A new dehydration method to improve the diffraction resolution of protein crystals is reported.
Keywords: protein crystal, dehydration, diffraction resolution, Cas5a, LptA
Abstract
The production of high-quality crystals is one of the major obstacles in determining the three-dimensional structure of macromolecules by X-ray crystallography. It is fairly common that a visually well formed crystal diffracts poorly to a resolution that is too low to be suitable for structure determination. Dehydration has proven to be an effective post-crystallization treatment for improving crystal diffraction quality. Several dehydration methods have been developed, but no single one of them is suitable for all crystals. Here, a new convenient and effective dehydration method is reported that makes use of a dehydrating solution that will not dry out in air for several hours. Using this dehydration method, the resolution of Archaeoglobus fulgidus Cas5a crystals has been increased from 3.2 to 1.95 Å and the resolution of Escherichia coli LptA crystals has been increased from <5 to 3.4 Å.
1. Introduction
The production of high-quality crystals is still a bottleneck in X-ray crystallography, the premier method for determining the three-dimensional structures of macromolecules. Crystal growth remains an empirical and tedious process. Moreover, obtaining a crystal is not always a guarantee of successful determination of the structure. A relatively frequent occurrence is that the crystal obtained is of poor quality (most commonly diffracting to only low resolution) and not suitable for X-ray diffraction studies. Before giving up on these poor-quality crystals, there are several quick and simple post-crystallization methods that are worth considering. Crystal annealing, dehydration, high-pressure cryocooling, post-crystallization soaking and cross-linking have been reported to dramatically improve the diffraction resolution of macromolecular crystals (Heras & Martin, 2005 ▸; Harp et al., 1998 ▸; Kriminski et al., 2002 ▸; Schick & Jurnak, 1994 ▸; Petock et al., 2001 ▸; Holyoak et al., 2003 ▸; Quiocho & Richards, 1964 ▸; Lusty, 1999 ▸; Kim et al., 2005 ▸).
Of all these treatments, dehydration has proven to be the most effective. Loose packing of molecules and high solvent content are common problems that result in poor-quality diffraction. Correspondingly, it is well established that in some cases reduction of solvent content can result in more closely packed and better ordered crystals that diffract to higher resolution (Salunke et al., 1985 ▸; Frey, 1994 ▸; Krauss et al., 2012 ▸; Newman, 2006 ▸; Heras et al., 2003 ▸). Several different dehydration protocols have been developed.
(i) The simplest method is air dehydration, in which a loop-mounted crystal is held in air for a short time before flash-cooling. During this time, the evaporation of water can be increased in a controlled manner by irradiation with an IR laser (Kiefersauer et al., 2014 ▸). Alternatively, the cover slip over a well can be removed and the droplet of mother liquor containing the crystal allowed to equilibrate against air for up to 4 h before the crystal is mounted (Samygina et al., 2000 ▸). A variant of this method is to add a large volume of cryoprotectant buffer to the drop before equilibrating against air (Haebel et al., 2001 ▸).
(ii) A crystal is picked up from the mother drop and soaked in a dehydrating solution for minutes to days. If the crystal is sensitive to osmotic shock, dehydration is carried out by a serial transfer of the crystal to droplets of dehydrating solution of increasing concentration. At each concentration, the incubation time varies from several minutes to days. The dehydrating solution is usually the original mother liquor either with a higher concentration of the precipitant or supplemented with cryoprotectants such as glycerol, ethylene glycol, MPD or PEG 400. Sometimes a precipitant other than that used in the mother liquor is included in the dehydrating solution (Schick & Jurnak, 1994 ▸; Bowman et al., 2004 ▸; Esnouf et al., 1998 ▸).
(iii) When a crystal is grown using the hanging-drop method, dehydration can be carried out by replacing the reservoir solution with dehydrating solution (Malay et al., 2005 ▸). This is the most widely used dehydration method. If the crystal is sensitive to fast dehydration, the method can be modified to a serial transfer of the cover slip holding the mother droplet over reservoirs containing increasing concentrations of dehydrating solution (Bailly et al., 2009 ▸; Wojdyla et al., 2009 ▸; Heras et al., 2003 ▸). A combined method may also be used in which equilibration of a hanging drop against dehydrating solution is followed by dipping a mounted crystal into another dehydrating solution (Igura et al., 2005 ▸).
(iv) Another method uses a special device to control the relative humidity around the crystal, and hence its dehydration, such as the Free Mounting System developed by the Huber group (Kiefersauer et al., 2000 ▸), which is now sold by Rigaku under the name Proteros, and the HC1b dehydration device developed at EMBL Grenoble (Sanchez-Weatherby et al., 2009 ▸; Russi et al., 2011 ▸).
