Abstract
Background and objectives
Data reported to the Organ Procurement and Transplantation Network (OPTN) are used in kidney transplant research, policy development, and assessment of center quality, but the accuracy of early post–transplant outcome measures is unknown.
Design, setting, participants, & measurements
The Deceased Donor Study (DDS) is a prospective cohort study at five transplant centers. Research coordinators manually abstracted data from electronic records for 557 adults who underwent deceased donor kidney transplantation between April of 2010 and November of 2013. We compared the post-transplant outcomes of delayed graft function (DGF; defined as dialysis in the first post–transplant week), acute rejection, and post–transplant serum creatinine reported to the OPTN with data collected for the DDS.
Results
Median kidney donor risk index was 1.22 (interquartile range [IQR], 0.97–1.53). Median recipient age was 55 (IQR, 46–63) years old, 63% were men, and 47% were black; 93% had received dialysis before transplant. Using DDS data as the gold standard, we found that pretransplant dialysis was not reported to the OPTN in only 11 (2%) instances. DGF in OPTN data had a sensitivity of 89% (95% confidence interval [95% CI], 84% to 93%) and specificity of 98% (95% CI, 96% to 99%). Surprisingly, the OPTN data accurately identified acute allograft rejection in only 20 of 47 instances (n=488; sensitivity of 43%; 95% CI, 17% to 73%). Across participating centers, sensitivity of acute rejection varied widely from 23% to 100%, whereas specificity was uniformly high (92%–100%). Six-month serum creatinine values in DDS and OPTN data had high concordance (n=490; Lin concordance correlation =0.90; 95% CI, 0.88 to 0.92).
Conclusions
OPTN outcomes for recipients of deceased donor kidney transplants have high validity for DGF and 6-month allograft function but lack sensitivity in detecting rejection. Future studies using OPTN data may consider focusing on allograft function at 6 months as a useful outcome.
Keywords: deceased donors, validation studies, acute rejection, adult, allografts, delayed graft function, humans, kidney transplantation, tissue donors, tissue and organ procurement
Introduction
Data reported by transplant centers in the United States to the Organ Procurement and Transplantation Network (OPTN) are necessary to detect national trends in transplant outcomes over time and evaluate variation in performance between centers (1). Although some outcomes, such as death and graft failure, are confirmed by linkage to national databases, including the Social Security Death Master File and the US Renal Data System, few studies have examined the validity of delayed graft function (DGF), allograft function, acute rejection, or other important early outcomes after kidney transplantation.
Understanding the validity of OPTN data is important, because the OPTN is the single largest source of United States transplant data. The United Network for Organ Sharing and the Scientific Registry of Transplant Recipients rely on these data to generate their Annual Data Report, which offers important insights into the overall state of solid organ transplantation in the United States. The Annual Data Report describes early post–transplant outcomes, including graft failure and function. Many researchers have used OPTN data to examine determinants of important outcomes after kidney transplantation, including death, graft failure, DGF, and acute rejection (2–7).
Transplant programs report outcomes to the OPTN at the time of transplantation and in routine follow-up forms, including forms submitted at 6 months. These data may contain errors for multiple reasons. In patients with DGF, defined by the OPTN as dialysis in the first 7 days after transplant (8), single dialysis treatments may not be well documented in the chart, or dialysis episodes may be performed just before transplant (but erroneously identified as DGF) or after discharge (and thereby, missed by administrative staff submitting OPTN reports). The ascertainment of rejection may require the expert and subjective interpretation of pathology data. Follow-up renal function requires measurement of serum creatinine, but in some cases, patients may have returned to the care of the referring nephrologist, have missed an appointment, or had data measured multiple times (for instance, when hospitalized). Additionally, administrative staff responsible for completing follow-up forms may have limited medical training, leading to errors.
Few studies have focused on assessing the validity of OPTN data elements (9–11). Israni et al. (9) found that, compared with source data, OPTN data for DGF had a false-negative rate of 0.44 and a false-positive rate of 0.05. In liver transplantation, the Adult-to-Adult Liver Transplantation Cohort Study compared OPTN data with data collected by onsite coordinators regarding candidates for and recipients of liver transplants as well as living donors. Gillespie et al. (10) reported that, for 29 baseline variables related to recipients of liver transplants, the majority of variables (including demographics, ABO blood group, and previous transplant) were identical in the two databases in 90% of the data. However, several recipient comorbidities, such as diabetes and coronary artery disease, were commonly not reported to the OPTN. Additionally, acute rejection after liver transplantation was missing in the OPTN data in 14% of patients (10). Stirneman et al. (12) compared the accuracy of immunosuppression reported in the OPTN database with pharmacy claims reported to the Centers for Medicare and Medicaid Services (CMS) and found that concordance was high for calcineurin inhibitors but poor for oral corticosteroids. Other investigators have merged OPTN data with CMS claims related to comorbidities to determine whether post-transplant outcomes could be more accurately predicted using this supplemental information (13). To our knowledge, however, no studies have estimated the validity of reported acute rejection and 6-month renal function in kidney transplantation.
