Abstract
Background and objectives
eGFR on the basis of creatinine (eGFRcre) associates differently with cardiovascular disease and mortality than eGFR on the basis of cystatin C (eGFRcys). This may be related to risk factors affecting the level of creatinine and cystatin C along non-GFR pathways, which may confound the association between eGFR and outcome. Nontraditional risk factors are usually not measured in epidemiologic studies of eGFR and cannot be adjusted for to reduce confounding. We examined whether the inflammatory markers soluble TNF receptor type 2 (sTNFR2), C-reactive protein (CRP), and fibrinogen associated differently with eGFR than with measured GFR (mGFR).
Design, setting, participants, & measurements
GFR was measured by iohexol clearance in 1627 middle-aged participants without kidney disease, diabetes, or cardiovascular disease enrolled in the Renal Iohexol Clearance Survey Study from the Sixth Tromsø Study between 2007 and 2009. Generalized estimating equations were used to assess the residual associations between eGFR (eGFRcre, eGFRcys, and eGFR on the basis of creatinine and cystatin C) and the inflammatory markers relative to mGFR.
Results
sTNFR2, CRP, and fibrinogen were associated with a higher eGFRcre after accounting for mGFR in multivariable-adjusted models (2.63 ml/min per 1.73 m2; 95% confidence interval [95% CI], 2.1 to 3.2 per SD increase in sTNFR2, 0.93 ml/min per 1.73 m2; 95% CI, 0.3 to 1.5 per SD increase in log CRP, and 1.19 ml/min per 1.73 m2; 95% CI, 0.6 to 1.8 per SD increase in fibrinogen). sTNFR2 and CRP were inversely associated with eGFRcys (−1.4 ml/min per 1.73 m2; 95% CI, −2.1 to −0.6 per SD increase in sTNFR2, and −0.76 ml/min per 1.73 m2; 95% CI, −1.4 to −0.1 per SD increase in log CRP).
Conclusions
eGFRcre and eGFRcys are associated with inflammatory factors after accounting for mGFR but in opposite directions. These non-GFR–related associations may bias risk estimates by eGFR and, in part, explain the different risks predicted by eGFRcre and eGFRcys in longitudinal studies.
Keywords: chronic kidney disease, glomerular filtration rate, cardiovascular disease, cystatin C, tumor necrosis factor, fibrinogen, C-reactive protein, humans, kidney function tests
Introduction
The eGFR is widely used in both the clinical assessment of CKD and epidemiologic studies. An eGFR<60 ml/min per 1.73 m2 is an established risk factor for cardiovascular disease (CVD), ESRD, and all-cause mortality (1). However, the associations between eGFR, CVD, and mortality are unclear for individuals in the near–normal GFR range and differ depending on whether cystatin C or creatinine is used to estimate the GFR. One recent study reported an increased hazard ratio for CVD and all-cause mortality with an eGFR<85 ml/min per 1.73 m2 on the basis of cystatin C, but when the eGFR was on the basis of creatinine, the hazard ratio increased when eGFR was <60 or >120 ml/min per 1.73 m2 (2). This differential risk prediction has commonly been explained by the different abilities of creatinine and cystatin C to estimate a true GFR. However, eGFR calculations on the basis of creatinine (eGFRcre) and eGFR calculations on the basis of cystatin C (eGFRcys) perform similarly when estimating a measured GFR (mGFR) in both the general population and patients with CKD (3,4). Alternatively, eGFRcre and eGFRcys could be influenced by risk factors for CVD and mortality that are not related to the true GFR, leading to a biased risk prediction in longitudinal studies (5–7). Chronic inflammation may affect muscle mass and thus, creatinine generation, and cystatin C concentration may increase in an inflammatory state (8). Bias from nontraditional risk factors, such as TNFα, fibrinogen, and C-reactive protein (CRP), may be of particular concern, because they are usually not measured in longitudinal studies of eGFR and outcome, and thus, adjustments to minimize confounding are not possible.
Inflammation and coagulopathy have been closely associated with the pathologic processes that underlie CVD, and in epidemiologic studies, the levels of the biomarkers TNFα receptors, fibrinogen, and CRP have been shown to predict CVD and mortality (9–13). Recently, soluble TNF receptor type 2 (sTNFR2), among several inflammatory biomarkers, including CRP, was found to be the strongest predictor for all-cause mortality in the community (10). In a prospective study of women with diabetes, an elevated sTNFR2 level was the only biomarker that was associated with an increased risk of coronary heart disease, even after multiple adjustments for traditional cardiovascular risk factors and CRP (13). Using a cross-sectional design, this study examined whether sTNFR2, fibrinogen, and CRP associated differently with eGFR on the basis of the equations from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) than with mGFR.
