Abstract
Background and objectives
Low serum bicarbonate associates with mortality in CKD. This study investigated the associations of bicarbonate and acid-base status with mortality in healthy older individuals.
Design, setting, participants, & measurements
We analyzed data from the Health, Aging, and Body Composition Study, a prospective study of well functioning black and white adults ages 70–79 years old from 1997. Participants with arterialized venous blood gas measurements (n=2287) were grouped into <23.0 mEq/L (low), 23.0–27.9 mEq/L (reference group), and ≥28.0 mEq/L (high) bicarbonate categories and according to acid-base status. Survival data were collected through February of 2014. Mortality hazard ratios (HRs; 95% confidence intervals [95% CIs]) in the low and high bicarbonate groups compared with the reference group were determined using Cox models adjusted for demographics, eGFR, albuminuria, chronic obstructive pulmonary disease, smoking, and systemic pH. Similarly adjusted Cox models were performed according to acid-base status.
Results
The mean age was 76 years, 51% were women, and 38% were black. Mean pH was 7.41, mean bicarbonate was 25.1 mEq/L, 11% had low bicarbonate, and 10% had high bicarbonate. Mean eGFR was 82.1 ml/min per 1.73 m2, and 12% had CKD. Over a mean follow-up of 10.3 years, 1326 (58%) participants died. Compared with the reference group, the mortality HRs were 1.24 (95% CI, 1.02 to 1.49) in the low bicarbonate and 1.03 (95% CI, 0.84 to 1.26) in the high bicarbonate categories. Compared with the normal acid-base group, the mortality HRs were 1.17 (95% CI, 0.94 to 1.47) for metabolic acidosis, 1.21 (95% CI, 1.01 to 1.46) for respiratory alkalosis, and 1.35 (95% CI, 1.08 to 1.69) for metabolic alkalosis categories. Respiratory acidosis did not associate with mortality.
Conclusions
In generally healthy older individuals, low serum bicarbonate associated with higher mortality independent of systemic pH and potential confounders. This association seemed to be present regardless of whether the cause of low bicarbonate was metabolic acidosis or respiratory alkalosis. Metabolic alkalosis also associated with higher mortality.
Keywords: bicarbonate; acid-base equilibrium; mortality; acidosis; body composition; follow-up studies; glomerular filtration rate; humans; prospective studies; renal insufficiency, chronic
Introduction
In CKD, low serum bicarbonate concentration is a risk factor for mortality and CKD progression (1–4). Low bicarbonate may also be a risk factor for eGFR decline and mortality in persons without CKD. In the non-CKD setting, the association between low bicarbonate and eGFR decline is more established (5–7). In the Health, Aging, and Body Composition (Health ABC) Study, participants with normal eGFR and bicarbonate <23.0 mEq/L had higher risk of incident CKD compared with those with normal bicarbonate (6). Shah et al. (7) reported a 54% higher risk of eGFR decline for those with bicarbonate ≤22 mEq/L compared with those with normal bicarbonate in a cohort of >5000 individuals, 91% of whom had normal eGFR at baseline. Regarding mortality, an analysis of the Third National Health and Nutrition Examination Survey (NHANES III) showed that participants with serum bicarbonate <22 mEq/L had 76% higher mortality than those with normal bicarbonate, and this association was not modified by CKD status (8). The association between bicarbonate and mortality has not been investigated in other cohorts with a low prevalence of CKD. Furthermore, a limitation of NHANES III is that systemic pH and Pco2 were not measured, which is important, because low bicarbonate in persons with preserved eGFR may not necessarily reflect primary metabolic acidosis as it does in CKD.
Therefore, the goals of this study were to investigate the relationships between bicarbonate and acid-base status and all-cause mortality in a cohort with a low prevalence of CKD. To accomplish this, we examined data from participants in the Health ABC Study who had arterialized venous blood gas (AVBG) measurements. Secondary analyses investigated the relationship between systemic pH and mortality and the associations between bicarbonate and cardiovascular and noncardiovascular mortality.
Materials and Methods
Study Population
The Health ABC Study enrolled 3075 black and white participants aged 70–79 years old from clinical sites in Memphis, Tennessee, and Pittsburgh, Pennsylvania from 1997 to 1998. Participants were eligible if they self-reported ability to walk one quarter of a mile, climb ten steps, perform basic activities of daily living without difficulty, and no life-threatening illnesses. Participants were evaluated at baseline, annually for the subsequent 5 years, and biannually thereafter. The Health ABC Study was overseen by institutional review boards at the University of Tennessee Health Science Center and the University of Pittsburgh and performed under the principles embodied in the Declaration of Helsinki.
AVBG samples were obtained at the year 3 visit. Of 2921 participants who attended the year 3 visit, 2287 underwent AVBG sampling and were included in this analysis; the year 3 visit served as baseline.
Measurements
AVBG samples were obtained from a cannulated hand or wrist vein placed in a warmer set to 42°C for ≥15 minutes before blood sampling. Samples were obtained after ≥2 hours of fasting and analyzed on the day of phlebotomy. pH, Pco2, and Po2 were measured in triplicate on a Radiometer ABL5 Blood Gas Analyzer (Radiometer, Brea, CA). Bicarbonate concentration was calculated using the Henderson–Hasselbalch equation. Average values of bicarbonate, pH, Pco2, and Po2 for each participant were used.