The Deceased Donor Study (DDS) is a prospective cohort study that focuses on predictors of post-transplant outcomes that can be identified among deceased kidney donors (14). Center-based coordinators manually abstracted key outcomes and patient characteristics from the charts of recipients of kidney transplants. The DDS data were linked to OPTN data, creating an opportunity for validation of the OPTN data against a high-quality standard. Therefore, the primary aim was to estimate the accuracy of OPTN data for the outcomes of recipient DGF, acute rejection, and renal function at 6 months post-transplant.
Materials and Methods
Overview
From April of 2010 to November of 2013, we assembled a cohort of deceased kidney donors at five organ procurement organizations and the recipients of these kidneys at five centers: the University of Pennsylvania (Pennsylvania), Mount Sinai Hospital (New York), Harper University Hospital (Michigan), Saint Barnabas Medical Center (New Jersey), and Yale-New Haven Hospital (Connecticut; the data coordinating center). The cohort was limited to recipients of kidneys from deceased donors whose surrogates consented to research. Consent from recipients of kidney transplants was not obtained. The scientific review boards of the organ procurement organizations and the institutional review boards at each transplant hospital approved the protocols. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Inclusion criteria included recipient age >16 years old. Exclusion criteria included recipients of (1) multiorgan transplants other than kidney/pancreas, (2) en bloc kidney transplants, and/or (3) kidneys from donors <5 years of age.
Data Sources
For DDS data collection, trained study coordinators at participating sites reviewed the electronic medical record to ascertain participant characteristics, treatments, and outcomes. The principal investigator at each site confirmed every DGF episode and etiology by reviewing dialysis and treating physician notes. At every site except Harper University Hospital, coordinators involved in data collection for DDS were not involved in administrative data reporting to OPTN.
For the OPTN data, we used data from transplant registration and 6-month follow-up forms. The minimum time between date of transplantation and follow-up (defined as date of merger of the OPTN and DDS datasets) was 330 days, providing sufficient opportunity for centers to submit 6-month forms to the OPTN. The OPTN data system includes data on all donors, waitlisted candidates, and recipients of transplants in the United States submitted by the members of the OPTN and has been described elsewhere (1,15). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight of the activities of the OPTN contractor.
In both datasets, DGF was defined as dialysis in the week after transplantation (OPTN variable FIRST_WK_DIAL). Allograft failure was defined as any cause leading to resumption of maintenance dialysis, new waitlisting, or repeat transplantation (16).
In both datasets, acute allograft rejection was defined as a clinical diagnosis by the transplant team, regardless of whether antirejection treatment was given. No center routinely performed protocol biopsies for all recipients of transplants during the study period. At two centers, a small number of patients (n=16) underwent protocol biopsies because of participation in other clinical studies or protocols. For the OPTN dataset, we identified acute rejection with data from both the recipient of transplant registration form (OPTN variable ACUTE_REJ_EPI_KI) and the recipient follow-up forms (OPTN variable ACUTE_REJ_EPI). For comparisons of 6-month creatinine values, we used OPTN variable CREAT from the follow-up forms.
Comparison of Study Cohort with National Cohort
To compare our cohort with the national experience, we generated a cohort of recipients of deceased donor kidney transplants who were >16 years of age, met other study inclusion/exclusion criteria, and underwent transplantation in the period from April of 2010 to November of 2013.
Statistical Analyses
Sensitivity, specificity, and positive and negative predictive values were estimated using conventional epidemiologic methods (17,18). For DGF, 95% confidence intervals (95% CIs) were calculated using Wilson score interval. For acute rejection, Wald 95% CIs were adjusted with a Pearson–based scale parameter to account for overdispersion because of heterogeneity among centers (19). Descriptive statistics were reported as mean (SD) or median (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. Using methods described by the OPTN, we calculated the kidney donor risk index (KDRI) (20,21). Also, using methods described by the OPTN, we converted KDRI scores to the kidney donor profile index with a scaling factor on the basis of the characteristics of deceased kidney donors in the United States from the year 2010 (20). For comparison of creatinine between the DDS and OPTN data, we calculated Lin concordance correlation among individuals with data available in both datasets. Scatter plots and Bland–Altman plots of serum creatinine were also constructed for descriptive analysis. Chi-squared tests were used to compare categorical variables. We used SAS 9.3 statistical software for Windows (SAS Institute Inc., Cary, NC). All statistical tests and 95% CIs were two sided with an α-level of 0.05.
Missing Data
Missing data were present in a small number of recipients in the OPTN and DDS datasets for serum creatinine and acute rejection. For comparisons of these outcomes, we restricted analyses to individuals without missing data in either dataset.