Dehydration can also be combined with other post-crystallization treatments such as annealing to improve diffraction quality (Abergel, 2004 ▸; Yang et al., 2002 ▸). It is worth noting that for a new crystal it cannot be predicted which dehydration method will work best (or at all), so trials of multiple methods and conditions will often be required.
Adaptive immune systems have recently been recognized in prokaryotic organisms where, in response to viral infection, they incorporate short fragments of invader-derived DNA into loci called clustered regularly interspersed short palindromic repeats (CRISPRs). The loci are then transcribed and the processed CRISPR transcripts are used to target invading viral DNA and RNA. The Archaeoglobus fulgidus ‘CRISPR-associated complex for antiviral defense’ (CASCADE) is central in targeting invading DNA. Cas5a is a key component of this CASCADE complex. In striving to collect diffraction data on crystals of Cas5a suitable for structure determination, we serendipitously found a new, convenient and effective dehydration method. Using this method, the resolution of data from Cas5a, as well as that from a second protein, LptA, which is a component of the system which transports lipopolysaccharides across a bacterial outer membrane, has been improved dramatically.
2. Materials and methods
2.1. Protein expression and purification
The cDNA encoding Cas5a (AF_1872) was PCR-amplified from the genomic DNA of A. fulgidus VC-16 and inserted into the expression vector pQE80. The recombinant plasmid pQE80-Cas5a was transferred into Escherichia coli BL21 (DE3) cells for protein expression by induction at 18°C for 20 h with 0.3 mM IPTG. Cells were harvested by centrifugation and were then resuspended in binding buffer (500 mM NaCl, 50 mM Tris–HCl pH 8.5, 5 mM imidazole) supplemented with 0.2 mM PMSF. Cell lysis was carried out by sonication. After centrifugation, the supernatant was applied onto a nickel-affinity column. After protein binding, the column was washed with 100 volumes of binding buffer followed by ten volumes