Year 3 creatinine and cystatin C measurements were used to estimate GFR using the Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C equation (9). Creatinine was measured using a colorimetric assay calibrated to isotope dilution mass spectrometry–traceable standards. Cystatin C was measured using a particle–enhanced immunonephelometric assay (10). Urine albumin was measured using a particle–enhanced turbidimetric inhibition immunoassay. A modified Jaffe method measured urine creatinine. Serum albumin was measured using the bromocresol green method. Blood concentrations of TNF-α, IL-6, and C-reactive protein (CRP) were measured in duplicate as described (11). Urine albumin and creatinine, serum albumin, TNF-α, CRP, and IL-6 measurements were not measured at year 3; however, they were measured at baseline.
Protein intake was evaluated at the year 2 visit using food frequency questionnaires developed by Block Dietary Systems (Berkeley, CA). Trained interviewers administered questionnaires in person using three–dimensional food models. Body composition was measured at year 3 using fan–beam dual–energy x-ray absorptiometry (DXA; Hologic QDR-4500A; Hologic, Bedford, MA) as described (12). Total nonbone lean mass was calculated by subtracting bone mineral content from total lean mass obtained by DXA (13). Spirometry was performed at year 1 using a horizontal dry rolling seal HF6 Spirometer (Sensor Medics Corporation, Yorba Linda, CA) according to standard guidelines (14).
Definitions
Race (black or white) was self-reported. CKD was defined as eGFR<60 ml/min per 1.73 m2. Participants were categorized as having low (<23.0 mEq/L), normal (23.0–27.9 mEq/L), or high (≥28.0 mEq/L) bicarbonate as previously performed in this cohort (6). Participants were categorized as having low (<7.39), normal (7.39–7.43), or high (>7.43) pH; normal pH was defined if the average pH was within 0.02 U of the median pH (7.41) in the cohort. Normal Pco2 was defined if the average was within 2 mmHg of the median Pco2 (40 mmHg) in the cohort. Participants were categorized into one of seven acid-base status categories as shown in Table 1. Normal acid-base status was defined if average pH, Pco2, and bicarbonate were each within the normal range. Remaining participants had at least one average value of pH, Pco2, or bicarbonate that was outside the normal range and were grouped into one of the abnormal acid-base categories as described in Table 1.
Table 1.
Definitions of acid-base status and observed values of pH, bicarbonate, and Pco2 in each category
| Acid-Base Status | pH | Bicarbonate, mEq/L | Pco2, mmHg | N |
|---|---|---|---|---|
| Normal | 7.39–7.43 | 23 to <28 | 38–42 | |
| Observed mean (minimum–maximum) | 7.41 (7.39–7.43) | 25.0 (23.0–27.7) | 40.0 (38.0–42.0) | 758 |
| Metabolic acidosis | ≤7.41 | <25.5 | <40 | |
| Observed mean (minimum–maximum) | 7.39 (7.28–7.41) | 21.8 (12.3–24.0) | 36.9 (22.0–39.7) | 211 |
| Respiratory acidosis | ≤7.41 | ≥25.5 | ≥40 | |
| Observed mean (minimum–maximum) | 7.39 (7.31–7.41) | 27.2 (25.5–34.0) | 45.7 (42.3–59.3) | 418 |
| Metabolic and respiratory acidosis | ≤7.41 | <25.5 | ≥40 | |
| Observed mean (minimum–maximum) | 7.38 (7.30–7.40) | 24.2 (19.0–25.3) | 42.2 (40.0–51.7) | 213 |
| Metabolic alkalosis | >7.41 | ≥25.5 | ≥40 | |
| Observed mean (minimum–maximum) | 7.43 (7.42–7.49) | 28.1 (26.0–33.0) | 43.0 (40.0–49.0) | 215 |
| Respiratory alkalosis | >7.41 | <25.5 | <40 | |
| Observed mean (minimum–maximum) | 7.44 (7.42–7.57) | 23.4 (17.0–25.3) | 35.5 (24.0–38.3) | 372 |
| Metabolic and respiratory alkalosis | >7.41 | ≥25.5 | <40 | |
| Observed mean (minimum–maximum) | 7.45 (7.43–7.59) | 26.3 (25.7–29) | 38.3 (27.3–39.7) | 100 |
Cardiovascular disease (CVD) was defined as a history of coronary artery bypass graft surgery or angioplasty, carotid endarterectomy, lower extremity bypass or angioplasty, aneurysm repair, myocardial infarction, angina pectoris, transient ischemic attack, or cerebrovascular accident. Congestive heart failure (CHF) was defined if a physician diagnosed the participant with CHF. Chronic obstructive pulmonary disease (COPD) was defined as a forced expiratory volume in 1 second <70% of the forced vital capacity.
Ascertainment of Mortality Data
Mortality was ascertained from death certificates, hospital records, and interview with next of kin through February 24, 2014 (representing 14 years of follow-up). A central committee adjudicated all deaths, including cause of death. Cardiovascular deaths included those caused by atherosclerotic CVD, cerebrovascular disease, atherosclerotic disease at noncoronary/noncerebrovascular sites, and other CVD. Other deaths were considered noncardiovascular.
Statistical Analyses
Continuous variables are presented as means with SDs, unless otherwise specified. Categorical variables are presented as percentages. Significance tests were performed using ANOVA for continuous variables and chi-squared tests for dichotomous variables. Pearson correlation coefficients were calculated for bicarbonate, pH, Po2, and Pco2.
All–cause mortality hazard ratios (HRs) were determined using Cox proportional hazards models. The normal bicarbonate and pH categories served as the reference group in the corresponding Cox models. Model 1 was unadjusted. Model 2 was adjusted for demographics (age, sex, race, and clinical site). Model 3 included model 2 variables, eGFR, urinary albumin-to-creatinine ratio (ACR), COPD, and smoking, which were considered to be the major potential confounders. Model 4 included model 3 variables and systemic pH (when bicarbonate was the predictor variable) to determine if the relationship between bicarbonate and mortality was independent of systemic pH. When pH was the predictor variable, model 4 included model 3 variables and bicarbonate.
