Abstract
Background:
Human papillomavirus (HPV) is the most common viral sexually-transmitted infection. Despite HPV infection is associated with several malignant disorders including penile and anal cancers, little is known about the epidemiology of HPV infection in males, particularly in developing countries.
Objectives:
The aim of this study was to determine the prevalence of HPV infection and its genotype distribution among Iranian males.
Patients and Methods:
Between March 2009 and April 2014, a total number of 483 males, referred to Iran University of Medical Sciences-affiliated sexually transmitted infections (STI) clinics, were enrolled in this study. Following DNA extraction, HPV detection and genotyping were performed using INNO-LiPA HPV Genotyping Extra assay. To analyze the association of HPV infection and age, the logistic regression was employed.
Results:
No statistical association between HPV infection and age was observed (P = 0.469). Furthermore, there was no statistically significant correlation between HR HPV infection and age (P = 0.330).
Conclusions:
In this investigation, the prevalence of HPV infection was relatively substantial. Totally, 17 different HPV genotypes were detected and the most frequently detected genotypes were HPV6, HPV11, HPV16, HPV18 and HPV52, respectively. The data from this study is essential for planning future public health strategies including HPV vaccination programs.
Keywords: Genotype, Infection, Human Papillomavirus
1. Background
Human papillomavirus (HPV) infection of the anogenital tract is the most prevalent viral sexually-transmitted infection in males and females, with a broad range of clinical manifestations and consequences varying from subclinical and self-limited to persistent and related with malignant progression (1, 2). Persistent infection with HPV is a well-established cause of cervical cancer and there has been massive advancements in the characterization of the natural history of cervical HPV infection in females (3-10). However, there is a lack of knowledge with regard to the natural history of HPV infection in males, especially in developing countries (2).
HPV infection is associated with several malignant disorders in males, including penile, anal, and oral cancers (11-14). Moreover, HPV is responsible for the development of condylomata acuminata (genital warts) and other nonmalignant diseases such as recurrent respiratory papillomatosis and oral papillomas. Most HPV-related cancers in males are associated with HPV16/18 and nearly all HPV-positive nonmalignant diseases are caused by HPV6/11 (15-18). Recently, it has been revealed that HPV infection in males has been associated with the increased risk of human immunodeficiency virus (HIV) infection acquisition (19). Taking into account that HPV is a sexually-transmitted virus, HPV infection in males may result in considerable diseases in females (2). Therefore, HPV infection in males is a significant clinical matter. With the advent of effective prophylactic vaccines against oncogenic HPV genotypes, understanding of the burden of HPV infection and its genotype-specific prevalence in males has attracted enormously growing attention (20, 21)
2. Objectives
The aim of this study was to assess the prevalence of genital HPV infection and its genotype distribution among Iranian males which might be useful for designing public health policies and prevention measures, including vaccination.
3. Patients and Methods
3.1. Patients
A total of 483 males, referred to Iran University of Medical Sciences-affiliated sexually transmitted infections (STI) clinics for genital HPV testing and genotyping during March 2009 through April 2014, were enrolled in this cross-sectional study. The inclusion criteria included the presence of a genital lesion, no symptoms and having an HPV-positive partner, no symptoms and a desire to screening for sexually-transmitted infections. This investigation was approved by the ethical committee of Tehran University of Medical Sciences and informed consents were obtained from all the participants. Exfoliated epithelial cells were collected from the urethra, penile shaft, glans, scrotum and anus with sterilized brush or Dacron swab (22). The samples were placed into standard transport medium and stored at -80°C prior to HPV detection and genotyping.