Results
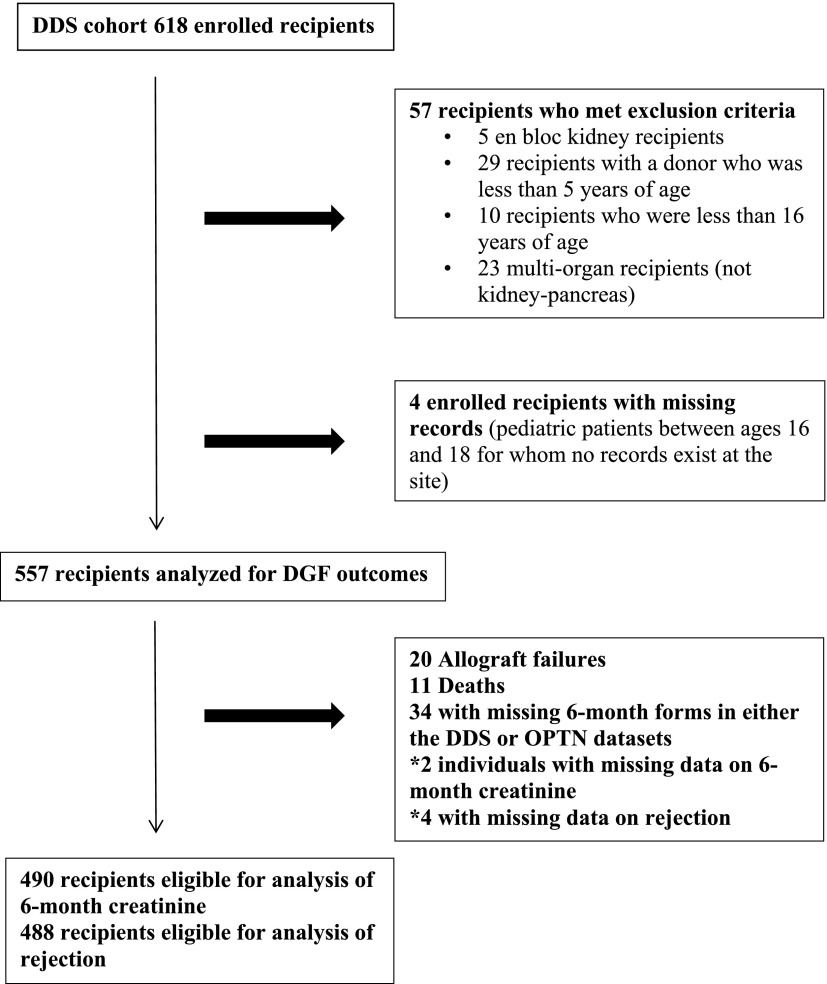
Cohort generation is shown in Figure 1. The cohort comprised 557 recipients of kidney transplants who received kidneys from 482 deceased donors. For analyses of rejection and allograft function, we excluded individuals with death and allograft failures that occurred by the 6-month anniversary of transplantation as well as individuals (n=34) with missing DDS and/or OPTN forms at the 6-month time point. In addition, we excluded two patients who had missing data on 6-month creatinine from analyses of creatinine concordance and four patients with missing data on rejection from analyses of rejection.
Figure 1.
Cohort generation. DDS, Deceased Donor Study; DGF, delayed graft function; OPTN, Organ Procurement and Transplantation Network.
As shown in Tables 1 and 2, the median KDRI was 1.22 (IQR, 0.97–1.53). The median recipient age was 55 years old (IQR, 46–63); 63% were men, 26% had diabetes listed as cause of ESRD, and 15% had a prior transplant.
Table 1.