of washing buffer (500 mM NaCl, 50 mM Tris–HCl pH 8.5, 40 mM imidazole). The protein was eluted with five volumes of elution buffer (200 mM NaCl, 300 mM imidazole–HCl pH 7.5). The eluate was concentrated and further purified using a Superdex 200 10/300 GL size-exclusion chromatography column (GE Healthcare, USA). The protein was eluted with an elution buffer consisting of 0.15 M NaCl, 5 mM Tris–HCl pH 7.5. The fractions containing the target protein were pooled, concentrated and stored at −80°C for subsequent use.
The cDNA encoding LptA (residues 27–185) was PCR-amplified from the genomic DNA of E. coli K-12 and inserted into the expression vector pQE80. The recombinant plasmid pQE80-LptA was transferred into E. coli BL21 (DE3) cells for protein expression by induction at 18°C for 20 h with 0.3 mM IPTG. The protein was purified according to the same protocol as used for Cas5a.
2.2. Crystallization
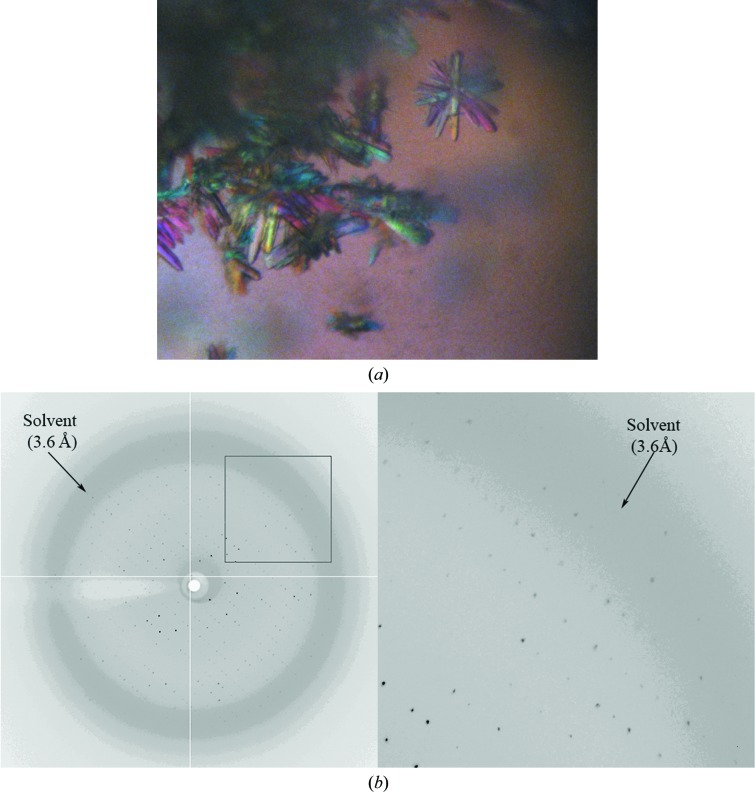
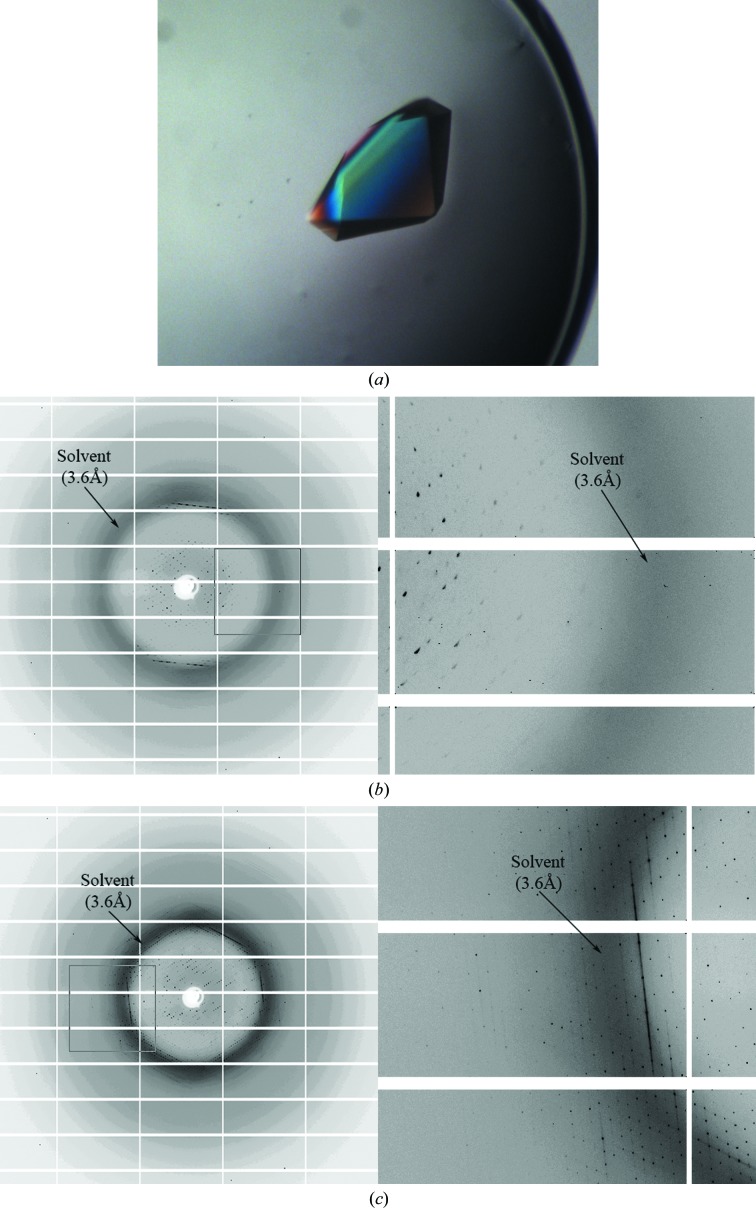
Cas5a and LptA were concentrated to 10 and 20 mg ml−1, respectively, in 150 mM NaCl, 5 mM Tris–HCl pH 7.5. Crystal screening was carried out using commercial crystallization kits (Crystal Screen and Crystal Screen 2 from Hampton Research, USA) and the self-made HQ192 kit. Tiny crystals of Cas5a were found in condition 167 of the HQ192 kit, which is composed of 35%(v/v) ethanol, 0.1 M sodium citrate pH 5.5. Small crystals of LptA were found in condition 79 of the HQ192 kit, which is composed of 1.0 M potassium sodium tartrate, 0.1 M HEPES–HCl pH 7.5. After optimization, crystals were grown by the hanging-drop vapour-diffusion method at 20°C. For Cas5a, 1.5 µl protein stock was mixed with 1.5 µl reservoir solution [30%(v/v) ethanol, 0.1 M sodium citrate pH 5.5]. Thin plate-shaped crystals (10 × 50 × 150 µm) were obtained one week later (Fig. 1 ▸ a). For LptA, 1.5 µl protein stock was mixed with 1.5 µl reservoir solution (0.4 M potassium sodium tartrate, 0.1 M HEPES–HCl pH 7.5). Large crystals (200 × 200 × 400 µm) were obtained one week later (Fig. 2 ▸ a).
Figure 1.
