Abstract
Background and objectives
Treatment of congenital nephrotic syndrome (CNS) and steroid–resistant nephrotic syndrome (SRNS) is demanding, and renal prognosis is poor. Numerous causative gene mutations have been identified in SRNS that affect the renal podocyte. In the era of high–throughput sequencing techniques, patients with nongenetic SRNS frequently escape the scientific interest. We here present the long-term data of the German CNS/SRNS Follow-Up Study, focusing on the response to cyclosporin A (CsA) in patients with nongenetic versus genetic disease.
Design, setting, participants, & measurements
Cross–sectional and longitudinal clinical data were collected from 231 patients with CNS/SRNS treated at eight university pediatric nephrology units with a median observation time of 113 months (interquartile range, 50–178). Genotyping was performed systematically in all patients.
Results
The overall mutation detection rate was high at 57% (97% in CNS and 41% in SRNS); 85% of all mutations were identified by the analysis of three single genes only (NPHS1, NPHS2, and WT1), accounting for 92% of all mutations in patients with CNS and 79% of all mutations in patients with SRNS. Remission of the disease in nongenetic SRNS was observed in 78% of patients after a median treatment period of 2.5 months; 82% of nongenetic patients responded within 6 months of therapy, and 98% of patients with nongenetic SRNS and CsA–induced complete remission (normalbuminemia and no proteinuria) maintained a normal renal function. Genetic SRNS, on the contrary, is associated with a high rate of ESRD in 66% of patients. Only 3% of patients with genetic SRNS experienced a complete remission and 16% of patients with genetic SRNS experienced a partial remission after CsA therapy.
Conclusions
The efficacy of CsA is high in nonhereditary SRNS, with an excellent prognosis of renal function in the large majority of patients. CsA should be given for a minimum period of 6 months in these patients with nongenetic SRNS. In genetic SRNS, response to CsA was low and restricted to exceptional patients.
Keywords: NPHS1; NPHS2; WT1; FSGS; steroid resistant nephrotic syndrome; cyclosporine A; humans; kidney failure, chronic; mutation
Introduction
Morbidity and economic burden of primary congenital nephrotic syndrome (CNS) and steroid–resistant nephrotic syndrome (SRNS) in children and adults are high. Whereas patients with steroid–sensitive nephrotic syndrome have a fortunate renal prognosis, SRNS, defined by the inability to achieve complete remission (CR) on corticosteroid therapy, is associated with a high risk of progressive renal failure. Treatment of SRNS is challenging and requires a specialist’s expertise (1,2). Therapeutic options are limited, but nonsteroid-based immunosuppression, including cyclosporin A (CsA), is effective in a significant number of patients (3,4). However, response rates to CsA treatment have been shown to vary considerably, depending on the study cohort. No remission (NR) induction by CsA in patients with SRNS was observed in early studies (5,6). Niaudet (7) observed remission in 42% of patients. Others reported CR in seven of 11 (64%), 18 of 23 (70%), and 31 of 35 (89%) patients with SRNS/steroid–dependent nephrotic syndrome (8–10). Most of these studies included only a small number of patients and did not discriminate between genetic and nongenetic SRNS. With the identification of gene mutations in NPHS2 in 10%–40% of patients with SRNS (11,12), a rapid development of genetic studies took place. To date, >24 single-gene causes of SRNS have been described, mostly affecting components of the podocyte actin cytoskeleton and signaling molecules (13–15). Although some of these mutations are very rare, mutations in NPHS1, NPHS2, and WT1 affect an important percentage of children and young adults with SRNS (11,16–19). These patients proved to be largely insensitive to intensified immunosuppression with CsA, which was shown in medium–sized cohort studies (12,16,20). Despite this growing knowledge and with few exceptions (12,16,20–23), many clinical studies still do not define genetic and nongenetic SRNS in patients. It is likely that this largely explains the variability in results among the different studies. We here present the genetic data and long-term outcomes of 231 children and young adults with CNS/SRNS.
Materials and Methods
Two hundred thirty-one patients (106 boys) of 219 families were included; of these, 62 were diagnosed with CNS (age at onset ≤3 months), and 169 were diagnosed with primary SRNS (age at onset >3 months). Data were provided by university pediatric nephrology units of Essen, Munster, Hanover, Cologne, Hamburg, Heidelberg, Munich, and Innsbruck. The median observation time was 113 months (interquartile range [IQR], 50–178). A subset of patients has been included in the works in refs. 16 and 24–26. Patients with an age at onset >20 years old, secondary nephrotic syndrome, or secondary steroid resistance were excluded. Age at onset, steroid responsiveness, dose of immunosuppressive medication and blood levels, time to remission, renal outcome, renal histology, and recurrence of the disease after renal transplantation (RTx) were assessed. Renal outcome was classified as normal renal function (GFR>90 ml/min per 1.73 m2), CKD stages 2–4 (GFR=15–90 ml/min per 1.73 m2), or ESRD (GFR<15 ml/min per 1.73 m2) at the end of observation. Standard steroid treatment and steroid response were defined according to the criteria of the German Pediatric Nephrology Association (GPN) (27) and the International Study of Kidney Disease in Children (28). CsA doses were titrated to achieve blood levels between 80 and 150 ng/ml. CR to CsA was defined as proteinuria <4 mg/m2 per hour, urinary protein to creatinine ratio <30 mg/mmol, or trace of protein on dipstick analysis and normalization of serum albumin (>3.5 g/dl). Partial remission (PR) was defined as proteinuria between 4 and 40 mg/m2 per hour and normalization of serum albumin (>3.5 g/dl). Steroid resistance was defined as failure of induction of CR after 4 weeks of standard steroid therapy. The study was performed according to the Declaration of Helsinki, and informed consent was obtained from all patients and the parents or legal guardians of children below the age of consent. The study protocol was approved by the GPN, the ethical committee of the Medical Faculty of the University Duisburg-Essen (09–3954), and the local committees of collaborating institutions.
