Abstract
Immunosuppressive agents are commonly used in the nephrologist’s practice in the treatment of autoimmune and immune-mediated diseases and transplantation, and they are investigational in the treatment of AKI and ESRD. Drug development has been rapid over the past decades as mechanisms of the immune response have been better defined both by serendipity (the discovery of agents with immunosuppressive activity that led to greater understanding of the immune response) and through mechanistic study (the study of immune deficiencies and autoimmune diseases and the critical pathways or mutations that contribute to disease). Toxicities of early immunosuppressive agents, such as corticosteroids, azathioprine, and cyclophosphamide, stimulated intense investigation for agents with more specificity and less harmful effects. Because the mechanisms of the immune response were better delineated over the past 30 years, this specialty is now bestowed with a multitude of therapeutic options that have reduced rejection rates and improved graft survival in kidney transplantation, provided alternatives to cytotoxic therapy in immune-mediated diseases, and opened new opportunities for intervention in diseases both common (AKI) and rare (atypical hemolytic syndrome). Rather than summarizing clinical indications and clinical trials for all currently available immunosuppressive medications, the purpose of this review is to place these agents into mechanistic context together with a brief discussion of unique features of development and use that are of interest to the nephrologist.
Keywords: GN, kidney transplantation, immunology, cytokines, cell, activation
Introduction
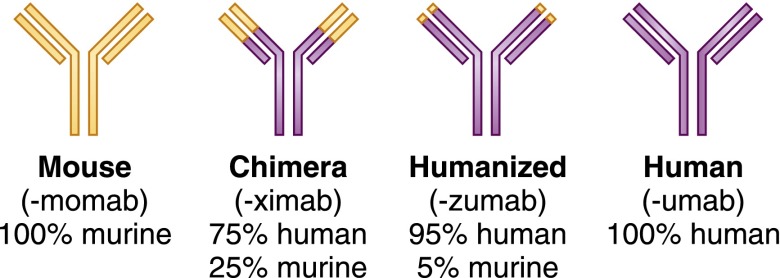
Immunosuppressive agents have a long history, with a recent acceleration in growth in number. After the discovery by Nobel Prize awardee Philip Hench that the corticosteroid cortisone had significant anti-inflammatory effects in patients with rheumatoid arthritis (RA) in 1949 (1) and the independent discoveries by Calne et al. (2), Murray et al. (3), and Zukoski et al. (4) that azathioprine (AZA) was an effective immunosuppressive agent in the prevention of kidney allograft rejection in the early 1960s (2–4), many of the mechanisms of the immune response remained opaque. The 1960s and 1970s were marked by a borrowing of cyclophosphamide from the developing field of cancer chemotherapy for use in immune diseases and transplantation, whereas the use of antilymphocyte serum as a lymphocyte-depleting agent gained favor in the developing field of kidney transplantation. The late 1970s and early 1980s brought revolutionary changes in drug development and discovery; two key developments were the technology to develop monoclonal antibodies (mAbs) for human therapeutic use and the discovery of the immunosuppressive effects of cyclosporin A from fermentation extracts of the fungal species Tolypocladium inflatum (5,6). The 1990s were a period of significant immunosuppressive drug development, because increased insight into B and T cell development, activation, and proliferation, cytokine and chemokine signaling, and complement activation led to targeted therapeutics, particularly mAbs that could later be humanized (Figure 1). In reciprocal fashion, drug discovery often led to further understanding of the mechanisms of the immune response. Similar to cyclosporin A, sirolimus (previously called rapamycin) was discovered and developed as an antifungal, but it was found to have antineoplastic and immunosuppressive properties, the mechanisms of which were only later appreciated and described as mammalian target of rapamycin (mTOR) pathways (7,8).
Figure 1.
Schematic representation and nomenclature of mAbs in clinical use. The suffix denotes of the degree of human versus nonhuman components.
In recent decades, immunosuppressive drug development has slowed from its accelerated pace in the late 1990s, but it still shows steady growth. With improvements in efficacy and specificity of existing agents, it is increasingly difficult to develop an agent that meets superiority and safety measures necessary to gain regulatory and public opinion approval. This is particularly true for diseases that the nephrologist may encounter: most uses of immunosuppressive agents are in rare, orphan category diseases that are difficult or unlikely to be studied in large multicenter trials. Thus, many of the newer agents that the nephrologist may encounter will inevitably be in off-label use stemming from experience in other fields, such as rheumatology and oncology. Exceptions to this generalization are emerging attempts to treat the inflammation identified in the settings of AKI and maintenance hemodialysis.
To provide a framework for understanding the multitude of immunosuppressive agents currently available and in late-stage development, this review will summarize key agents commonly encountered in nephrology practice by immune cell target rather than disease state or clinical indication. Together with the previous reviews within this Renal Immunology Series, it is hoped that the reader will be able to intertwine the science with its clinical applications.
T Cell–Directed Therapy
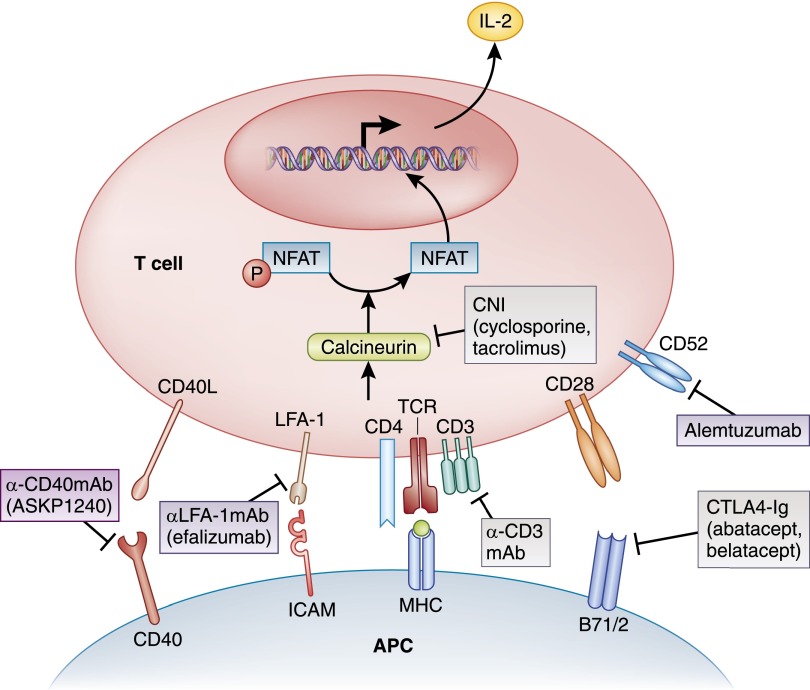
Therapeutic agents that target T cell function can be separated into those that inhibit signal 1 (the interaction of the T cell receptor [TCR] complex with an antigen-presenting cell [APC] either carrying antigen or in the case of transplantation, acting as antigen itself) and its resulting intracellular signaling and those that inhibit signal 2 (the costimulatory signal provided by additional T cell/APC interaction that leads to full activation of the T cell) (Figure 2). Agents that inhibit further downstream activation and proliferation (occasionally referred to as signal 3) are typically driven by cytokine production and signaling and will be discussed in later sections.
Figure 2.
