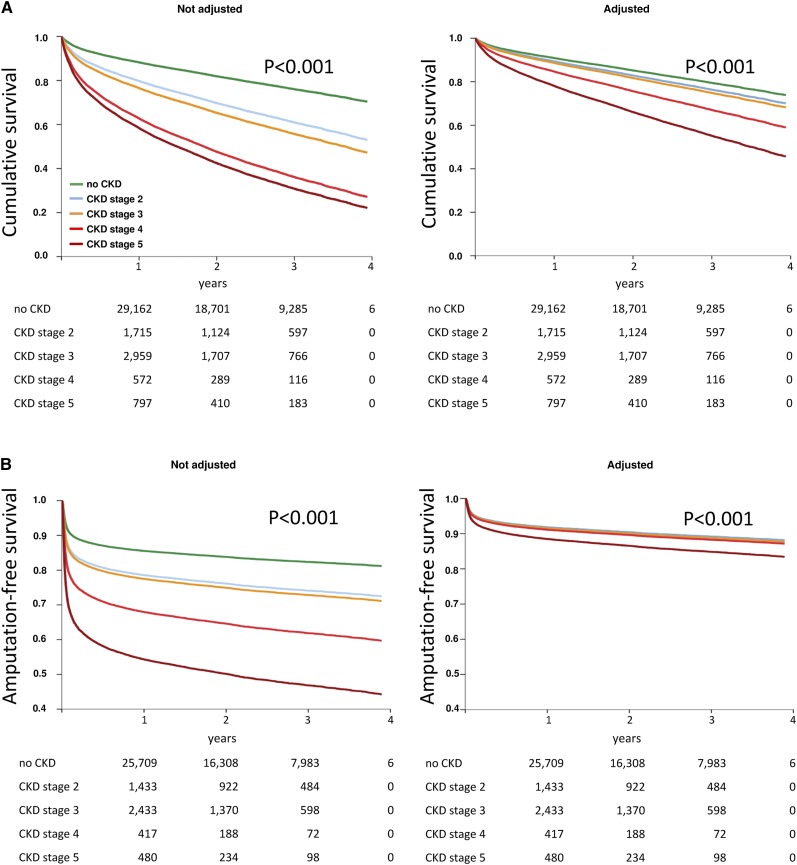
Mortality and amputation risks were also analyzed during a follow-up period of up to 4 years (median, 775 days; 25th–75th percentiles, 469–1120 days). In the staged Cox regression analysis adjusted for comorbidities and baseline parameters (Figure 1, Table 3), CKD was a significant predictor of long-term mortality and, in the case of CKD stage 5, of amputation. The adjusted hazard ratios for long-term mortality were associated with higher CKD stages, to a maximum of 2.59 (95% confidence interval, 2.21 to 2.78; P<0.001) in CKD stage 5. Mortality rates after 4 years were 27% in patients without known CKD and 46%, 52%, 72%, and 78% in patients with CKD stages 2, 3, 4, and 5, respectively. The projected amputations showed also a highly significant association in CKD stage 5: hazard ratio, 1.43 (95% confidence interval, 1.31 to 1.55; P<0.001). The proportions with amputations after 4 years were 18% in patients without known CKD and 27%, 28%, 40%, and 56% in patients with CKD stages 2, 3, 4, and 5, respectively.
Figure 1.
CKD and survival and amputation. During follow-up, CKD was a high significant independent predictor of long-term mortality and amputation (P<0.001). Cox regression analysis adjusted for additional comorbidities and baseline parameters is presented for death (A) and amputation (B). Highly significant differences were observed among the CKD stages (P<0.001).
Table 3.
Multivariable Cox regression analysis of death and amputation during follow-up
| Variable | Death | Amputation | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | P Value | Hazard Ratio (95% Confidence Interval) | P Value | |
| No CKD | 1 (Reference) | 1 (Reference) | ||
| CKD stage 2 | 1.17 (1.09 to 1.26) | <0.001 | 0.99 (0.91 to 1.09) | 0.90 |
| CKD stage 3 | 1.26 (1.19 to 1.34) | <0.001 | 1.03 (0.96 to 1.11) | 0.35 |
| CKD stage 4 | 1.74 (1.59 to 1.91) | <0.001 | 1.08 (0.96 to 1.22) | 0.19 |
| CKD stage 5 | 2.59 (2.41 to 2.78) | <0.001 | 1.43 (1.31 to 1.55) | <0.001 |
| Age | 1.06 (1.06 to 1.06) | <0.001 | 1.00 (1.00 to 1.00) | 0.04 |
| Women | 1.19 (1.14 to 1.24) | <0.001 | 1.32 (1.25 to 1.38) | <0.001 |
| Hypertension | 0.83 (0.79 to 0.86) | <0.001 | 0.91 (0.87 to 0.96) | <0.001 |
| Obesity | 0.91 (0.84 to 0.99) | 0.03 | 0.95 (0.87 to 1.04) | 0.25 |
| Dyslipidemia | 0.75 (0.71 to 0.78) | <0.001 | 0.84 (0.79 to 0.89) | <0.001 |
| Smoking | 1.07 (0.98 to 1.16) | 0.12 | 0.84 (0.76 to 0.92) | <0.001 |
| Diabetes | 1.04 (1.00 to 1.09) | 0.04 | 1.49 (1.43 to 1.57) | <0.001 |
| PAD Rutherford stage | ||||
| 1–3 | 1 (Reference) | 1 (Reference) | ||
| 4 | 2.01 (1.88 to 2.14) | <0.001 | 3.00 (2.66 to 3.38) | <0.001 |
| 5 | 2.46 (2.32 to 2.61) | <0.001 | 9.12 (8.30 to 10.02) | <0.001 |
| 6 | 3.59 (3.40 to 3.78) | <0.001 | 28.28 (25.93 to 30.83) | <0.001 |
| Coronary artery disease | 1.22 (1.17 to 1.28) | <0.001 | 0.99 (0.94 to 1.05) | 0.80 |
| Chronic heart failure | 1.63 (1.55 to 1.71) | <0.001 | 1.12 (1.05 to 1.19) | 0.001 |
| Malignancies | 2.20 (2.00 to 2.43) | <0.001 | 0.92 (0.79 to 1.07) | 0.28 |
PAD, peripheral arterial disease.