Mutational Analyses
Mutational analysis was performed for NPHS1 (NG_013356.1), NPHS2 (NM_014625.2), exons 8 and 9 of WT1 (NG_009272.1), LAMB2 (NM_002292.3), TRPC6 (NM_004621.5), and PLCE1 (NM_016341.3) depending on an age-related algorithm. All patients received diagnostic testing of NPHS2 and WT1. Patients without pathogenic mutations in WT1 or NPHS2 received analysis of NPHS1 (age at onset <6 years old) or TRPC6 (age at onset ≥6 years old). Analysis of PLCE1 was performed in patients with diffuse mesangial sclerosis (DMS) and analysis of LAMB2 was performed in patients with DMS and ocular symptoms. In 68 patients, panel diagnostics was performed including the following genes: NPHS1, NPHS2, WT1, TRPC6, PLCE1, LAMB2, ACTN4 (NM_004924.4), CD2AP (NM_012120.2), COQ6 (NM_182476.2), and INF2 (NM_022489.3). Novel mutations were excluded in ≥100 healthy individuals, and in silico testing was performed with MutationTaster (http://mutationtaster.org/), PolyPhen-2 (http://genetics.bwh.havard.edu/pph/), and SIFT (http://sift.jcvi.org/).
Statistical Analyses
Data are given as the medians (IQRs) and/or means±SDs. Comparisons of CsA response rates and renal survival between different patients groups were performed using Fisher exact tests. The level of statistical significance was predefined as P≤0.05. Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA).
Results
Sixty-two of 231 (27%) patients presented with CNS, and 169 of 231 (73%) patients presented with primary SRNS (Table 1).
Table 1.
Clinical data
| Clinical Parameter | Nongenetic (n=100) | Genetic (n=131) | ||
|---|---|---|---|---|
| CNS (n=2) | SRNS (n=98) | CNS (n=60) | SRNS (n=71) | |
| Age at onset median (IQR), mo | CNS | 61 (32–103) | CNS | 42 (15–96) |
| CsA therapy, % | No | 85 | 16 | 47 |
| Response to CsA, n/n | NA | 79% (64/81); 60% CR (49/81); 19% PR (15/81) | 11% (1/9); 11% CR (1/9); 0% PR (0/9) | 19% (6/32); 3% CR (1/32); 16% PR (5/32) |
| Time to CR median (IQR), mo | NA | 2.5 (1–5) | 2 (n=1) | 2.5 (n=1) |
| ESRD, % | 100 | 27 | 83 | 66 |
| Time to ESRD median (IQR), mo | 1 | 36 (16–101) | 24 (7–40) | 44 (5–76) |
| Renal histology | ||||
| FSGS, %; n/n | NA | 69; 68/98 | 21; 8/38 | 66; 43/65 |
| MCN, %; n/n | NA | 24; 23/98 | 13; 5/38 | 17; 11/65 |
| DMS, %; n/n | 1/1 | 1; 1/98 | 40; 15/38 | 14; 9/65 |
| Finnish type, %; n/n | NA | 0; 0/98 | 21; 8/38 | 0; 0/65 |
| Other, %; n/n | NA | 6; 6/98 | 5; 2/38 | 3; 2/65 |
| Not done | n=1 | n=1 | n=22 | n=6 |
CNS, congenital nephrotic syndrome; SRNS, steroid–resistant nephrotic syndrome; IQR, interquartile range; CsA, cyclosporin A; NA, not applicable; CR, complete remission; PR, partial remission; MCN, minimal change nephropathy; DMS, diffuse mesangial sclerosis.