Characteristics of recipients of deceased donor kidney transplants
| Variable | DDS (%) | Eligible Recipients at Participating Centers Not Included in the DDS Cohort (%)a | All Eligible Recipients at Participating Centers (%) |
|---|---|---|---|
| n | 557 | 682 | 1239 |
| Age, yr [IQR] | 55 [46–63] | 54 [45–62] | 55 [45–62] |
| Sex | |||
| Women | 206 (37) | 271 (40) | 476 (38) |
| Men | 351 (63) | 411 (60) | 763 (62) |
| Black | |||
| Yes | 261 (47) | 294 (43) | 555 (45) |
| No | 296 (53) | 388 (57) | 684 (55) |
| Listed cause of ESRD | |||
| Diabetes | 143 (26) | 228 (33) | 406 (33) |
| Hypertension | 143 (26) | 174 (26) | 319 (26) |
| GN | 75 (13) | 112 (16) | 209 (17) |
| CAKUT/congenital uropathy | 42 (8) | 66 (10) | 110 (9) |
| Failed transplant | 85 (15) | 17 (2) | 29 (2) |
| Other | 69 (12) | 85 (12) | 166 (13) |
| History of diabetes | |||
| No | 330 (59) | 399 (59) | 726 (59) |
| Yes | 226 (41) | 278 (41) | 501 (40) |
| Unknown | 1 (0) | 5 (1) | 12 (1) |
| Hepatitis C seropositive | |||
| No | 517 (93) | ||
| Yes | 38 (7) | 31 (5) | 60 (5) |
| Unknown | 2 (0) | ||
| Panel reactive antibody, % | |||
| 0 | 363 (65) | 473 (69) | 836 (67) |
| 1–10 | 27 (5) | 26 (4) | 53 (4) |
| 11–80 | 86 (15) | 95 (14) | 181 (15) |
| >80 | 81 (15) | 88 (13) | 169 (14) |
| Prior transplant | |||
| No | 470 (85) | 606 (89) | 1093 (88) |
| Yes | 85 (15) | 76 (11) | 146 (12) |
| Unknown | 1 (0) | 0 | 0 |
| HLA antigen mismatches | |||
| 0 | 20 (4) | 32 (5) | 53 (4) |
| 1–2 | 24 (4) | 27 (4) | 49 (4) |
| 3–4 | 214 (39) | 257 (38) | 481 (39) |
| 5–6 | 297 (54) | 366 (54) | 656 (53) |
| Duration of dialysis, mo [IQR] | 53 [27–74] | 54 [34–77] | 53 [32–74] |
| HIVb | |||
| No | 539 (97) | ||
| Yes | 17 (3) | ||
| Unknown | 1 (0) | ||
| Pretransplant dialysisb | |||
| Hemodialysis | 444 (80) | ||
| Peritoneal | 70 (13) | ||
| No dialysis | 39 (7) |
DDS, Deceased Donor Study; IQR, interquartile range; CAKUT, congenital abnormalities of the kidney and urinary tract.
These recipients met inclusion criteria, except that they received kidneys from deceased donors whose surrogates had not provided consent to research, and hence, they were not included in the DDS cohort.
Information for these variables was primarily obtained by manual chart review by DDS coordinators, and hence, these data were only reported for the DDS cohort. Other variables were obtained through the Organ Procurement and Transplantation Network dataset.
Table 2.
Characteristics of deceased donor kidney allografts
| Variable | DDS (%) | Eligible Donors at OPOs Not Included in DDS Cohort (%)a | All Eligible Donors at Participating OPOs (%) |
|---|---|---|---|
| n | 557 | 682 | 1239 |
| Age, yr [IQR] | 43 [27–53] | 41 [26–53] | 42 [27–53] |
| Sex | |||
| Women | 211 (38) | 257 (38) | 468 (38) |
| Men | 346 (62) | 425 (62) | 771 (62) |
| Black | |||
| Yes | 96 (17) | 118 (17) | 213 (17) |
| No | 461 (83) | 564 (83) | 1026 (83) |
| History of diabetes | |||
| No | 499 (90) | 620 (91) | 1114 (90) |
| Yes | 58 (10) | 57 (8) | 115 (9) |
| Unknown | 0 | 5 (1) | 10 (1) |
| Donation after cardiac death | |||
| No | 457 (82) | 530 (78) | 987 (80) |
| Yes | 100 (18) | 152 (22) | 252 (20) |
| Terminal serum creatinine [IQR] | 1 [0.7–1.4] | 0.9 [0.7–1.2] | 0.9 [0.7–1.3] |
| Donor hepatitis C seropositivity | |||
| No | 541 (97) | 665 (98) | 1206 (97) |
| Yes | 16 (3) | 17 (2) | 33 (3) |
| Kidney donor risk index [IQR] | 1.22 [0.97–1.53] | 1.19 [0.95–1.49] | 1.2 [0.95–1.5] |
| Kidney pumpedb | |||
| No | 339 (61) | ||
| Yes | 218 (39) | ||
| Biopsy performedb | |||
| No | 304 (55) | ||
| Yes | 253 (45) |
In total, 482 donors provided 557 kidneys to recipients in the cohort. DDS, Deceased Donor Study; OPO, organ procurement organization; IQR, interquartile range.
These donors met inclusion criteria, except that surrogates did not provide research consent, and hence, they were not included in the DDS cohort.
Information for these variables was primarily obtained by manual chart review by DDS coordinators, and hence, these data were only reported for the DDS cohort. Other variables were obtained through the Organ Procurement and Transplantation Network dataset.
Supplemental Table 1 shows that the DDS cohort was similar to the national cohort of adults receiving decease donor kidney transplants during the same period in terms of age, sex, elevated panel reactive antibody (PRA), and prior transplant. The DDS cohort had a greater percentage of black recipients (47%) than the national population (32%).
Thirty-nine (7%) recipients had preemptive kidney transplants. Comparing OPTN data with chart review done for the DDS, we found that 11 (2%) recipients were incorrectly listed in the OPTN dataset as having undergone preemptive transplants but had actually received pretransplant dialysis.