Materials and Methods
Study Population and Data Collection
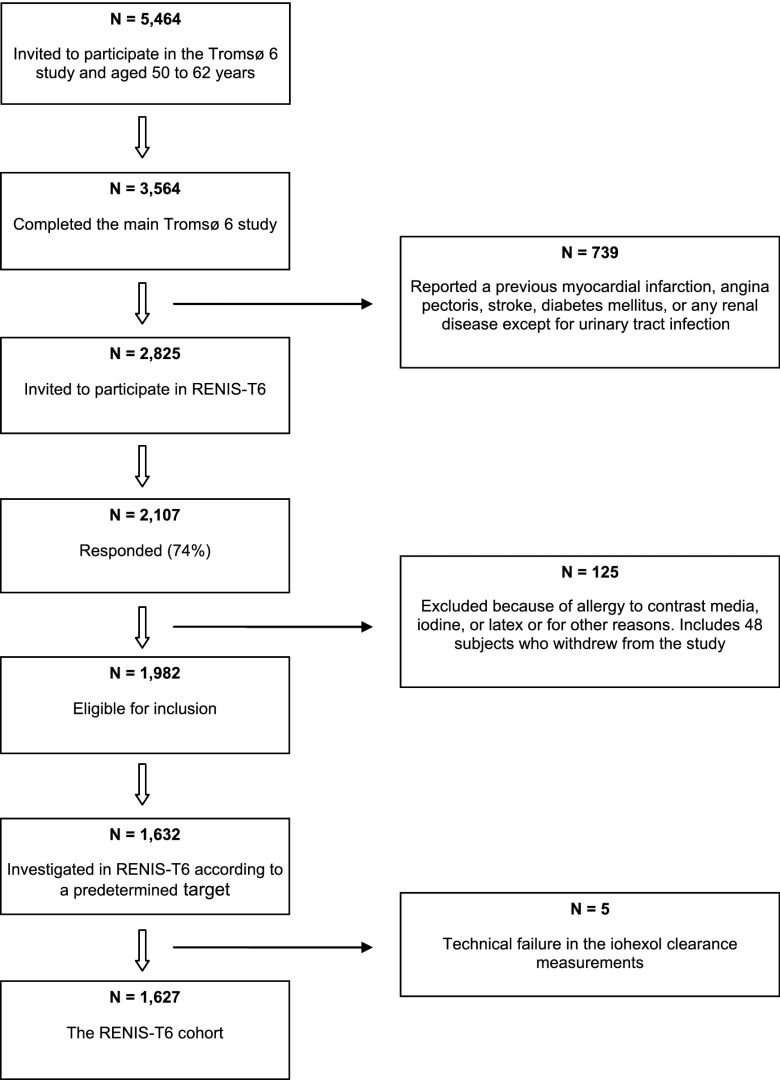
The Renal Iohexol Clearance Survey in the Sixth Tromsø Study (RENIS-T6) is a substudy of the population–based Sixth Tromsø Study (Tromsø 6) and was conducted from November of 2007 to June of 2009. The Tromsø 6 Study included an age–stratified representative sample of 12,984 inhabitants of the municipality of Tromsø in northern Norway and was conducted from October of 2007 to December of 2008 (14). All inhabitants ages 60–62 years old and 40% of the inhabitants ages 50–59 years old were invited to participate, and 3564 (65%) from these two age groups completed the main part of the Tromsø 6 Study . Of these, 739 were excluded after self-reporting previous myocardial infarction, angina pectoris, stroke, diabetes mellitus, or renal disease (Figure 1). The remaining 2825 individuals were invited to participate in the RENIS-T6, and 2107 (75%) agreed. In total, 77 participants were excluded because of allergies to contrast media, iodine, or latex or for other reasons, and 48 participants did not appear at their appointments. Thus, 1632 participants of the remaining 1982 were included according to a predetermined cohort target size and stratified by sex and age groups. Five of these participants were excluded because of technical failures with the iohexol clearance measurements, leaving 1627 participants in the RENIS-T6 cohort. The characteristics of the study cohort and the eligible group were comparable (15). A more detailed description of the RENIS-T6 has been published previously (3).
Figure 1.
