Abstract
Objective
To use a human factors perspective to examine how older adult patients with heart failure use cognitive artifacts for medication management.
Methods
We performed a secondary analysis of data collected from 30 patients and 14 informal caregivers enrolled in a larger study of heart failure self-care. Data included photographs, observation notes, interviews, video recordings, medical record data, and surveys. These data were analyzed using an iterative content analysis.
Results
Findings revealed that medication management was complex, inseparable from other patient activities, distributed across people, time, and place, and complicated by knowledge gaps. We identified fifteen types of cognitive artifacts including medical devices, pillboxes, medication lists, and electronic personal health records used for: 1) measurement/evaluation; 2) tracking/communication; 3) organization/administration; and 4) information/sensemaking. These artifacts were characterized by fit and misfit with the patient’s sociotechnical system and demonstrated both advantages and disadvantages. We found that patients often modified or “finished the design” of existing artifacts and relied on “assemblages” of artifacts, routines, and actors to accomplish their self-care goals.
Conclusions
Cognitive artifacts are useful but sometimes are poorly designed or are not used optimally. If appropriately designed for usability and acceptance, paper-based and computer-based information technologies can improve medication management for individuals living with chronic illness. These technologies can be designed for use by patients, caregivers, and clinicians; should support collaboration and communication between these individuals; can be coupled with home-based and wearable sensor technology; and must fit their users’ needs, limitations, abilities, tasks, routines, and contexts of use.
Keywords: human factors, heart failure, aging, medication management, cognitive artifacts, health information technology
INTRODUCTION
The scientific and practice-based discipline human factors engineering uses data, theory, design principles, and various methods to optimize interactions between people and other elements of a system to improve human performance and well-being [1,2]. Central to the human factors profession is a “systems” orientation, which states that human performance occurs within the context of a sociotechnical system [3]. Cognitive artifacts, tools and technologies that aid the mind in the performance of cognitive work, are an essential part of sociotechnical systems [4,5] as are people, tasks, the organization, and the internal and external environments [6]. These elements all interact, are interdependent, and act together [7]. Emphasis on interactions in context, as opposed to isolated system elements, distinguishes human factors from other disciplines and professions [8,9]. A human factors analysis of cognitive artifacts—our present aim—examines both the artifacts themselves and how they interact with different people, tasks, other artifacts, and organizational and environmental factors. To put it another way, a human factors analysis looks at how cognitive artifacts fit in their surrounding sociotechnical system [10,11] to inform system (re)design that optimizes performance and well-being [12,13].
Human factors and patient work performance
Applying human factors methods and theories to health and healthcare dates back to the 1960s. It accelerated at the turn of the century due in part to the call by the Institute of Medicine for a human factors approach to achieving patient safety [13]. Healthcare professionals (i.e., clinicians) and their work have been the aim of the vast majority of applications of human factors in healthcare. Some have noted additional opportunity to apply human factors to understand and improve patient work [6,14]. Patient work is effortful, goal-driven, health-related activity performed by patients, families, and other nonprofessionals [15]. The need to study and improve patient work stems from several converging factors:
-
a)
A realization that most care takes place in homes and communities, not in formal healthcare delivery settings [16];
-
b)
The rising volume and expense of clinical care and interventions, combined with concerns about a clinical workforce that will not match future demands [17];
-
c)
Perceptions of the financial value that patients and families can provide through self-care and preventive health behaviors [18];
-
d)
Increased expectations for patients and families to engage in health-related tasks such as information seeking and self-care [19]; and
-
e)
Newly available personal and clinical technologies that make it possible for people to manage health outside of formal clinical settings (e.g., home dialysis, mobile devices, tele-medicine, online medical knowledge bases) [20].
Cognitive artifacts for patient work
We conceptualize cognitive artifacts as digital or non-digital artificial devices that maintain, display, or operate upon information through representations and that shape human cognitive performance [21]. Norman [5] describes cognitive artifacts bridging two gaps that jeopardize task performance. Artifacts bridging the gap of execution (action) provide alternative ways to act upon the real world (e.g., controls); representational artifacts bridging the gap of evaluation (interpreting effects) represent the real world (e.g., displays) [22]. Cognitive artifacts extended human performance by externalizing or offloading information processing to the environment [23]. They can also change the nature of the task itself [22]. Artifacts improve performance to the extent that they: a) address the important and leave out irrelevant information; b) fit the task, goals, and skills of their users; c) represent the properties or attributes of the represented entity; and d) use perceptual-spatial properties analogous to the real world [22].
Hutchins [24] argues that cognitive artifacts cannot be separated from the human operator, task, or the environment and have no inherent separate value. The emergent coordination and functioning of those elements together determine performance [25]. Thus, cognitive artifacts are best studied in a relational context, rather than by the analysis of individual attributes alone [26].
Study of cognitive artifacts used by older adults for heart failure related medication management
This study used a human factors lens to examine the cognitive artifacts of older adults with heart failure. Specifically, identified cognitive artifacts in use, who used them, and how they facilitated or impeded successful medication management. In taking a human factors approach, we were attentive to how older adults’ artifacts fit within the broader sociotechnical system.
Medication management for patients with heart failure is an important daily, lifelong process. However, reported heart failure medication adherence rates are 40–60% [27,28]. Medication non-adherence can be intentional non-use of medications or unintentional errors such as lapses in medication taking, adding doses, or mixing up pills. Therefore, cognitive artifacts and other strategies that support memory and performance, mitigate errors, or help people recover from errors, could address medication non-adherence [29]. This may be particularly true among older adults, who are at risk for age-related cognitive decline and take a multitude of medications, including ones that may affect their cognition [30]. Several studies report patients with heart failure using cognitive artifacts, including paper records, notes, pillboxes, and kitchen cabinets, to manage medication-related activities [31,32]. Studies have also introduced cognitive artifacts such as charts, organizers, that improved medication adherence among older adults [33]. However, there remains a need to describe patients’ cognitive artifacts in more detail, examine how artifacts fit into patients’ sociotechnical systems, identify their strengths and limitations, and propose consequent design and policy recommendations.