3.2. DNA Extraction and INNO-LiPA HPV Genotyping Assay
Total DNA was extracted using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. HPV genotyping was carried out using the INNO-LiPA HPV genotyping extra assay (Innogenetics NV, Ghent, Belgium), according to the manufacturer’s instructions. The INNO-LiPA HPV assay is one of the most widely used HPV genotyping tests based on the concept of reverse hybridization, planned for the identification of 28 different genotypes of HPV including 15 HR genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82 ), three probable HR (pHR) genotypes (26, 53 and 66), seven LR genotypes (6, 11, 40, 43, 44, 54 and 70) and three genotypes (69, 71 and 74) which are not categorized as HR, pHR or LR genotypes, based on Munoz et al. (23). A 65 bp region of HPV L1 gene was amplified using consensus SPF10 primers, followed by denaturation and hybridization of the resulting biotinylated amplicons, with specific oligonucleotide probes fixed on membrane strips (24). To control the specimen quality and DNA extraction an additional primer pair targeting, the human HLA-DPB1 gene was included.
After PCR amplification using the INNO-LiPA HPV Genotyping Extra Amp, the amplified biotinylated product was denatured using an alkaline solution. The denatured PCR product was then hybridized to probes immobilized on membrane strips. Thereafter, streptavidin-conjugated alkaline phosphatase was added, which binds to any biotinylated PCR product/probe hybrid previously formed. Finally, the PCR product bound to a specific probe was detected by adding BCIP/NBT chromogen. The results were interpreted visually using the interpretation chart provided.
3.3. Statistical Analysis
Data analysis was performed using SPSS version 16 software (SPSS Inc., Chicago, IL, USA). The association of HPV infection and age was evaluated using the logistic regression test. A P value < 0.05 was considered as statistically significance. 95% CI for prevalence estimations was performed using an online confidence intervals calculator (https://www.mccallum-layton.co.uk).
4. Results
A total of 483 males were enrolled in this investigation. The mean age of the participants was 34.4 ± 8.5 (range: 15 - 76). Table 1 shows the prevalence of HPV genotypes. Totally, HPV DNA was detected in 269 (55.7%, 95% CI: 51.2 - 60.1%) of the subjects. HPV6 was the predominant HPV genotype detected overall (46.2%). HPV11 was confirmed as the second most common genotype (8.1%).
Table 1. The Genital Prevalence of Human Papillomavirus Genotypes in Iranian Males (n = 483)a.
| HPV Genotypes | No. | Percent (95% CI) |
|---|---|---|
| Any HPV genotypes | 269 | 55.7 (51.2 - 60.1) |
| High-risk | 46 | 9.5 (7.2 - 12.5) |
| 16 | 11 | 2.3 (1.2 - 4) |
| 18 | 9 | 1.9 (1 - 3.5) |
| 31 | 3 | 0.6 (0.2 - 1.8) |
| 33 | 3 | 0.6 (0.2 - 1.8) |
| 39 | 3 | 0.6 (0.2 - 1.8) |
| 45 | 1 | 0.2 (0.04 - 1.2) |
| 51 | 4 | 0.8 (0.3 - 2.1) |
| 52 | 9 | 1.9 (1 - 3.5) |
| 58 | 2 | 0.4 (0.1 - 1.5) |
| 82 | 1 | 0.2 (0.04 - 1.2) |
| Probable high-risk | 4 | 0.8 (0.3 - 2.1) |
| 53 | 1 | 0.2 (0.04 - 1.2) |
| 66 | 3 | 0.6 (0.2 - 1.8) |
| Low-risk | 268 | 55.5 (51.1 - 59.9) |
| 6 | 223 | 46.2 (41.8 - 50.1) |
| 11 | 39 | 8.1 (6 - 10.8) |
| 43 | 1 | 0.2 (0.04 - 1.2) |
| 44 | 4 | 0.8 (0.3 - 2.1) |
| 54 | 1 | 0.2 (0.04 - 1.2) |
aAbbreviation: HPV, human papillomavirus.
Forty six (9.5%, 95% CI: 7.2 - 12.5%) males were positive for HR HPV genotypes. The most prevalent HR genotype was HPV16 (2.3%), followed by HPV18 (1.9%) and HPV52 (1.9%). LR HPV genotypes were detected in 268 (55.5%, 95% CI: 51.1 - 59.9%) subjects. The most common LR genotypes were HPV6 and HPV11. The prevalence of infection with multiple HPV genotypes or coinfection determined by two or more HPV genotypes is shown is Table 2. Infection with multiple HPV genotypes was observed in 38 (7.9%, 95% CI: 5.8 - 10.6%) of the study participants (14.1% (95% CI: 10.5 - 18.8%) of HPV-positive cases). Of those with multiple HPV genotypes infection, 31 (6.4%) had coinfection with two genotypes, 4 (0.8%) had coinfection with three genotypes, 2 (0.4%) had coinfection with four genotypes, and only 1 (0.2%) had coinfection with five genotypes.