Immunosuppressive agents targeting T cell/antigen-presenting cell interaction and early T cell activation. This depicts agents that inhibit signal 1 and signal 2 in T cell activation. APC, antigen-presenting cell; CNI, calcineurin inhibitor; CTLA4, cytotoxic T lymphocyte–associated protein 4; ICAM, intracellular adhesion molecule 1; LFA-1, lymphocyte function–associated antigen-1; NFAT, nuclear factor of activated T cells; TCR, T cell receptor.
Agents Targeting Signal 1
Anti-TCR Agents.
Inhibition of the first point of antigen presentation (the MHC/TCR complex) has been an attractive target in transplant immunosuppression. The murine anti-CD3 mAb Muromonab-CD3 (OKT3) was the first mAb approved as a drug for human use in 1986 for the prevention of rejection in renal, heart, and liver transplants (9). It targeted the CD3 subunit of the TCR complex and led to rapid elimination of functional T cells. It is now no longer in production because of waning utilization, primarily because of significant side effects related to the mitogenicity associated with its murine source. This early experience led to the development of humanized forms of anti-TCR–based agents in an effort to reduce this mitogenicity as well as other anti-TCR mAbs that targeted other receptor subunits (10–12). These next generation therapeutics were subsequently forwarded for the treatment of new-onset diabetes and as induction agents in kidney transplantation but have been hindered by ongoing safety and efficacy issues.
Calcineurin Inhibitors (Cyclosporin and Tacrolimus).
After initial TCR binding, a calcineurin-dependent signaling pathway is induced that leads to initial T cell gene transcription necessary for additional activation. Two commonly used calcineurin inhibitors (CNIs; cyclosporin and tacrolimus) and one investigational agent (voclosporin) inhibit the ability of calcineurin to dephosphorylate nuclear factor (NF) of activated T cells (NFAT), required for translocation from cytoplasm to nucleus, and prevent calcineurin-dependent gene transcription (13,14). In the early 1980s, cyclosporin transformed the field of transplantation with dramatic reductions in acute rejection rates, and it has been shown to be effective in a number of immune diseases, including a number of glomerulopathies (15,16). In the late 1990s, tacrolimus was introduced in kidney transplantation and over time, has shown to be more potent in reducing the rates of acute rejection (17,18). Growing experience in glomerular disease suggests its use to be of similar value as cyclosporin (19). One potential nonimmunosuppressive mechanism that could explain CNI efficacy in glomerular disease is the ability to inhibit synaptopodin degradation in the podocyte, thereby stabilizing the actin cytoskeleton and reducing proteinuria (20).
Tacrolimus and cyclosporin share the side effect of CNI-induced vascular constriction that contributes to an increase in BP as well as diminished renal perfusion. This is primarily a dose-dependent phenomenon; it can result in renal ischemia and acute tubular necrosis in the acute setting, and with prolonged ischemia, it can result in chronic kidney injury. Aside from the direct vascular effect on renal blood flow, the potential direct nephrotoxic effects of CNI agents remain an active area of debate and research (21). To address these issues of toxicity and side effect profiles (including post-transplant diabetes), alternative formulations of tacrolimus (extended release) as well as the novel CNI voclosporin have been developed and are approved or in late-phase clinical trials in transplantation (22–24).
Agents Targeting Signal 2
Costimulation Blockade by CD80/86:CD28 Targeting (Abatacept and Belatacept).
The interaction of CD80/86 on the APC with CD28 on the T cell (costimulation) is required for optimal T cell activation. After upregulation and the generation of an effective immune response, the T cell expresses the cell surface molecule cytotoxic T lymphocyte–associated protein 4 (CTLA4), which competitively binds to CD80/86 and downregulates the T cell response. To mimic this downregulatory effect, human IgG heavy chains were linked with CTLA4 to create a fusion protein for clinical use. The first generation CTLA4-Ig that was clinically developed, abatacept, is approved for the treatment of RA and is under investigation in other autoimmune diseases, including lupus (25). Recently, abatacept has been proposed to be of potential value in the treatment of FSGS in a small series of cases, in which immunostaining of podocytes is positive for CD80 (B7–1) (26). Abatacept was ineffective in preclinical primate studies in the prevention of kidney transplant rejection, leading to development of another CTLA4-Ig with significantly higher affinity for CD80/86 for transplantation (belatacept) (27). This agent is Food and Drug Administration (FDA) indicated for use as a substitute for CNIs at the time of transplant (28).
Costimulation Blockade by CD154:CD40 Targeting (Anti-CD40 mAb).
The CD154 (also known as CD40L; present on activated T cells): CD40 (on APCs) interaction is a critical step in T cell costimulatory signaling, because this interaction leads to the upregulation of CD80/86 on APCs. Targeting the induced surface molecule CD154 on activated T cells was a focus of drug development until it was recognized that CD154 was also present on platelets, and agents binding this cell surface molecule led to an increase in thrombotic events in both primate and early-phase human trials (29). Attention has, thus, turned to targeting CD40. A number of mAbs against CD40 are in development, with a fully human anti-CD40 (ASKP1240; Astellas) under study in phase 2 clinical trials in kidney transplantation (30).
B Cell–Directed Therapy
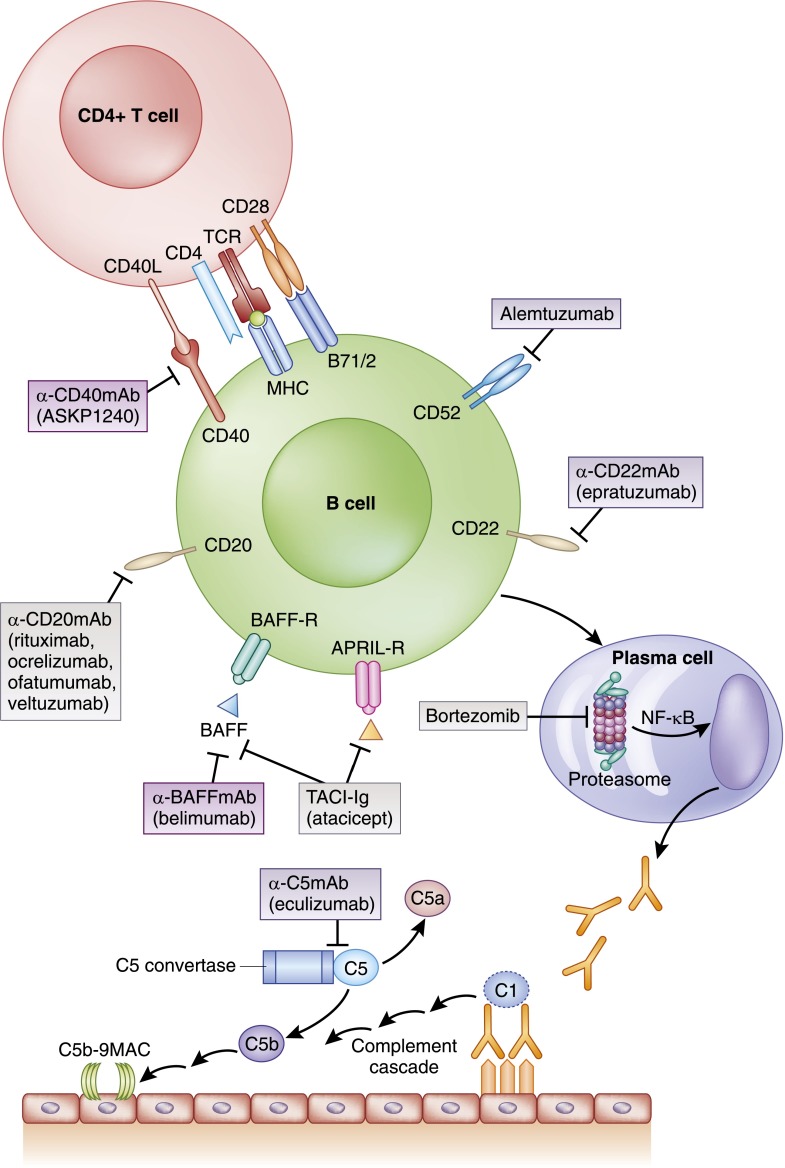
The goals of B cell inhibition include inhibiting not only the humoral response to auto- or alloantigen but also, the APC function and B/T cell interactions that lead to efficient T cell activation and proliferation. B cell therapies can be considered in the context of the agents that inhibit maturation and differentiation of the resting B cell throughout its development to a highly active antibody-producing plasma cell (Figure 3).