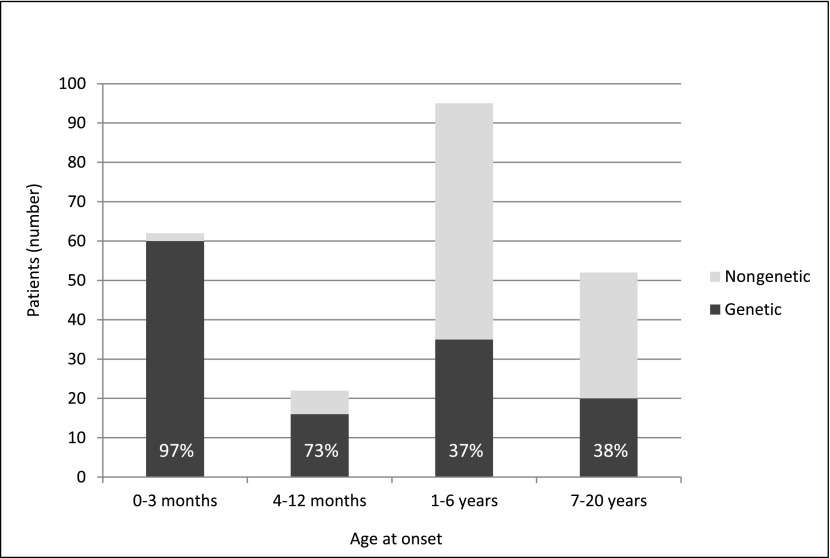
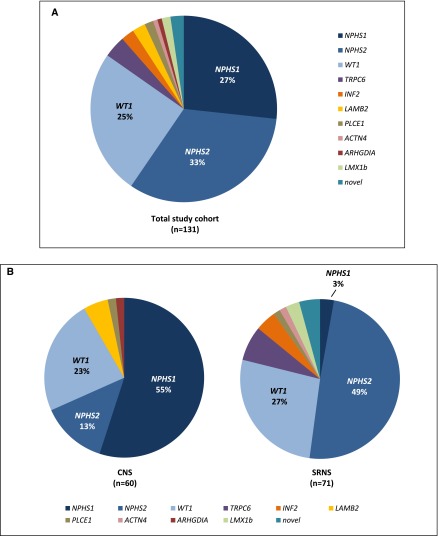
The overall mutation detection rate (MDR) was 57% (131 of 231): 97% (60 of 62) in patients with CNS and 42% (71 of 169) in patients with SRNS (Supplemental Table 1). The MDR was higher in patients with younger age at onset but still significant in school-aged children (CNS, 97%; age 4 to ≤12 months old, 73%; age 1–6 years old, 37%; age 7–18 years old, 38%) (Figure 1). Mutations were detected in NPHS1 (n=35), NPHS2 (n=43), WT1 (n=33), LAMB2 (n=3), PLCE1 (n=2), TRPC6 (n=5), ACTN4 (n=1), INF2 (n=3), ARHGDIA (n=1), LMX1B (n=2), and novel podocyte genes (n=3; unpublished data). No mutations were identified in CD2AP and COQ6. Mutations in NPHS1, NPHS2, and WT1 accounted for 92% of mutations in patients with CNS and 79% of mutations in patients with SRNS (94% in patients with SRNS with an age at onset of 4–12 months old, 77% in patients with an age at onset of 1–6 years old, and 70% in patients with an age at onset of ≥6 years old) (Figure 2).
Figure 1.
Age at onset of congenital nephrotic syndrome/steroid–resistant nephrotic syndrome in age groups. Patients with genetic disease are depicted in black; patients with nongenetic disease are depicted in light gray. Percentages refer to the fraction of patients with genetic disease.
Figure 2.
Distribution of podocyte gene mutations in patients with genetic congenital nephrotic syndrome (CNS) and steroid–resistant nephrotic syndrome (SRNS). (A) Gene mutations in the total study cohort of patients with genetic disease (n=131). 85% of mutations were identified in NPHS1, NPHS2, and WT1. All other gene mutations were rare events affecting a small number of patients. (B) Distribution of podocyte gene mutations in patients with genetic CNS (left panel; n=60) and genetic SRNS (right panel; n=71).
Stratification of Study Cohort
Patients were stratified according to a nongenetic or genetic background and further classified with respect to their age at onset into CNS and SRNS.
Patients without Mutations in the Analyzed Genes
One hundred of 231 (43%) patients presented without mutations in the analyzed genes (SRNS, n=98; CNS, n=2).
Data of Two Patients with CNS without Mutations.
Both were not treated with CsA and progressed to ESRD within weeks. Renal biopsy revealed DMS in one patient. RTx in both was without recurrence of the disease. Unexpectedly, no disease-causing mutation was identified by panel or whole-exome sequencing.
Data of Patients with SRNS without Mutations.
Median age at onset in patients with SRNS was 61 months (IQR, 32–103). Age at onset between 4 and 12 months old was observed in 6% of patients, between 1 and 6 years old was observed in 60% of patients, and between 7 and 18 years old was observed in 34% of patients. Kidney biopsies were performed in all patients and showed FSGS in 69%, minimal change nephropathy (MCN) in 24%, and DMS in 1% (Table 1).
Therapy.