Table 2. The Prevalence of Infection With Multiple Human Papillomavirus Genotypes in Iranian Males (n = 483)a.
| No. | Percent (95% CI) | |
|---|---|---|
| Multiple HPV genotypes infection | 38 | 7.9 (5.8 - 10.6) |
| Multiple HR HPV genotypes infection | 4 | 0.8 (0.3 - 2.1) |
| Multiple LR HPV genotypes infection | 9 | 1.9 (0.1 - 3.5) |
| Infection with both HPV16 and HPV18 genotypes | None | 0 (0) |
| Infection with both HPV6 and HPV11 genotypes | 6 | 1.2 (0.6 - 2.7) |
| Infection with HPV6, HPV11, HPV16 and HPV18 genotypes | None | 0 (0) |
| Number of HPV genotypes | ||
| 1 | 231 | 47.8 (43.4 - 52.3) |
| 2 | 31 | 6.4 (4.6 - 9) |
| 3 | 4 | 0.8 (0.3 - 2.1) |
| 4 | 2 | 0.4 (0.1 - 1.5) |
| 5 | 1 | 0.2 (0.04 - 1.2) |
aAbbreviations: HPV, human papillomavirus; HR, high-risk; LR, low-risk.
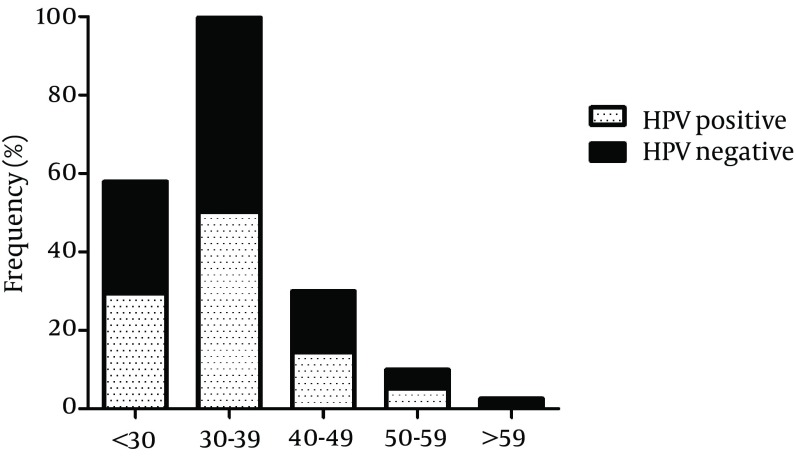
Overall, 42 cases (8.7%, 95% CI: 6.5 - 11.5%) were infected with at least one HR HPV genotype and at least one LR HPV genotype was detected in 258 (53.4%) of the participants. Multiple HR HPV genotypes and multiple LR HPV genotypes infections were found in 4 (0.8%, 95% CI: 0.3 - 2.1%) and 9 (1.9%, 95% CI: 0.1 - 3.5%) of the study samples, respectively. In particular, 20 (4.1%, 95% CI: 2.7 - 6.3%) cases were infected by HPV16 or HPV18. However, concomitant infection with both HPV16 and HPV18 was not observed. In 250 (51.8%, 95% CI: 47.3 - 56.2%) cases, infections with HPV6 or HPV11 were found, while coinfection with both HPV6 and HPV11 was observed in only 6 (1.2%, 95% CI: 0.6 - 2.7%) subjects. pHR genotypes were detected in 4 (0.8%, 95% CI: 0.3 - 2.1%) cases and not-categorized genotypes were not found in any of the cases. Figure 1 shows the age-specific prevalence of HPV infection. The highest prevalence of HPV was detected in males aged between 30 - 39 years and the lowest prevalence was seen in those more than 59 years old. However, using logistic regression, there was no statistical association between HPV infection and age (P = 0.469). In addition, there was no significant correlation between age and HR HPV infection (P = 0.330) or LR HPV infection (P = 0.346).