Figure 3.
Immunosuppressive agents targeting T cell/B cell interaction, plasma cell function, and complement-mediated injury. APRIL, proliferation-inducing ligand; BAFF, B cell–activating factor; MAC, membrane attack complex; TACI, transmembrane activator and CAML interactor; TCR, T cell receptor.
B Cell Targeting
Anti-CD20 Targeting: Rituximab, Ocrelizumab, Ofatumumab, and Veltuzumab.
CD20 is a transmembrane protein present on pre-B and mature B lymphocytes, but it is not present on stem cells, normal plasma cells, or other cell lines. Its role in B cell development includes regulation of activation for cell cycling and B cell differentiation. The first agent to target CD20, rituximab, is a chimeric anti-CD20 mAb (30% murine and 70% human) that leads to B cell depletion through a number of mechanisms, including complement-dependent cytotoxicity, growth arrest, and apoptosis (31). This depletion is durable, with B cell counts suppressed for up to 6–9 months and occasionally, beyond. Efficacy data pertinent to nephrology practice include the treatment of ANCA-associated vasculitis, with supportive data in antibody-mediated rejection and forms of nephrotic syndrome (32–34). The chimeric nature of the antibody leads to side effects attributable to cytokine release, such as fever, bronchospasm, and hypotension. Agents that are humanized (ocrelizumab) or fully humanized (ofatumumab) have been developed to minimize these untoward infusion reactions. However, ocrelizumab development in RA has been discontinued because of an increased risk of serious infections (35). A phase 1/2 trial of ofatumumab in RA has shown preliminary efficacy, with mild/moderate infusion reactions still prevalent (36). All anti-CD20 therapy carries a risk of hepatitis B reactivation in patients positive for hepatitis B surface antigen or hepatitis B core antibody (37). Therefore, before starting treatment, patients should be screened for hepatitis B surface antigen and hepatitis B core antibody.
Anti-CD22 Targeting: Epratuzumab.
CD22 is expressed on B cells during B cell maturation and loss of CD20 expression. B cell receptor signaling is modulated by phosphorylation of CD22, which regulates B cell activation. Epratuzumab is a humanized anti-CD22 mAb that inhibits B cell activation and has a more modest depleting effect on B cells than rituximab. It is currently in phase 3 trials in patients with moderate to severe SLE after phase 2 trials suggested a low rate of adverse events, similar to placebo (38,39).
Targeting B Cell Differentiation: Belimumab and Atacicept
A key pathway for differentiation of B cells is the binding of the cytokine B cell–activating factor (BAFF; also referred to BlyS) to its B cell receptors [(BAFF-R, B cell maturation (BCMA), and transmembrane activator and CAML interactor (TACI)] and the binding of the cytokine proliferation–inducing ligand to its B cell receptors (BCMA and TACI). These interactions lead to increases in NF-κβ, which in turn, promote B cell differentiation and inhibit apoptosis. Belimumab is a humanized anti-BAFF/BlyS mAb that interferes with ligand/receptor binding and inhibits this maturation. It is currently approved for use in active SLE (40). However, patients with severe active lupus nephritis were excluded from the pivotal trials, and post hoc analyses of renal outcomes were inconclusive in showing improvement in clinically relevant renal outcomes (41). Atacicept is a recombinant fusion protein that inhibits both BlyS and proliferation-inducing ligand. In phase 2 trials in RA, efficacy was not shown (42), whereas in a phase 2/3 trial in patients with lupus nephritis, the trial was terminated after pronounced reduction in Ig levels and the occurrence of severe pneumonia, leaving in question the further development of this agent (43).
Plasma Cell Targeting: Bortezomib
All B cell agents previously described have no direct activity against plasma cells, and thus, for diseases in which plasma cell maturation and antibody production are felt to be a primary pathogenic mechanism, these previous agents are expected to have limited efficacy. Critical to the function of highly metabolic cells, such as plasma cells, is the ability to regulate the degradation of proteins through the proteasome, which is present in all eukaryotic cells. Inhibition of the proteasome leads to inhibition of cell cycling and induction of apoptosis (44). Bortezomib is a proteasome inhibitor that was found to be particularly effective in treatment of the plasma cell dyscrasia multiple myeloma and indicated by the FDA for the treatment of advanced myeloma in 2003 (45). Subsequent studies showed a benefit in myeloma with renal involvement and antibody-mediated rejection of kidney allografts by targeting antibody-producing plasma cells (46,47). Side effects of peripheral neuropathy, cytopenias, and gastrointestinal effects occur in a dose-dependent fashion.
Complement Inhibition (Eculizumab)
The role of complement in renal disease is increasingly appreciated and contributes to disease by either direct activation of complement or initial antibody fixation and subsequent activation of complement. There is direct evidence of its role in the thrombotic microangiopathic changes seen in atypical hemolytic uremic syndrome (aHUS), antibody-mediated injury of the kidney allograft, and C3 GN (previously called dense deposit disease or membranoproliferative GN type 2) (48–50). To date, one agent is available for clinical use. Eculizumab is a humanized mAb to C5 that effectively inhibits its cleavage to C5a and C5b. Because C5a is a neutrophil chemoattractant and because C5b is required to form the C5b-9 membrane attack complex, inhibition of this enzymatic step results in blockade of proinflammatory, prothrombotic, and lytic functions of complement (Figure 3). Its efficacy is most apparent for cases of aHUS in which a complement factor mutation has been identified. However, therapy is recommended in all patients with aHUS who are at risk for (or suffering from) renal failure given the potential of an unidentified complement mutation as a contributing factor (51,52). Although data regarding the use of eculizumab for the treatment of antibody-mediated rejection are currently at the level of case reports, its use pretransplant for the prevention of antibody-mediated rejection as part of a desensitization protocol has been shown to be of benefit (53). The cost of eculizumab remains a significant barrier to use. Inhibition of the complement cascade increases the risk of serious infection from encapsulated bacteria; thus, vaccination for Neisseria meningitis, Streptococcus pneumonia, and Haemophilus influenza type b should be performed before therapy.
Additional indications for complement inhibition may be in the treatment or prevention of ischemia/reperfusion injury (AKI in the native kidney and delayed graft function in the transplanted kidney) (54,55). A multicenter trial investigating the use of eculizumab in the prevention of delayed graft function is underway, and numerous compounds targeting the complement pathway are in preclinical investigation.