Inclusion of the study participants from the main Tromsø 6 Study. RENIS-T6, Renal Iohexol Clearance Survey in the Sixth Tromsø Study.
Measurements
All measurements were made at the Clinical Research Unit at the University Hospital of North Norway. Participants arrived between 08:00 and 10:00. They were instructed to avoid large meals with meat and any nonsteroid inflammatory drugs during the 48 hours before the examination. They were also instructed to fast from midnight and abstain from tobacco use but were advised to drink two to three glasses of water before arrival. A new appointment was made if the participant developed an acute illness.
Five milliliters iohexol (Omnipaque, 300 mg I/ml; Amersham Health, London, United Kingdom) was injected intravenously. The optimal times for measuring iohexol were calculated using the method by Jacobsson (16) on the basis of eGFRcre. Iohexol concentrations were measured by HPLC. The interassay coefficient of variation (CV) during the study period was 3.0%. GFR was calculated using the formulas described by Jacobsson (16). A detailed description of the iohexol clearance measurements has been published (3).
Serum sTNFR2 levels were measured by quantitative sandwich ELISA with a QuantiKine Kit from R&D Systems (Minneapolis, MN). The serum was stored at −80°C and thawed at the time of analysis. The color intensity was measured on a Microplate Spectrophotometer (BioTek Instruments, Winooski, VT). The inter- and intra-assay CVs were 6.0% and 3.0%, respectively.
Fasting glucose, creatinine, triglycerides, and cholesterol were analyzed on the same day. Both fibrinogen and high-sensitivity CRP were measured and analyzed in the Tromsø 6 Study a few months earlier. The interassay CV was 4.2% for fibrinogen, and the inter- and intra-assay CVs for CRP were 2.8% and 1.1%, respectively. Serum samples for measurement of sTNFR2 were collected in the RENIS-T6. A questionnaire was used for additional information about smoking habits, leisure time physical exercise, and any family history of early myocardial infarctions. Smoking status was classified as current smoker or nonsmoker. A family history of early myocardial infarction was defined as a first-degree relative with a myocardial infarction before the age of 60 years old. Physical exercise was dichotomized as never exercise to low-intensity exercise and moderate- to high-intensity exercise as previously reported (15). The data on physical exercise contained 60 missing values (3.7%).
Plasma creatinine was analyzed using a standardized enzymatic assay (CREA Plus; Roche Diagnostics, Indianapolis, IN). External quality assessment was provided by Labquality (Helsinki, Finland). The interassay CV during the study period was 2.3%. Cystatin C was analyzed using a particle–enhanced turbidimetric immunoassay with reagents from Gentian (Moss, Norway) and a Modular E Analyzer (Roche Diagnostics). The interassay CV during the study was 3.1%. External quality control was provided by Equalis (www.equalis.se). In 2013, 300 random samples were reanalyzed with the same cystatin C assay, which had been calibrated to the international standard (17). The interassay CV was 2.9%. All of the cystatin C levels were then recalculated to standardized the values as previously reported for the RENIS-T6 cohort (6).
eGFRcre, eGFRcys, and eGFR on the basis of both creatinine and cystatin C (eGFRcrecys) were calculated using the CKD-EPI equations (4).
Three samples of first–void morning spot urine were collected on separate days. Urinary albumin and creatinine were measured with commercial kits as previously described (18). The albumin-to-creatinine ratio was calculated for each urine sample, and mean value was used for the analyses.
In addition to conventional BP, ambulatory BP (ABP) was measured with a Spacelab 90207 device (Spacelabs Healthcare, Redmond, WA) at 20-minute intervals from 08.00 to 22:00. The daytime mean systolic and diastolic ABPs were calculated as the weighted means of the measurements from 10:00 to 20:00. Details of these measurements have been published elsewhere (19).
Statistical Analyses
Means (SDs) or medians (interquartile ranges) for skewed data were estimated for the study population characteristics and presented by sex. An office diastolic BP was substituted for 19 participants whose ABPs were missing. All of the inflammatory markers were modeled continuously. CRP was modeled with a natural logarithmic transformation because of its skewed distribution.