Figure 1. Age-Specific Prevalence of Genital Human Papillomavirus Infection in Iranian Males.
5. Discussion
The identification of HPV infection and its genotyping description in males is a serious clinical issue due to the strong association of persistent HPV infection and several cancers in males. In addition, males have an important role in the transmission of HPV to females (1). The knowledge of HPV infection in males seems to be necessary for public health policies and males’ vaccination with HPV vaccine. However, data about the epidemiology of HPV infection in Iranian males are considerably low. The present cross-sectional study described the prevalence of genital HPV infection and HPV genotype distribution in Iranian males. To our knowledge, this was one of the largest epidemiological studies reporting the prevalence of HPV infection and its genotype distribution in males.
In this study, the prevalence of HPV infection (any genotype) was relatively high (54.8%). This finding is in agreement with other published reports, which showed the prevalence of about 50% in STI clinic attendees (25, 26). Totally, 17 different HPV genotypes were detected in the study population. The most prevalent HPV genotypes were HPV6 (46.2%) and HPV11 (8.1%), in line with previous reports (15, 27, 28). In agreement with several previous studies investigating the prevalence and genotype distribution of HPV in male genital warts (15, 27, 29-31), HPV16 was confirmed as the most common HR HPV genotype and the third most common HPV genotype detected after HPV6 and HPV11 in this study. Furthermore, these findings are consonant with those of Freire et al. (32) who assessed participants similar with to our study samples. The results also placed HPV18 and HPV52 as the second most prevalent HR genotypes. Therefore, it could be claimed that vaccine-targeted HPV genotypes (HPV6, HPV11, HPV16, and HPV18) were among the commonly detected HPV genotypes in our investigation. Potentially, the availability of an effective vaccine against theses genotypes may allow us to prevent the most common HPV genotypes in Iranian males.
Infection with multiple HPV genotypes which was found to associate with the increased risk of HPV persistence (33) was relatively low in the present study. We detected infection with multiple HPV genotypes in 14.1% of HPV-positive cases. This finding is in disagreement with several reports, showing the rate of infection with multiple HPV genotypes to be 33.8% (15), 56.7% (27) and 59.7% (32). This variation between the studies could be explained by differences in sampling approaches, the HPV detection protocols employed, and geographical variations in HPV genotypes distribution. While the prevalence of HPV infection was the highest among males aged 30 - 39 years, no significant association was identified between HPV infection and age in this investigation (P = 0.469). The absence of association between HPV infection and age was also reported by several studies (14, 32, 34-36).
This study provided beneficial information about the epidemiology of genital HPV infection in Iranian males, which should be applied for evaluating the efficiency of HPV vaccines for the prevention of vaccine-targeted HPV genotypes. However, this report should be interpreted with caution, due to the STI clinic setting of this investigation. Therefore, this data could not be generalizable to the general population of Iranian males. In conclusion, the prevalence of HPV infection was relatively high. Totally, 17 different HPV genotypes were detected and the most frequently detected genotypes were HPV6, HPV11, HPV16, HPV18 and HPV52, respectively.
Acknowledgments
The authors would like to thank the directors and staff of Iran University of Medical Sciences-affiliated STI clinics for their assistance in sample collection.
Footnotes
Authors’ Contribution:Mostafa Salehi-Vaziri, Farzin Sadeghi and Farah Bokharaei-Salim developed the study concept, performed the experimental protocols and prepared the manuscript. Hossein Keyvani, Sarang Younesi, Samaneh Alinaghi and Seyed Hamidreza Monavari carried out administrative, technical, and material support.
Funding/Support:This study was financially supported by a grant from Tehran university of medical sciences (Project code: 92-01-30-21080).