Interaction of the relationship between all-cause mortality and (1) bicarbonate or (2) pH by CKD status was investigated by including a multiplicative interaction term in model 4. A cubic spline regression analysis adjusted for model 4 variables was performed using bicarbonate as the predictor variable. Knots were placed at quartiles of bicarbonate concentration, and 25 mEq/L was the reference point. Proportional hazards assumptions were evaluated using a formal significance test on the basis of the unscaled and scaled Schoenfeld residuals and a graphical assessment of log-log survival curves. None of the variables violated proportional hazards assumptions.
The association between bicarbonate and (1) cardiovascular or (2) noncardiovascular mortality was explored using Cox models adjusted for model 4 variables.
Sensitivity analyses, using bicarbonate category as the predictor variable, were performed by adding to model 4 (1) CVD, CHF, systolic BP, and diabetes; (2) use of medications that can affect bicarbonate concentration (diuretics, angiotensin–converting enzyme inhibitors, or angiotensin receptor blockers) (15); (3) nutritional factors associated with bicarbonate concentration (body mass index, serum albumin concentration, and daily protein intake) (15,16); (4) height–adjusted total nonbone lean mass, because acidosis promotes muscle proteolysis in CKD (17); and (5) IL-6, CRP, and TNF-α concentrations, because low bicarbonate associates with greater inflammation (18). Po2 was included in another sensitivity analysis, because pH, Pco2, and bicarbonate may have been affected by the degree of arterialization of the AVBG.
The association between acid-base status and mortality was investigated by adjusting for (1) model 3 variables and (2) model 3 variables plus all of the sensitivity analysis variables. Normal acid-base status served as the reference group.
Analyses were performed using Stata 11 (StataCorp., College Station, TX). P values <0.05 were considered statistically significant.
Results
Participant Characteristics
Table 2 presents characteristics of 2287 Health ABC Study participants with AVBG measurements. In general, there were higher percentages of men, whites, and chronic comorbidities in the low bicarbonate category compared with the other groups. Dietary protein intake was higher and diuretic use was lower in the low bicarbonate category compared with the other groups. pH was statistically different across the bicarbonate categories, although mean values were within the normal range. The percentage of participants with pH<7.39 was highest in the low bicarbonate category, and the percentage of participants with pH>7.43 was highest in the high bicarbonate category. Among participants in the normal bicarbonate category, pH was <7.39 in 20% and >7.43 in 16%.
Table 2.
Baseline characteristics
| Characteristics | Entire Cohort (n=2287) | Serum Bicarbonate Category | P Value | ||
|---|---|---|---|---|---|
| <23.0 mEq/L (n=246) | 23.0–27.9 mEq/L (n=1804) | ≥28.0 mEq/L (n=237) | |||
| Demographics | |||||
| Age (yr), mean (SD) | 76 (3) | 76 (3) | 76 (3) | 76 (3) | 0.25 |
| Sex, no. (%) | |||||
| Women | 1163 (51) | 109 (44) | 905 (50) | 149 (63) | <0.001 |
| Men | 1124 (49) | 137 (56) | 899 (50) | 88 (37) | |
| Race/ethnicity, no. (%) | |||||
| Black | 869 (38) | 96 (39) | 653 (36) | 120 (51) | <0.001 |
| White | 1418 (62) | 150 (61) | 1151 (64) | 117 (49) | |
| Clinical characteristics | |||||
| Cardiovascular disease, no. (%) | 569 (25) | 87 (35) | 430 (24) | 52 (22) | <0.001 |
| Congestive heart failure, no. (%) | 52 (2) | 9 (4) | 34 (2) | 9 (4) | 0.05 |
| Diabetes, no. (%) | 326 (14) | 50 (20) | 242 (13) | 34 (14) | 0.02 |
| Systolic BP (mmHg), mean (SD) | 135.3 (20.3) | 134.3 (22.4) | 135.2 (20) | 137.1 (21.0) | 0.31 |
| CKD,a no. (%) | 242 (12) | 61 (28) | 160 (10) | 21 (10) | <0.001 |
| Chronic obstructive pulmonary disease,b no. (%) | 456 (21) | 68 (29) | 341 (20) | 47 (22) | <0.01 |
| Current smoker, no. (%) | 175 (8) | 36 (15) | 129 (7) | 10 (4) | <0.001 |
| Body mass index (kg/m2), mean (SD) | 27.3 (4.8) | 27.1 (4.7) | 27.3 (4.7) | 27.2 (5.4) | 0.77 |
| ACE-I or ARB use, no. (%) | 537 (23) | 70 (28) | 406 (23) | 61 (26) | 0.08 |
| Diuretic use, no. (%) | 662 (29) | 45 (18) | 485 (27) | 132 (56) | <0.001 |
| Protein intake (g/d), mean (SD) | 66.9 (28.9) | 68.3 (32.9) | 67.4 (28.6) | 62.1 (25.2) | 0.02 |
| Laboratory data | |||||
| eGFR (ml/min per 1.73 m2), mean (SD) | 82.1 (18.3) | 73.0 (20.9) | 83.2 (17.7) | 82.7 (17.8) | <0.001 |
| Urine ACR (mg/g), median (IQR) | 8.1 (4.4–19.3) | 10.7 (5.6–27.5) | 7.7 (4.2–18.4) | 9.5 (5.2–22.8) | <0.001 |
| Serum albumin (g/dl), mean (SD) | 4.0 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 0.27 |
| Arterialized venous pH, mean (SD) | 7.41 (0.03) | 7.40 (0.04) | 7.41 (0.02) | 7.41 (0.03) | <0.001 |
| pH<7.39, no. (%) | 502 (22.0) | 94 (38.2) | 360 (20.0) | 48 (20.3) | <0.001 |
| pH=7.39–7.43, no. (%) | 1400 (61.2) | 111 (45.1) | 1155 (64.0) | 134 (56.5) | |
| pH>7.43, no. (%) | 385 (16.8) | 41 (16.7) | 289 (16.0) | 55 (23.2) | |
| Arterialized venous Pco2 (mmHg), mean (SD) | 40.4 (3.9) | 35.4 (3.3) | 40.4 (3.0) | 46.0 (3.6) | <0.001 |
| Arterialized venous Po2 (mmHg), mean (SD) | 53.2 (10.9) | 55.0 (11.9) | 53.5 (10.6) | 49.0 (11.6) | <0.001 |
ACE-I, angiotensin–converting enzyme inhibitor; ARB, angiotensin receptor blocker; ACR, albumin-to-creatinine ratio; IQR, interquartile range.