Multiple linear regression models were used to explore the association between the mGFR and eGFR as dependent variables and fibrinogen, log CRP, and sTNFR2 as independent variables. We adjusted for age, sex, and use of angiotensin–converting enzyme inhibitors or angiotensin receptor blockers in model 1 and additionally, body mass index (BMI), diastolic BP, HDL- and LDL-cholesterol, triglycerides, fasting glucose, smoking (yes/no), albumin-to-creatinine ratio, and having a first-degree relative with a myocardial infarction at <60 years of age in model 2. Generalized estimating equations were used to assess the residual associations between eGFR and fibrinogen, sTNFR2, and log CRP after accounting for mGFR as described by Rule et al. (7). In the analyses, mGFR, eGFRcre, eGFRcys, and eGFRcrecys were regressed simultaneously on fibrinogen, sTNFR2, or log CRP, with adjustment for the same variables as described above. To detect a significant deviation of the risk factor’s association with eGFR compared with mGFR, the interaction between the risk factor and an indicator variable for eGFR method was tested. We also adjusted for the non-GFR–related effects of other risk factors, such as smoking and BMI, as previously reported (5,7) by adjusting for the interactions between eGFR method and all other independent variables. A statistically significant interaction was interpreted as a non-GFR–related association with the eGFR.
Analyses were performed with Stata 13 (StataCorp., College Station, TX). Statistical significance was defined as P<0.05. The study adhered to the Declaration of Helsinki and was approved by the Regional Ethics Committee of Northern Norway. All participants gave informed written consent.
Results
There were 1627 participants in the RENIS-T6 cohort. Table 1 shows the characteristics of the study sample by sex. The mean mGFR was 87.8 ml/min per 1.73 m2 for women and 95.7 ml/min per 1.73 m2 for men. There were 12 missing fibrinogen and four missing sTNFR2 values. The results in Tables 2 and 3 are presented per SD higher concentrations of sTNFR2, log CRP, and fibrinogen.
Table 1.
Study population characteristics of the Renal Iohexol Clearance Survey in the Sixth Tromsø Study
| Characteristics | Women (n=826) | Men (n=801) |
|---|---|---|
| Age, yr | 58.1 (3.9) | 58.0 (3.8) |
| Body mass index, kg/m2 | 26.8 (4.4) | 27.8 (3.5) |
| Systolic BP, mmHga | 126.3 (12.2) | 134.2 (12.9) |
| Diastolic BP, mmHga | 79.0 (8.0) | 85.3 (8.2) |
| HDL-cholesterol, mmol/L | 1.67 (0.41) | 1.39 (0.38) |
| LDL-cholesterol, mmol/L | 3.63 (0.87) | 3.71 (0.84) |
| Fasting glucose, mmol/L | 5.2 (0.51) | 5.5 (0.55) |
| Smoking (yes/no), % | 23 | 19 |
| Triglycerides, mmol/L | 1.0 (0.7–1.3) | 1.1 (0.8–1.6) |
| Albumin-to-creatinine ratio, mg/mmol | 0.37 (0.22–0.65) | 0.29 (0.15–0.53) |
| sTNFR2, pg/ml | 2628 (698) | 2719 (602) |
| CRP, mg/L | 1.10 (0.61–2.24) | 1.26 (0.72–2.25) |
| Fibrinogen, g/L | 3.55 (0.67) | 3.36 (0.63) |
| ACEi (yes/no), % | 1.6 | 2.0 |
| ARB (yes/no), % | 7.3 | 9.9 |
| Waist-to-hip ratio | 0.87 (0.06) | 0.95 (0.06) |
| Moderate to high intensity exercise, % | 52.7 | 57.5 |
| Measured GFR, ml/min per 1.73 m2 | 87.8 (14.0) | 95.7 (13.7) |
| eGFR by CKD-EPI equation, ml/min per 1.73 m2 | ||
| eGFRcre | 94.4 (10.0) | 95.3 (9.0) |
| eGFRcys | 102.2 (12.1) | 108.6 (11.7) |
| eGFRcrecys | 101.4 (11.9) | 104.6 (10.7) |
Numbers are means (SDs), percentages, or medians (interquartile ranges). sTNFR2, soluble TNF receptor type 2; CRP, C-reactive protein; ACEi, angiotensin–converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFRcre, eGFR calculations on the basis of creatinine; eGFRcys, eGFR calculations on the basis of cystatin C; eGFRcrecys, eGFR calculations on the basis of creatinine and cystatin C.
Ambulatory BP.
Table 2.