Agents Targeting Cytokines
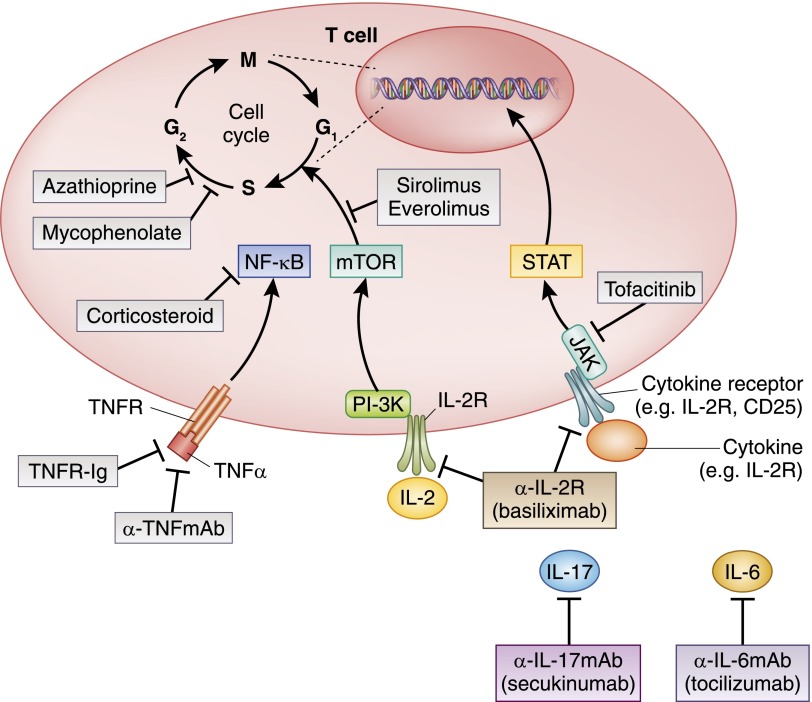
Cytokines are proteins that are secreted by a variety of cell types and function to direct the initiation, differentiation, and up- and downregulation of the immune response. Pharmacologic targeting of specific cytokines is expected to redirect or inhibit an untoward immune response (Figure 4).
Figure 4.
Immunosuppressive agents targeting later stages of T cell differentiation and proliferation and selected cytokines. This depicts agents that inhibit signal 3, T cell proliferation. JAK, Janus kinase; mTOR, mammalian target of rapamycin; PI-3K, phosphoinositide 3-kinase; R, receptor; STAT, signal transducer and activator of transcription.
Nonspecific Cytokine Inhibition
Corticosteroids.
Corticosteroids bind to the intracellular glucocorticoid receptor and modulate a multitude of cellular functions by binding to glucocorticoid-responsive elements in the nucleus. Effects on the immune system are also numerous but most clearly related to inhibition of all cytokine transcription by blocking transcription factors, such as NF-κβ and activator protein-1 (56). This has numerous downstream effects, such as (1) depletion of T cells because of inhibition of IL-2, inhibition of Th1 differentiation, and induction of apoptosis, (2) eosinophil apoptosis (either directly or by inhibition of IL-5), and (3) macrophage dysfunction because of inhibition of IL-1 and TNF-α. Its effect on neutrophil function is modest; however, neutrophil migration to sites of inflammation is impaired, bone marrow secretion of neutrophils is increased, and apoptosis is decreased, all of which contribute to leukocytosis (57). Similarly, B cells are not significantly inhibited by corticosteroids, with only mild decreases in Ig production. Its side effect profile is well appreciated clinically and frequently maligned as a chronic therapy (58). Thus, despite its efficacy in a wide range of immune and inflammatory conditions, a primary focus of many clinical development programs and clinical trials is to find agents with similar efficacy but greater specificity in immunosuppression without the attendant side effects of corticosteroids.
Janus Kinase Inhibition (Tofacitinib).
Janus kinases are cytoplasmic tyrosine kinases that mediate signaling from cytokine receptors to phosphorylation of signal transducers and activators of transcription, enabling signal transducers and activators of transcription to enter the nucleus and regulate gene expression and transcription. An inhibitor of Janus kinase, tofacitinib, inhibits cytokine receptor signaling from a number of cytokines, including IL-2, -4, -7, -9, -15, and -21, and has shown efficacy in psoriatic arthritis and RA (59). Its development as an alternative for CNI in kidney transplantation was halted after a phase 2b trial showed similar rejection rates, better GFR, lower rates of post-transplant diabetes, and higher rates of cytomegalovirus and BK virus infection and post-transplant lymphoproliferative disease (60).
Specific Cytokine Inhibition
IL-2 Receptor Antagonist (Basiliximab).
Activated T cells produce IL-2 and express the α-subunit of the IL-2 receptor, rendering it fully functional. After T cells become activated in response to signals 1 and 2 activation, IL-2 binding and subsequent intracellular signaling lead to proliferation of T cells. Humanized antibodies to the α-subunit of the IL-2 receptor (IL-2 receptor antagonists basiliximab and daclizumab) limit proliferation of activated T cells and have been approved for the prevention of acute rejection in kidney transplantation (the latter is no longer in production), primarily in patients with lower immunologic risk (61).
Targeting TNF-α.
TNF-α is an acute-phase cytokine released by macrophages, T cells, B cells, neutrophils, natural killer cells, mast cells, and some nonimmune cell types (smooth muscle and epithelial cells) in response to tissue injury (62). Well described roles for TNF-α include its release by Th1 T cells to promote ongoing expansion of Th1 cells and proinflammatory responses and its release by synovial macrophages to induce synovial cells to increase collagenase production, thus promoting bone and joint destruction. TNF-α binds to its receptors (TNF receptor 1 [TNFR1] and TNFR2) and stimulates apoptotic pathways as well as NF-κβ signaling, which partially explains its diverse physiologic effects. Currently, there are five TNF inhibitors clinically available and indicated for the treatment of a variety of rheumatic diseases. Infliximab, adalimumab, golimumab, and certolizumab are mAbs to TNF-α that differ in the degree of chimerism and route of administration (intravenous versus subcutaneous) (63). Etanercept is a TNFR fusion protein bound to IgG. All carry the risk of increased susceptibility to intracellular pathogens, such as tuberculosis, coccidiomycosis, and Cryptococcus (64).
IL-1 Inhibition (Anikinra, Rilonacept, and Canakinumab).
IL-1 is a signature proinflammatory cytokine and a primary effector of many inflammatory conditions, including RA and adult-onset Still’s disease. Two isoforms have been identified: IL-1α and IL-1β. IL-1α is constitutively expressed, present intracellularly in vascular endothelium, mucosal epithelium, keratinocytes, liver, lung, and kidney, and released on cell necrosis (e.g., from injury/ischemia). IL-1β is induced by macrophage/monocytes in response to TNF and other proinflammatory cytokines (65). Inhibition of IL-1 has been an attractive target not only for states of dysregulated inflammation but also, in the mitigation of injury in response to ischemic events, including postmyocardial infarction. At present, three agents are clinically available: anikinra (an IL-1 receptor antagonist), rilonacept (a soluble decoy receptor), and canakinumab (an anti–IL-1β mAb). Of interest, IL-1β is elevated in patients on maintenance hemodialysis and may contribute to the chronic inflammation noted in this population. Preliminary studies have examined the feasibility of targeted inhibition of IL-1 in patients on chronic hemodialysis, with additional studies ongoing (66).