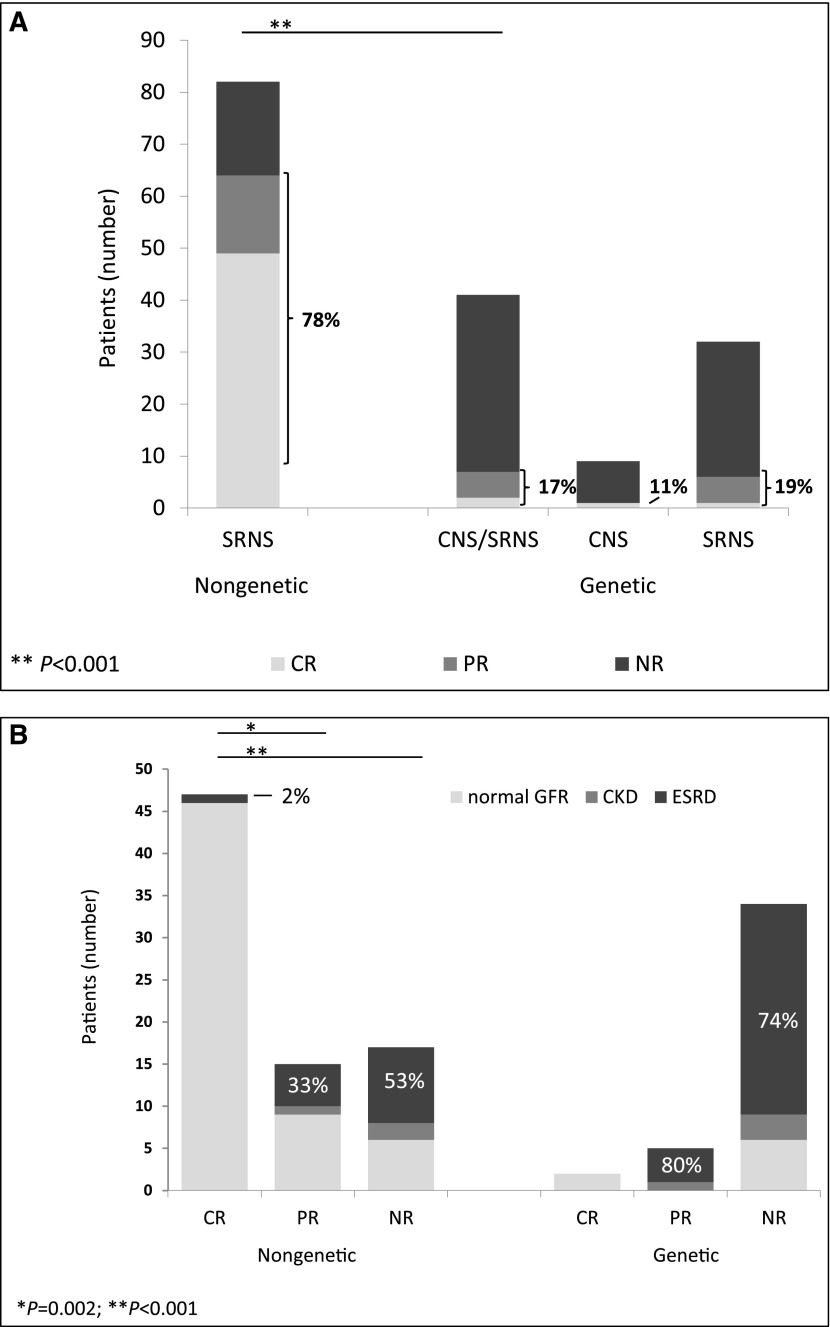
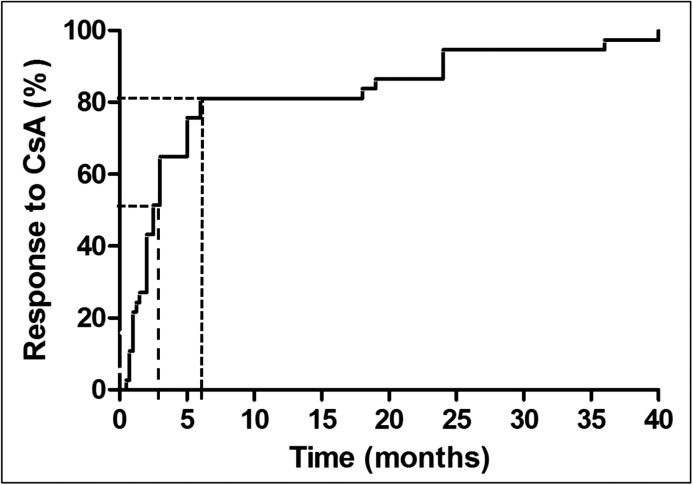
Eighty-two of 96 (85%; no data, n=2) patients received CsA after diagnosis of steroid resistance; 64 of 81 (79%; no data, n=1) patients responded to CsA, with CR in 49 of 81 (60%) and PR in 15 of 81 (19%) patients (Figure 3A). Median time to CR was 2.5 months (IQR, 1–5); 31 of 38 (82%; no data, n=11) patients responded within the first 6 months of therapy (Figure 4). Time to PR ranged from 3 to 37 months, with a median of 10.5 months (IQR, 4–25; no data, n=9); 18 of 82 (22%) patients showed NR. Eighty of 94 (85%; no data, n=4) patients received angiotensin–converting enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs) as antiproteinuric treatment (of these, 10 patients without CsA therapy).
Figure 3.
Response to cyclosporin A (CsA) treatment and renal outcome in patients with either nongenetic or genetic disease. (A) Response to CsA treatment. Patients (numbers) with complete remission (CR) are depicted in light gray, patients with partial remission (PR) are depicted in gray, and patients with no remission (NR) are depicted in black. The group of patients with genetic congenital nephrotic syndrome (CNS) /steroid–resistant nephrotic syndrome (SRNS) is further divided into patients with CNS and patients with SRNS (on the right). The two patients with nongenetic CNS were not exposed to CsA and are, therefore, not depicted in this graph. Percentages refer to CR and PR. **P<0.001. (B) Renal function in patients with either nongenetic or genetic disease depending on their response to CsA treatment. Patients (numbers) with a normal renal function at the end of the observation time are shown in light gray, patients with chronic CKD are shown in gray, and patients with ESRD are shown in black. *P=0.002; **P<0.001.
Figure 4.
Time to response to cyclosporin A (CsA) in nongenetic patients with complete remission. 50% of patients responded within 2.5 months of treatment (median; dashed line), and 82% of patients responded within 6 months of treatment (dotted line).
Renal Function.
Sixty-nine of 96 (72%; no data, n=2) patients had a normal renal function after a median follow-up of 94 months (IQR, 41–159). Three of 96 (3%) patients developed CKD, and 24 of 96 (25%) patients developed ESRD. Median time to ESRD was 36 months (IQR, 16–101); 46 of 47 (98%; no data, n=2) patients with CsA-induced CR preserved a normal renal function compared with nine of 15 (60%) with PR and six of 17 (35%) with NR (Figure 3B). In total, 2% of patients with CR, 33% with of patients PR, and 53% of patients with NR developed ESRD.