Multiple linear regressions of measured GFR and eGFR on risk factors
| Dependent Variable and Risk Factora per SD | Model 1b | Model 2c | ||||
|---|---|---|---|---|---|---|
| Estimate (ml/min per 1.73 m2) | 95% CI | P Value | Estimate (ml/min per 1.73 m2) | 95% CI | P Value | |
| mGFR | ||||||
| Fibrinogen | −0.37 | −1.04 to 0.31 | 0.30 | −0.74 | −1.46 to −0.01 | 0.05 |
| sTNFR2 | −4.24 | −4.87 to −3.60 | <0.001 | −4.81 | −5.47 to −4.15 | <0.001 |
| Log CRP | −0.37 | −1.04 to 0.30 | 0.27 | −0.73 | −1.45 to −0.02 | 0.05 |
| eGFRcre | ||||||
| Fibrinogen | 0.55 | 0.10 to 1.00 | 0.02 | 0.44 | −0.04 to 0.92 | 0.08 |
| sTNFR2 | −1.80 | −2.24 to −1.36 | <0.001 | −2.09 | −2.54 to −1.64 | <0.001 |
| Log CRP | 0.25 | −0.21 to 0.69 | 0.29 | 0.17 | −0.31 to 0.65 | 0.49 |
| eGFRcys | ||||||
| Fibrinogen | −1.86 | −2.42 to −1.30 | <0.001 | −0.85 | −1.43 to −0.26 | <0.01 |
| sTNFR2 | −6.48 | −6.93 to −6.02 | <0.001 | −6.10 | −6.61 to −5.64 | <0.001 |
| Log CRP | −2.56 | −3.09 to −2.0 | <0.001 | −1.55 | −2.14 to −0.98 | <0.001 |
| eGFRcrecys | ||||||
| Fibrinogen | −0.85 | −1.38 to −0.31 | 0.002 | −0.27 | −0.85 to 0.30 | 0.30 |
| sTNFR2 | −5.07 | −5.54 to −4.62 | <0.001 | −5.00 | −5.49 to −4.51 | <0.001 |
| Log CRP | −1.47 | −1.99 to −0.93 | <0.001 | −0.89 | −1.45 to −0.32 | 0.002 |
95% CI, 95% confidence interval; mGFR, measured GFR; sTNFR2, soluble TNF receptor type 2; CRP, C-reactive protein; eGFRcre, eGFR calculations on the basis of creatinine; eGFRcys, eGFR calculations on the basis of cystatin C; eGFRcrecys, eGFR calculations on the basis of creatinine and cystatin C.
The overall SDs are fibrinogen =0.66, sTNFR2=654, and log CRP =0.98.
Adjusted for age, sex, and use of angiotensin–converting enzyme inhibitor or angiotensin receptor blocker.
Adjusted for age, sex, use of angiotensin–converting enzyme inhibitor or angiotensin receptor blocker, body mass index, daily smoking (yes/no), diastolic BP, HDL-cholesterol, LDL-cholesterol, triglycerides, having a first-degree relative with myocardial infarction at <60 years old, fasting glucose, and albumin-to-creatinine ratio.
Table 3.
Generalized estimated equations showing residual associations between risk factor and eGFR after accounting for measured GFR
| Dependent Variable and Risk Factora per SD | Model 1: Adjusted for Age, Sex, and Use of ACEis or ARBs | Model 2: Fully Adjustedb | ||||
|---|---|---|---|---|---|---|
| Estimatec (ml/min per 1.73 m2) | 95% CI | P Valued | Estimatec (ml/min per 1.73 m2) | 95% CI | P Valued | |
| eGFRcre | ||||||
| Fibrinogen | 0.92 | 0.38 to 1.47 | 0.001 | 1.19 | 0.60 to 1.80 | <0.00le |
| sTNFR2 | 2.44 | 1.91 to 2.97 | <0.001 | 2.63 | 2.09 to 3.17 | <0.001 |
| Log CRP | 0.62 | 0.06 to 1.18 | 0.03 | 0.93 | 0.33 to 1.54 | 0.002 |
| eGFRcys | ||||||
| Fibrinogen | −1.50 | −2.10 to −0.90 | <0.001 | −0.06 | −0.68 to 0.55 | 0.80 |
| sTNFR2 | −2.24 | −3.03 to −1.45 | <0.001 | −1.35 | −2.09 to −0.60 | <0.001 |
| Log CRP | −2.16 | −2.76 to −1.57 | <0.001 | −0.76 | −1.38 to −0.14 | 0.02 |
| eGFRcrecys | ||||||
| Fibrinogen | −0.48 | −0.99 to 0.03 | 0.06 | 0.50 | −0.05 to 1.04 | 0.07 |
| sTNFR2 | −0.83 | −0.47 to −0.19 | 0.01 | −0.25 | −0.87 to 0.37 | 0.40 |
| Log CRP | −1.08 | −1.59 to −0.57 | <0.001 | −0.09 | −0.64 to 0.45 | 0.74 |
Generalized estimating equations with eGFR and measured GFR (mGFR) as stacked dependent variables regressed on each independent variable to compare the difference in eGFR and mGFR regression coefficients. ACEi, angiotensin–converting enzyme inhibitor; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval; eGFRcre, eGFR calculations on the basis of creatinine; sTNFR2, soluble TNF receptor type 2; C-reactive protein; eGFRcys, eGFR calculations on the basis of cystatin C; eGFRcrecys, eGFR calculations on the basis of creatinine and cystatin C.