Defined as eGFR<60 ml/min per 1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C equation (9). eGFR was available in 219, 1645, and 221 participants in the low, normal, and high bicarbonate categories (total with eGFR=2085).
Defined as forced expiratory volume in 1 second <70% of the forced vital capacity. Spirometry was performed in 236, 1700, and 215 participants in the low, normal, and high bicarbonate categories (total with spirometry =2151).
Correlations of Bicarbonate, pH, Pco2, and Po2
Table 3 presents Pearson correlation coefficients for bicarbonate, pH, Pco2, and Po2. All correlations were statistically significant. However, correlation coefficients were low for all comparisons, except bicarbonate and Pco2, pH and Pco2, pH and Po2, and Po2 and Pco2.
Table 3.
Pearson correlation coefficients for bicarbonate, pH, Po2, and Pco2
| Variable | Bicarbonate, mEq/L | pH | Po2, mmHg | Pco2, mmHg |
|---|---|---|---|---|
| Bicarbonate, mEq/L | 1.00 | |||
| pH | 0.16 | 1.00 | ||
| Po2, mmHg | −0.17 | 0.41 | 1.00 | |
| Pco2, mmHg | 0.77 | −0.49 | −0.42 | 1.00 |
P<0.01 for all correlations.
Association between Bicarbonate Concentration and All-Cause Mortality
The mean (SD) follow-up time was 10.3 (3.9) years (median, 12.1 years). During follow-up, 1326 (58%) participants died. The mortality rate was 56 (95% confidence interval [95% CI], 53 to 60) per 1000 person-years. Mortality rates in the low, normal, and high bicarbonate categories were 76 (95% CI, 65 to 88), 53 (95% CI, 50 to 57), and 61 (95% CI, 52 to 72) per 1000 person-years, respectively.
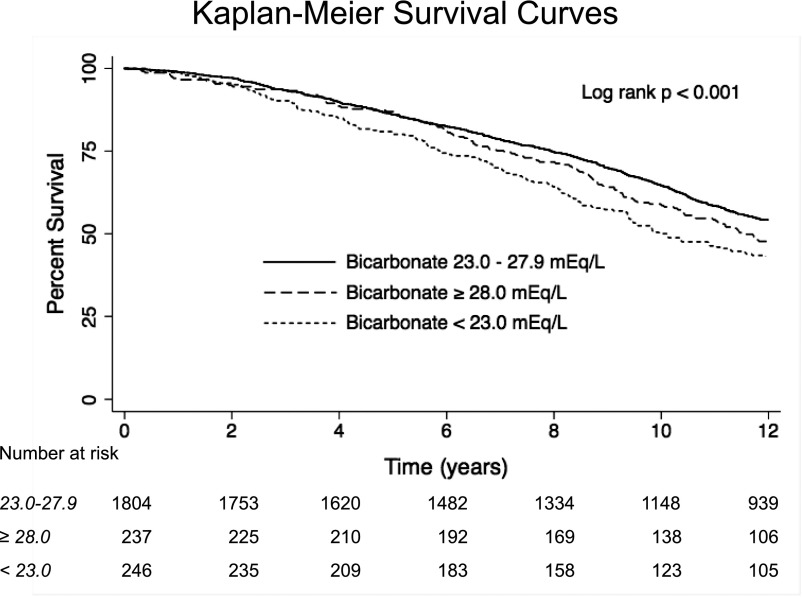
Figure 1 presents the unadjusted survival by bicarbonate category, and Table 4 presents unadjusted and adjusted all–cause mortality HRs using the normal bicarbonate category as the reference group. After adjusting for demographics, eGFR, ACR, COPD, and smoking (model 3), mortality was 22% higher in the low bicarbonate category compared with the reference group. Mortality in the high bicarbonate category was similar to the reference group. Adding pH (model 4) did not substantially change the results in the low (HR, 1.24; 95% CI, 1.02 to 1.49) or (HR, 1.03; 95% CI, 0.84 to 1.26) high bicarbonate groups. There was no evidence that the association between bicarbonate categories and mortality varied by CKD status (interaction P=0.74).
Figure 1.
Unadjusted survival in the study population according to bicarbonate category.
Table 4.