RTx.
Of those patients who received an RTx (21 of 96; no data, n=2), eight of 16 (50%) experienced a recurrence of the disease (no data, n=5).
Patients with a Hereditary Background of the Disease
One hundred thirty-one of 231 (57%) patients presented with a genetic background of the disease (Supplemental Table 1). Age at onset was ≤3 months of age in 46% (n=60), 4–12 months of age in 12% (n=16), 1–6 years of age in 27% (n=35), and 7–18 years of age in 15% of patients (n=20).
Data of Patients with CNS with Mutations.
Thirty-eight of 60 (63%) patients received a renal biopsy, with DMS in 40% (n=15), Finnish type in 21% (n=8), FSGS in 21% (n=8), and MCN in 13% (n=5).
Therapy.
The exposure rate to CsA was low at 16% (nine of 56). One patient, affected by compound heterozygous NPHS1 mutations (p.Asp310Asn, c.2816–3T>G), responded to CsA with CR within 2 months and preserved a normal renal function. PR was not observed in any patient, and eight of nine patients did not respond to CsA (NR). Overall, 40 of 52 (77%; no data, n=8) patients received ACEIs and/or ARBs (of these, 32 without concomitant CsA therapy).
Renal Function.
Fifty of 60 (83%) patients with CNS progressed to ESRD, with a median time to ESRD of 24 months (IQR, 7–40). Four of 60 (7%) patients developed CKD, and only six of 60 (10%) patients had a preservation of renal function after a median follow-up of 147 months (IQR, 27–205).
RTx.
Forty-one of 60 patients received an RTx without recurrence of proteinuria within the observation time (no data, n=10).
Data of Patients with SRNS with Mutations.
Median age at onset was 42 months (IQR, 15–96); 65 of 71 patients received a renal biopsy, with FSGS in 66% (n=43), MCN in 17% (n=11), and DMS in 14% (n=9). As expected, no Finnish type histology was observed in this cohort.
Therapy.
Thirty-two of 68 (47%) patients received CsA. Response was CR in one of 32 within 2.5 months (3%), PR in five of 32 (16%) after 1.5, 6, and 8 months (no data, n=2), and NR in 26 of 32 (81%) patients (Figure 3A). Overall, 51 of 64 (80%; no data, n=7) patients received ACEIs and/or ARBs (of these, 23 without CsA).
Renal Function.
Forty-six of 70 (66%; no data, n=1) progressed to ESRD within a median time of 44 months (IQR, 5–76); six of 70 (9%) patients developed CKD, and 18 of 70 (26%) patients had a preservation of renal function after a median follow-up of 115 months (IQR, 77–165). The patient with CsA-induced CR affected by an ACTN4 mutation (p.Arg674Cys) showed a preservation of renal function. Of five patients with PR, three patients had pathogenic mutations in NPHS2 (p.Leu156Phefs*11 [homozygous (H)]; p.Arg138Gln [H]; p.Val290Met, p.Arg138Gln), and two patients had pathogenic mutations in WT1 (p.His465Pro; c.1432+4C>T). Although reduction of proteinuria was observed in these patients, all developed chronic renal insufficiency or ESRD (CKD in one of five and ESRD in four of five after a median time of 72 months [IQR, 30–124]); 74% of 26 genetic patients with NR developed ESRD after a median time of 42 months (IQR, 12–73) (Figure 3B).
Of those patients with ACEI/ARB therapy without concomitant CsA (n=24), 54% developed ESRD (n=13); in patients with ACEI/ARB and CsA therapy (n=28), 71% developed ESRD (n=20).
RTx.
Forty-one of 71 patients received an RTx. Two of 29 patients with SRNS (7%; no data, n=12) experienced recurrence of the disease (affected by NPHS2-p.Arg138Gln [H] and NPHS1-p.Ser220Ala, p.Ala916Ser, respectively).
Immunosuppression of the Total Study Cohort
Exposure rates to CsA differed significantly between patients with genetic and nongenetic CNS/SRNS (33% versus 85%; P<0.001). Whereas most of the patients with nongenetic disease responded to therapy with CR (CR, 60%; PR, 18%), it was rarely observed in the group of patients with genetic disease (CR, 5%; PR, 12%; P<0.001) (Figure 3A).
Renal Function of the Total Study Cohort
Patients with nongenetic disease developed ESRD less frequently than patients with genetic disease (27% versus 74%; P<0.001 and 3% versus 8% CKD; P=0.16). At the end of observation, a normal renal function was preserved in 70% of patients with nongenetic disease compared with 19% with genetic disease (P<0.001; median renal survival, 205 months in patients with nongenetic disease versus 48 months in patients with genetic disease; P<0.001; hazard ratio, 3.48; 95% confidence interval, 2.36 to 5.14); 98% of patients with nongenetic disease and CR after CsA maintained a normal renal function (Figure 3B). No statistically significant sex–specific differences were observed.
Discussion
Recent studies applying next generation sequencing (NGS) techniques have identified single-gene mutations in a significant percentage of patients with CNS and SRNS (13,25,29). However, the available clinical data in these large consortium studies are scarce and heterogeneous, especially with respect to the response to treatment and renal outcome. More importantly, patients with nonhereditary SRNS escape the scientific focus in the era of molecular genetics. Still, their clinical course can be severe, especially if they do not respond to intensified immunosuppression.