METHODS
We conducted a secondary analysis of data from 30 patients, and 14 informal caregivers enrolled in a larger study of heart failure self-care. Patient participants were aged ≥65, lived in a 200-mile radius of Nashville, Tennessee, and received continuing outpatient care in a cardiology clinic specializing in heart failure. Table 1 describes participant characteristics (see also [32]). Participants provided consent and permission for scholarly use of audiovisual data. The study paid participants up to $65 for completing all study phases. The Vanderbilt University Institutional Review Board and Human Research Protection Program reviewed and approved the study.
Table 1.
Patient participant characteristics (N=30).
| Age | M=74.0 (SD=6.5) (range 65–86) |
| Sex | 17 male / 13 female (57% / 43%) |
| Race | 18 White non-Hispanic (60%), 10 Black (33%), 2 Mixed-race (7%) |
| Marital status | 16 (53%) married, 7 (23%) widowed, 5 (17%) separated or divorced, 2 (7%) single |
| Caregivers | 14 informal caregivers consented to participate: 6 spouses, 8 adult children |
| Education | 10 (33%) completing 12 years, 11 (37%) >12 years, 9 (30%) <12 years |
|
Annual household incomea |
7 (25%) ≤ $15,000, 15 (53%) ≤ $25,000, 21 (75%) ≤ $50,000 |
| Employmenta | 26 (87%) retired, 3 (10%) disabled/unable to work, 1 (3%) part-time |
| Insurancea | 100% Medicare, 17% Medicaid, 10% military, 87% private supplement |
|
Heart failure type/severitya,b |
9 (30%) systolic, 13 (43%) diastolic, 8 (27%) systolic and diastolic; NYHA Class: 11 (37%) II or “mild,” 18 (60%) III or “mild/moderate” |
| Comorbidities | 80% hyperlipidemia, 83% hypertension, 53% diabetes mellitus |
If known
NYHA Class=New York Heart Association functional classification; NYHA classes I and IV were excluded.
Data—originally collected in 2012–2013—included verbatim transcription of clinic visit observations, short (30-minute) interviews, and follow-up (90-minute) interviews. Photos of cognitive artifacts extracted from in-home and in-clinic video recordings, electronic medical records, and self-administered standardized surveys (100% response rate) provided additional data.
NVivo 10 qualitative data analysis software was used for descriptive qualitative content analysis with iterative category development [34]. These methods systematically derive trends, patterns, and themes from large amounts of textual data revealing the underlying meaning [55]. Close analysis of words and photos directly depicting a participant’s life is a way to achieve a rich, contextualized, participant-centered understanding of a phenomenon [35]. During first-pass structural coding [36], researchers identified broad passages of data mentioning a cognitive artifact used in medication self-management. We defined medication self-management as the processes by which prescribed medications are administered by patients or their caregivers in a manner optimal for achieving treatment goals including activities related to planning, sensemaking, organizing, tracking, problem-solving, communicating and coordinating [37–39]. Next, during second-pass analysis, authors RSM and RJH assigned thematic codes to structurally coded passages related to the artifact’s functional category [22], fit or misfit within the patient’s broader sociotechnical [32], and observed advantages and disadvantages. The third pass involved data-driven, discussion-based thematic and category development using preliminary categories and exemplars [40]. The senior researcher (RJH) facilitated analytic convergence and presided over any analytic disagreements [41]. Passages and still photographs were selected to illustrate and enrich analytic themes [42]. In the final step, we assembled an illustrative case from one participant’s data.
RESULTS
We first describe observations about the nature of medication management and related knowledge gaps among participants. Next, we describe identified cognitive artifacts and their uses. Lastly, we discuss the fit between used artifacts and the patient’s sociotechnical system and the artifacts’ advantages and disadvantages.
Nature of medication management among older adult patients with heart failure
Heart failure is a progressive disease prevalent in older adults [43], characterized by the heart’s diminished ability to fill or to pump blood to the body resulting in symptoms such as shortness of breath, fatigue, and peripheral swelling [44]. The goals of pharmacologic therapy are to improve the pumping effectiveness of the heart and to control fluid build-up [45] achieved through a multitude of medications impacting a variety of physiological systems to control: blood pressure; heart rate and rhythm; fluid balance; clot formation; and lipid blood levels [44].
We observed that medication management in heart failure patients was complex, inseparable from other patient activities, and distributed across people, time, and place. These properties are described in Table 2 and elsewhere [31,32,46].
Table 2.
Observed properties of medication management.
Complex
|
Inseparable from other patient activities
|
Distributed across people, time, and place
|
A striking observation was that many patients, caregivers, and clinicians had incomplete or incompatible knowledge regarding medication management. Patients do not always know what medications they were taking, their medications names, directions for use, or what effects to expect (Table 3a). Several patients lacked knowledge about the relationship between medications and symptoms, and, therefore, when it was appropriate to take medications. Informal caregivers also lacked knowledge about symptoms, medications, and the relationship between the two.
Table 3.
Selected examples of observed knowledge gaps.
|
|
|
Clinicians could not guarantee patients were taking their medications as directed. Clinicians struggled to get accurate accounts of patients’ current medications. Few patients brought their medications or medication lists to clinic visits as requested, and over half relied on memory (Table 3b).
While patients often represented their knowledge of medications based on pill size, shape, and corresponding condition or organ (water pill, kidney medicine), clinicians almost always used the brand or generic medication names when speaking to patients (Table 3c).
Cognitive artifacts used by older adult patients with heart failure
Patients and their caregivers used multiple cognitive artifacts to achieve medication management goals of measurement and evaluation, tracking and communication, organization and administration, and information and sense making. Table 4 provides a case example of artifacts used by one patient.
Table 4.