IL-6 Inhibition (Tocilizumab).
IL-6 is expressed in response to inflammatory stimuli and contributes to CD8 T cell differentiation, B cell differentiation, and activation of the hepatic acute-phase response. Increased circulating IL-6 has been associated with mortality in AKI and ESRD, malnutrition in ESRD, and rejection in recipients of kidney transplants (67). The humanized mAb tocilizumab is an inhibitor of IL-6 and has shown efficacy in RA (68). A phase 1/2 study in highly sensitized patients awaiting kidney transplantation (NCT01594424) is currently evaluating safety and effect on donor-specific anti-HLA antibodies, with other kidney transplant studies planned (NCT02108600), but currently, no studies in native acute or chronic renal disease are forthcoming.
IL-17 Inhibition (Secukinumab).
IL-17 is a cytokine produced by CD4 T cells, but it is also secreted by CD8 T cells, eosinophils, monocytes, and neutrophils. IL-17 functions to increase inflammatory cell migration by stimulating chemokine release, and it increases APC activity to enhance adaptive immune responses. IL-17 has been shown to be a key mediator of injury in a number of autoimmune diseases, including ankylosing spondylitis, psoriasis, and multiple sclerosis (MS). A human anti–IL-17A mAb (secukinumab) has been developed for clinical use, and recently, two phase 3 trials in psoriasis now show greater efficacy compared with placebo or the TNF-α inhibitor etanercept (69).
Agents Targeting Chemokines and Cell Adhesion
At present, there are a handful of agents that target specific chemokines or their receptors that have been approved for clinical use, although a multitude of others have been tested in early clinical development (70). Difficulties in successful drug development targeting chemokines can be attributed to poorly predictive preclinical models, a redundancy in chemokine signaling that circumvents targeted therapy, and an incomplete understanding of the signals that up- or downregulate chemokines that can occasionally appear paradoxical. Those agents that have found clinical applicability reflect this diversity. For example, approved chemokine receptor antagonists include the CCR5 receptor antagonist maraviroc used in the treatment of HIV, the CXCR4 antagonist plerixafor approved for hematopoietic stem cell mobilization, and the CCR4 humanized mAb mogamulizumab approved for the treatment of T cell lymphoma. Relevant to nephrology practice, emapticap is an inhibitor to CCL2 (also known as monocyte chemotactic protein 1), which has been studied in phase 1 and 2 trials in diabetic nephropathy, with proof-of-concept studies showing a reduction in albuminuria presumably by inhibiting inflammatory cell migration into the kidney (71).
Antibodies targeting adhesion molecules have had a circuitous and tenuous route to clinical use. The agent FTY720 (fingolimod) is a sphingosine 1-phosphate (S1P) receptor modulator that binds to S1P receptors on lymphocytes, preventing lymphocyte migration from lymph node to the vasculature. Clinical trials in kidney transplantation were halted after no improvement over mycophenolate was noted together with untoward side effects of prolonged QT interval, bradycardia (caused by S1P receptor binding on cardiomyocytes), and macular edema. However, S1P receptors are present on neural cells and seem to be critical in neural cell migration during central inflammation in MS; fingolimod has now gained approval for the treatment of relapsing/remitting MS (72). Similarly, efalizumab is a humanized mAb to leukocyte function–associated antigen-1 that prevents lymphocyte activation and cell migration from the vasculature into tissues. It had been shown to be effective in moderate/severe plaque psoriasis, islet transplantation, and kidney transplant, but it has been withdrawn from the United States market after high rates of post-transplant lymphoproliferative disease and brain infections, including progressive multifocal leukoencephalopathy, were reported (73,74). Finally, natalizumab, a humanized mAb against the adhesion molecule α-4 integrin, blocks lymphocyte migration from vasculature to tissues and was approved by the FDA in 2004 for MS and later, Crohn’s disease; however, similar to efalizumab, there have been concerns regarding serious infections, such as progressive multifocal leukoencephalopathy, and it has been withdrawn and returned to the market, with strict monitoring programs in place (75).
Pooled Polyclonal Antibodies as Immunosuppressive Agents
Immune Globulins
Intravenous Ig.
Intravenous Ig (IVIG) is an Ig extract pooled from several thousand plasma donors to create a product that is IgG rich. Although IVIG was initially used to provide passive immunity in patients with immune deficiencies (with doses of 500 mg/kg monthly), ongoing experience and research suggest a very diverse immunomodulatory and anti-inflammatory role of IVIG therapy noted with high-dose therapy (1–2 g/kg) (76). Although the mechanisms underlying these effects are broad and may differ in each disease state in which a benefit has been reported, a few common mechanisms often cited include (1) direct binding to natural antibodies, immunomodulatory proteins (e.g., cytokines), or superantigens and pathogens, (2) inhibition of complement fixation on target tissues by acting as a complement sink, (3) Fc receptor (FcR) binding and subsequent inhibition of the FcR-mediated recycling of native IgG, and (4) stimulation of FcR-induced anti-inflammatory pathways. Its use in the nephrology specialties is primarily in the setting of kidney transplant for desensitization (inhibition and elimination of preformed HLA or blood group (ABO) antibodies to achieve a negative cross-match and permit transplant) and treatment of antibody-mediated rejection (77). Although effective as monotherapy, the addition of other modalities, including plasmapheresis, rituximab, and bortezomib, provides greater immunomodulatory effects and improved clinical outcomes (78). There are different products available that differ in their concentration of IgG, stabilizers, osmolality, and IgA content. The latter is important in that rare patients who suffer from severe IgA deficiency may produce anti-IgA antibodies and suffer anaphylactic reactions when receiving IVIG products. Side effects of IVIG include infusion-related effects (including hives, fever, and anaphylactoid reactions), headaches (including aseptic meningitis), thrombotic complications (including myocardial infarction), and AKI (predominantly seen with sucrose-containing preparations) (79).
Polyclonal Antithymocyte Globulin.
Therapeutic antibodies to human lymphocyte antigens have been created by a number of techniques: by immunizing rabbits with human thymocytes (Thymoglobulin), immunizing horses with human thymocytes (Atgam), or immunizing rabbits with lymphocytes from a Jurkat T cell leukemia line (Fresenius antithymocyte globulin [ATG]). The resulting IgG fraction from sera is then purified and pasteurized for use. The resulting antibodies are polyclonal (i.e., all cell surface molecules presented on the infused thymocytes may lead to a humoral response in the immunized source, and the final preparation contains a vast array of diverse antibodies to various antigens). Although the Igs in these cases are anti–T cell predominant, there are many shared cell surface antigens among T cells and other immune cells; thus, the ATG products also have activity against B cells, monocytes, and neutrophils to lesser degrees. The primary mechanism of action of ATGs is lymphocyte depletion, predominantly by complement-dependent lysis and T cell activation–induced apoptosis (80). The two rabbit ATG products are most widely used at present for the treatment and prevention of acute kidney allograft rejection (81). When compiling small head-to-head trials of Thymoglobulin versus ATG Fresenius, Thymoglobulin may be considered more potent in terms of both efficacy and untoward effects (82). All ATG products, as nonhumanized Igs, are prone to symptoms consistent with cytokine release (fever, chills, hypotension, and pulmonary edema) related to natural killer cell and macrophage/monocyte binding of FcR binding as well as cellular cytotoxicity (83).