Mutation Analyses
In this study, the overall causative MDR was high at 57%. In other studies, MDR in patients with CNS/SRNS was substantially lower, ranging from 19% (13) to 23.6% (25) and 29.5% (29). The strict definition of primary CNS/SRNS, the high quality of clinical data, and the relevant percentage of 27% patients with CNS in our cohort might contribute to these differences. However, even in patients with CNS, the MDR was considerably higher in our study than in former studies (97% versus 66% [25], 69% [29], and 81% [30]). As has been reported by Sadowski et al. (29), the MDR declines with growing age at onset. However, in our study cohort, the MDR was still high at 38% in age group 7–18 years old. The overall MDR might even be higher, because it cannot be excluded that mutations were missed in genes beyond the applied diagnostic algorithm or novel, so far unidentified genes. This holds especially true for two patients with CNS in whom no pathogenic mutations were detected and generally, all patients without response to CsA or without recurrence of the disease after RTx. Of note, 85% of all mutations were identified by the analysis of the genes NPSH1, NPHS2, and WT1. A similar observation has been made by Santín et al. (18), with 89% of all mutations identified in NPSH1, NPHS2, and WT1. All other gene mutations are rare events, affecting per gene only a small number of patients each (in total, amounting to approximately 25% in children with an age at onset >1 year old). This implies that restricted testing of NPHS1, NPHS2, and WT1 can be efficient to diagnose hereditary CNS and early-onset SRNS. This observation is especially important for those countries with limited resources of genetic testing. Genetic panels are useful alternatives, and NGS can provide supplementary data in the remaining unsolved patients.
Immunosuppressive Treatment and Renal Outcome
Response to intensified immunosuppression has been reported to be associated with a favorable outcome in children and adults with SRNS (3,4,31).
In total, 56% of our patients received CsA, with significantly lower treatment rates in genetic than in nongenetic disease (33% versus 85%, respectively). Reasons for this include the high percentage of congenital disease, the knowledge of a hereditary background, and/or the rapid progression to ESRD. A significant benefit of CsA was observed in nongenetic disease (CR, 60%; PR, 18%), and 98% of patients with CsA-induced CR and nongenetic disease maintained a normal renal function. This emphasizes the significance of calcineurin inhibitors (CNIs) in the management of nongenetic SRNS and underscores the importance of genetic testing not only for the identification of genetic disease but also, on the contrary, for the identification of patients with nongenetic SRNS. These patients frequently experience excellent results after CsA treatment, and we believe that this is an important result of this study.
In CsA-induced PR, nine of 15 patients with nongenetic disease maintained a normal renal function in contrast to zero of five patients with genetic disease. The patient numbers are small and without statistical power but indicate that PR in genetic disease might be biologically different from PR in nongenetic disease. Only very scarce data are available on the prognostic value of PR in genetic SRNS/FSGS. Small patient numbers and genetic heterogeneity render prospective studies impossible. In adult patients with FSGS, one large study reported a slower rate of renal function decline and a reduced risk for renal failure in PR compared with NR (32). However, the comparability to our study is limited, because only adult patients were included, CsA treatment rates were low (4%), and genetic testing was not performed. A meta-analysis of all data might be helpful to resolve the effect and prognostic value of PR in genetic SRNS.
Time to Response
Median time to CR to CsA in nongenetic SRNS was 2.5 months, and 82% of patients responded within the first 6 months of therapy. From these data, it can be concluded that patients with nonhereditary SRNS should not be spared from an effective treatment with CNIs. The minimum period of treatment should be at least 6 months. This is in accordance with the Kidney Disease Improving Global Outcomes guidelines (1), and the probability of preserving a good renal function is high in these patients. In patients responding to CsA, the possibility of discontinuation after several years of treatment has been discussed recently (26). In a single-center study, 11 of 15 patients with SRNS showed no relapse after tapering of CsA with a median follow-up of 10 years (26). In nonresponders, long–term CsA therapy should be avoided to minimize renal toxicity and side effects (33). In our cohort, 15% of patients with nongenetic SRNS did not receive an immunosuppressive treatment with CsA after diagnosis of steroid resistance. Reasons for nontreatment were early onset of the disease at <1 year old and/or rapid development of ESRD.
Although being highly effective in nonhereditary SRNS, an intensified immunosuppression with CsA in genetic FSGS/SRNS is not beneficial in most patients and puts the patients at risk. In our study, remission (PR or CRI) to CNI therapy was reported in seven patients with apparently genetic SRNS. Two patients experienced CR to CsA and maintained a normal renal function. Five other patients responded partially to CsA but then, went rapidly into CKD or ESRD (83%). These observations deserve clinical attention, and few other genetic patients with a (partial) remission to intensified immunosuppression have been described in the literature (34,35). Off-target effects of immunosuppressants on the podocyte cytoskeleton (36,37) can be one aspect of improved podocyte function in these patients. It remains unclear why these patients differ from other patients with genetic SRNS. CNI use in rare genetic forms (e.g., ACTN4- or TRPC6-associated nephropathy) has not been systematically analyzed so far, and the available data are scarce. In the future, it will be important to study in more detail the biology, pathophysiology, and drugability of these exceptional patients.
Overall, the renal prognosis was poor in hereditary CNS/SRNS, with ESRD in 74% of patients, irrespective of CsA (76% ESRD without and 71% ESRD with CsA treatment, respectively).