References
- 1.Giuliano AR, Anic G, Nyitray AG. Epidemiology and pathology of HPV disease in males. Gynecol Oncol. 2010;117(2 Suppl):S15–9. doi: 10.1016/j.ygyno.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palefsky JM. Human papillomavirus-related disease in men: not just a women's issue. J Adolesc Health. 2010;46(4 Suppl):S12–9. doi: 10.1016/j.jadohealth.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Who Ico Information Centre on HPV, Cervical C. HPV and cervical cancer in the 2007 report. Vaccine. 2007;25 Suppl 3:C1–230. doi: 10.1016/S0264-410X(07)01183-8. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22(12):2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 5.Kjaer SK, Tran TN, Sparen P, Tryggvadottir L, Munk C, Dasbach E, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196(10):1447–54. doi: 10.1086/522863. [DOI] [PubMed] [Google Scholar]
- 6.Roteli-Martins CM, de Carvalho NS, Naud P, Teixeira J, Borba P, Derchain S, et al. Prevalence of human papillomavirus infection and associated risk factors in young women in Brazil, Canada, and the United States: a multicenter cross-sectional study. Int J Gynecol Pathol. 2011;30(2):173–84. doi: 10.1097/PGP.0b013e3181f38dfe. [DOI] [PubMed] [Google Scholar]
- 7.Ting J, Kruzikas DT, Smith JS. A global review of age-specific and overall prevalence of cervical lesions. Int J Gynecol Cancer. 2010;20(7):1244–9. doi: 10.1111/igc.0b013e3181f16c5f. [DOI] [PubMed] [Google Scholar]
- 8.Natphopsuk S, Settheetham-Ishida W, Pientong C, Sinawat S, Yuenyao P, Ishida T, et al. Human papillomavirus genotypes and cervical cancer in northeast Thailand. Asian Pac J Cancer Prev. 2013;14(11):6961–4. doi: 10.7314/apjcp.2013.14.11.6961. [DOI] [PubMed] [Google Scholar]
- 9.Hamzi Abdul Raub S, Isa NM, Zailani HA, Omar B, Abdullah MF, Mohd Amin WA, et al. Distribution of HPV genotypes in cervical cancer in multi- ethnic Malaysia. Asian Pac J Cancer Prev. 2014;15(2):651–6. doi: 10.7314/apjcp.2014.15.2.651. [DOI] [PubMed] [Google Scholar]
- 10.Niakan M, Yarandi F, Entezar M. Human papillomavirus (HPV) detection in biopsies from cervical cancer patients; A population–based study from Iran. Arch Clin Infect Dis. 2009;4(1):35–7. [Google Scholar]
- 11.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 12.Ryan DP, Mayer RJ. Anal carcinoma: histology, staging, epidemiology, treatment. Curr Opin Oncol. 2000;12(4):345–52. doi: 10.1097/00001622-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116(4):606–16. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 14.Anic GM, Giuliano AR. Genital HPV infection and related lesions in men. Prev Med. 2011;53 Suppl 1:S36–41. doi: 10.1016/j.ypmed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubin F, Pretet JL, Jacquard AC, Saunier M, Carcopino X, Jaroud F, et al. Human papillomavirus genotype distribution in external acuminata condylomata: a Large French National Study (EDiTH IV). Clin Infect Dis. 2008;47(5):610–5. doi: 10.1086/590560. [DOI] [PubMed] [Google Scholar]
- 16.Donne AJ, Hampson L, Homer JJ, Hampson IN. The role of HPV type in Recurrent Respiratory Papillomatosis. Int J Pediatr Otorhinolaryngol. 2010;74(1):7–14. doi: 10.1016/j.ijporl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Menton JF, Cremin SM, Canier L, Horgan M, Fanning LJ. Molecular epidemiology of sexually transmitted human papillomavirus in a self referred group of women in Ireland. Virol J. 2009;6:112. doi: 10.1186/1743-422X-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartwig S, Syrjanen S, Dominiak-Felden G, Brotons M, Castellsague X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer. 2012;12:30. doi: 10.1186/1471-2407-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin-Hong PV, Husnik M, Cranston RD, Colfax G, Buchbinder S, Da Costa M, et al. Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS. 2009;23(9):1135–42. doi: 10.1097/QAD.0b013e32832b4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–67. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Karimi Zarchi M, Behtash N, Chiti Z, Kargar S. Cervical cancer and HPV vaccines in developing countries. Asian Pac J Cancer Prev. 2009;10(6):969–74. [PubMed] [Google Scholar]