Case example of a patient and his family using multiple artifacts (underlined).
| Bill Smith is retired firefighter in his 80s who lives in the city with his wife of 60 years. He developed heart failure about 5 years ago following a heart attack and cardiac bypass surgery. He is also diabetic and has poor vision. Bill has difficulty walking due to his shortness of breath and chronic vertigo. He uses a walker at home and a wheelchair when away from home. One of his adult children lives nearby and helps Bill and his wife with their medication management tasks such as picking up medications, maintaining medication lists, and accompanying them to clinic appointments. Because Bill’s wife also has several chronic illnesses, the kitchen table is the center of health-related activities for both. Bill regularly uses various devices to measure his blood pressure, weight, and blood sugar. He records these measures on a paper log. Bill is visibly upset by the frequent burden of these activities and says he does not see their benefit. “It aggravates the fool out of me,” he tells his nurse practitioner (NP). “I started coming out here, taking my blood pressure, taking my weight, and sugar count, so forth ‘til I feel like a secretary.” Recently, he bought a new digital weight scale because he could not see the numbers on the old scale. He does not appear to view rapid weight gain as a seriously concerning indicator of fluid retention. When his NP asks what he would do if he rapidly gained weight, he replies, “I’d stop, I’d back off from the table (is) the first thing.” Bill does not know his medications by name and depends on a medication list maintained by his adult child. When his NP asks if he is taking Lasix, he responds, “Whatever, whatever it (medication list) says, yeah.” His child keeps track of medication changes and once a week helps Bill set up his pillbox, which he uses to administer his daily medications. Bill’s poor vision makes it difficult for him to read prescription drug labels. Also, different family members fill Bill’s pillbox at different times. Therefore, Bill has developed a strategy for labeling the tops of prescription pill bottles. For each medication, Bill used a bold marker to write the number of pills to take and an abbreviation indicating the time of administration (M for morning, N for noon, B for bedtime). He explains, “When I get my prescription filled from the drug store, I take (its) top off. I put (the marked top) on the new bottle.” This way, he can use the same tops even after refilling the medications. This strategy has simplified the process of identifying medications and filling his pillbox, as his child explains: “We done it too because there was two or three of us at one time trying to fill his pill bottle and (his wife’s) pill bottles and when they were both down, we were trying and I would be over there and I would try to do it vice versa, so when he come up with this system here, it just really made it easy.” Bill uses his social support system and a spatial arrangement strategy for medication refills. “If I get a pill bottle and I look into there and I say, uh, well I’ve got six pills. I’ve got pills filled out for this week. I set it over here on the turntable… on my little table and my wife calls the drugstore and says fill this prescription. And, and, and then she picks it up you know, if it has to be called in (authorized), it tells me on the bottle. They’ll call in and when it gets filled, my pharmacy, they will call and tell us your, your prescription is ready… She (child) goes and picks it up or (wife) will come by and go get it. And I, then I’ll take the top off of this one and change the top, I got it marked.” Bill’s case is an example of a system of people, cognitive artifacts, and places that assemble and adapt to accomplish medication management goals in the context of limitations, challenges, and available resources. |
Artifacts used for monitoring and measurement helped externalize the patient’s condition and provided data for interpretation and action, including medication taking. These artifacts resembled those used by clinicians, were rarely modified, and were embedded in daily routines. Most patients owned scales (97%) and weighed themselves daily (77%). Most used their scales during their morning routine and in the bathroom. Clinicians instructed patients to use scales to monitor for weight gain over time (e.g., 5lbs in 3 days) or above a personal threshold value (e.g., >185lbs) and to either take extra diuretic medication (33%) or call the clinician.
Many patients (70%) owned blood pressure (BP) cuffs, and some (60%) used them daily for BP and heart rate readings. Patients kept BP cuffs in various places in their homes. Some models were portable for travel. Three patients (10%) used pulse oximeters, which are small sensors that clip to the index finger or earlobe and display indirect measures of oxygen in the blood. Two patients used these on physician recommendation. The other purchased one after observing its use in others; he used it several times per day and took his extra diuretic at oxygen saturation < 96%. Two patients (7%) participated in a left atrial pressure (LAP) monitoring clinical trial. Patients placed a patient advisor module (PAM) on the chest over an implanted sensor twice a day. The PAM would give a LAP measure and recommended to patients the diuretic dose for that time. The device also wirelessly transmitted data such as weight and temperature to the clinic.
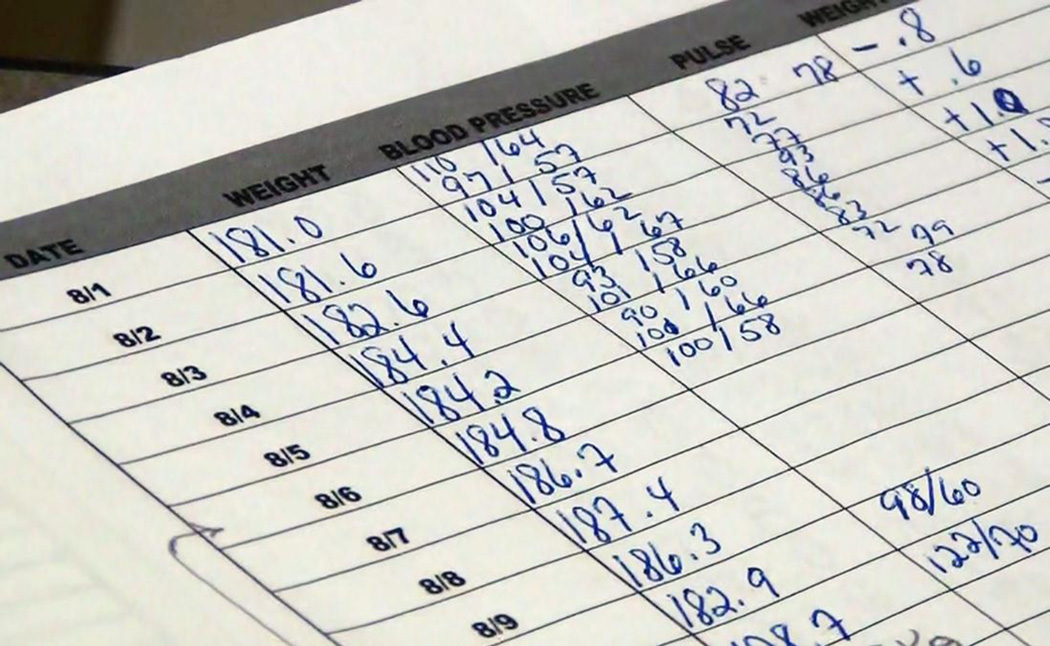
Tracking and communication artifacts were among the most useful types of patient artifacts. Because months separated clinic visits, clinician awareness of patients’ status and event occurrences depended on patients tracking, detecting, and communicating trends and deviations. Fewer than half of patients documented weight (43%) and BP (37%) daily. Clinicians gave patients a paper form for this task, but some used homemade forms. Patients modified the forms to fit their needs. One added his weight and BP measurements to a form designed to record blood sugar. Patients kept logs in the area of their home where they took the measures. Logs gave patients feedback on their condition over time and allowed them to follow trends and note changes such as taking additional medications. A 68-year-old White female described using her log (Figure 1): “You see how my weight constantly kept going down… You can see here where I went up a little bit and took those pills and dropped.”