DGF
Tables 3 and 4 shows that DGF ascertainment by the OPTN dataset was highly accurate, with sensitivity and specificity of 89% (95% CI, 84% to 93%) and 98% (95% CI, 96% to 99%), respectively. Positive and negative predictive values were 97% (95% CI, 94% to 99%) and 94% (95% CI, 91% to 96%), respectively. As shown in Supplemental Table 2, the most common cause of DGF (among individuals who received one dialysis session) was hyperkalemia followed by presumed uremia (i.e., no reason specified) and volume overload. In 138 recipients (64% of those with DGF), there were multiple sessions of dialysis within the first week after receiving a deceased donor kidney transplant. Notably, among 45 recipients with DGF who only had dialysis once and the treatment was on postoperative day 1, the OPTN dataset did not record 13 (29%) of these DGF events.
Table 3.
Comparison of delayed graft function events identified in the Deceased Donor Study with events recorded in the Organ Procurement and Transplantation Network database
| OPTN DGF | DDS DGF | ||
|---|---|---|---|
| Yes | No | Total | |
| Yes | 191 | 6 | 197 |
| No | 23 | 337 | 360 |
| Total | 214 | 343 | 557 |
Please see Table 4 for data on diagnostic performance of the OPTN DGF variable. OPTN, Organ Procurement and Transplantation Network; DDS, Deceased Donor Study; DGF, delayed graft function.
Table 4.
Statistical performance of the delayed graft function variable reported in the OPTN dataset
| Performance Measure | Estimate (95% CI) |
|---|---|
| Sensitivity, % | 89 (84 to 93) |
| Specificity, % | 98 (96 to 99) |
| Positive predictive value, % | 97 (94 to 99) |
| Negative predictive value, % | 94 (91 to 96) |
OPTN, Organ Procurement and Transplantation Network; 95% CI, 95% confidence interval.
Post–Transplant 6-Month Serum Creatinine
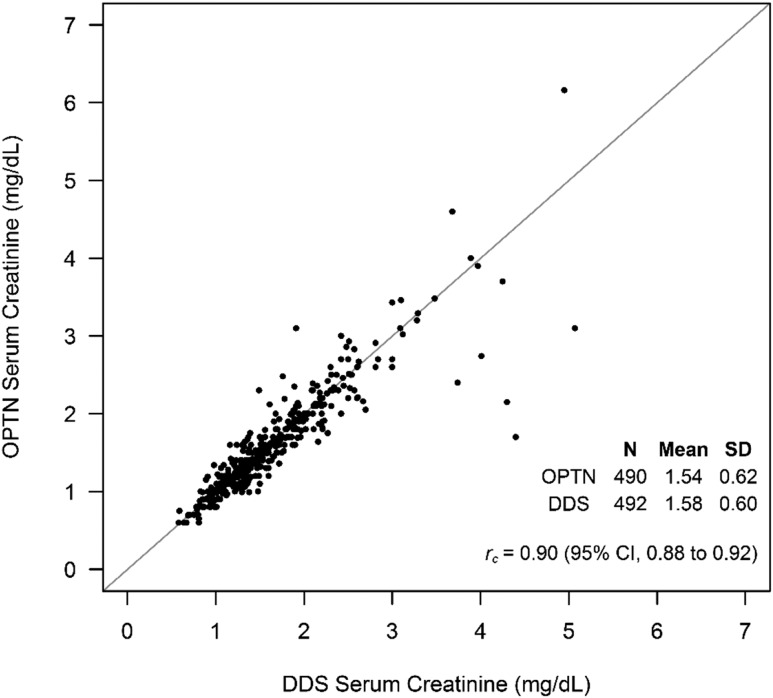
Figure 2 compares 6-month post–transplant creatinine values reported to the OPTN and collected for the DDS. Mean 6-month serum creatinine according to OPTN data was 1.54 (SD=0.62) mg/dl versus 1.58 (SD=0.60) mg/dl in the DDS, with a Lin concordance correlation of 0.90 (95% CI, 0.88 to 0.92). A Bland–Altman plot, shown in Supplemental Figure 1, was constructed for visual inspection of the correlation. When examining the difference between OPTN 6-month creatinine values and DDS creatinine values, we found only 17 (3.5%) for which differences exceeded 0.50 mg/dl and 13 (2.7%) for which differences exceeded 2 SDs of the mean (0.54 mg/dl).
Figure 2.
Scatter plot comparing 6-month post–transplant creatinine values collected for the Deceased Donor Study (DDS) with creatinine values recorded in the Organ Procurement and Transplantation Network (OPTN) database (n=490). 95% CI, 95% confidence interval.
Acute Rejection
Rejection episodes were often not captured within the OPTN database, resulting in a sensitivity of 43% (95% CI, 17% to 73%) at 6 months (Tables 5 and 6). Specificity for OPTN–reported acute rejection was 99% (95% CI, 94% to 100%) at 6 months, and positive and negative predictive values were 83% (95% CI, 64% to 93%) and 94% (95% CI, 90% to 97%), respectively. In three instances, no data were detected by the DDS staff, but they were reported to the OPTN as no rejection.
Table 5.
