ABSTRACT
Medical therapy is the first-line option in glaucoma management, with benzalkonium chloride (BAC) being the most frequently used preservative in antiglaucoma medications. Its use is however, known to be associated with deleterious effects on the ocular surface. This review is an attempt to critically evaluate whether BAC really is indispensable for better bioavailability of antiglaucoma drugs and consequently, better IOP control.
How to cite this article: Louati Y, Shaarawy T. Controversy: Is Benzalkonium Chloride Necessary in Antiglaucoma Drops? J Current Glau Prac 2012;6(3):104-107.
Keywords: BAK, Ocular surface disease and glaucoma, Bioavailability, Preservatives in glaucoma medication, Preservative free glaucoma medication.
INTRODUCTION
Medical therapy using ocular drops is in most cases the first-line option in glaucoma management, with benzalkonium chloride (BAC) being the most frequently used preservative in glaucoma preparations. But increasing evidence of BAC toxicity on ocular surface has progressively led to the emergence of a whole new generation of BAC-free antiglaucoma medication whose efficacy is still questioned by many physicians, a majority of glaucoma patients throughout the world continuing to be prescribed BAC-preserved eye drops, with subsequent fierce debate on BAC necessity in ophthalmic drops.
The aim of the present review is to try to determine whether BAC really is useful/indispensable for better penetrance and better intraocular pressure (IOP) control based on updated literature and evidence.
What is BAC?
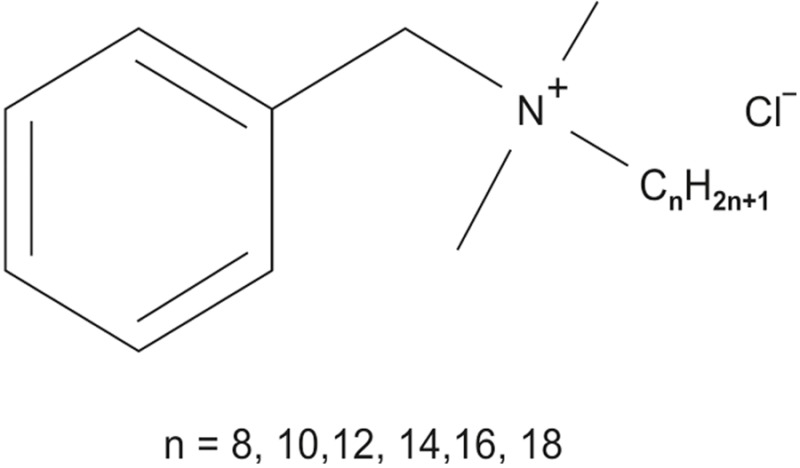
BAC is a nitrogenous cationic surface-acting agent belonging to the quaternary ammonium group that has an extremely wide range of applications with three major categories of use: As a biocide, as a cationic surfactant, as a phase transfer agent. BAC is used in skin antiseptics, hand sanitizers, high-level surgical instrument sterilizing and disinfection solutions, air and surface sprayable disinfectants, over-the-counter herpes cold sore and fever blister single-application treatments and eye and nasal drops as a preservative.
BAC use in Ophthalmology
Bottled antiglaucoma topical medications contain numerous ingredients including the drug itself, its vehicle, a preservative, as well as other chemicals preventing the drug from binding to the inner surface of the plastic container. The need for sterility in multidose eye drops bottles requires the inclusion of an antimicrobial preservative. BAC is a highly effective preservative and the most commonly used in antiglaucoma medications with concentrations ranging from 0.004 to 0.025% (Table 1).