Figure 1.
A completed weight, blood pressure, and heart rate log. The patient has noted extra medications taken and absolute daily changes in her weight.
All patient received a printed medication list at the end of each clinic visit from the reconciled electronic health record (EHR) medication list. Patients added handwritten notes to these lists depending on what information was important to the patient.
EHR-generated medications lists did not always meet the patient’s needs. The listed medications were uncategorized and ordered by when last prescribed, listed along with alternative names, dose, frequency, route, and directions. In contrast, patients organized medication administration by time. Consequently, some patients (23%) made their own computer-generated or handwritten medication list and revised the EHR-generated list (Figure 2).
Figure 2.
Patient-made medication list ordered by time, updated and annotated by hand.
Two patients tracked their medication history: one patient made a list of medications he had issues with; and one made a chart of all the medications he had ever taken, discontinue date (if applicable), the name of the provider that wrote the prescription, the prescription number, and when the next refill was due. Hand-made lists gave insights into informational gaps in EHR-generated lists.
Patients brought a medication list to 37% of observed clinic appointments and medication bottles to 17%. These helped communicate the current medication regimen to clinicians. Over half (60%) relied on memory alone to communicate about medications. Some patients (17%) also carried notepads or other portable objects like appointment books to note the information they needed to communicate to clinicians or to track information. One patient’s wife used a calendar to track changing Coumadin doses.
All patients had access to the medical center’s web-based patient portal, and 27% used it. Users had access to secure messaging with clinicians, appointment scheduling, laboratory and test results, clinical summaries, and problem lists including current medications. Some were unaware the portal existed (13%), some did not own a computer (27%), and some (13%) did not like to use computers, or felt they had insufficient computer skills.
Patients organized medication administration using unique systems that fit their regimen, lifestyle, skills and limitations, and circumstances. Pharmacies put medications in labeled plastic containers. Labels could deteriorate over time: one patient described taking a medication she thought was for constipation, but could not read the name on the label. Sometimes patients used these labels as an organizational system, arranging bottles by administration time in a cabinet or drawer. However, 73% used pill organizers (pillboxes) to reduce the burden of reading labels, opening bottles, and taking medications out of bottles several times a day (Figure 3). Patients would fill the pillboxes on a most often once a week, regular schedule, thus batching what would otherwise be a twice-daily cognitive activity. Pillboxes had various separate compartments for time of day (e.g., morning, noon, night). Some patients checked whether these compartments were empty or full to verify if they took their medications. Patients also used paper bags dividing morning and evening medications, tinfoil bags when traveling outside the house, and a variety of containers such as toiletry bags, baskets, drawers, and cabinets (Figure 3). Pillboxes were located in an area of the house dedicated to health-related activities, the place of one’s morning routine (e.g., in the bathroom), and in a visible area to serve as a reminder. Baskets, drawers, and cabinets also served to separate medications taken by cohabitants.
Figure 3.
A typical patient pillbox (left) and medications stored in a kitchen drawer (right).
The main source of information for patients regarding medications was their clinician or pharmacist, but some used additional information artifacts to make sense of their medications and condition. Some patients (27%) described using written information such as booklets and brochures provided by clinicians. Most received a binder containing patient-centered educational information about heart failure and self-care. One patient had medical and pharmaceutical books in his home. Some patients (10%) mentioned reading package inserts from the pharmacy that came with their medications. A third of patients looked up information about their disease and medications on the Internet. Some did this daily and others infrequently. Patients (17%) mentioned receiving health and medication information from television shows and advertising. Some did not trust the information and others considered it reliable and useful. After hearing on a television advertisement that all heart patients should take aspirin a patient taking an anticoagulant consulted his clinician and learned this did not apply to him.
Fit between artifacts and the patient’s sociotechnical system
We observed several instances of “misfit,” in which artifacts were incompatible with patients, other artifacts, routines, and environments of use. Instances of artifact-artifact misfit included differences between patients’ and clinicians’ artifacts. For example, patients’ scales or BP cuffs produced readings different from their clinics’. Patients’ homemade or modified medication lists often differed from those generated by the EHR. Artifact-person misfit occurred when cognitive artifacts were ill-suited for older users, their experiences, mental models, limitations, and daily routines. For example, prescription labels using small text or websites with multiple navigation options were challenging for those with visual acuity and less computer experience, respectively. Artifact-task misfit occurred when daily measures were taken at home but not communicated directly to clinicians, except in summary form during visits spaced months apart. Another example was medication lists that were not organized by time of day or were missing information on indication or brand name. Artifact-context misfit included lack of access and portability. For example, patient portals required computer, Internet, and e-mail access, but 27% of patients did not own a computer. Pillboxes, scales, and other artifacts were not portable.
Advantages and disadvantages of cognitive artifact use
For each of the 15 artifacts identified, Table 5 summarizes their advantages and disadvantages. We identified three major advantages. First, cognitive artifacts facilitated clinician-patient communication, particularly outside of clinic appointments. With daily electronic transmission of patient data, interpretation and action were no longer dependent only on patients’ knowledge, memory, and skills. Clinicians could intervene early, and patients could receive rapid feedback. Through the patient portal, patients could review past health information, upcoming appointments, and medication list with ease and email clinicians with questions and refill requests.
Table 5.