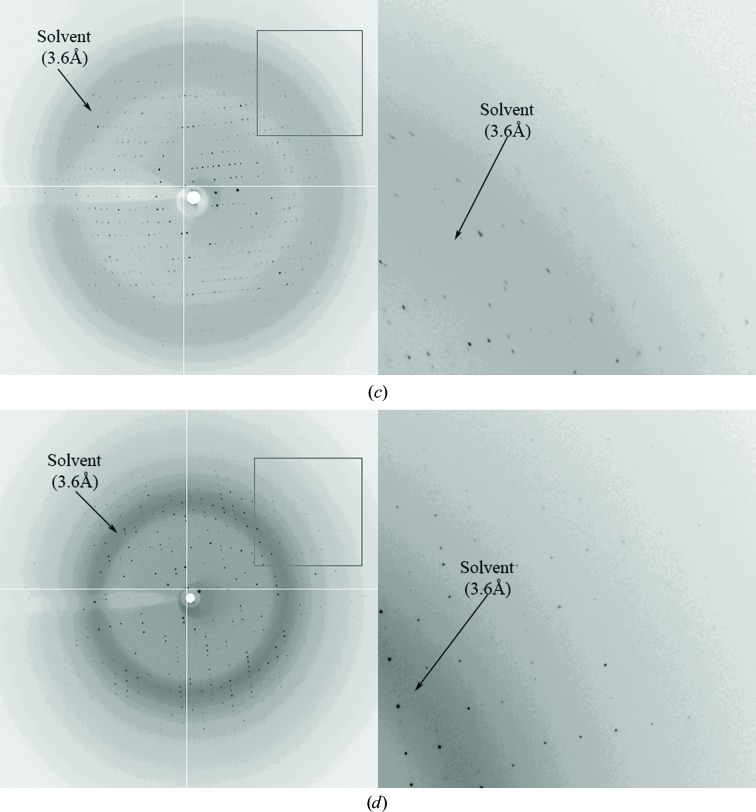
Diffraction patterns from Cas5a crystals. (a) Crystals of Cas5a grown in 30% ethanol, 0.1 M sodium citrate pH 5.5. (b)–(d) Diffraction patterns of Cas5a crystals soaked in the dehydrating solution (22.5% ethanol, 0.075 M sodium citrate pH 5.5, 25% glycerol) in air for 0 min (b), 30 min (c) and 3 h (d).
Figure 2.
Diffraction patterns from LptA crystals. (a) Crystals of LptA grown in 0.4 M potassium sodium tartrate, 0.1 M HEPES–HCl pH 7.5. (b, c) Diffraction patterns of LptA crystals soaked in the dehydrating solution (0.3 M potassium sodium tartrate, 0.075 M HEPES–HCl pH 7.5, 25% glycerol) in air for 0 min (b) and 1 h (c).
2.3. Dehydration and data collection
Various methods and solutions were employed to prepare crystals for data collection, as described below in §3.1. In the most successful method, a dehydrating solution for Cas5a crystals was made by mixing 75 µl reservoir solution with 25 µl glycerol (22.5% ethanol, 0.075 M sodium citrate pH 5.5, 25% glycerol). Several crystals were transferred with a loop to a droplet (∼5 µl) of the dehydrating solution sitting on a cover slip in the open air at room temperature. At time intervals of 0, 30 min, 1 h, 3 h, 6 h and overnight, one crystal was picked up with a cryoloop for diffraction data collection. LptA crystals were handled in the same manner, except that the dehydrating solution, made by mixing 75 µl reservoir solution with 25 µl glycerol, had the composition 0.3 M potassium sodium tartrate, 0.075 M HEPES–HCl pH 7.5, 25% glycerol. During the time that they were exposed to air, the droplets of dehydrating solution did not dry out and shrinkage of their volume was not apparent.
Diffraction data were collected at 100 K on beamline A1 at MacCHESS. The diffraction data were processed using HKL-2000 (Otwinowski & Minor, 1997 ▸); the statistics of data collection and processing are summarized in Table 1 ▸.
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell. N/D, not determined.