Length of Hospital Stay and Reimbursement Costs
The median length of in-hospital stay for the PAD subgroup without known CKD was 7 days (25th–75th percentiles, 2–13 days). The length of stay among patients with CKD was on average nearly 1.4-fold higher (15.4 days; P<0.001) (Table 2).
Median costs for the hospitalization of a patient with PAD without known CKD were €4065 (25th–75th percentiles, €2174–€6442). Median costs for a hospitalization with CKD were on average 40% higher (€534; P<0.001) (Table 2).
Discussion
The results of this study highlight several important aspects of the comorbidity of PAD and CKD. First, coexistence of CKD is associated with more severe PAD: Patients with CKD had a nearly 2-fold higher prevalence of ischemic ulcerations or gangrene (Rutherford stages 5 and 6) compared with those without known CKD. In line with our findings, several case series and one meta-analysis demonstrated that CKD coincides with severe PAD (2,8–11). However, those studies evaluated only a subset of patients with CKD (those with established kidney failure). The data presented here extend those findings by quantifying the detrimental association of the entire spectrum of CKD with the outcome regarding amputation and mortality on the different stages of PAD.
Second, despite the evidence-based preventive effect of revascularization on limb amputation in patients with critical limb ischemia, it is surprising that in particular patients with Rutherford category 5 and 6 PAD had lower rates of revascularization than asymptomatic patients and those with claudication. This finding related not only to surgical but also to endovascular procedures. This lack of adherence to therapeutic measures recommended by the specific guidelines was recently demonstrated by Reinecke et al. (7). In a meta-analysis, Garimella et al. (2) also reported that CKD is associated not only with severe PAD but also with higher rates of limb amputation. Thus, this observation gives rise to questions about the status quo and standards for revascularization indications in patients with PAD and CKD; this procedure is obviously still underused.
Third, CKD in patients with PAD predicted higher rates of in-hospital complications, such as ischemic myocardial and cerebrovascular events as well as infections and sepsis. Our data demonstrate a two-fold higher risk for an infection, septic course, or acute renal failure when patients with PAD also have CKD.
Previous studies have shown that CKD and PAD independently predict cardiovascular ischemic events (3,12). Our data additionally demonstrate substantially higher rates of myocardial infarction (2.5-fold higher) and ischemic stroke (1.6-fold higher) in patients with both diseases than in those who have PAD without CKD. The ischemic event rates were associated with higher CKD stages. Similar data are available only from cohorts consisting of patients with ESRD. The influence of PAD was considered. These data demonstrate a nearly six-fold higher rate of ischemic stroke in this cohort compared with those without CKD (12,13).
The most striking finding of this study is the outcome regarding limb amputation and mortality: Coexistence of PAD and CKD is associated with distinctly higher rates of limb amputation as well as higher rates of in-hospital and long-term mortality. CKD was a significant predictor of long-term mortality and amputation in patients with PAD. Liew et al. (14) described an overall 1.5-fold higher 6-year mortality rate in patients with both CKD and PAD than in patients with either disease alone. Pasqualini et al. (15) ascertained a 2.2-fold higher 4-year mortality rate in patients with both CKD and PAD than in patients without CKD. Our data indicate markedly higher mortality rates in patients with PAD and CKD compared with data reported by Liew et al. (14) and Pasqualini et al. (15). In our cohort, CKD was associated with a nearly three-fold higher in-hospital mortality rate and long-term mortality rate in patients with PAD.
We also found an association of in-hospital mortality and higher CKD stage, with an over two-fold higher mortality in CKD stage 4 compared with CKD stage 3. Comparative data are still lacking. The frequency of amputation in PAD patients with CKD was 1.8-fold higher than in patients with PAD without known CKD. This is in line with the findings of Garimella et al. (2) and O’Hare et al. (16), who described this finding exclusively in patients with established kidney failure.