Advantages and disadvantages of fifteen types of cognitive artifacts used by patients.
| Artifact | Advantages | Disadvantages |
|---|---|---|
| Blood pressure cuff | Easy to use, numbered scale | Need to interpret, calibration issues |
| Scale | Familiar; available; easy to use; numbered scale; clear rules for action (if known) | Rules for action not always known; difficult to use if physical disability or vision problem; calibration issues |
| Pulse oximeter | Numbered scale, small and portable | Need to interpret; reliability issues |
| Left atrial pressure monitor | Suggests action; uses real time, personalized, and longitudinal data | Requires surgical implant; must be trained to use; may promote overdependence |
| Health telemetry | Real time output; efficient and low-burden | Requires special equipment, training, and staff effort |
| Paper weight & blood pressure logs | Adaptable; longitudinal; inexpensive | Burdensome; rely on memory and motivation; can be lost; not real time; provide no action/decision support |
| Paper medication lists (printed, handwritten) | Useful; flexible; portable; easily updated or recreated | Legibility issues; multiple versions; not always clear; must be updated; may promote overdependence |
| Appointment books, calendars, notes | Easily available; flexible; personal | Not permanent; not standardized; not easily shared; hard to search |
| Patient portal/personal health records | Speed of access; fast, secure communication; connected to verified health data | Requires computer skills; access issues; use of clinical language |
| Prescription medication bottles | Accurate; up to date; standardized | Difficult to open; label visibility and legibility |
| Pill Organizer | Reduces effort; provides feedback; simplifies refill planning | Not adaptable; feedback is delayed, passive; pills in box become separated from original containers |
| Other containers: baskets, Bags, drawers, cabinets | Flexible; sometimes portable; usually available; personalized | Large, take up space; larger containers are less portable |
| Medication inserts; books; brochures | Available; inexpensive; sometimes accurate | Can be out-of-date; can be lost, damaged; require health literacy |
| Internet | Flexible; useful; high volume of information; potential for interaction | Credibility issues; access and cost issues; requires skills; not personalized; unfiltered; commercial |
| Television advertisements | Accessible; clear; often repeated | Credibility issues; not personalized; hard to interpret; commercial |
Second, artifacts engaged patients in medication management. Patients using logs could identify abnormalities or trends as well as explore possible causes and solutions. When artifacts were used with knowledge of action or decision rules, such as extra medications taken above a certain threshold, patients could be more active rather than passive recipients of care.
Third, when cognitive artifacts were easy to use, they reduced complexity and task burden. Electronic transmission of measures eliminated daily logging and the need to bring logs to appointments. Pillboxes helped to batch cognitive activity and may have reduced the risk of error. They also supplemented memory and calculations regarding administration and refills. Patient portals made information retrieval and refill requests less effortful.
There were also four major disadvantages. The first was related to integrating or reconciling multiple representations. Clinic visit communication was rarely structured around patient artifacts such as personal medication, lists and both clinicians and patients showed difficulty understanding each other’s lists. The multitude of lists and frequent updates was challenging, with some patients using outdated or incorrect lists. Once a pillbox was filled, it took effort to verify and identify the dispensed medications; patients described medication errors due to similar-looking medications or misfiling the pillbox.
Second, patients used cognitive artifacts designed for clinicians. Patients accessed information with clinician-oriented language and formatting. Information important to the patients (e.g., a medication’s purpose) was sometimes overlooked, and patients and caregivers needed to add it later. Some misperceived or misunderstood the implications of clinical device data; for example, one patient self-administered extra diuretics based on oxygen saturation values deemed “normal” by his cardiologist.
Third, artifacts did not always filter or sort information based on attributes such as importance or accuracy. Although most patients regarded clinicians as the primary source of medication information, some relied on other pervasive sources such as television advertising and the Internet. The challenge for the patient was judging the credibility and interpreting this information. Some patients accepted television advertising as credible but misunderstood the information presented. Patients had few opportunities to validate this information with the clinician and could omit or start medications based on misinformation.
Fourth, data were sometimes lost when, as described earlier, they were not recorded or communicated between patients and clinicians.
DISCUSSION
We used a human factors lens to focus on cognitive artifacts and explore older adults’ management of medications, a phenomenon of particular importance among those living with heart failure. Patients used multiple artifacts for multiple functions, yet artifacts were not always well designed, appropriately used, or compatible with patients’ broader sociotechnical systems. Consequently, artifacts appeared to be both helpful for coping with complex regimens and knowledge gaps and potentially harmful by increasing the risk of misinformation, misinterpretation, and overdependence.
For example, pillboxes reduced the burden of daily medication administration but separated medications from important information such as name, dose, prescribing clinician, and special instructions. Not surprisingly, many patients knew what their medications looked like and when to take them, but not their names or uses. Clinical practice could accommodate these common representations through the use of visual identifiers, written or pictorial, in medication lists and instructions. Informational pharmacy-printed stickers could be included for affixing to the bottom surface of a pillbox. These suggestions could also improve clinical medication reconciliation. Any newly introduced cognitive artifacts should be usable and acceptable to older adults; they must therefore consider physical (manual dexterity, ability to stand, walk), sensory (vision and hearing), cognitive (working memory and attention), and skill (computer literacy) limitations of older adults as well as their tasks (goals, strategies, constraints) and environments of use [47,48]. They should also be flexible enough to accommodate customization and ad-hoc data entry, to supports users’ needs and mental models [5,22]. New systems must also focus on affordability, compared to comprehensive, subscription-based medication management products such as the Philips Medication Dispensing Device.
We noted missed opportunities for artifact use for a) monitoring and recording data in a timely—if not real time—manner and b) bidirectional communication between patients and clinicians about new data, interpretation, and related actions. Data often ended up unused or communicated based on memory in summary fashion. A promising solution is remote health monitoring with well-calibrated telemetry devices, proper training, and timely feedback from clinicians [49]. Self-management software applications using self-directed learning or intelligent agents (e.g., avatars) may be a more patient-engaged and cost-efficient solution. We suggest that these patient-facing technologies should not only deliver education but also support problem-solving, sensemaking, and communication. There is also growing potential to harness home-based sensor technology and commercial wearable health-monitoring devices as part of a model of connected health.