Table 1: BAC concentration in antiglaucoma drops (%)
| Generic (Trade name) | Manufacturer | Preservative | |||
| Apraclonidine (Iopidine) | Alcon | 0.01% BAC | |||
| Betaxolol (Betoptic S suspension) | Alcon | 0.01% BAC | |||
| Bimatoprost (Lumigan 0.03%) | Allergan | 0.005% BAC | |||
| Bimatoprost (Lumigan 0.01%) | Allergan | 0.02% BAC | |||
| Bimatoprost/Timolol (Ganfort) | Allergan | 0.005% BAC | |||
| Brimonidine (Alphagan) | Allergan | 0.005% BAC | |||
| Brimonidine/Timolol (Combigan) | Allergan | 0.005% BAC | |||
| Brinzolamide (Azopt suspension) | Alcon | 0.01% BAC | |||
| Brinzolamide/Timolol (Azarga suspension) | Alcon | 0.01% BAC | |||
| Carteolol (Arteoptic) | Bausch & Lomb | 0.005% BAC | |||
| Dorzolamide (Trusopt) | MSD | 0.0075% BAC | |||
| Dorzolamide/Timolol (Cosopt) | MSD | 0.0075% BAC | |||
| Latanoprost (Xalatan) | Pfizer | 0.02% BAC | |||
| Latanoprost/Timolol (Xalacom) | Pfizer | 0.02% BAC | |||
| Levobunolol (Vistagan) | Allergan | 0.005% BAC | |||
| Timolol (Timoptic) | MSD | 0.01% BAC | |||
| Travoprost (Travatan) | Alcon | 0.015% BAC | |||
| Unoprostone (Rescula) | Novartis | 0.015% BAC |
Biological Activity
BAC biocidal activity is thought to be due to disruption of intermolecular interaction and dissociation of cellular membrane lipid bilayers which compromise cellular permeability control inducing leakage of cellular contents. BAC antimicrobial action is by means of dissolution of bacteria walls and membrane of their cellular contents that is unfortunately nonselective, exerting a toxic effect on human cells as well, even at low concentration (0.01%).1,2
Safety Questioned
A proper ophthalmic preparation must ensure drug penetration into the globe, adequate efficacy, acceptable side effects and measures to prevent microbial conta-mination.3
Preservatives acting as detergents, such as BAC, not only help maintaining ophthalmic bottles sterility. They also disrupt the superficial lipid layer of the precorneal tear film allowing for subsequent evaporation of the aqueous layer that shortens break up time. They reduce the number of goblets cells resulting in failure of corneal epithelial wetting and thus, precorneal tear film thinning and malfunction, superficial punctate epithelial erosions and even ulcers, especially in patients with preexisting dry eye syndrome (DES). Swan4 for example found that repeated use of BAC at concentrations of 1:5,000 (0.02%) or stronger can denature corneal protein and cause irreversible damage to the eye (Table 2).
Table 2: Frequency of symptoms reported by patients with preserved and preservative-free eyedrops at first visit33
|
Preserved eyedrops (n = 3469) |
Preservative-free eyedrops (n = 552) |
||||
| Discomfort upon instillation | 43% | 17%* | |||
| Foreign body sensation | 31% | 14% | |||
| Stinging or burning sensation | 40% | 22%* | |||
| Dry eye sensation | 23% | 14% | |||
| Tearing | 21% | 14% | |||
| Eyelid itching | 18% | 10% | |||
| Presence of symptoms of irritation between instillations | 61% | 36% |
*Preservative-free vs preserved comparison: p < 0.001 (χ2-test)
Even though DES is an age-associated condition like glaucoma itself with a reported prevalence of DES varying from 5.5. Up to 33.7%,5-11 symptoms of dry eye are reported in more than 60% of patients suffering from open-angle glaucoma suggesting higher incidence within this population.30,32 Described symptoms consist of foreign body sensation (31% in preserved group vs 14% in preserved-free), dry eyes (23% vs 14%), tearing (21% vs 14%), itchy eyelids (18% vs 10%), all of which are overall more frequent in patients taking preserved medications, observations that have been confirmed by very large-scale studies.12,33
Toxic and allergic conjunctivitis have also been reported with BAC use: Toxic effect by loss of contact between adjacent epithelial cells and cell death resulting from BAC insertion in cell membrane that reduces ionic resistance and increases water and ions influx leading to edema and cell damage, hence cell desquamation and ulcer.3 BAC also generates superoxide anions formation and immune inflammatory process involving Langerhaans cells that leads to reversible conjunctiva fibrosis13 that is associated with failure of glaucoma filtration surgery,14-17 because of excessive fibrotic postoperative wound healing induced by BAC.