| Protein | Cas5a | LptA | ||
|---|---|---|---|---|
| Conditions | Dehydrated, cryoprotected | Cryoprotected | Room temperature | Dehydrated, cryoprotected |
| Space group | P21 | P21 | P21 | P3221 |
| Unit-cell parameters | ||||
| a (Å) | 75.92 | 76.70 | 76.01 | 146.98 |
| b (Å) | 42.54 | 44.47 | 43.81 | 146.98 |
| c (Å) | 89.64 | 91.39 | 90.11 | 193.12 |
| α (°) | 90 | 90 | 90 | 90 |
| β (°) | 111.85 | 110.34 | 112.04 | 90 |
| γ (°) | 90 | 90 | 90 | 120 |
| Resolution (Å) | 50–1.94 (1.97–1.94) | <3.5 | <4 | 50–3.40 (3.46–3.40) |
| Unique reflections | 39309 | N/D | N/D | 63515 |
| Completeness (%) | 99.4 (92.6) | N/D | N/D | 99.5 (99.5) |
| Multiplicity | 3.8 (2.3) | N/D | N/D | 4.5 (4.4) |
| 〈I/σ(I)〉 | 12.82 (1.17) | N/D | N/D | 12.30 (2.61) |
| R merge | 0.11 (0.60) | N/D | N/D | 0.17 (0.54) |
| Solvent content (%) | 42.8 | 47.4 | 44.7 | 52.9 |
2.4. Dehydrating solutions that do not dry up
To investigate what other cryoprotectant-containing dehydrating solutions might not dry up when open to air, several conditions from the Crystal Screen Cryo kit (Hampton Research, USA) that include glycerol were selected. A droplet (2.5 µl) of each selected condition was pipetted onto a glass cover slip in the open air at room temperature. The cover slip was left undisturbed on the laboratory bench. After various intervals (0, 1 h, 3 h, 6 h and overnight), the droplet was visually inspected under an optical microscope for signs that the droplet had dried out (i.e. become turbid), phase separation had occurred or any salt crystals had appeared. Additional dehydrating solutions were made starting from conditions without cryoprotectant chosen from commercial crystallization kits. 75 µl of the initial solution was mixed with 25 µl cryoprotectant (glycerol, MPD, PEG 400 or ethylene glycol). The resulting solutions were inspected as described above.
3. Results and discussion
3.1. Improved diffraction resolution of Cas5a using the new dehydration method
Crystals were flash-cooled in a cryogenic nitrogen-gas stream and diffraction data were collected at 100 K. Initially, a cryoprotectant solution made by mixing 75 µl reservoir solution with 25 µl cryoprotectant (glycerol, MPD, PEG 400 or ethylene glycol) was used. However, none of the crystals treated with this cryoprotectant solution diffracted to better than 3.2 Å resolution. A modified cryoprotectant solution consisting of the reservoir solution supplemented with 25% cryoprotectant was no more successful. The intrinsic diffraction quality of the crystal was then tested: a cryoloop holding a crystal was inserted into a plastic capillary with a small amount of reservoir solution at the other end, and diffraction was measured at room temperature. Of the several crystals tested, none diffracted beyond 3.2 Å resolution. Therefore, the poor diffraction from Cas5a crystals was owing to intrinsic low quality rather than an unsuitable cryoprotectant solution.
Dehydration has proven to be a most effective, and relatively simple, method for improving the diffraction quality of macromolecular crystals. Several dehydration methods were tried to improve the diffraction quality of Cas5a.
(i) Cas5a crystals were soaked in 40%(v/v) ethanol, 0.1 M sodium citrate pH 5.5 for 10–30 min and then in 50%(v/v) ethanol, 0.1 M sodium citrate pH 5.5 for 10–30 min. None of these crystals diffracted to better than 3.2 Å resolution.
(ii) The cover slip holding the crystal droplet was removed and 100 µl ethanol was added to the well (containing 1000 µl reservoir solution). The cover slip was then replaced on the well to let the droplet containing crystals equilibrate against this new reservoir solution (1100 µl, ∼36% ethanol) for 1 h. This procedure was repeated three times, so that the final concentration of ethanol in the well solution was 50%. Unfortunately, none of crystals treated in this way diffracted better than untreated crystals.
(iii) The cover slip holding the crystal droplet was removed and the well solution was replaced with 1000 µl dehydrating solution (40% PEG 4000, 3.5 M ammonium sulfate, 4.5 M NaCl, 6.0 M NaBr or saturated K2SO4). The cover slip was then replaced on the well and the droplet containing crystals was equilibrated against this new reservoir solution overnight. This treatment also did not yield any better diffraction than that from untreated crystals.
Crystal annealing was also tried to improve the diffraction quality of Cas5a crystals. A cryocooled Cas5a crystal was removed from the cryostream and placed back into a droplet of cryoprotectant solution. After various equilibration times (several seconds to 3 min), the crystal was replaced in the cryostream for X-ray examination. The ‘flash’ annealing method was also tested: the cryostream was blocked for 2–5 s three times, with intervals of 5–10 s between each thawing step. None of the annealing treatments improved the diffraction over that from untreated crystals. In fact, most of the crystals were damaged by annealing to some extent, resulting in lower resolution.