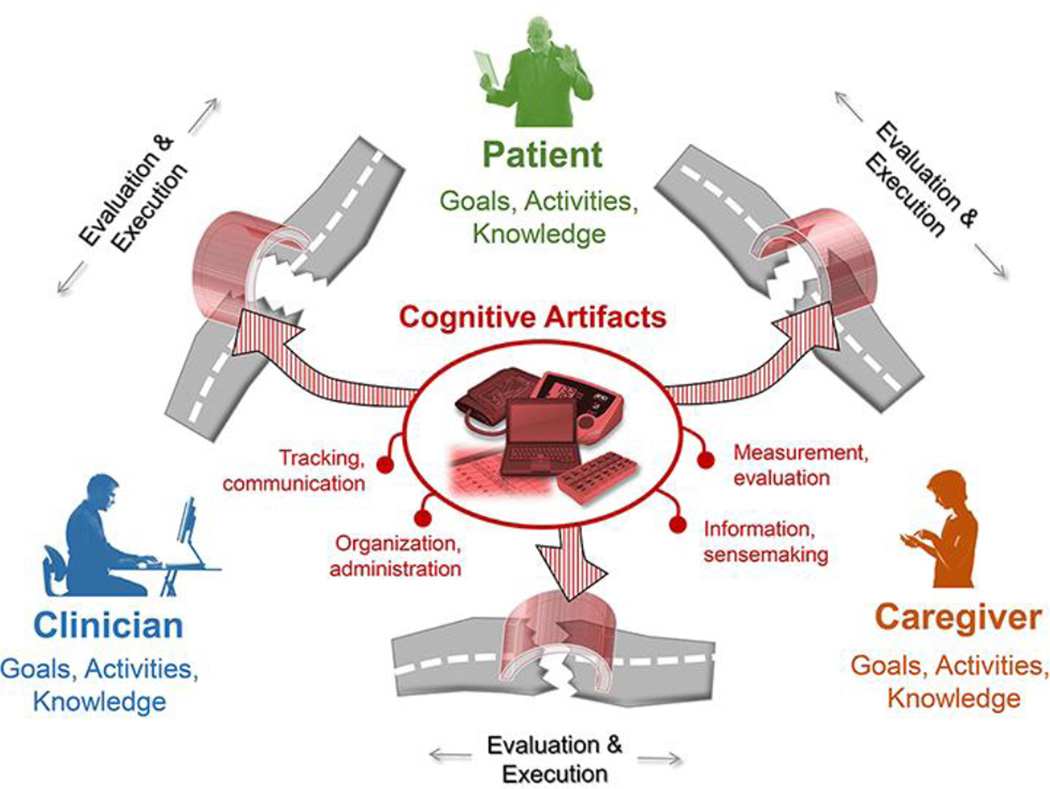
Patient work, especially among patients with heart failure, is a distributed and cooperative activity delegated among patient, informal caregivers, clinicians, and artifacts [31,46]. Designers of artifacts and information systems should be aware that everything designed for the patient may also include that patient’s family members or close friends. The work is also situated in the larger context of life, in which symptoms and health status may compete for time and priority [15]. For new artifacts to integrate into this context and support daily living, as opposed to adherence to discrete disease-management tasks, designers and policy makers will need to be aware of the full complexity of so-called patient work system and work processes that govern patients’ lives [6,15,32]. The data from this study supports the view of cognitive artifacts as effective or ineffective mediators of patient and collaborative work [54], bridging the barriers to the execution and evaluation of the goal-related activities. For medication management these activities include measurement and evaluation, tracking and communication, organization and administration, and information and sense making (Figure 4).
Figure 4.
Cognitive artifacts bridge gulfs of evaluation and execution in patient and collaborative medication management work.
As patients in our study performed the bulk of their health-related activity at home yet relied considerably on their clinicians, our findings support continued efforts to promote patient-centered care and appropriately balanced patient-clinician relationships. We endorse the metaphorical “pilot’s role” for patients, as articulated by Wagner et al. [50]. Under this view, patients with chronic disease work with “co-pilot” caregivers and “air traffic controller” clinicians. They also require appropriate “cockpit technology” to connect these actors, especially when separated by time and space. The role of technology in patient-centered care can be transformative [20,51]. Processes such as shared decision-making could co-evolve with shared cognitive artifacts used by both patients and clinicians and therefore a sort of common ground. Coordinated care could be better achieved if plans of care, changes, and communications were centralized in one system, accessible to all stakeholders. Patients’ goals could be better managed and accessed if they were electronically available and modifiable by patients and shareable with clinicians. In short, there are limitless opportunities for technology to support new and emerging models of care.
Methodological considerations and future directions
A limitation of the study was that the older heart failure patients and informal caregivers were from one region of the US recruited from clinics at one academic medical center. The basis of findings was extensive interviewing and short periods of nonrandom observation; therefore, findings are limited to what patients could or would self-report and shown researchers. Furthermore, the data represent patient and caregiver perspectives, but not clinicians’. The effectiveness and usability of observed artifacts were not assessed objectively because the study objective was to identify and describe, not formally evaluate, artifacts. A clinical view of patient medication work dominated the design of artifacts used by patients in this study. The use of ethnographic [31], cognitive task analysis [14], and participatory ergonomics methods [53] for patient medication work research would be useful for uncovering needs and goals from a patient perspectives.
Additionally, our participant sample consisted of adults with heart failure. The ways in which this group of patients uses objects and artifacts may limit the findings of this study. It is unknown if the properties of cognitive artifacts described here extrapolate to other patient groups with complex routines, such as those with dementia, diabetes, cancer, or pediatric patients. Follow-up research should test the transferability of our findings and investigate the ways, if any, that use of cognitive artifacts varies by treatment, disease, or patient type.
Another promising direction is to consider the patient’s broader life context and consider technologies and artifacts with which patients interact that are not always directly related to treatment. This might include, for example, personal phones, diaries, wearable sensor systems, and the Internet, whose functions may not be cleanly divided into health-related or unrelated-to-health. This perspective may be especially important for considering primary prevention and treatment during early disease onset, as personal technologies become increasingly used for health. Additionally, other social actors in the patient’s immediate network, besides primary caregivers and clinicians, are worth investigating in future studies of cognitive artifacts for patient work. A clear takeaway from our findings is that multiple roles that artifacts and objects play, and that these objects can be seen as a bundle of values and potential uses. This concept can be advanced through future research unpacking what design decisions influence specific values and uses by a patient. For example an experiment using A/B testing where the same cognitive artifact (e.g., a digital bodyweight scale) with different design choices are compared. The different uses of experimentally assigned artifacts may reveal a lot about the nature of artifacts and their use.
Additional future research might focus on unbundling the requirements of cognitive artifacts. We show that an artifact like the personal health record has many implicit requirements: Internet access, computer access, health literacy, and computer literacy, to name a few. These requirements might not be explicit to healthcare professionals, policy makers, or designers.