Comparison of acute rejection within 6 months of deceased donor kidney transplantation collected for the Deceased Donor Study with rejection recorded in the Organ Procurement and Transplantation Network database
| OPTN Rejection | DDS Rejection | ||
|---|---|---|---|
| Yes | No | Total | |
| Yes | 20 | 4 | 24 |
| No | 27 | 437 | 464 |
| Total | 47 | 441 | 488 |
Patients who experienced allograft failure or died by 6 months were excluded from this analysis along with patients with missing 6-month forms for either study. One patient in the OPTN dataset and three patients in the DDS dataset had no outcome reported on acute rejection in the 6-month form. Please see Table 6 for data on diagnostic performance of the OPTN acute rejection variable. OPTN, Organ Procurement and Transplantation Network; DDS, Deceased Donor Study.
Table 6.
Statistical performance of the acute rejection variable reported in the OPTN dataset
| Performance Measure | Estimate (95% CI) |
|---|---|
| Sensitivity, % | 43 (17 to 73) |
| Specificity, % | 99 (94 to 100) |
| Positive predictive value, % | 83 (64 to 93) |
| Negative predictive value, % | 94 (90 to 97) |
OPTN, Organ Procurement and Transplantation Network; 95% CI, 95% confidence interval.
We also compared the sensitivity and specificity of OPTN–reported acute rejection for recipients at the different DDS transplant centers. Specificity was high (range, 92%–100%) across all five centers. However, sensitivity varied from 23% to 100% across four centers (at one center, sensitivity could not be calculated, because no episodes of rejection were recorded in either the OPTN database or the DDS database).
Sensitivity Analyses
We performed a sensitivity analysis after excluding Harper University Hospital, because the coordinator at Harper University Hospital was involved in reporting data to the OPTN as well as abstracting data for the DDS. We found no meaningful difference in operating characteristics of OPTN-reported outcomes after excluding Harper University Hospital. These results are presented in Supplemental Tables 3–5.
Discussion
Little is known about the validity of early outcomes reported to the OPTN after kidney transplantation. Using carefully abstracted source data, we estimated the accuracy of OPTN-reported DGF, acute rejection, and allograft function at five transplant centers. Our results indicate that OPTN data are highly sensitive and specific for DGF, and 6-month serum creatinine has excellent accuracy compared with manually chart–abstracted data. However, at some centers, data on acute rejection in the first year post-transplant were under-reported to the OPTN.
Every United States transplant center is mandated by the OPTN to report data on waitlisted candidates, recipients, and live donors (15). Centers must report 6-month follow-up forms within 30 days of the 6-month anniversary of transplantation. The OPTN also defines timely data submission as submission of 95% of forms within 3 months of the form’s due date (22). To facilitate accurate reporting, the OPTN provides data dictionaries for every field in the transplant forms and has, for some variables, implemented range limits into UNet. The OPTN performs internal validation studies and conducts site audits of centers with a high percentage of missing data. Despite these measures, prior investigators have identified some inaccuracies in the reporting of DGF after kidney transplantation and the reporting of deaths after removal from the liver transplant waiting list (9–11). Our study provides new evidence of limited ascertainment of acute rejection within the first 6 months of kidney transplantation.
The reporting of acute rejection poses particular challenges because of the different methods (clinical versus biopsy proven) for confirming the diagnosis and because of the subjective nature of pathology interpretation. Treating clinicians may disagree about the nature of the rejection (which may be cellular or humoral and may have acute and/or chronic elements), and treatment options are often customized for each patient. It is plausible that acute rejection is under-reported by the staff who enter these data for the OTPN and might have limited medical knowledge. For the DDS, acute rejection was defined by clinical diagnosis, regardless of whether antirejection treatment was given, and only a small percentage of patients underwent protocol biopsy (23–25). We did not require biopsy for the definition of acute rejection in the DDS, because the OPTN fields related to acute rejection that were used for this study also do not specify biopsy findings.
Our study has limitations. The findings may have limited generalizability, because the DDS cohort was assembled at five transplant programs that were not randomly selected, whereas there are >200 adult kidney transplant programs across the United States. However, these five programs are located across multiple states and have diverse patient populations. Additionally, our cohort resembled the national cohort in terms of age, sex, and prior transplantation. Another limitation is that data collection took place up to 6 months post-transplantation, limiting inferences about accuracy of OPTN data beyond that time period. However, the outcomes of DGF, acute rejection, and 6-month post–transplant serum creatinine may predict longer-term outcomes. The population was limited to recipients of kidneys from deceased donors whose surrogates provided research consent; recipients of live kidney donor transplants were also not included. However, estimates of data accuracy reported here would likely apply to these excluded recipients as well, because transplant administrators did not know which candidates would be included in the study at the time of reporting data to the OPTN (22).
The study’s strengths include a protocol designed to maximize DDS data accuracy. Study coordinators underwent training to ensure accurate recording of specific data elements. DGF episodes were individually verified by principal investigators at each site before recording in the DDS database. In addition, study coordinators and staff took part in monthly conference calls with site principal investigators when missing data elements were identified, and routine data reviews were implemented to ensure accuracy.