Furthermore, studies have demonstrated that BAC use was associated with direct trabecular meshwork toxicity with significant cell death within 10 minutes of exposure to as little as 0.0001% BAC (1/100th of BAC concentration used in ophthalmic)18 leading to reduction of trabecular function and potentially worsening of the condition. These findings are of particular concern since we now know that trabecular meshwork cells within the meshwork were found to be statically lower in patients with primary open-angle glaucoma.19
BAC has also been incriminated in the development of cataract with higher incidence in the eyes exposed to preserved topical glaucoma therapy as compared to preservative free in a large prospective randomized study as well as to postoperative cystoid macular edema after cataract surgery.20,21,34,35
Necessity Questioned
It has been suggested that through its detergent activity BAC facilitates drug penetration into the eye and thus enhances its efficacy. But many studies have now shown equal efficacy between same class preserved and preservative-free antiglaucoma medication and equal or improved tolerance22-26 making this past belief close to obsolete.
Besides industries have already developed alternatives to BAC, ranging from apparently less toxic preservatives (Polyquad, Purite, Sofzia…) to subtle mechanisms that already guaranty multi dose bottles sterility in antiallergic and lubricant preparations (ABAK® COMOD® antibacterial film, AADSTM® silver coil combined with airless pump, VISMED® system), through a whole new generation of preservative free single-dose units of antiglaucoma preparations.
Alternatives to BAC
Clinical studies have now demonstrated that preservative-free formulations of antiglaucoma medications have the same efficacy as preserved formulations, achieving equivalent reductions of intraocular pressure (Table 3).23-26
Table 3: Currently available preservative-free and BAC-free antiglaucoma preparations
| Beta blockers: Timolol, carteolol, betaxolol, levobunolol | Miotic: Pilocarpine | ||
| Carbonic anhydrase inhibitors: Dorzolamide | Alpha-agonists: Apraclonidine, brimonidine | ||
| Combination: Cosopt (dorzolamide + timolol) | Prostaglandins: Tafluprost, travoprost |
Besides, Jong et al reported that switching patients with glaucoma from preserved to preservative-free medication reduced the permeability of the corneal epithelium, suggesting improvement in epithelial function.
Ammar et al20 demonstrated that substitution of BAC from topical ophthalmic drugs results in greater viability of cultured trabecular meshwork cells, suggesting better trabeculum meshwork function in patients in whom aqueous outflow is already compromised.
A recent large European Study assessed ocular symptoms in a total of 9,658 patients before and after switching from preserved to preservative-free eyedrops and demonstrated that stinging or burning sensation occurred in 48% of patients receiving preserved eyedrops compared with only 20% of those who received preservative-free eyedrops, whereas dry eye sensation was reported in 35 and 16% of the 2 groups, respectively. Similar reductions in the incidence of reported symptoms occurred in patients who reduced their exposure to benzalkonium.12
Perspective
Studies have already shown solid evidence that commercially available preservative-free antiglaucoma formulations do offer clinical benefits to patients in term of safety and efficacy. Care should therefore be taken from now on to avoid long-term use of preservatives when possible, single dose units manufacturing and packaging still make them expensive and more difficult to use as compared to multiple dose bottles especially for older patients (hand arthritis for instance). Otherwise preparations with less toxic preservative should be developed especially for patients with the greatest exposure to high doses and/or prolonged treatments, for those suffering from preexisting ocular surface disease and those experiencing side-effects related to the ocular surface because of their current treatment.