Fortunately, a new dehydration method was serendipitously discovered to improve the diffraction resolution of Cas5a crystals dramatically. In the process of flash-cooling, several Cas5a crystals were transferred from the mother drop to a 5 µl droplet of cryoprotectant solution which had been made by mixing 75 µl reservoir solution with 25 µl glycerol. One of the crystals was picked up for X-ray examination, while the others were left in the droplet, open to air, overnight. Next morning, it was a surprise to find that the droplet was still clear, with no salt crystals or precipitate. The volume of the droplet had shrunk only slightly, and the Cas5a crystals were still there, with no visible change after inspection under the optical microscope. These dehydrated crystals were picked up directly for diffraction data collection at 100 K. All of them diffracted to around 2.1 Å resolution, with diffraction from the best crystal extending to 1.95 Å resolution.
To examine the relationship between dehydration time and diffraction quality, several crystals were transferred from the mother drop to a 5 µl droplet of cryoprotectant solution. After various time intervals (0, 30 min, 1 h, 3 h, 6 h and overnight), one crystal was picked up for X-ray examination. As shown in Fig. 1 ▸, the diffraction quality was improved slightly at 30 min and 1 h and dramatically at 3 h. Prolonging the dehydration time (to 6 h and overnight) did not improve the diffraction quality further. Therefore, under these conditions, 3 h of dehydration time was sufficient to improve the diffraction quality of Cas5a crystals.
3.2. A dehydrating solution that will not dry up
Having established that a cryoprotectant solution comprising 22.5% ethanol, 0.075 M sodium citrate pH 5.5, 25% glycerol would not dry out after being left open to air overnight and could be used as an effective dehydrating solution for a Cas5a crystal, we investigated whether this was a unique case. Further study showed that it was not. As shown in Table 2 ▸, there are many glycerol-containing dehydrating solutions that will not dry out (become turbid) or precipitate buffer components when left open in air overnight and exhibit only a slight volume shrinkage. All of these conditions use salts (such as the popular salts for macromolecule crystallization potassium sodium tartrate, ammonium phosphate, sodium formate, sodium citrate and sodium chloride) or volatile organic agents (for example 2-propanol and ethanol, which are also popular in macromolecular crystallization conditions) as the primary precipitant. In contrast, when ammonium sulfate, lithium sulfate or sodium potassium phosphate was used as the primary precipitant the dehydrating solution dried out and salt crystals appeared. When ammonium tartrate was the primary precipitant, the dehydrating solution did not dry out overnight, but salt crystals appeared. Dehydrating solutions using PEG 4K, PEG 8K or PEG 3350, the most popular precipitants for macromolecule crystallization as the primary precipitant, dried out quickly.
Table 2. Observation of dehydrating solutions in open air.
| Dehydrating solution | 1 h | 3 h | Overnight |
|---|---|---|---|
| 2 (0.26 M potassium/sodium tartrate, 35% glycerol)† | Clear | Clear | Clear |
| 3 (0.26 M NH4H2PO4, 35% glycerol)† | Clear | Clear | Clear |
| 4 [1.5 M (NH4)2SO4 pH 8.5, 25% glycerol]† | Dried out quickly | Many salt crystals | |
| 6 (24% PEG 4K pH 8.5, 0.16 M MgCl2, 20% glycerol)† | Dried out slowly | ||
| 7 (0.98 M sodium acetate pH 6.5, 30% glycerol)† | Clear | Clear | Clear |
| 8 (21% 2-propanol pH 6.5, 0.14 M sodium citrate, 30% glycerol)† | Clear | Clear | Clear |
| 16 (1.125 M Li2SO4 pH 7.5, 25% glycerol)† | Dried out slowly | Some salt crystals | |
| 34 (1.4 M sodium formate pH 4.6, 30% glycerol)† | Clear | Clear | Clear |
| 35 (0.6 M NaH2PO4/K2HPO4 pH 7.5, 25% glycerol)† | Dried out slowly | Some salt crystals | |
| 38 (1.26 M sodium citrate pH 7.5, 10% glycerol)† | Clear | Clear | Clear |
| 0.75 M NH4H2PO4 pH 4.5, 25% glycerol | Clear | Clear | Clear |
| 0.75 M ammonium tartrate pH 4.6, 25% glycerol | Clear | Clear | Some salt crystals |
| 26.5% Tacsimate pH 7.0, 25% glycerol | Clear | Clear | Clear |
| 1.875 M sodium formate pH 8.0, 25% glycerol | Clear | Clear | Clear |
| 25% ethanol, 0.1 M sodium citrate, 25% glycerol | Clear | Clear | Clear |
| 15% PEG 8K pH 8.0, 20% glycerol | Dried out slowly | ||
| 12% PEG 3350, 0.08 M MgCl2, 20% glycerol | Dried out quickly | ||
| 12% PEG 3350, 0.08 M MgCl2, 20% MPD | Dried out quickly | ||
| 12% PEG 3350, 0.08 M MgCl2, 20% PEG 400 | Dried out quickly | ||
| 12% PEG 3350, 0.08 M MgCl2, 20% ethylene glycol | Clear | Clear | Clear |
| 0.26 M potassium/sodium tartrate, 25% MPD | Dried out and phase separation | ||
| 0.26 M potassium/sodium tartrate, 25% ethylene glycol | Clear | Clear | Clear |
These conditions were selected from the Crystal Screen Cryo kit (Hampton Research) and are numbered accordingly.