Immunosuppressive Agents with Multiple Cellular Targets
Panlymphocyte Depleting Agents: Anti-CD52 (Alemtuzumab)
Anti-CD52 (Campath 1H and alemtuzumab) is a humanized mAb that binds to CD52, an antigen of unclear physiologic significance that is present on both B and T cells. Ligation of CD52 results in depletion of both lymphoid cell lines. Its ability to induce prolonged, significant lymphopenia for up to 6–12 months after dosing led to its use in refractory chronic lymphocytic leukemia (84). As a humanized antibody, fewer infusion-related side effects are noted than with other depleting agents, such as ATG. In kidney transplantation, growth in off-label use as an induction agent had grown over the last decade but recently, was abruptly diminished subsequent to manufacturer removal from the United States market in preparation for relabeling for use in MS (85). Kidney transplant trials suggest equivalence to other depleting agents in the prevention of rejection and efficacy in corticosteroid-withdrawal regimens, but the long-term effect of prolonged lymphopenia on the risk for infection or post-transplant lymphoproliferative disorder is not determined (86,87).
Antiproliferative Agents: mTOR Inhibitors (Sirolimus and Everolimus)
In lymphoid cells, the mTOR pathway leads to cell cycle progression from G1 to S phase and proliferation in response to cytokine stimulation, including but not limited to IL-2 receptor binding (Figure 4). Inhibitors of mTOR that are clinically available include sirolimus, everolimus, and temsirolimus; the primary immunosuppressive mechanism of action of these agents has been attributed largely to inhibition of lymphocyte proliferation (7). However, mTOR signaling is not isolated to lymphocytes, and this intracellular signaling pathway has been described in monocytes/macrophages, dendritic cells, natural killer cells, and endothelial cells (8). Thus, inhibition of mTOR may be expected to lead to a number of clinically relevant effects related to its antiproliferative, antiviral, anti-inflammatory, and antitumor effects as well as a diverse side effect profile (88). mTOR inhibitors have been evaluated for their ability to inhibit cyst growth in autosomal dominant polycystic kidney disease, with conflicting and modest results in large multicenter trials (89,90), have been effective in reducing intimal proliferation and obliterative vasculopathy in heart transplantation (91), have shown efficacy in the treatment of angiomyolipomas (92), and have been approved for use in the treatment of advanced renal cell, breast, and other malignancies (93,94).
Antimetabolites: Inhibition of DNA Synthesis
AZA.
AZA is an analog of 6-mercaptopurine; the metabolites of these agents act as both purine analogs (interfering with de novo purine synthesis and thus, DNA and RNA synthesis) and immunomodulatory agents (contributing to S-G2 cell cycle arrest in addition to other anti-inflammatory effects) (95). Toxicities that are often noted include bone marrow suppression and gastrointestinal intolerance (primarily upper gastrointestinal symptomatology). The xanthine oxidase inhibitors allopurinol and febuxostat slow elimination of 6-mercaptopurine and exacerbate the risk of these side effects (96). Use of AZA has dramatically decreased in kidney transplantation and rheumatologic diseases with the introduction of mycophenolate (discussed below), except in the setting of pregnancy planning. AZA has not been associated with teratogenicity, unlike mycophenolate (97).
Mycophenolate.
Mycophenolate is an inhibitor of IMPDH, the rate-limiting enzyme of guanine nucleotide synthesis critical for de novo purine synthesis and thus, DNA synthesis. T cells (and B cells) are dependent on the de novo pathway for DNA synthesis. Similar to AZA, primary side effects are gastrointestinal and hematopoetic. Its efficacy in the prevention of rejection compared with AZA together with better tolerability than mTOR inhibitors have led to its use as the primary antimetabolite in transplantation, despite a lack of definitive long-term data showing improved graft outcomes. Its efficacy in autoimmune diseases and other glomerular diseases is increasingly appreciated (98,99). Therapeutic drug monitoring has not revealed a clear relationship between mycophenolate exposure and prevention of rejection in recipients of kidney transplants or clinical efficacy parameters in rheumatologic diseases (100). Two formulations are available, mycophenolate mofetil and enteric-coated mycophenolate sodium, with generic formulations available for both. Despite generic availability, cost still is significantly greater than AZA.
Leflunomide.
Leflunomide is a pyrimidine antagonist that blocks DNA synthesis and cell cycling from S to G2 phase. Its specific mechanism of action entails inhibition of the key rate–limiting enzyme dihydro-orotate dehydrogenase critical for de novo pyrimidine synthesis (101). It is approved for use in RA, but its in vitro activity against cytomegalovirus and BK virus has prompted its off-label use in kidney transplantation for its potential dual antiviral and anti-inflammatory properties (102). A very long half-life (>14 days), hepatic and bone marrow toxicities, and a lack of compelling data supporting an advantage over reduction in immunosuppression alone in the clinical management of BK virus have reduced interest in leflunomide for this purpose, but it still is used off label in circumstances of drug-resistant cytomegalovirus infection (103).
Cytotoxic Agents (Cyclophosphamide) as Immunosuppressive Agents
A brief mention of cyclophosphamide is necessary given its use as an immunosuppressant in life-threatening or severe rheumatologic and renal diseases, including ANCA-related vasculitis, lupus nephritis, and other systemic vasculidites. Cyclophosphamide is an alkylating agent that is toxic to all human cells to differing degrees, with hematopoetic cells forming a particularly sensitive target (104). Primary toxicities, such as bladder toxicity, gonadal toxicity, and later malignancy, have led to attempts to minimize exposure (<250–300 mg/kg cumulative dose to avoid gonadal toxicity and <360 mg/kg cumulative dose to minimize the risk of malignancy), attempts to use intermittent intravenous rather than daily oral therapy to minimize exposure, and search for alternative agents (for example, mycophenolate mofetil in lupus nephritis and rituxan in ANCA-related vasculitis) (105–107).