The overall SDs are fibrinogen =0.66, sTNFR2=654, and log CRP =0.98.
Additionally adjusted for body mass index, daily smoking (yes/no), diastolic BP, HDL- and LDL-cholesterol, triglycerides, having a first-degree relative with myocardial infarction at <60 years old, fasting glucose, and albumin-to-creatinine ratio.
The difference in eGFR and mGFR regression coefficients.
Statistical significance determined by the statistical interaction between each risk factor and eGFR relative to mGFR.
The interaction between fibrinogen and age was significant (P=0.02). The estimate for the interaction was −0.24 ml/min per 1.73 m2 per year (95% CI, −0.44 to −0.04).
Multiple linear regression models with mGFR and eGFR as dependent variables in separate models and fibrinogen, sTNFR2, and log CRP as independent variables are shown in Table 2. Higher concentrations of fibrinogen were associated with lower mGFR and eGFRcys but not lower eGFRcre in the fully adjusted model (Table 2, model 2). Higher concentrations of sTNFR2 were associated with lower mGFR and eGFR in all of the estimating formulas. Higher CRP was associated with lower eGFRcys and slightly higher eGFRcre, whereas there was no association between CRP and mGFR.
Table 3 shows the residual association between the eGFR equations and the independent variables after accounting for mGFR. Both eGFRcre and eGFRcys were associated with fibrinogen, sTNFR2, and CRP after accounting for mGFR and adjusting for age, sex, angiotensin–converting enzyme inhibitors, and angiotensin receptor blockers (Table 3, model 1). Higher concentrations of fibrinogen, sTNFR2, and CRP were associated with higher eGFRcre but lower eGFRcys. In the fully adjusted model (Table 3, model 2), eGFRcre remained associated with fibrinogen, sTNFR2, and CRP, whereas only sTNFR2 and CRP were associated with eGFRcys, again in the opposite direction from eGFRcre. The residual association of fibrinogen and eGFRcre was attenuated with higher age (interaction estimate: −0.24 ml/min per 1.73 m2; 95% confidence interval, −0.44 to −0.04), with no association above a threshold of approximately 56 years old.
The combined eGFRcrecys equation showed no statistically significant residual association between any of the inflammatory or hemostatic markers in the multivariable adjusted model (Table 3).
We repeated the analysis with additional adjustments for waist-to-hip ratio and leisure time physical exercise, because these factors may correlate with muscle mass and thus, influence eGFRcre. The estimates remained similar as those reported in Tables 2 and 3 (data not shown).
Discussion
In this middle-aged population, we found that fibrinogen, sTNFR2, and CRP differed significantly in their associations with eGFR relative to mGFR. eGFRcre was positively associated with all markers after multivariable adjustment and accounting for mGFR. eGFRcys was associated with sTNFR2 and CRP but in an opposite direction compared with eGFRcre. Non-GFR–related factors of eGFRcre and eGFRcys have been reported in prior studies (5–7,20,21). In particular, cystatin C has been associated with cardiovascular risk factors and CRP as a marker of inflammation. In a recent study, Rule et al. (7) found CRP, BMI, and hypertension to be associated with eGFRcys, whereas only urinary creatinine was associated with eGFRcre after accounting for mGFR. Accordingly, Rule et al. (7) argued that eGFRcre is superior in reflecting true GFR in association studies of eGFR and outcome.