Cox model results showing the all–cause mortality hazard ratios and 95% confidence intervals by serum bicarbonate category in the Health, Aging, and Body Composition Study
| All-Cause Mortality | Bicarbonate Categories HR (95% CI) | ||
|---|---|---|---|
| <23.0 mEq/L | 23.0–27.9 mEq/L | ≥28.0 mEq/L | |
| Model 1 | 1.48 (1.26 to 1.73) | Reference | 1.14 (0.96 to 1.36) |
| Model 2 | 1.41 (1.20 to 1.66) | Reference | 1.10 (0.92 to 1.31) |
| Model 3 | 1.22 (1.01 to 1.47) | Reference | 1.04 (0.85 to 1.27) |
| Model 4a | 1.24 (1.02 to 1.49) | Reference | 1.03 (0.84 to 1.26) |
| Model 4 + CVD, CHF, diabetes, and SBP | 1.22 (1.01 to 1.47) | Reference | 1.03 (0.84 to 1.26) |
| Model 4 + diuretics, ACE-I, and ARB | 1.26 (1.04 to 1.52) | Reference | 1.00 (0.81 to 1.23) |
| Model 4 + serum albumin, BMI, and daily protein intake | 1.22 (1.00 to 1.48) | Reference | 1.04 (0.84 to 1.27) |
| Model 4 + nonbone lean mass | 1.23 (1.01 to 1.48) | Reference | 1.03 (0.84 to 1.26) |
| Model 4 + IL-6, TNF-α, and CRP | 1.26 (1.03 to 1.54) | Reference | 0.97 (0.78 to 1.21) |
| Model 4 + Po2 | 1.26 (1.04 to 1.53) | Reference | 0.98 (0.80 to 1.21) |
| Model 4 + all variablesb | 1.26 (1.02 to 1.56) | Reference | 0.95 (0.76 to 1.19) |
Model 1: unadjusted. Model 2: adjusted for age, sex, race, and clinical site. Model 3: adjusted for model 2 variables, eGFR, urinary albumin-to-creatinine ratio, chronic obstructive pulmonary disease, and smoking. Model 4: adjusted for model 3 variables and systemic pH. HR, hazard ratio; 95% CI, 95% confidence interval; CVD, cardiovascular disease; CHF, congestive heart failure; SBP, systolic BP; ACE-I, angiotensin–converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C-reactive protein.
P=0.74 for interaction between bicarbonate categories and mortality by CKD status.
Additional variables were CVD, CHF, diabetes, SBP, use of diuretics, ACE-Is, or ARBs, serum albumin, BMI, daily protein intake, nonbone lean mass, IL-6, TNF-α, CRP, and Po2.
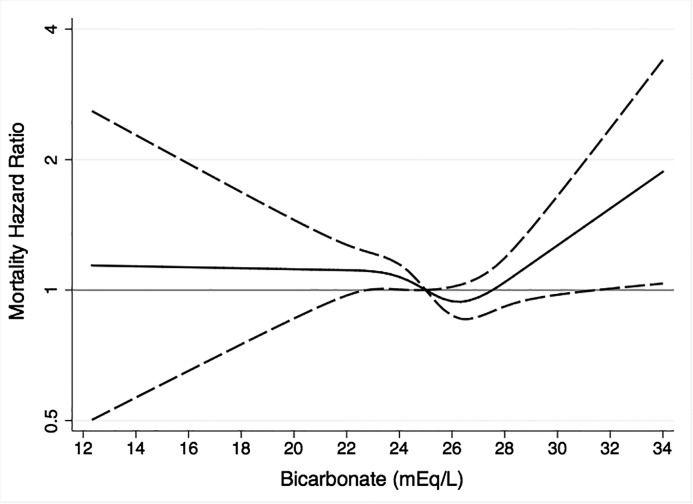
Figure 2 presents the cubic spline regression results adjusted for model 4 variables. Mortality was lowest at bicarbonate concentration approximately 26 mEq/L. Mortality was higher at <26 mEq/L, peaked at approximately 23 mEq/L, and remained flat below this. Mortality was also higher at >26 mEq/L and reached statistical significance at approximately 32 mEq/L.
Figure 2.
Cubic spline regression analysis of the relationship between serum bicarbonate and mortality. Adjusted for age, sex, race, clinical site, eGFR, urinary albumin-to-creatinine ratio, chronic obstructive pulmonary disease, smoking, and pH. The line represents the hazard ratio point estimate, and the dashed lines represent the 95% confidence limits. Serum bicarbonate concentration of 25 mEq/L was the reference.
In sensitivity analyses, the all–cause mortality HRs were not substantially different after CVD, CHF, systolic BP, diabetes, medications, nutritional factors, total nonbone mass, inflammatory markers, and Po2 were added to model 4. A final sensitivity analysis, which added all of these variables to model 4, did not meaningfully alter the results (Table 4).
Associations between Bicarbonate Concentration and Cardiovascular and Noncardiovascular Mortality
There were 372 cardiovascular deaths, 866 noncardiovascular deaths, and 88 unclassified deaths. Cardiovascular mortality rates in the low, normal, and high bicarbonate categories were 19 (95% CI, 15 to 26), 15 (95% CI, 14 to 17), and 16 (95% CI, 12 to 22) per 1000 person-years, respectively. Noncardiovascular mortality rates in the low, normal, and high bicarbonate categories were 51 (95% CI, 42 to 61), 35 (95% CI, 32 to 37), and 41 (95% CI, 34 to 51) per 1000 person-years, respectively.