Preservative-free drops emergence represents a real hope for global improvement in glaucoma patients care because of equally efficacious, less toxic and therefore more tolerated treatment possibilities, as compared to preserved drops, and thus increased likelihood of adherence to the treatment prescribed, all of which is responsible for better visual health, better quality of life and less use of health care resources.3,12,14,26-31
Footnotes
Source of support: Nil
Conflict of interest: None declared
Contributor Information
Y Louati, Clinical Fellow, University of Geneva Hospitals, Glaucoma Sector, Geneva Switzerland.
T Shaarawy, Assistant Physician and Head, Department of Ophthalmology, University Hospitals of Geneva Glaucoma Sector, Geneva, Switzerland.
REFERENCES
- 1.Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004 Jul;23(5):490–496. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- 2.Bernal LD, Ubels JL. Quantitative evaluation of the corneal epithelial barrier: effect of artificial tears and preservatives. Curr Eye Res. 1991 Jul;10(7):645–656. doi: 10.3109/02713689109013856. [DOI] [PubMed] [Google Scholar]
- 3.Hopes M, Broadway DC. Preservative-free treatment in glaucoma is a sensible and realistic aim for the future. Eur Ophthalmol Rev. 2010;4:23–28. [Google Scholar]
- 4.Swan KC. Reactivity of the ocular tissues to wetting agents. Am J Ophthalmol. 1944;27:118. [Google Scholar]
- 5.Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye study. Ophthalmology. 2003 Jun;110(6):1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 6.Moss SE, Klein R, Klein BE. Prevalence and risk factors for dry eye syndrome. Arch Ophthalmol. 2000 Sep;118(9):1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 7.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003 Aug;136(2):318–322. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, Tan DT. Prevalence and risk factors associated with dry eye symptoms: a population-based study in Indonesia. Br J Ophthalmol. 2002 Dec;86(12):1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of a dry eye in Melbourne, Australia. Ophthalmology. 1998 Jun;105(6):1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 10.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye study. Clin Experiment Ophthalmol. 2003 Jun;31(3):229–232. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 11.Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997 Dec;124(6):723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 12.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007 May-Jun;17(3):341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 13.Broadway DC, Grierson I, Stürmer J, Hitchings RA. Reversal of topical antiglaucoma medication effects on the conjunctiva. Arch Ophthalmol. 1996;114:262–267. doi: 10.1001/archopht.1996.01100130258004. [DOI] [PubMed] [Google Scholar]
- 14.Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994 Nov;112(11):1437–1445. doi: 10.1001/archopht.1994.01090230051020. [DOI] [PubMed] [Google Scholar]
- 15.Baudouin C, Pisella PJ, Fallacier K, Goldschild M, Becquet F, De Saint Jean M, Béchetoille A. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999 Mar;106(3):556–563. doi: 10.1016/S0161-6420(99)90116-1. [DOI] [PubMed] [Google Scholar]
- 16.Aritürk N, Oge I, Baris S, Erkan D, Süllü Y, Koc F. The effects of antiglaucomatous agents on conjunctiva used for various durations. Int Ophthalmol 1996-1997. 20(1-3):57–62. doi: 10.1007/BF00212947. [DOI] [PubMed] [Google Scholar]
- 17.Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994 Nov;112(11):1446–1454. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- 18.Ammar D, Kahook MY. Effects of glaucoma medications and preservatives on cultured human trabecular meshwork and non-pigmented ciliary epithelial cell lines. Br J Ophthalmol. 2011 Oct;95(10):1466–1469. doi: 10.1136/bjophthalmol-2011-300012. [DOI] [PubMed] [Google Scholar]