In order to find a suitable cryoprotectant for crystallization conditions using high-molecular-weight PEG as the primary precipitant, additives including MPD, PEG 400 and ethylene glycol were tested. As shown in Table 2 ▸, the original reservoir solution (12% PEG 3350, 0.08 M MgCl2) supplemented with 20% glycerol, 20% MPD or 20% PEG 400 dried out quickly n less than 1 h. In contrast, the original reservoir solution supplemented with 20% ethylene glycol did not dry out overnight.
Therefore, if a salt is used as the primary precipitant in the crystallization condition, the recommended dehydrating solution is the original reservoir solution supplemented with 20–30% glycerol. If a high-molecular-weight PEG (such as PEG 4K, PEG 8K or PEG 3350) is used as the primary precipitant in the crystallization condition, the recommended dehydrating solution is the original reservoir solution supplemented with 20–30% ethylene glycol.
3.3. Dehydration tests on other crystals
The new dehydration method was tested with other crystals. LptA protein was crystallized using sodium potassium tartrate as the precipitant. The crystals were flash-cooled using glycerol, PEG 400, MPD or ethylene glycol as a cryoprotectant. However, none of those crystals diffracted to better than 5 Å resolution. Dehydration was performed using a dehydrating solution made by mixing 75 µl reservoir solution with 25 µl glycerol. Several crystals were transferred to a droplet of this dehydrating solution in the open air. After dehydration for 1 h, the diffraction resolution was improved dramatically, with reflections visible to 2.5 Å resolution (Fig. 2 ▸). The data were anisotropic and were processed to only 3.4 Å resolution, which is still a substantial improvement over 5 Å. On extending the dehydration time to 3 h, overnight or even two weeks the droplet remained clear, with no salt crystals, but the diffraction resolution of LptA crystals did not improve further. Dehydration for less than 1 h (30 min) produced only a slight improvement in resolution.
Crystals of the uncharacterized protein AF_2146 were grown in 35% Tacsimate pH 7.5. They diffracted to around 8 Å resolution using the normal flash-cooling method. For dehydration, crystals were transferred to a droplet of dehydrating solution (26% Tacsimate pH 7.5, 25% glycerol), open to air, for various times and then examined by X-ray diffraction. The droplet remained clear, no salt crystals appeared and the dehydrated crystals diffracted to around 7 Å resolution, which is a slight improvement over the original resolution, although in this instance not sufficient for structure solution.
4. Conclusion
Dehydration is an effective post-crystallization treatment in improving the diffraction quality of macromolecular crystals. However, there is neither a single outstanding dehydration procedure nor a single outstanding dehydrating agent. For each new crystal a range of dehydration procedures must be considered. Here, we describe a new dehydration method, which has proven to be effective and convenient in improving the diffraction quality of Cas5a and LptA crystals. This method requires only one crystal transfer beyond normal mounting and is complete in less than 3 h, without any need for extremely precise control of air-exposure time. As 20–30% cryoprotectant is included in the dehydrating solution, after dehydration the crystal can be flash-cryocooled directly. Like other dehydration methods, this new dehydration method does not work for all crystals, but it is a useful addition to the options for improving crystal quality. Tests on crystals of additional proteins, which did not succeed in improving the diffraction, allow us to estimate that the method will be useful in approximately one out of five cases.
Acknowledgments
This work is based upon research conducted at the Cornell High Energy Synchrotron Source (CHESS), which is supported by the National Science Foundation and the National Institutes of Health/National Institute of General Medical Sciences under NSF award DMR-1332208, using the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by award GM-103485 from the National Institute of General Medical Sciences, National Institutes of Health. We are grateful to Candice Klug and colleagues for providing initial samples of LptA protein.