Disclosures
A.C.W. has served as a consultant for Astellas, Tolera, and Veloxis and currently receives research/grant support from Alexion, Bristol Meyer Squibb, and Novartis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hench PS, Kendall EC, Slocumb CH, Polley HF: The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Ann Rheum Dis 8: 97–104, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calne RY, Alexandre GP, Murray JE: A study of the effects of drugs in prolonging survival of homologous renal transplants in dogs. Ann N Y Acad Sci 99: 743–761, 1962 [DOI] [PubMed] [Google Scholar]
- 3.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ: Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med 268: 1315–1323, 1963 [DOI] [PubMed] [Google Scholar]
- 4.Zukoski CF, Lee HM, Hume DM: The prolongation of functional survival of canine renal homografts by 6-mercaptopurine. Surg Forum 11: 470–472, 1960 [PubMed] [Google Scholar]
- 5.Köhler G, Milstein C: Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495–497, 1975 [DOI] [PubMed] [Google Scholar]
- 6.Borel JF: History of the discovery of cyclosporin and of its early pharmacological development. Wien Klin Wochenschr 114: 433–437, 2002 [PubMed] [Google Scholar]
- 7.Shimobayashi M, Hall MN: Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 15: 155–162, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Ferrer IR, Araki K, Ford ML: Paradoxical aspects of rapamycin immunobiology in transplantation. Am J Transplant 11: 654–659, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortho Multicenter Transplant Study Group: A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. N Engl J Med 313: 337–342, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA: Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346: 1692–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352: 2598–2608, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Flechner SM, Mulgoankar S, Melton LB, Waid TH, Agarwal A, Miller SD, Fokta F, Getts MT, Frederick TJ, Herrman JJ, Puisis JP, O’Toole L, Sung R, Shihab F, Wiseman AC, Getts DR: First-in-human study of the safety and efficacy of TOL101 induction to prevent kidney transplant rejection. Am J Transplant 14: 1346–1355, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble S, Markham A: Cyclosporin. A review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (Neoral). Drugs 50: 924–941, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Peters DH, Fitton A, Plosker GL, Faulds D: Tacrolimus. A review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs 46: 746–794, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL; North America Nephrotic Syndrome Study Group: Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484–1490, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Braun N, Schmutzler F, Lange C, Perna A, Remuzzi G, Risler T, Willis NS: Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev (3): CD003233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS: A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 63: 977–983, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study: Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Praga M, Barrio V, Juárez GF, Luño J; Grupo Español de Estudio de la Nefropatía Membranosa: Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int 71: 924–930, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naesens M, Kuypers DR, Sarwal M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Busque S, Cantarovich M, Mulgaonkar S, Gaston R, Gaber AO, Mayo PR, Ling S, Huizinga RB, Meier-Kriesche HU; PROMISE Investigators: The PROMISE study: A phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am J Transplant 11: 2675–2684, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Ho ET, Wong G, Craig JC, Chapman JR: Once-daily extended-release versus twice-daily standard-release tacrolimus in kidney transplant recipients: A systematic review. Transplantation 95: 1120–1128, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Bunnapradist S, Ciechanowski K, West-Thielke P, Mulgaonkar S, Rostaing L, Vasudev B, Budde K; MELT investigators: Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): The phase III randomized MELT trial. Am J Transplant 13: 760–769, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxwell L, Singh JA: Abatacept for rheumatoid arthritis. Cochrane Database Syst Rev 4: CD007277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW, 3rd, Mundel P: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369: 2416–2423, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ: Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 5: 443–453, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP: A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 10: 535–546, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Sidiropoulos PI, Boumpas DT: Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus 13: 391–397, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Okimura K, Maeta K, Kobayashi N, Goto M, Kano N, Ishihara T, Ishikawa T, Tsumura H, Ueno A, Miyao Y, Sakuma S, Kinugasa F, Takahashi N, Miura T: Characterization of ASKP1240, a fully human antibody targeting human CD40 with potent immunosuppressive effects. Am J Transplant 14: 1290–1299, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pescovitz MD: Rituximab, an anti-cd20 monoclonal antibody: History and mechanism of action. Am J Transplant 6: 859–866, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Fessler BJ, Ding L, Viviano L, Tchao NK, Phippard DJ, Asare AL, Lim N, Ikle D, Jepson B, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh K, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Mueller M, Sejismundo LP, Mieras K, Stone JH; RAVE-ITN Research Group: Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 369: 417–427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha A, Bagga A: Rituximab therapy in nephrotic syndrome: Implications for patients’ management. Nat Rev Nephrol 9: 154–169, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM: A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant 8: 2607–2617, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Genovese MC, Kaine JL, Lowenstein MB, Del Giudice J, Baldassare A, Schechtman J, Fudman E, Kohen M, Gujrathi S, Trapp RG, Sweiss NJ, Spaniolo G, Dummer W; ACTION Study Group: Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum 58: 2652–2661, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Østergaard M, Baslund B, Rigby W, Rojkovich B, Jorgensen C, Dawes PT, Wiell C, Wallace DJ, Tamer SC, Kastberg H, Petersen J, Sierakowski S: Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: Results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum 62: 2227–2238, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Martin ST, Cardwell SM, Nailor MD, Gabardi S: Hepatitis B reactivation and rituximab: A new boxed warning and considerations for solid organ transplantation. Am J Transplant 14: 788–796, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, Kilgallen B, Bongardt S, Barry A, Kelley L, Gordon C: Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: Results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis 73: 183–190, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace DJ, Gordon C, Strand V, Hobbs K, Petri M, Kalunian K, Houssiau F, Tak PP, Isenberg DA, Kelley L, Kilgallen B, Barry AN, Wegener WA, Goldenberg DM: Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: Results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatology (Oxford) 52: 1313–1322, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Manzi S, Sánchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, Ginzler EM, D’Cruz DP, Doria A, Cooper S, Zhong ZJ, Hough D, Freimuth W, Petri MA; BLISS-52 and BLISS-76 Study Groups: Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: Combined results from two phase III trials. Ann Rheum Dis 71: 1833–1838, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dooley MA, Houssiau F, Aranow C, D’Cruz DP, Askanase A, Roth DA, Zhong ZJ, Cooper S, Freimuth WW, Ginzler EM; BLISS-52 and -76 Study Groups: Effect of belimumab treatment on renal outcomes: Results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 22: 63–72, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Richez C, Truchetet ME, Schaeverbeke T, Bannwarth B: Atacicept as an investigated therapy for rheumatoid arthritis. Expert Opin Investig Drugs 23: 1285–1294, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, Singer NG: Atacicept in combination with MMF and corticosteroids in lupus nephritis: Results of a prematurely terminated trial. Arthritis Res Ther 14: R33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cenci S: The proteasome in terminal plasma cell differentiation. Semin Hematol 49: 215–222, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Palumbo A, Anderson K: Multiple myeloma. N Engl J Med 364: 1046–1060, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Gaballa MR, Laubach JP, Schlossman RL, Redman K, Noonan K, Mitsiades CS, Ghobrial IM, Munshi N, Anderson KC, Richardson PG: Management of myeloma-associated renal dysfunction in the era of novel therapies. Expert Rev Hematol 5: 51–66, quiz 67–68, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Walsh RC, Alloway RR, Girnita AL, Woodle ES: Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int 81: 1067–1074, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valenzuela NM, McNamara JT, Reed EF: Antibody-mediated graft injury: Complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant 19: 33–40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bu F, Borsa N, Gianluigi A, Smith RJ: Familial atypical hemolytic uremic syndrome: A review of its genetic and clinical aspects. Clin Dev Immunol 2012: 370426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Zuber J, Le Quintrec M, Krid S, Bertoye C, Gueutin V, Lahoche A, Heyne N, Ardissino G, Chatelet V, Noël LH, Hourmant M, Niaudet P, Frémeaux-Bacchi V, Rondeau E, Legendre C, Loirat C; French Study Group for Atypical HUS: Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant 12: 3337–3354, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM: Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11: 2405–2413, 2011 [DOI] [PubMed] [Google Scholar]