In this study, we investigated the role of sTNFR2, a reliable marker of TNFα activity, as a non-GFR–related factor of both eGFRcys and eGFRcre. Unlike CRP, which is an acute-phase protein and regarded as a nonspecific inflammatory marker, TNFα plays a key role in inflammation, and its receptors are stable over time within individuals (22–24). Moreover, our study suggests that sTNFR2, CRP, and fibrinogen are also non-GFR factors of eGFRcre. These novel findings indicate similar problems for eGFRcre as for eGFRcys and suggest that eGFRcre does not necessarily reflect the associations with true GFR better than eGFRcys. In the Framingham Heart Study, sTNFR2 was the only inflammatory biomarker that improved the discriminatory ability of the model to predict all-cause mortality beyond traditional risk factors (10). sTNFR2 and CRP showed the same associations with cardiovascular risk, although the risk estimates were relatively low. In a previous prospective cohort of women with diabetes, sTNFR2 was independently associated with the risk of coronary heart disease, and the estimate was sustained after multiple adjustments, including CRP (13). Interestingly, after adjustments for sTNFR2 and traditional cardiovascular risk factors, CRP was not significantly associated with a risk of coronary heart disease, which suggests that CRP may, in part, be dependent on TNFα activity.
eGFR is extensively used in epidemiologic studies. The non-GFR–related factors of eGFR documented by us and others imply that caution should be exercised in interpreting increased risk attributable to eGFR equations as true effects of GFR. Even small non-GFR–related effects might result in statistically significant spurious associations in large epidemiologic studies. To illustrate this, we found a non-GFR–related effect of 1.19 ml/min per 1.73 m2 in eGFRcre per SD increase in fibrinogen. According to two meta-analyses on fibrinogen and outcome, a 1-SD increase in fibrinogen corresponds to an adjusted hazard ratio of 1.5 (95% confidence interval, 1.3 to 1.9) for CVD and 1.6 (95% confidence interval, 1.5 to 1.6) for all-cause mortality (11,25). Whether the magnitudes of our observed non–GFR associations are substantial enough to cause major bias in longitudinal risk estimates is uncertain. However, the total effect of several non–GFR factors may cause considerable bias.
Moreover, our findings indicate that the non-GFR–related associations of sTNFR2 and fibrinogen operate in opposite directions for eGFRcre and eGFRcys. Similarly, opposite non-GFR–related associations for eGFRcre and eGFRcys were shown for smoking and asymmetric dimethylarginine in two previous studies (5,6). Taken together, these findings may, in part, explain the different risk predictions with eGFRcre and eGFRcys in epidemiologic studies (2). Indeed, most identified non-GFR–related factors of cystatin C, such as smoking, obesity, hypertension, CRP, and sTNFR2, are associated with higher cystatin C (lower eGFRcys), whereas the opposite is the case for creatinine (higher eGFRcre) for smoking, asymmetric dimethylarginine, sTNFR2, CRP, and fibrinogen (5–7).
Several studies have reported an increased risk of mortality in subjects with high eGFRcre (2,26). This has generally been explained by muscle wasting related to chronic diseases, although a high eGFRcre, possibly a marker of hyperfiltration, was recently associated with mortality, even after adjusting for muscle mass (27). Our findings suggest that other factors associated with chronic diseases, such as increased levels of inflammatory markers and fibrinogen, may bias risk prediction in the same direction as low muscle mass and similarly confound longitudinal studies using eGFR. Of note, our participants had normal kidney function and were relative healthy, without CVD or diabetes, and free from acute illness at the time of GFR measurement. Thus, the non-GFR–related associations with inflammation or hemostatic disturbances might be of even greater significance in other populations.
There were no statistically significant residual associations between eGFRcrecys and the markers evaluated. This may be because of the opposite associations of the non-GFR determinants of eGFRcre and eGFRcys, which may neutralize each other. Similar findings were suggested in a previous study of nontraditional cardiovascular risk factors (6). Thus, the combined eGFRcrecys equation may be less biased than eGFRcre or eGFRcys in epidemiologic studies. Still, eGFRcre seems to be the least biased equation with regard to traditional CVD risk factors, such as obesity, smoking, and hypertension (7). Studies of eGFR and outcome commonly adjust for traditional CVD risk factors, thereby reducing, although not eliminating, problems arising from non-GFR–related bias.
The main strength of this study was the measurement of GFR according to iohexol clearance in a large sample from the general population. Single–sample iohexol clearance has been shown to be a reliable measure of GFR (28,29). Furthermore, the measurements of creatinine and cystatin C were both calibrated to international standards, and the serum levels of sTNFR2 were measured with a reliable commercial ELISA kit. The cross-sectional design of our study was also appropriate to examine the non-GFR–related factors of eGFR. There are also limitations with this study. All participants were white and middle aged. Therefore, caution should be used for comparisons with other ethnic or age groups. We were not able to adjust our analysis for dietary factors or measurements of muscular mass. Muscular mass is probably the most important non–GFR determinant of eGFRcre. Recently, an association between inflammation and low muscular strength/mass in patients on dialysis was reported (30). Thus, low muscle mass may, in part, explain the influence of CRP and sTNFR2 on eGFRcre. However, our population was relative healthy, and we obtained similar results after adjusting for waist-to-hip ratio and physical exercise, which both correlate with muscle mass.