An interesting finding worth further exploration is the concept of the configuration [6] or assemblage [52], illustrated in the case of “Bill Smith” (Table 4), whose medication management relied on an apparently coincidental but likely purposeful combination of tools, routines, and human relationships. These heterogeneous human and non-human elements assemble to form a meaningful whole maintained through repeat enacted practices and stabilizing and destabilizing forces [52]. Future studies may further examine the nature of these assemblages and how they form, evolve, and support or jeopardize medication management. Other research directions include systematic examination of specific strategies patients develop to use or modify artifacts for specific purposes, from common techniques such as annotating EHR-generated medication lists to creative strategies such as labeling medication bottle tops. Such an examination would identify how patients, to use a human factors saying, “finish the design” started by artifact developers [53]. A final direction is to explore combinations of patient- and clinician-facing information technology and sensor-based data toward a vision of connected, coordinated, and closed-loop health and disease management.
ACKNOWLEDGEMENTS
We thank the patient, caregiver, and clinician participants in this study. We thank Dr. Matt Weinger for his mentorship and feedback, Dr. Doug Sawyer for his mentorship and clinical insights and Drs. Kevin Johnson, Russell Rothman, and Jack Schnelle for their mentorship and assistance. Several clinicians helped us to understand the clinical domain: Melissa Smith and Connie Lewis. Thank you to Amanda McDougald Scott and Courtney Thomas for assisting with data collection. We thank the reviewers for their helpful feedback. This study and RJH were sponsored by grants from the National Institute on Aging (NIA) of the US National Institutes of Health (NIH) (K01AG044439) and grants UL1 TR000445 and KL2 TR000446 from the National Center for Advancing Translational Sciences (NCATS/NIH) through the Vanderbilt Institute of Clinical and Translational Research (VICTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Ethical approval for the study was granted by the Vanderbilt University Institutional Review Board, Behavioral Sciences Committee (IRB# 120950). The study and author RJH were supported by grants from the National Institute on Aging (NIA) of the US National Institutes of Health (NIH) (K01AG044439) and grants UL1 TR000445 and KL2 TR000446 from the National Center for Advancing Translational Sciences (NCATS/NIH) through the Vanderbilt Institute of Clinical and Translational Research (VICTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests to declare.
WORKS CITED
- 1.Association IE. The discipline of ergonomics. 2000 URL: http://wwwieacc/-01_what/What%20is%20Ergonomicshtml (Stand: 1911 2012)
- 2.Dul J, Bruder R, Buckle P, Carayon P, Falzon P, Marras WS, et al. A strategy for human factors/ergonomics: developing the discipline and profession. Ergonomics. 2012;55(4):377–395. doi: 10.1080/00140139.2012.661087. [DOI] [PubMed] [Google Scholar]
- 3.Carayon P. Human factors of complex sociotechnical systems. Applied ergonomics. 2006;37(4):525–535. doi: 10.1016/j.apergo.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Hollnagel E. Cognitive ergonomics: it's all in the mind. Ergonomics. 1997;40(10):1170–1182. [Google Scholar]
- 5.Norman DA. The design of everyday things: Basic books. 2002 [Google Scholar]
- 6.Holden RJ, Carayon P, Gurses AP, Hoonakker P, Hundt AS, Ozok AA, et al. SEIPS 2.0: a human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics. 2013;56(11):1669–1686. doi: 10.1080/00140139.2013.838643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasmore WA. Designing effective organizations: The sociotechnical systems perspective. John Wiley & Sons Inc; 1988. [Google Scholar]
- 8.Wilson JR. Fundamentals of ergonomics in theory and practice. Applied ergonomics. 2000;31(6):557–567. doi: 10.1016/s0003-6870(00)00034-x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JR. Fundamentals of systems ergonomics/human factors. Applied ergonomics. 2014;45(1):5–13. doi: 10.1016/j.apergo.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Holden RJ, Karsh B-T. A review of medical error reporting system design considerations and a proposed cross-level systems research framework. Human Factors: The Journal of the Human Factors and Ergonomics Society. 2007;49(2):257–276. doi: 10.1518/001872007X312487. [DOI] [PubMed] [Google Scholar]
- 11.Holden RJ, Karsh B-T. A theoretical model of health information technology usage behaviour with implications for patient safety. Behaviour & Information Technology. 2009;28(1):21–38. [Google Scholar]
- 12.Karsh B, Holden R, Alper S, Or C. A human factors engineering paradigm for patient safety: designing to support the performance of the healthcare professional. Quality and Safety in Health Care. 2006;15(suppl 1):i59–i65. doi: 10.1136/qshc.2005.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human:: Building a Safer Health System. National Academies Press; 2000. [PubMed] [Google Scholar]
- 14.Unruh KT, Pratt W. Patients as actors: the patient's role in detecting, preventing, and recovering from medical errors. International Journal of Medical Informatics. 2007;76:S236–S244. doi: 10.1016/j.ijmedinf.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Valdez RS, Holden RJ, Novak LL, Veinot TC. Transforming consumer health informatics through a patient work framework: connecting patients to context. Journal of the American Medical Informatics Association. 2014 doi: 10.1136/amiajnl-2014-002826. amiajnl-2014-002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asch DA, Muller RW, Volpp KG. Automated hovering in health care—watching over the 5000 hours. New England Journal of Medicine. 2012;367(1):1–3. doi: 10.1056/NEJMp1203869. [DOI] [PubMed] [Google Scholar]
- 17.Juraschek SP, Zhang X, Ranganathan V, Lin VW. United States registered nurse workforce report card and shortage forecast. American Journal of Medical Quality. 2012;27(3):241–249. doi: 10.1177/1062860611416634. [DOI] [PubMed] [Google Scholar]
- 18.Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Affairs. 1999;18(2):182–188. doi: 10.1377/hlthaff.18.2.182. [DOI] [PubMed] [Google Scholar]
- 19.Sofaer S, Schumann M. Fostering Successful Patient and Family Engagement: Nursing’s Critical Role. Nursing Alliance for Quality Care. 2013 2013. [Google Scholar]