Our findings have potential implications for future research. Given the accuracy of OPTN-reported variables of DGF and serum creatinine in the first 6 months, these variables could be used to evaluate the effects of treatments given to deceased donors on outcomes experienced by recipients of kidney transplants. Six-month creatinine could be used to evaluate recipient outcomes after treatments given to recipients in the peritransplant period. Other investigators (26–29) have linked databases from trials to the OPTN database for the purpose of ascertaining post-trial outcomes. For example, Brennan and colleagues (27,28) used OPTN mortality data to evaluate long-term effects of antibody induction therapy on recipients of kidney transplants, whereas Wiesner and colleagues (26,29) estimated late acute rejection for recipients of liver transplants who received mycophenolate mofetil.
This study reveals that some centers need to make substantial improvements in the reporting of acute rejection data to the OPTN. First, the OPTN may consider providing more specific guidance about how centers should identify rejection and other data elements that may be interpreted differently across centers. Centers should also report dates of acute rejection to the OPTN, which will improve analyses that aim to understand and reduce acute rejection after kidney transplantation. Second, the OPTN may consider focusing on data quality for this outcome during site visits. The OPTN might direct attention to reviewing the charts of patients with a higher probability of experiencing rejection, such as young recipients or those with elevated PRA (30,31). In addition, future research should evaluate other OPTN variables that may require clinical expertise to report accurately, such as PRA. Integration of transplant center electronic medical record systems with the OPTN database and reduction of the total burden of collected variables might also help in improving accuracy and reducing missingness of outcomes, such as rejection. These proposals have been considered previously and deserve new attention (32).
In conclusion, the results of our study indicate that OPTN data have high validity for DGF and 6-month serum creatinine. However, at some centers, early acute rejection was under-reported. Larger, multicenter studies should be performed to confirm the findings of this work. New efforts by centers and the OPTN may be warranted to improve the quality of reporting early acute rejection events.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors acknowledge the advice of Jennifer Wainright and Stacey Doll in providing information about data reporting to the Organ Procurement and Transplantation Network (OPTN).
This work was supported by National Institutes of Health Grants R01DK-93770 and K24DK090203, a Roche Organ Transplantation Research Foundation Award (to C.R.P.), an award from the American Heart Association (to I.E.H.), and Health Resources and Services Administration Contract 234-2005-37011C.
Preliminary results have been presented at the American Transplant Congress on May 4, 2015 (Philadelphia, PA).
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation. The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06950615/-/DCSupplemental.
References
- 1.Dickinson DM, Bryant PC, Williams MC, Levine GN, Li S, Welch JC, Keck BM, Webb RL: Transplant data: Sources, collection, and caveats. Am J Transplant 4[Suppl 9]: 13–26, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Axelrod DA, Lentine KL, Xiao H, Bubolz T, Goodman D, Freeman R, Tuttle-Newhall JE, Schnitzler MA: Accountability for end-stage organ care: Implications of geographic variation in access to kidney transplantation. Surgery 155: 734–742, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S: Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: An analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis 56: 1127–1139, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Locke JE, James NT, Mannon RB, Mehta SG, Pappas PG, Baddley JW, Desai NM, Montgomery RA, Segev DL: Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation 97: 446–450, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Mohan S, Tanriover B, Ali N, Crew RJ, Dube GK, Radhakrishnan J, Hardy MA, Ratner LE, McClellan W, Cohen D: Availability, utilization and outcomes of deceased diabetic donor kidneys; analysis based on the UNOS registry. Am J Transplant 12: 2098–2105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpkins CE, Montgomery RA, Hawxby AM, Locke JE, Gentry SE, Warren DS, Segev DL: Cold ischemia time and allograft outcomes in live donor renal transplantation: Is live donor organ transport feasible? Am J Transplant 7: 99–107, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Tanriover B, Zhang S, MacConmara M, Gao A, Sandikci B, Ayvaci MU, Mete M, Tsapepas D, Rajora N, Mohan P, Lakhia R, Lu CY, Vazquez M: Induction therapies in live donor kidney transplantation on tacrolimus and mycophenolate with or without steroid maintenance. Clin J Am Soc Nephrol 10: 1041–1049, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallon DH, Summers DM, Bradley JA, Pettigrew GJ: Defining delayed graft function after renal transplantation: Simplest is best. Transplantation 96: 885–889, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Israni AK, Li N, Cizman BB, Snyder J, Abrams J, Joffe M, Rebbeck T, Feldman HI: Association of donor inflammation- and apoptosis-related genotypes and delayed allograft