In the low bicarbonate category, the adjusted cardiovascular mortality HR was 1.23 (95% CI, 0.86 to 1.75), and the adjusted noncardiovascular mortality HR was 1.15 (95% CI, 0.91 to 1.46). In the high bicarbonate category, the adjusted cardiovascular mortality HR was 1.09 (95% CI, 0.75 to 1.59), and the adjusted noncardiovascular mortality HR was 1.03 (95% CI, 0.80 to 1.32).
Association between pH and All-Cause Mortality
Table 5 presents unadjusted and adjusted all–cause mortality HR using the normal pH category as the reference. There was no statistically significant association between pH and mortality after adjusting for demographics, eGFR, ACR, COPD, and smoking (model 3). The results were the same after including bicarbonate in the model. The P value for interaction by CKD status was not significant.
Table 5.
Cox model results showing the all–cause mortality hazard ratios and 95% confidence intervals by pH category in the Health, Aging, and Body Composition Study
| All-Cause Mortality | pH Categories HR (95% CI) | ||
|---|---|---|---|
| <7.39 | 7.39–7.43 | >7.43 | |
| Model 1 | 1.19 (1.05 to 1.36) | Reference | 1.02 (0.88 to 1.19) |
| Model 2 | 1.10 (0.97 to 1.26) | Reference | 1.05 (0.90 to 1.22) |
| Model 3 | 0.97 (0.83 to 1.13) | Reference | 1.05 (0.89 to 1.25) |
| Model 4 | 0.97 (0.83 to 1.13) | Reference | 1.05 (0.89 to 1.25) |
Model 1: unadjusted. Model 2: adjusted for age, sex, race, and clinical site. Model 3: adjusted for model 2 variables, eGFR, urinary albumin-to-creatinine ratio, chronic obstructive pulmonary disease, and smoking. Model 4: adjusted for model 3 variables and systemic pH. P=0.35 for interaction between pH categories and mortality by CKD status. HR, hazard ratio; 95% CI, 95% confidence interval.
Association between Acid-Base Status and All-Cause Mortality
Table 1 presents the mean, minimum, and maximum values for pH, Pco2, and bicarbonate and the number of participants in each acid-base category. Table 6 presents the adjusted Cox model results. Mortality was higher for both metabolic acidosis and respiratory alkalosis compared with those with normal acid-base status, although this did not reach statistical significance in the metabolic acidosis group. Metabolic alkalosis but not respiratory acidosis associated with higher mortality. Combined metabolic and respiratory disorders did not associate with mortality.
Table 6.
Cox model results showing the all–cause mortality hazard ratios and 95% confidence intervals by acid-base status
| Acid-Base Status | Model 1 HR (95% CI) | Model 2 HR (95% CI) |
|---|---|---|
| Normal | Reference | Reference |
| Metabolic acidosis | 1.17 (0.94 to 1.47) | 1.25 (0.98 to 1.61) |
| Respiratory acidosis | 1.10 (0.91 to 1.32) | 1.12 (0.90 to 1.39) |
| Metabolic and respiratory acidosis | 1.05 (0.84 to 1.32) | 1.13 (0.87 to 1.45) |
| Metabolic alkalosis | 1.35 (1.08 to 1.69) | 1.38 (1.06 to 1.78) |
| Respiratory alkalosis | 1.21 (1.01 to 1.46) | 1.29 (1.05 to 1.59) |
| Metabolic and respiratory alkalosis | 0.88 (0.62 to 1.25) | 0.93 (0.62 to 1.39) |
Model 1: adjusted for age, sex, race, clinical site, eGFR, urinary albumin-to-creatinine ratio, chronic obstructive pulmonary disease, and smoking. Model 2: adjusted for model 1 variables, cardiovascular disease, congestive heart failure, diabetes, systolic BP, use of diuretics, angiotensin–converting enzyme inhibitors, or angiotensin receptor blockers, serum albumin, body mass index, daily protein intake, nonbone lean mass, IL-6, TNF-α, C-reactive protein, and Po2. HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
In this cohort of generally healthy older individuals with a low prevalence of CKD, participants with low bicarbonate had 24% higher risk of all-cause mortality than those with normal bicarbonate, and CKD status did not modify this association. These results are consistent with the findings in the NHANES III (8) and support the notion that low bicarbonate concentration is a risk factor for poor outcomes (5–7) in persons with preserved renal function. The blood gas measurements in the Health ABC Study allowed us to address a limitation of prior research in this area, and we make the novel contribution of determining that the association between low bicarbonate and mortality was independent of systemic pH, suggesting that low bicarbonate associated with mortality, regardless of whether the underlying acid-base disorder was metabolic acidosis or respiratory alkalosis. This was reinforced by the higher HR for those with metabolic acidosis and respiratory alkalosis compared with the normal acid-base group.
Although metabolic acidosis did not have a statistically significant association with mortality in this cohort, metabolic acidosis contributes to several negative consequences. These include tubulointerstitial fibrosis (19–21), bone demineralization (22), protein catabolism and sarcopenia (23), inflammation (24), stimulation of the renin-angiotensin system (25) and adrenocortiocotrophic hormone (26), and resistance to growth hormone and IGF (27). However, including some of these factors in the Cox models did not attenuate the association between low bicarbonate and mortality. Nevertheless, correction of metabolic acidosis with alkali seems to ameliorate many of these effects (28–31), although it is uncertain if it improves survival.