Renal Biopsy
In nongenetic disease, FSGS was the most prevalent histology, diagnosed in 69% of patients followed by MCN in 23% of patients. In hereditary disease, FSGS was identified in 21% and 66% of patients with CNS and patients with SRNS, respectively, and DMS was identified in 40% and 14% of patients with CNS and patients with SRNS, respectively. A percentage of 26.9% DMS has been reported in infants with hereditary SRNS by Sadowski et al. (29), but a much lower percentage in the study by Giglio et al. (20) (11%) in genetic SRNS (excluding patients with CNS) was comparable with our results. We observed Finnish type histology only in patients with genetic disease, all affected by a mutation in NPHS1, whereas Machuca et al. (30) describe Finnish type histology also in two patients with NPHS2 mutations. Summarizing these observations, the value of renal histology with respect to the nature of the disease seems restricted to the specific diagnoses of Finnish type and DMS, which highly suggest a genetic origin. In patients with FSGS or MCN, however, the prognostic value is limited: 57% and 59% were of nongenetic origin and 43% and 41% were of genetic origin in patients with FSGS and patients with MCN, respectively. This highlights the importance of genetic testing in these patients to differentiate between hereditary and nonhereditary disease.
The available data underscore that CNS and SRNS are not homogeneous clinical syndromes but have to be subdivided into distinct clinical entities associated with different outcomes and requiring specific treatment modalities. These observations support the importance of early genetic testing to identify those patients who benefit from an effective CNI treatment. Patients with genetic SRNS require alternative treatment strategies depending on their genetic mutation. Prospective treatment studies are well advised to differentiate between genetic and nongenetic SRNS. The application of NGS–based panel diagnostics will facilitate this task significantly.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the patients and their families for participating in this study and Berthold Hauffa for statistical advice.
This work was supported by the German Society of Pediatric Nephrology, the German Research Association (Deutsche Forschungsgemeinschaft), and the Forschungsunterstützungskreis Kindernephrologie e.V.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07370715/-/DCSupplemental.
References
- 1.Lombel RM, Hodson EM, Gipson DS, Kidney Disease: Improving Global Outcomes : Treatment of steroid-resistant nephrotic syndrome in children: New guidelines from KDIGO. Pediatr Nephrol 28: 409–414, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N: Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int 72: 1429–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North America Nephrotic Syndrome Study Group : A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int 56: 2220–2226, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, Ghio L, Lusvarghi E, Gusmano R, Locatelli F, Pasquali S, Castellani A, Casa-Alberighi OD: A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 43: 1377–1384, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Garin EH, Orak JK, Hiott KL, Sutherland SE: Cyclosporine therapy for steroid-resistant nephrotic syndrome. A controlled study. Am J Dis Child 142: 985–988, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Melocoton TL, Kamil ES, Cohen AH, Fine RN: Long-term cyclosporine A treatment of steroid-resistant and steroid-dependent nephrotic syndrome. Am J Kidney Dis 18: 583–588, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Niaudet P, French Society of Pediatric Nephrology : Treatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. J Pediatr 125: 981–986, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Hino S, Takemura T, Okada M, Murakami K, Yagi K, Fukushima K, Yoshioka K: Follow-up study of children with nephrotic syndrome treated with a long-term moderate dose of cyclosporine. Am J Kidney Dis 31: 932–939, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Smoyer WE, Gregory MJ, Bajwa RS, Johnson KJ, Bunchman TE: Quantitative morphometry of renal biopsies prior to cyclosporine in nephrotic syndrome. Pediatr Nephrol 12: 737–743, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Hamasaki Y, Yoshikawa N, Nakazato H, Sasaki S, Iijima K, Nakanishi K, Matsuyama T, Ishikura K, Ito S, Kaneko T, Honda M, for Japanese Study Group of Renal Disease in Children : Prospective 5-year follow-up of cyclosporine treatment in children with steroid-resistant nephrosis. Pediatr Nephrol 28: 765–771, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Weber S, Gribouval O, Esquivel EL, Morinière V, Tête MJ, Legendre C, Niaudet P, Antignac C: NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66: 571–579, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F, Arbeitsgemeinschaft Für Pädiatrische Nephrologie Study Group : Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732, 2004 [DOI] [PubMed] [Google Scholar]