- 20.Finkelstein J, Knight A, Marinopoulos S, Gibbons MC, Berger Z, Aboumatar H, et al. AHRQ Publication No. 12-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Jun, Enabling Patient-Centered Care Through Health Information Technology. Evidence Report/Technology Assessment No. 206. (Prepared by Johns Hopkins University Evidence-based Practice Center under Contract No. 290-2007-10061-I.) 2012. Report No. [PMC free article] [PubMed] [Google Scholar]
- 21.Norman DA. In: Cognitive artifacts. Carroll JM, editor. New York: Cambridge University Press; 1991. [Google Scholar]
- 22.Norman DA. Things that make us smart. Reading, MA: Addison-Wesley; 1993. [Google Scholar]
- 23.Dror IE, Harnad S. Cognition distributed: How cognitive technology extends our minds. John Benjamins Publishing; 2008. [Google Scholar]
- 24.Hutchins E. Cognition in the Wild. MIT press; 1995. [Google Scholar]
- 25.Hollan J, Hutchins E, Kirsh D. Distributed cognition: toward a new foundation for human-computer interaction research. ACM Transactions on Computer-Human Interaction (TOCHI) 2000;7(2):174–196. [Google Scholar]
- 26.Berg M. Patient care information systems and health care work: a sociotechnical approach. International journal of medical informatics. 1999;55(2):87–101. doi: 10.1016/s1386-5056(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 27.van der Wal MHL, Jaarsma T. Adherence in heart failure in the elderly: Problem and possible solutions. Int J Cardiol. 2008;125:203–208. doi: 10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Moser DK, Watkins JF. Conceptualizing self-care in heart failure: A life course model of patient characteristics. J Cardiovasc Nurs. 2008;23:205–218. doi: 10.1097/01.JCN.0000305097.09710.a5. [DOI] [PubMed] [Google Scholar]
- 29.Furniss D, Barber N, Lyons I, Eliasson L, Blandford A. Unintentional non-adherence: Can a spoon full of resilience help the medicine go down? BMJ Qual Saf. 2014;23:95–98. doi: 10.1136/bmjqs-2013-002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park DC, Willis SL, Morrow D, Diehl M, Gaines CL. Cognitive function and medication usage in older adults. Journal of Applied Gerontology. 1994;13(1):39–57. [Google Scholar]
- 31.Palen L, Aaløkke S. Of pill boxes and piano benches: "Home-made" methods for managing medication. Banff, Alberta, Canada. Proceedings of the 2006 conference on Computer Supported Cooperative Work (CSCW); 2006. pp. 79–88. 1180888: ACM. [Google Scholar]
- 32.Holden RJ, Schubert CC, Mickelson RS. The patient work system: An analysis of self-care performance barriers among elderly heart failure patients and their informal caregivers. Appl Ergon. 2015;47:133–150. doi: 10.1016/j.apergo.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park DC, Morrell RW, Frieske D, Kincaid D. Medication adherence behaviors in older adults: effects of external cognitive supports. Psychol Aging. 1992;7(2):252–256. doi: 10.1037//0882-7974.7.2.252. [DOI] [PubMed] [Google Scholar]
- 34.Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. 3rd ed. Thousand Oaks, CA: SAGE; 2014. [Google Scholar]
- 35.Pope C, Mays N, editors. Qualitative Research in Health Care. 3rd ed. Malden, MA: Blackwell; 2006. [Google Scholar]
- 36.Saldaña J. The coding manual for qualitative researchers. Sage; 2012. [Google Scholar]
- 37.Corbin J, Strauss A. Managing chronic illness at home: Three lines of work. Qualitative sociology. 1985;8(3):224–247. [Google Scholar]
- 38.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Annals of behavioral medicine. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 39.Swanlund SL. Successful cardiovascular medication management processes as perceived by community-dwelling adults over age 74. Applied Nursing Research. 2010;23(1):22–29. doi: 10.1016/j.apnr.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Miles MB, Huberman AM, Saldaña J. Qualitative data analysis: A methods sourcebook. SAGE Publications, Incorporated; 2013. [Google Scholar]
- 41.Berends L, Johnston J. Using multiple coders to enhance qualitative analysis: The case of interviews with consumers of drug treatment. Addiction Research & Theory. 2005;13(4):373–381. [Google Scholar]
- 42.Bell P. Content analysis of visual images. Handbook of visual analysis. 2001:10–34. [Google Scholar]
- 43.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Remme W, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. European heart journal. 2001;22(17):1527–1560. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 46.Mickelson R, Holden R. Assessing the distributed nature of home-based heart failure medication management in older adults; Proceedings of the Human Factors and Ergonomics Society Annual Meeting; 2013. SAGE Publications. [Google Scholar]
- 47.Fisk AD, Rogers WA, Charness N, Czaja SJ, Sharit J. Designing for Older Adults: Principles and Creative Human Factors Approaches. 2nd ed. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- 48.Or CKL, Karsh B. A systematic review of patient acceptance of consumer health information technology. J Am Med Inform Assoc. 2009;16:550–560. doi: 10.1197/jamia.M2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dierckx R, Pellicori P, Cleland JG, Clark AL. Telemonitoring in heart failure: Big Brother watching over you. Heart Fail Rev. 2015;20(1):107–116. doi: 10.1007/s10741-014-9449-4. [DOI] [PubMed] [Google Scholar]
- 50.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: Translating evidence into action. Health Aff. 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 51.Institute of Medicine. Health IT and Patient Safety: Building Safer Systems for Better Care. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 52.DeLanda M. A New Philosophy of Society: Assemblage Theory and Social Complexity. New York, NY: Bloomsbury Academic; 2006. [Google Scholar]
- 53.Rasmussen J. Information Processing and Human-Machine Interaction: An Approach to Cognitive Engineering. New York, NY: Elsevier Science; 1986. [Google Scholar]
- 54.Xiao Y. Artifacts and collaborative work in healthcare: methodological, theoretical, and technological implications of the tangible. J of Biomedi Inform. 2005;1(38):26–33. doi: 10.1016/j.jbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Krippendorff K. Content analysis. In: Barnouw E, Gerbner G, Schramm W, Worth TL, Gross L, editors. International Encyclopedia of Communication. New York, NY: Oxford University Press; 1989. pp. 403–407. [Google Scholar]