With respect to respiratory alkalosis, hypoxemia is a strong stimulus and could explain the association with mortality. However, adjusting for common hypoxemic conditions (CHF and COPD) did not attenuate the results. Respiratory alkalosis can result from pain, which could increase mortality by promoting autonomic dysfunction (32,33), and anxiety, which associates with higher cardiovascular mortality in women (34). The mortality associated with respiratory alkalosis may relate to the tendency toward alkalemia, because metabolic alkalosis also associated with mortality in this cohort. In critically ill patients, mortality is higher with higher pH (35), and displacement of the hemoglobin-oxygen dissociation curve (36) and ionized hypocalcemia are likely mediators. Chronic metabolic alkalosis may increase mortality by inducing vascular calcification (37). These associations between acid-base status and mortality are intriguing and should be confirmed and further investigated.
To broadly elucidate potential causes of death associated with bicarbonate concentration, we explored the relationship between bicarbonate and cardiovascular and noncardiovascular mortality. Individuals in the low bicarbonate category had 23% higher risk of cardiovascular death and 15% higher risk of noncardiovascular death than those with normal bicarbonate, although these were not statistically significant for either outcome. These data suggest that low bicarbonate associates with both cardiovascular and noncardiovascular mortality; however, this requires additional exploration.
Several interventional studies have investigated and are investigating the potential benefits of normalizing low bicarbonate with alkaline therapy in patients with CKD (28,38–40). This has not been evaluated in persons without CKD. However, results from studies in healthy individuals not known to have low bicarbonate suggest that oral potassium bicarbonate supplementation raises bicarbonate concentration and maintains bone health and muscle mass (41–44). The results from this study suggest that raising low serum bicarbonate may improve clinical outcomes in persons with preserved eGFR. However, the results also caution against raising bicarbonate too high, because this might raise mortality risk if metabolic alkalosis develops. The results presented in Figure 2 suggest that targeting a serum bicarbonate concentration of approximately 26 mEq/L is reasonable.
The blood gas measurements are a significant strength of this study. Another is that bicarbonate was measured at the point of care, which is important, because measurement delays can result in falsely low values (45). Also, mortality event rates were high in this cohort. Creatinine and cystatin C were used to estimate GFR, which is more accurate in individuals with normal eGFR and helped minimize residual confounding by CKD (9). The Health ABC Study also collected valuable data, such as DXA and systemic inflammatory markers, which allowed us to explore whether nonbone lean mass, a surrogate of skeletal muscle mass, and inflammation attenuated the relationship between bicarbonate and mortality. Despite these strengths and others, this study also has limitations. AVBG measurements were performed once, and certain measurements, such as urinary albumin and spirometry, were not performed contemporaneously with the AVBG. GFR was not measured, and residual confounding remains possible. Total CO2 is usually measured in clinical practice; hence, the bicarbonate concentrations in this study underestimated total CO2.
In summary, the association between low bicarbonate and mortality in healthy older individuals was independent of pH, suggesting that this relationship did not depend on whether metabolic acidosis or respiratory alkalosis was the underlying disorder. Analyses by acid-base status reinforced this notion. Bicarbonate >32 mEq/L and metabolic alkalosis also associated with mortality. Although alkaline therapy is a promising strategy to improve clinical outcomes, high serum bicarbonate and metabolic alkalosis should probably be avoided.
Disclosures
None.
Supplementary Material
Acknowledgments
The Health, Aging, and Body Composition Study was funded by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA Grant R01-AG028050; National Institute of Nursing Research Grant R01-NR012459; and the Intramural Research Program of the National Institutes of Health, NIA. K.L.R. received support from US Department of Veterans Affairs Clinical Sciences Research and Development Service Career Development Award IK2 CX000537, a Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Award, and National Institutes of Diabetes, Digestive, and Kidney Disease (NIDDK) Grant 1U01DK099933. M.G.S. received NIDDK Grants 1R01DK098234 and R01AG027002. M.J.S. received NIDDK Grant R01AG027002. J.H.I. received NIDDK Grant 1R01DK098234 and American Heart Association Established Investigator Award 14EIA18560026.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06200615/-/DCSupplemental.
References
- 1.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, Ojo A, Scialla J, Steigerwalt S, Teal V, Wolf M, Rahman M, CRIC Investigators : Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 62: 670–678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Wehbe E, Raina R, Simon JF, Srinivas TR, Jain A, Schreiber MJ, Jr., Nally JV, Jr.: Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol 6: 2395–2402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driver TH, Shlipak MG, Katz R, Goldenstein L, Sarnak MJ, Hoofnagle AN, Siscovick DS, Kestenbaum B, de Boer IH, Ix JH: Low serum bicarbonate and kidney function decline: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 64: 534–541, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenstein L, Driver TH, Fried LF, Rifkin DE, Patel KV, Yenchek RH, Harris TB, Kritchevsky SB, Newman AB, Sarnak MJ, Shlipak MG, Ix JH, Health ABC Study Investigators : Serum bicarbonate concentrations and kidney disease progression in community-living elders: The Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis 64: 542–549, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am J Kidney Dis 54: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raphael KL, Zhang Y, Wei G, Greene T, Cheung AK, Beddhu S: Serum bicarbonate and mortality in adults in NHANES III. Nephrol Dial Transplant 28: 1207–1213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibbins-Domingo K, Chertow GM, Fried LF, Odden MC, Newman AB, Kritchevsky SB, Harris TB, Satterfield S, Cummings SR, Shlipak MG: Renal function and heart failure risk in older black and white individuals: The Health, Aging, and Body Composition Study. Arch Intern Med 166: 1396–1402, 2006 [DOI] [PubMed] [Google Scholar]