Finally, the lengths of hospital stay and reimbursement costs were nearly 1.4-fold higher in patients with PAD and CKD than in patients with PAD who did not have CKD. Meyer et al. (4) and Laliberté et al. (17) showed that CKD leads to markedly higher in-hospital costs in treatment of patients with established cardiovascular disease. Our data underline these findings and demonstrate explicitly that CKD is associated with significantly higher cost of treatment in patients with PAD.
In summary, our data demonstrate the additive detrimental association of CKD with morbidity, complications, long-term outcome, and costs of patients with PAD.
Several limitations of our study should be mentioned. Diagnosis and procedure–based reports depend fundamentally on coding behavior. Therefore, we just analyzed end points such as amputation, myocardial infarction, ischemic stroke, and death, which have a low likelihood of being miscoded. Likewise, factors enhancing the reimbursement costs (CKD, diabetes, chronic heart failure, PAD, malignancies, acute renal failure, in-hospital infections, and sepsis) are unlikely to be omitted because complete coding is obligatory for correct reimbursement and therefore very important for the hospitals. Thus, completeness of these codiagnoses and procedures could be expected to be high. In contrast, characteristics with no effect on reimbursement costs (hypertension, obesity, dyslipidemia, and smoking) are more prone to miscoding and therefore might be underestimated in this analysis.
The precise coding rules for ICD-10-GM and OPS have been used for >10 years in Germany. They were not changed with regard to the subject of this study. Because only hospitalized patients were analyzed, the epidemiologic characteristics of the ambulatory PAD population and its economic impact were not studied. Moreover, they present only a selection of patients with conceivably more severe disease. Finally, this study is purely observational.
Disclosures
None.
Acknowledgments
We are indebted to Dirk Jürgen and the team of BARMER GEK for providing the data and for support during data analysis. We thank Susanne Schüler for her excellent support in manuscript preparation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hirsch AT, Duval S: The global pandemic of peripheral artery disease. Lancet 382: 1312–1314, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Garimella PS, Hart PD, O’Hare A, DeLoach S, Herzog CA, Hirsch AT: Peripheral artery disease and CKD: A focus on peripheral artery disease as a critical component of CKD care. Am J Kidney Dis 60: 641–654, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Drey N, Roderick P, Mullee M, Rogerson M: A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Meyer A, Bunzemeier H, Hausberg M, Walter M, Roeder N, Breithardt G, Reinecke H: Impact of different stages of chronic kidney disease on in-hospital costs in patients with coronary heart disease. Nephrol Dial Transplant 23: 1955–1960, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, Gebauer K, Roeder N, Berger K, Malyar NM: Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur Heart J 36: 932–938, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Albers M, Romiti M, De Luccia N, Brochado-Neto FC, Nishimoto I, Pereira CA: An updated meta-analysis of infrainguinal arterial reconstruction in patients with end-stage renal disease. J Vasc Surg 45: 536–542, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Reddan DN, Marcus RJ, Owen WF, Jr, Szczech LA, Landwehr DM: Long-term outcomes of revascularization for peripheral vascular disease in end-stage renal disease patients. Am J Kidney Dis 38: 57–63, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Ramdev P, Rayan SS, Sheahan M, Hamdan AD, Logerfo FW, Akbari CM, Campbell DR, Pomposelli FB, Jr: A decade experience with infrainguinal revascularization in a dialysis-dependent patient population. J Vasc Surg 36: 969–974, 2002 [DOI] [PubMed] [Google Scholar]
- 11.O’Hare AM, Sidawy AN, Feinglass J, Merine KM, Daley J, Khuri S, Henderson WG, Johansen KL: Influence of renal insufficiency on limb loss and mortality after initial lower extremity surgical revascularization. J Vasc Surg 39: 709–716, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, Powe NR, Parekh RS: Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: The choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis 54: 468–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyoda K, Fujii K, Ando T, Kumai Y, Ibayashi S, Iida M: Incidence, etiology, and outcome of stroke in patients on continuous ambulatory peritoneal dialysis. Cerebrovasc Dis 17: 98–105, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Liew YP, Bartholomew JR, Demirjian S, Michaels J, Schreiber MJ, Jr: Combined effect of chronic kidney disease and peripheral arterial disease on all-cause mortality in a high-risk population. Clin J Am Soc Nephrol 3: 1084–1089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasqualini L, Schillaci G, Pirro M, Vaudo G, Siepi D, Innocente S, Ciuffetti G, Mannarino E: Renal dysfunction predicts long-term mortality in patients with lower extremity arterial disease. J Intern Med 262: 668–677, 2007 [DOI] [PubMed] [Google Scholar]
- 16.O’Hare AM, Vittinghoff E, Hsia J, Shlipak MG: Renal insufficiency and the risk of lower extremity peripheral arterial disease: Results from the Heart and Estrogen/Progestin Replacement Study (HERS). J Am Soc Nephrol 15: 1046–1051, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Laliberté F, Bookhart BK, Vekeman F, Corral M, Duh MS, Bailey RA, Piech CT, Lefebvre P: Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: A managed care perspective. J Manag Care Pharm 15: 312–322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]