We conclude that sTNFR2, fibrinogen, and CRP are all non-GFR factors of eGFRcre and that sTNFR2 and CRP are non-GFR factors of eGFRcys. The opposite non–GFR directions of the estimates may, in part, explain the different risks predicted by eGFRcre and eGFRcys in longitudinal studies.
Disclosures
None.
Acknowledgments
We thank Britt-Ann Winther Eilertsen, Bjørg Skog Høgseth, Saskia van Heusden, and the rest of the staff at the Clinical Research Unit (University Hospital of North Norway) for accomplishment of the study; Harald Strand and the staff at the Department of Medical Biochemistry (University Hospital of North Norway) for HPLC analyses of iohexol; and Inger Sperstad and Ingrid Dorthea Sandstad at the Clinical Research Centre (University Hospital of North Norway) for database support.
This study was financed by The North Norwegian Regional Health Authority.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT, CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I: Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 78: 1305–1311, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO: Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 22: 927–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melsom T, Fuskevåg OM, Mathisen UD, Strand H, Schei J, Jenssen T, Solbu M, Eriksen BO: Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol 41: 7–15, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST: Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 83: 1169–1176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB, Shlipak MG: Kidney function and markers of inflammation in elderly persons without chronic kidney disease: The health, aging, and body composition study. Kidney Int 71: 239–244, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V: C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 350: 1387–1397, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, Rong J, Levy D, Keaney JF, Jr., Wang TJ, Murabito JM, Vasan RS, Benjamin EJ: Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol 33: 1728–1733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer IE, Pasterkamp G, Prins MW, Roest M: Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One 8: e62080, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shishehbor MH, Oliveira LP, Lauer MS, Sprecher DL, Wolski K, Cho L, Hoogwerf BJ, Hazen SL: Emerging cardiovascular risk factors that account for a significant portion of attributable mortality risk in chronic kidney disease. Am J Cardiol 101: 1741–1746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shai I, Schulze MB, Manson JE, Rexrode KM, Stampfer MJ, Mantzoros C, Hu FB: A prospective study of soluble tumor necrosis factor-alpha receptor II (sTNF-RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes Care 28: 1376–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njølstad I: Cohort profile: The Tromso Study. Int J Epidemiol 41: 961–967, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melsom T, Mathisen UD, Eilertsen BA, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO: Physical exercise, fasting glucose, and renal hyperfiltration in the general population: The Renal Iohexol Clearance Survey in Tromsø 6 (RENIS-T6). Clin J Am Soc Nephrol 7: 1801–1810, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsson L: A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 3: 297–305, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I; IFCC Working Group on Standardisation of Cystatin C (WG-SCC): First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 48: 1619–1621, 2010 [DOI] [PubMed]
- 18.Solbu MD, Kronborg J, Eriksen BO, Jenssen TG, Toft I: Cardiovascular risk-factors predict progression of urinary albumin-excretion in a general, non-diabetic population: A gender-specific follow-up study. Atherosclerosis 201: 398–406, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I, Eriksen BO: Ambulatory blood pressure is associated with measured glomerular filtration rate in the general middle-aged population. J Hypertens 30: 497–504, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS: Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75: 652–660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR: Tumor necrosis factor receptors: Biology and therapeutic potential in kidney diseases. Am J Nephrol 36: 261–270, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Al-Lamki RS, Mayadas TN: TNF receptors: Signaling pathways and contribution to renal dysfunction. Kidney Int 87: 281–296, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D’Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Piñango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssönen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Després JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A, Fibrinogen Studies Collaboration : Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA 294: 1799–1809, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J: Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol 20: 2214–2222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ: Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol 26: 1426–1433, 2015 [DOI] [PMC free article] [PubMed]
- 28.Bird NJ, Peters C, Michell AR, Peters AM: Comparison of GFR measurements assessed from single versus multiple samples. Am J Kidney Dis 54: 278–288, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Bàràny P, Heimbürger O, Cederholm T, Stenvinkel P, Carrero JJ: Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 9: 1720–1728, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]