- 11.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES, Health Aging and Body Composition Study : Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One 6: e19687, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visser M, Fuerst T, Lang T, Salamone L, Harris TB: Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study–Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985) 87: 1513–1520, 1999 [DOI] [PubMed]
- 13.Fuller NJ, Laskey MA, Elia M: Assessment of the composition of major body regions by dual-energy X-ray absorptiometry (DEXA), with special reference to limb muscle mass. Clin Physiol 12: 253–266, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Waterer GW, Wan JY, Kritchevsky SB, Wunderink RG, Satterfield S, Bauer DC, Newman AB, Taaffe DR, Jensen RL, Crapo RO, Health ABC Study : Airflow limitation is underrecognized in well-functioning older people. J Am Geriatr Soc 49: 1032–1038, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Raphael KL, Zhang Y, Ying J, Greene T: Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology (Carlton) 19: 648–654, 2014 [DOI] [PubMed]
- 16.Gennari FJ, Hood VL, Greene T, Wang X, Levey AS: Effect of dietary protein intake on serum total CO2 concentration in chronic kidney disease: Modification of Diet in Renal Disease study findings. Clin J Am Soc Nephrol 1: 52–57, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE: The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 97: 1447–1453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farwell WR, Taylor EN: Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey. CMAJ 182: 137–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesson DE, Dolson GM: Endothelin-1 increases rat distal tubule acidification in vivo. Am J Physiol 273: F586–F594, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Wesson DE, Simoni J: Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int 75: 929–935, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Bushinsky DA, Chabala JM, Gavrilov KL, Levi-Setti R: Effects of in vivo metabolic acidosis on midcortical bone ion composition. Am J Physiol 277: F813–F819, 1999 [DOI] [PubMed] [Google Scholar]
- 23.May RC, Kelly RA, Mitch WE: Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest 79: 1099–1103, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L: Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-kappaB activation. J Biol Chem 273: 5086–5092, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Ng HY, Chen HC, Tsai YC, Yang YK, Lee CT: Activation of intrarenal renin-angiotensin system during metabolic acidosis. Am J Nephrol 34: 55–63, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Wood CE, Isa A: Intravenous acid infusion stimulates ACTH secretion in sheep. Am J Physiol 260: E154–E161, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Ordóñez FA, Santos F, Martínez V, García E, Fernández P, Rodríguez J, Fernández M, Alvarez J, Ferrando S: Resistance to growth hormone and insulin-like growth factor-I in acidotic rats. Pediatr Nephrol 14: 720–725, 2000 [DOI] [PubMed] [Google Scholar]
- 28.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litzow JR, Lemann J, Jr., Lennon EJ: The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J Clin Invest 46: 280–286, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Löfberg E, Gutierrez A, Anderstam B, Wernerman J, Bergström J, Price SR, Mitch WE, Alvestrand A: Effect of bicarbonate on muscle protein in patients receiving hemodialysis. Am J Kidney Dis 48: 419–429, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D: Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: A prospective randomized single blind controlled trial. Ren Fail 28: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Koenig J, Loerbroks A, Jarczok MN, Fischer JE, Thayer JF: Chronic pain and heart rate variability in a cross-sectional occupational sample: Evidence for impaired vagal control [published online ahead of print April 28, 2015]. Clin J Pain [DOI] [PubMed] [Google Scholar]
- 33.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG: Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC Study. Atherosclerosis Risk In Communities. Circulation 102: 1239–1244, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Butnoriene J, Bunevicius A, Saudargiene A, Nemeroff CB, Norkus A, Ciceniene V, Bunevicius R: Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 190: 360–366, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Anderson LE, Henrich WL: Alkalemia-associated morbidity and mortality in medical and surgical patients. South Med J 80: 729–733, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Bellingham AJ, Detter JC, Lenfant C: Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis. J Clin Invest 50: 700–706, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Solis AJ, González-Pacheco FR, Deudero JJ, Neria F, Albalate M, Petkov V, Susanibar L, Fernandez-Sanchez R, Calabia O, Ortiz A, Caramelo C: Alkalinization potentiates vascular calcium deposition in an uremic milieu. J Nephrol 22: 647–653, 2009 [PubMed] [Google Scholar]
- 38.Gaggl M, Cejka D, Plischke M, Heinze G, Fraunschiel M, Schmidt A, Hörl WH, Sunder-Plassmann G: Effect of oral sodium bicarbonate supplementation on progression of chronic kidney disease in patients with chronic metabolic acidosis: Study protocol for a randomized controlled trial (SoBic-Study). Trials 14: 196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Witham MD, Band MM, Littleford RC, Avenell A, Soiza RL, McMurdo ME, Sumukadas D, Ogston SA, Lamb EJ, Hampson G, McNamee P, BiCARB Study Group : Does oral sodium bicarbonate therapy improve function and quality of life in older patients with chronic kidney disease and low-grade acidosis (the BiCARB trial)? Study protocol for a randomized controlled trial. Trials 16: 326, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buehlmeier J, Frings-Meuthen P, Remer T, Maser-Gluth C, Stehle P, Biolo G, Heer M: Alkaline salts to counteract bone resorption and protein wasting induced by high salt intake: Results of a randomized controlled trial. J Clin Endocrinol Metab 97: 4789–4797, 2012 [DOI] [PubMed] [Google Scholar]
- 42.He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA: Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension 55: 681–688, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Ceglia L, Harris SS, Abrams SA, Rasmussen HM, Dallal GE, Dawson-Hughes B: Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J Clin Endocrinol Metab 94: 645–653, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frassetto L, Morris RC, Jr., Sebastian A: Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in postmenopausal women. J Clin Endocrinol Metab 90: 831–834, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kirschbaum B: Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis 35: 1068–1071, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.