- 22.Barzon L, Militello V, Pagni S, Franchin E, Dal Bello F, Mengoli C, et al. Distribution of human papillomavirus types in the anogenital tract of females and males. J Med Virol. 2010;82(8):1424–30. doi: 10.1002/jmv.21733. [DOI] [PubMed] [Google Scholar]
- 23.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 24.Poljak M, Kocjan BJ. Commercially available assays for multiplex detection of alpha human papillomaviruses. Expert Rev Anti Infect Ther. 2010;8(10):1139–62. doi: 10.1586/eri.10.104. [DOI] [PubMed] [Google Scholar]
- 25.Wikstrom A, Lidbrink P, Johansson B, von Krogh G. Penile human papillomavirus carriage among men attending Swedish STD clinics. Int J STD AIDS. 1991;2(2):105–9. doi: 10.1177/095646249100200205. [DOI] [PubMed] [Google Scholar]
- 26.Svare EI, Kjaer SK, Worm AM, Osterlind A, Meijer CJ, van den Brule AJ. Risk factors for genital HPV DNA in men resemble those found in women: a study of male attendees at a Danish STD clinic. Sex Transm Infect. 2002;78(3):215–8. doi: 10.1136/sti.78.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan PK, Luk AC, Luk TN, Lee KF, Cheung JL, Ho KM, et al. Distribution of human papillomavirus types in anogenital warts of men. J Clin Virol. 2009;44(2):111–4. doi: 10.1016/j.jcv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Afshar RM, Mollaie HR, Fazlalipour M, Arabzadeh SA. Prevalence and type distribution of human papillomavirus infection using the INNo-Lipa assay, Kerman, Southeast Iran. Asian Pac J Cancer Prev. 2013;14(9):5287–91. doi: 10.7314/apjcp.2013.14.9.5287. [DOI] [PubMed] [Google Scholar]
- 29.Anic GM, Lee JH, Stockwell H, Rollison DE, Wu Y, Papenfuss MR, et al. Incidence and human papillomavirus (HPV) type distribution of genital warts in a multinational cohort of men: the HPV in men study. J Infect Dis. 2011;204(12):1886–92. doi: 10.1093/infdis/jir652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37(10):3316–22. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandepapeliere P, Barrasso R, Meijer CJ, Walboomers JM, Wettendorff M, Stanberry LR, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192(12):2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- 32.Freire MP, Pires D, Forjaz R, Sato S, Cotrim I, Stiepcich M, et al. Genital prevalence of HPV types and co-infection in men. Int Braz J Urol. 2014;40(1):67–71. doi: 10.1590/S1677-5538.IBJU.2014.01.10. [DOI] [PubMed] [Google Scholar]
- 33.Lajous M, Mueller N, Cruz-Valdez A, Aguilar LV, Franceschi S, Hernandez-Avila M, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1710–6. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 34.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194(8):1044–57. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 35.Hagensee ME, Kiviat N, Critchlow CW, Hawes SE, Kuypers J, Holte S, et al. Seroprevalence of human papillomavirus types 6 and 16 capsid antibodies in homosexual men. J Infect Dis. 1997;176(3):625–31. doi: 10.1086/514082. [DOI] [PubMed] [Google Scholar]
- 36.Slavinsky J3, Kissinger P, Burger L, Boley A, DiCarlo RP, Hagensee ME. Seroepidemiology of low and high oncogenic risk types of human papillomavirus in a predominantly male cohort of STD clinic patients. Int J STD AIDS. 2001;12(8):516–23. doi: 10.1258/0956462011923615. [DOI] [PubMed] [Google Scholar]