- 19.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984 Jun;91(6):564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekaran S, Cumming RG, Rochtchina E, Mitchell P. Associations between elevated intraocular pressure and glaucoma, use of glaucoma medications and 5-year incident cataract: the Blue Mountains Eye Study. Ophthalmology. 2006 Mar;113(3):417–424. doi: 10.1016/j.ophtha.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Herman DC, Gordon MO, Beiser JA, Chylack LT Jr, Lamping KA, Schein OD, Soltau JB, Kass MA. Topical ocular hypotensive medication and lens opacification: evidence from the ocular hypertension treatment study. Am J Ophthalmol. 2006 Nov;142(5):800–810. doi: 10.1016/j.ajo.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Easty DL, Nemeth-Wasmer G, Vounatsos JP, Girard B, Besnainou N, Pouliquen P, Delval L, Rouland JF. Comparison of a non-preserved 0.1% T-Gel eye gel (single dose unit) with preserved 0.1% T-Gel eye gel (multidose) in ocular hypertension and glaucomatous patient. Br J Ophthalmol. 2006 May;90(5):574–578. doi: 10.1136/bjo.2005.080424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998 Jan;82(1):39–42. doi: 10.1136/bjo.82.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis RA, Katz GJ, Weiss MJ, Landry TA, Dickerson JE, James JE, Hua SY, Sullivan EK, Montgomery DB, Wells DT et al. Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007 Jan;16(1):98–103. doi: 10.1097/01.ijg.0000212274.50229.c6. [DOI] [PubMed] [Google Scholar]
- 25.Uusitalo H, Chen E, Pfeiffer N, Brignole-Baudouin F, Kaarniranta K, Leino M, Puska P, Palmgren E, Hamacher T, Hofmann G. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 2010 May;88(3):329–336. doi: 10.1111/j.1755-3768.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamacher T. Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: results from a pharmacodynamics analysis. Acta Ophthalmol Suppl (Oxf) 2008;242:14–19. doi: 10.1111/j.1755-3768.2008.01381.x. [DOI] [PubMed] [Google Scholar]
- 27.Nordmann JP, Auzanneau N, Ricard S, Berdeaux G. Vision related quality of life and topical glaucoma treatment side effects. Health Qual Life Outcomes. 2003 Dec;1:75. doi: 10.1186/1477-7525-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levrat F, Pisella PJ, Baudouin C. Clinical tolerance of antiglaucoma eyedrops with and without a preservative. Results of an unpublished survey in Europe. J Fr Ophthalmol. 1999 Mar;22(2):186–191. (Fre). [PubMed] [Google Scholar]
- 29.Clouzeau C, Godefroy D, Riancho L, Rostène W, Baudouin C, Brignole-Baudouin F. Hyperosmolarity potentiates toxic effects of benzalkonium chloride on conjunctival epithelial cells in vitro. Mol Vis. 2012 Apr;18:851–863. [PMC free article] [PubMed] [Google Scholar]
- 30.Baudouin C. The ocular surface in glaucoma. Cornea. 2009 Oct;28(9 suppl):14S–19S. [Google Scholar]
- 31.Samples JR, Binder PS, Nayak S. The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells. Exp Eye Res. 1989 Jul;49(1):1–12. doi: 10.1016/0014-4835(89)90071-7. [DOI] [PubMed] [Google Scholar]
- 32.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008 Aug;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 33.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002 Apr;86(4):418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyake K, Ibaraki N, Goto Y, Oogiya S, Ishigaki J, Ota I, Miyake S. ESCRS Binkhorst lecture 2002: Pseudophakic preservative maculopathy. J Cataract Refract Surg. 2003 Sep;29(9):1800–1810. doi: 10.1016/s0886-3350(03)00560-1. [DOI] [PubMed] [Google Scholar]
- 35.Miyake K, Ota I, Ibaraki N, Akura J, Ichihashi S, Shibuya Y, Maekubo K, Miyake S. Enhanced disruption of the blood-aqueous barrier and the incidence of angiographic cystoid macular edema by topical timolol and its preservative in early postoperative pseudophakia. Arch Ophthalmol. 2001 Mar;119(3):387–394. doi: 10.1001/archopht.119.3.387. [DOI] [PubMed] [Google Scholar]