- 54.McCullough JW, Renner B, Thurman JM: The role of the complement system in acute kidney injury. Semin Nephrol 33: 543–556, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damman J, Daha MR, van Son WJ, Leuvenink HG, Ploeg RJ, Seelen MA: Crosstalk between complement and Toll-like receptor activation in relation to donor brain death and renal ischemia-reperfusion injury. Am J Transplant 11: 660–669, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Rhen T, Cidlowski JA: Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 353: 1711–1723, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Fauci AS, Dale DC, Balow JE: Glucocorticosteroid therapy: Mechanisms of action and clinical considerations. Ann Intern Med 84: 304–315, 1976 [DOI] [PubMed] [Google Scholar]
- 58.Matas AJ: Minimization of steroids in kidney transplantation. Transpl Int 22: 38–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS; ORAL Solo Investigators: Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367: 495–507, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Vincenti F, Tedesco Silva H, Busque S, O’Connell P, Friedewald J, Cibrik D, Budde K, Yoshida A, Cohney S, Weimar W, Kim YS, Lawendy N, Lan SP, Kudlacz E, Krishnaswami S, Chan G: Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: Efficacy, renal function and safety at 1 year. Am J Transplant 12: 2446–2456, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Gralla J, Wiseman AC: The impact of IL2ra induction therapy in kidney transplantation using tacrolimus- and mycophenolate-based immunosuppression. Transplantation 90: 639–644, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Feldmann M: Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol 2: 364–371, 2002 [DOI] [PubMed] [Google Scholar]
- 63.van Schouwenburg PA, Rispens T, Wolbink GJ: Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 9: 164–172, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V: Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295: 2275–2285, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Dinarello CA, Simon A, van der Meer JW: Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11: 633–652, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hung AM, Ellis CD, Shintani A, Booker C, Ikizler TA: IL-1β receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol 22: 437–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones SA, Fraser DJ, Fielding CA, Jones GW: Interleukin-6 in renal disease and therapy. Nephrol Dial Transplant 30: 564–574, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Singh JA, Beg S, Lopez-Olivo MA: Tocilizumab for rheumatoid arthritis: A Cochrane systematic review. J Rheumatol 38: 10–20, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C; ERASURE Study Group; FIXTURE Study Group: Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med 371: 326–338, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Solari R, Pease JE, Begg M: “Chemokine receptors as therapeutic targets: why aren’t there more drugs?”. Eur J Pharmacol 746: 363–367, 2015 [DOI] [PubMed] [Google Scholar]
- 71. Haller H, et al.: CCL2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Presented at the 51st ERA-EDTA Congress, June 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L; TRANSFORMS Study Group: Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362: 402–415, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Vincenti F, Mendez R, Pescovitz M, Rajagopalan PR, Wilkinson AH, Butt K, Laskow D, Slakey DP, Lorber MI, Garg JP, Garovoy M: A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant 7: 1770–1777, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering BJ, Bluestone JA, Stock PG: Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 10: 1870–1880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta S, Weinstock-Guttman B: Natalizumab for multiple sclerosis: Appraising risk versus benefit, a seemingly demanding tango. Expert Opin Biol Ther 14: 115–126, 2014 [DOI] [PubMed] [Google Scholar]
- 76.Gelfand EW: Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med 367: 2015–2025, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Jordan SC, Toyoda M, Kahwaji J, Vo AA: Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant 11: 196–202, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C: Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9: 1099–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Vo AA, Cam V, Toyoda M, Puliyanda DP, Lukovsky M, Bunnapradist S, Peng A, Yang K, Jordan SC: Safety and adverse events profiles of intravenous gammaglobulin products used for immunomodulation: A single-center experience. Clin J Am Soc Nephrol 1: 844–852, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, Bozorgzadeh A, Sanz I, Briggs BJ: Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation 79: 1507–1515, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, Dolan S, Kano JM, Mahon M, Schnitzler MA, Woodward R, Irish W, Singer GG: A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation 67: 1011–1018, 1999 [DOI] [PubMed] [Google Scholar]
- 82.Gharekhani A, Entezari-Maleki T, Dashti-Khavidaki S, Khalili H: A review on comparing two commonly used rabbit anti-thymocyte globulins as induction therapy in solid organ transplantation. Expert Opin Biol Ther 13: 1299–1313, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Büchler M, Hurault de Ligny B, Madec C, Lebranchu Y; French Thymoglobuline Pharmacovigilance Study Group: Induction therapy by anti-thymocyte globulin (rabbit) in renal transplantation: A 1-yr follow-up of safety and efficacy. Clin Transplant 17: 539–545, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Alinari L, Lapalombella R, Andritsos L, Baiocchi RA, Lin TS, Byrd JC: Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene 26: 3644–3653, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Freedman MS, Kaplan JM, Markovic-Plese S: Insights into the mechanisms of the therapeutic efficacy of alemtuzumab in multiple sclerosis. J Clin Cell Immunol 4: 2013 [PMC free article] [PubMed] [Google Scholar]
- 86.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, Croy R, Holman J; INTAC Study Group: Alemtuzumab induction in renal transplantation. N Engl J Med 364: 1909–1919, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, Potdar S, Shapiro R, Dharnidharka VR, Kauffman HM: Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant 7: 2619–2625, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peddi VR, Wiseman A, Chavin K, Slakey D: Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev (Orlando) 27: 97–107, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 90.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Crespo-Leiro MG, Marzoa-Rivas R, Barge-Caballero E, Paniagua-Martín MJ: Prevention and treatment of coronary artery vasculopathy. Curr Opin Organ Transplant 17: 546–550, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, Salisbury S, Franz DN: Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358: 140–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN: Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366: 520–529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ; Global ARCC Trial: Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Elion GB: The purine path to chemotherapy. Science 244: 41–47, 1989 [DOI] [PubMed] [Google Scholar]
- 96.Berns A, Rubenfeld S, Rymzo WT, Jr., Calabro JJ: Hazard of combining allopurinol and thiopurine. N Engl J Med 286: 730–731, 1972 [DOI] [PubMed] [Google Scholar]
- 97.Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT: Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 82: 1698–1702, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Appel AS, Appel GB: An update on the use of mycophenolate mofetil in lupus nephritis and other primary glomerular diseases. Nat Clin Pract Nephrol 5: 132–142, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 Annual Data Report: Kidney. Am J Transplant 14[Suppl 1]: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 100.Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, Tönshoff B, Holt DW, Chapman J, Gelder T; Transplantation Society (TTS) Consensus Group on TDM of MPA: Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol 5: 341–358, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G, Fox R, Moreland L, Olsen N, Furst D, Caldwell J, Kaine J, Sharp J, Hurley F, Loew-Friedrich I; Leflunomide Rheumatoid Arthritis Investigators Group: Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Arch Intern Med 159: 2542–2550, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Chacko B, John GT: Leflunomide for cytomegalovirus: Bench to bedside. Transpl Infect Dis 14: 111–120, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Chon WJ, Josephson MA: Leflunomide in renal transplantation. Expert Rev Clin Immunol 7: 273–281, 2011 [DOI] [PubMed] [Google Scholar]
- 104.Hengstler JG, Hengst A, Fuchs J, Tanner B, Pohl J, Oesch F: Induction of DNA crosslinks and DNA strand lesions by cyclophosphamide after activation by cytochrome P450 2B1. Mutat Res 373: 215–223, 1997 [DOI] [PubMed] [Google Scholar]
- 105.Boumpas DT, Austin HA, 3rd, Vaughn EM, Klippel JH, Steinberg AD, Yarboro CH, Balow JE: Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 340: 741–745, 1992 [DOI] [PubMed] [Google Scholar]
- 106.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sánchez-Guerrero J, Solomons N, Wofsy D; Aspreva Lupus Management Study Group: Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 20: 1103–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U; RAVE-ITN Research Group: Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]