function after kidney transplantation. Am J Kidney Dis 52: 331–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillespie BW, Merion RM, Ortiz-Rios E, Tong L, Shaked A, Brown RS, Ojo AO, Hayashi PH, Berg CL, Abecassis MM, Ashworth AS, Friese CE, Hong JC, Trotter JF, Everhart JE, A2ALL Study Group : Database comparison of the adult-to-adult living donor liver transplantation cohort study (A2ALL) and the SRTR U.S. Transplant Registry. Am J Transplant 10: 1621–1633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg D, French B, Trotter J, Shetty K, Schiano T, Reddy KR, Halpern SD: Underreporting of liver transplant waitlist removals due to death or clinical deterioration: Results at four major centers. Transplantation 96: 211–216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stirnemann PM, Takemoto SK, Schnitzler MA, Brennan DC, Abbott KC, Salvalaggio P, Burroughs TE, Gavard JA, Willoughby LM, Lentine KL: Agreement of immunosuppression regimens described in Medicare pharmacy claims with the Organ Procurement and Transplantation Network survey. J Am Soc Nephrol 17: 2299–2306, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Machnicki G, Pinsky B, Takemoto S, Balshaw R, Salvalaggio PR, Buchanan PM, Irish W, Bunnapradist S, Lentine KL, Burroughs TE, Brennan DC, Schnitzler MA: Predictive ability of pretransplant comorbidities to predict long-term graft loss and death. Am J Transplant 9: 494–505, 2009 [DOI] [PubMed] [Google Scholar]
- 14.US National Institutes of Health: Deceased Donor Biomarkers and Recipient Outcomes (DDS): 2013. Available at: https://clinicaltrials.gov/ct2/show/NCT01848249. Accessed August 23, 2015
- 15.Health Resources and Services Administration (HRSA), Department of Health and Human Services (HHS) : Organ procurement and transplantation network. Final rule. Fed Regist 78: 40033–40042, 2013 [PubMed] [Google Scholar]
- 16.Messa P, Ponticelli C, Berardinelli L: Coming back to dialysis after kidney transplant failure. Nephrol Dial Transplant 23: 2738–2742, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Altman DG, Bland JM: Diagnostic tests. 1: Sensitivity and specificity. BMJ 308: 1552, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordis L: Epidemiology, Philadelphia, W.B. Saunders, 1996 [Google Scholar]
- 19.Agresti A: Categorical Data Analysis, Hoboken, NJ, Wiley, 2013 [Google Scholar]
- 20.Organ Procurement and Transplantation Network: A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI). Available at: http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf. Accessed August 21, 2015
- 21.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Organ Procurement and Transplantation Network: Organ Procurement and Transplantation Network Policies: Policy 18. Data Submission Requirements. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf#nameddest=Policy_18. Accessed August 17, 2015
- 23.Rush DN, Henry SF, Jeffery JR, Schroeder TJ, Gough J: Histological findings in early routine biopsies of stable renal allograft recipients. Transplantation 57: 208–211, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Scholten EM, Rowshani AT, Cremers S, Bemelman FJ, Eikmans M, van Kan E, Mallat MJ, Florquin S, Surachno J, ten Berge IJ, Bajema IM, de Fijter JW: Untreated rejection in 6-month protocol biopsies is not associated with fibrosis in serial biopsies or with loss of graft function. J Am Soc Nephrol 17: 2622–2632, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Shapiro R, Jordan ML, Scantlebury VP, Vivas CA, Jain A, McCauley J, Egidi MF, Randhawa P, Chakrabarti P, Corry RJ: Renal allograft rejection with normal renal function in simultaneous kidney/pancreas recipients: Does dissynchronous rejection really exist? Transplantation 69: 440–441, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Wiesner RH, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Lake JR: Mycophenolate mofetil combination therapy improves long-term outcomes after liver transplantation in patients with and without hepatitis C. Liver Transpl 11: 750–759, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D, Thymoglobulin Induction Study Group : Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 355: 1967–1977, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Brennan DC, Schnitzler MA: Long-term results of rabbit antithymocyte globulin and basiliximab induction. N Engl J Med 359: 1736–1738, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Wiesner RH, Steffen BJ, David KM, Chu AH, Gordon RD, Lake JR: Mycophenolate mofetil use is associated with decreased risk of late acute rejection in adult liver transplant recipients. Am J Transplant 6: 1609–1616, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Andreoni KA, Forbes R, Andreoni RM, Phillips G, Stewart H, Ferris M: Age-related kidney transplant outcomes: Health disparities amplified in adolescence. JAMA Intern Med 173: 1524–1532, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Levine MH, Reese PP, Wood A, Baluarte JH, Huverserian A, Naji A, Abt PL: Inferior allograft outcomes in adolescent recipients of renal transplants from ideal deceased donors. Ann Surg 255: 556–564, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasiske BL, McBride MA, Cornell DL, Gaston RS, Henry ML, Irwin FD, Israni AK, Metzler NW, Murphy KW, Reed AI, Roberts JP, Salkowski N, Snyder JJ, Sweet SC: Report of a consensus conference on transplant program quality and surveillance. Am J Transplant 12: 1988–1996, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.