- 13.McCarthy HJ, Bierzynska A, Wherlock M, Ognjanovic M, Kerecuk L, Hegde S, Feather S, Gilbert RD, Krischock L, Jones C, Sinha MD, Webb NJ, Christian M, Williams MM, Marks S, Koziell A, Welsh GI, Saleem MA, RADAR the UK SRNS Study Group : Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 8: 637–648, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoit G, Machuca E, Nevo F, Gribouval O, Lepage D, Antignac C: Analysis of recessive CD2AP and ACTN4 mutations in steroid-resistant nephrotic syndrome. Pediatr Nephrol 25: 445–451, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Büscher AK, Weber S: Educational paper: The podocytopathies. Eur J Pediatr 171: 1151–1160, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Büscher AK, Kranz B, Büscher R, Hildebrandt F, Dworniczak B, Pennekamp P, Kuwertz-Bröking E, Wingen AM, John U, Kemper M, Monnens L, Hoyer PF, Weber S, Konrad M: Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 5: 2075–2084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Büscher AK, Konrad M, Nagel M, Witzke O, Kribben A, Hoyer PF, Weber S: Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clin Nephrol 78: 47–53, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Santín S, Bullich G, Tazón-Vega B, García-Maset R, Giménez I, Silva I, Ruíz P, Ballarín J, Torra R, Ars E: Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 6: 1139–1148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philippe A, Nevo F, Esquivel EL, Reklaityte D, Gribouval O, Tête MJ, Loirat C, Dantal J, Fischbach M, Pouteil-Noble C, Decramer S, Hoehne M, Benzing T, Charbit M, Niaudet P, Antignac C: Nephrin mutations can cause childhood-onset steroid-resistant nephrotic syndrome. J Am Soc Nephrol 19: 1871–1878, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giglio S, Provenzano A, Mazzinghi B, Becherucci F, Giunti L, Sansavini G, Ravaglia F, Roperto RM, Farsetti S, Benetti E, Rotondi M, Murer L, Lazzeri E, Lasagni L, Materassi M, Romagnani P: Heterogeneous genetic alterations in sporadic nephrotic syndrome associate with resistance to immunosuppression. J Am Soc Nephrol 26: 230–236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiggeri GM, Catarsi P, Scolari F, Caridi G, Bertelli R, Carrea A, Sanna-Cherchi S, Emma F, Allegri L, Cancarini G, Rizzoni GF, Perfumo F: Cyclosporine in patients with steroid-resistant nephrotic syndrome: An open-label, nonrandomized, retrospective study. Clin Ther 26: 1411–1418, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Bullich G, Trujillano D, Santín S, Ossowski S, Mendizábal S, Fraga G, Madrid Á, Ariceta G, Ballarín J, Torra R, Estivill X, Ars E: Targeted next-generation sequencing in steroid-resistant nephrotic syndrome: Mutations in multiple glomerular genes may influence disease severity. Eur J Hum Genet 23: 1192–1199, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovric S, Fang H, Vega-Warner V, Sadowski CE, Gee HY, Halbritter J, Ashraf S, Saisawat P, Soliman NA, Kari JA, Otto EA, Hildebrandt F, Nephrotic Syndrome Study Group : Rapid detection of monogenic causes of childhood-onset steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 9: 1109–1116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehnhardt A, Karnatz C, Ahlenstiel-Grunow T, Benz K, Benz MR, Budde K, Büscher AK, Fehr T, Feldkötter M, Graf N, Höcker B, Jungraithmayr T, Klaus G, Koehler B, Konrad M, Kranz B, Montoya CR, Müller D, Neuhaus TJ, Oh J, Pape L, Pohl M, Royer-Pokora B, Querfeld U, Schneppenheim R, Staude H, Spartà G, Timmermann K, Wilkening F, Wygoda S, Bergmann C, Kemper MJ: Clinical and molecular characterization of patients with heterozygous mutations in wilms tumor suppressor gene 1. Clin J Am Soc Nephrol 10: 825–831, 2015 [DOI] [PMC free article] [PubMed]
- 25.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, Anarat A, Caliskan S, Emma F, Gellermann J, Oh J, Baskin E, Ksiazek J, Remuzzi G, Erdogan O, Akman S, Dusek J, Davitaia T, Özkaya O, Papachristou F, Firszt-Adamczyk A, Urasinski T, Testa S, Krmar RT, Hyla-Klekot L, Pasini A, Özcakar ZB, Sallay P, Cakar N, Galanti M, Terzic J, Aoun B, Caldas Afonso A, Szymanik-Grzelak H, Lipska BS, Schnaidt S, Schaefer F, PodoNet Consortium : Spectrum of steroid-resistant and congenital nephrotic syndrome in children: The PodoNet registry cohort. Clin J Am Soc Nephrol 10: 592–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaassen I, Özgören B, Sadowski CE, Möller K, van Husen M, Lehnhardt A, Timmermann K, Freudenberg F, Helmchen U, Oh J, Kemper MJ: Response to cyclosporine in steroid-resistant nephrotic syndrome: Discontinuation is possible. Pediatr Nephrol 30: 1477–1483, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Ehrich JMM, Brodehl J: Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 152: 357–361, 1993 [DOI] [PubMed] [Google Scholar]
- 28.International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Hildebrandt F, SRNS Study Group : A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 26: 1279–1289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machuca E, Benoit G, Nevo F, Tête MJ, Gribouval O, Pawtowski A, Brandström P, Loirat C, Niaudet P, Gubler MC, Antignac C: Genotype-phenotype correlations in non-Finnish congenital nephrotic syndrome. J Am Soc Nephrol 21: 1209–1217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, Gibson K, Thomas DB: Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol 21: 344–349, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group : Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud I, Basuni F, Sabry A, El-Husseini A, Hassan N, Ahmad NS, Elbaz M, Moustafa F, Sobh M: Single-centre experience with cyclosporin in 106 children with idiopathic focal segmental glomerulosclerosis. Nephrol Dial Transplant 20: 735–742, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Gellermann J, Stefanidis CJ, Mitsioni A, Querfeld U: Successful treatment of steroid-resistant nephrotic syndrome associated with WT1 mutations. Pediatr Nephrol 25: 1285–1289, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Malina M, Cinek O, Janda J, Seeman T: Partial remission with cyclosporine A in a patient with nephrotic syndrome due to NPHS2 mutation. Pediatr Nephrol 24: 2051–2053, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeruschke S, Büscher AK, Oh J, Saleem MA, Hoyer PF, Weber S, Nalbant P: Protective effects of the mTOR inhibitor everolimus on cytoskeletal injury in human podocytes are mediated by RhoA signaling. PLoS One 8: e55980, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.