References
- Abergel, C. (2004). Acta Cryst. D60, 1413–1416. [DOI] [PubMed]
- Bailly, M., Blaise, M., Lorber, B., Thirup, S. & Kern, D. (2009). Acta Cryst. F65, 577–581. [DOI] [PMC free article] [PubMed]
- Bowman, G. D., O’Donnell, M. & Kuriyan, J. (2004). Nature (London), 429, 724–730. [DOI] [PubMed]
- Esnouf, R. M., Ren, J., Garman, E. F., Somers, D. O’N., Ross, C. K., Jones, E. Y., Stammers, D. K. & Stuart, D. I. (1998). Acta Cryst. D54, 938–953. [DOI] [PubMed]
- Frey, M. (1994). Acta Cryst. D50, 663–666. [DOI] [PubMed]
- Haebel, P. W., Wichman, S., Goldstone, D. & Metcalf, P. (2001). J. Struct. Biol. 136, 162–166. [DOI] [PubMed]
- Harp, J. M., Hanson, B. L., Timm, D. E. & Bunick, G. J. (1999). Acta Cryst. D55, 1329–1334. [DOI] [PubMed]
- Heras, B., Edeling, M. A., Byriel, K. A., Jones, A., Raina, S. & Martin, J. L. (2003). Structure, 11, 139–145. [DOI] [PubMed]
- Heras, B. & Martin, J. L. (2005). Acta Cryst. D61, 1173–1180. [DOI] [PubMed]
- Holyoak, T., Fenn, T. D., Wilson, M. A., Moulin, A. G., Ringe, D. & Petsko, G. A. (2003). Acta Cryst. D59, 2356–2358. [DOI] [PubMed]
- Igura, M., Ose, T., Obita, T., Sato, C., Maenaka, K., Endo, T. & Kohda, D. (2005). Acta Cryst. F61, 514–517. [DOI] [PMC free article] [PubMed]
- Kiefersauer, R., Grandl, B., Krapp, S. & Huber, R. (2014). Acta Cryst. D70, 1224–1232. [DOI] [PMC free article] [PubMed]
- Kiefersauer, R., Than, M. E., Dobbek, H., Gremer, L., Melero, M., Strobl, S., Dias, J. M., Soulimane, T. & Huber, R. (2000). J. Appl. Cryst. 33, 1223–1230.
- Kim, C. U., Kapfer, R. & Gruner, S. M. (2005). Acta Cryst. D61, 881–890. [DOI] [PubMed]
- Kriminski, S., Caylor, C. L., Nonato, M. C., Finkelstein, K. D. & Thorne, R. E. (2002). Acta Cryst. D58, 459–471. [DOI] [PubMed]
- Lusty, C. J. (1999). J. Appl. Cryst. 32, 106–112.
- Malay, A. D., Allen, K. N. & Tolan, D. R. (2005). J. Mol. Biol. 347, 135–144. [DOI] [PubMed]
- Newman, J. (2006). Acta Cryst. D62, 27–31. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Petock, J. M., Wang, Y.-F., DuBois, G. C., Harrison, R. W. & Weber, I. T. (2001). Acta Cryst. D57, 763–765. [DOI] [PubMed]
- Quiocho, F. A. & Richards, F. M. (1964). Proc. Natl Acad. Sci. USA, 52, 833–839. [DOI] [PMC free article] [PubMed]
- Russi, S., Juers, D. H., Sanchez-Weatherby, J., Pellegrini, E., Mossou, E., Forsyth, V. T., Huet, J., Gobbo, A., Felisaz, F., Moya, R., McSweeney, S. M., Cusack, S., Cipriani, F. & Bowler, M. W. (2011). J. Struct. Biol. 175, 236–243. [DOI] [PubMed]
- Russo Krauss, I., Sica, F., Mattia, C. A. & Merlino, A. (2012). Int. J. Mol. Sci. 13, 3782–3800. [DOI] [PMC free article] [PubMed]
- Salunke, D. M., Veerapandian, B., Kodandapani, R. & Vijayan, M. (1985). Acta Cryst. B41, 431–436.
- Samygina, V. R., Antonyuk, S. V., Lamzin, V. S. & Popov, A. N. (2000). Acta Cryst. D56, 595–603. [DOI] [PubMed]
- Sanchez-Weatherby, J., Bowler, M. W., Huet, J., Gobbo, A., Felisaz, F., Lavault, B., Moya, R., Kadlec, J., Ravelli, R. B. G. & Cipriani, F. (2009). Acta Cryst. D65, 1237–1246. [DOI] [PubMed]
- Schick, B. & Jurnak, F. (1994). Acta Cryst. D50, 563–568. [DOI] [PubMed]
- Wojdyla, J. A., Manolaridis, I., Snijder, E. J., Gorbalenya, A. E., Coutard, B., Piotrowski, Y., Hilgenfeld, R. & Tucker, P. A. (2009). Acta Cryst. D65, 1292–1300. [DOI] [PMC free article] [PubMed]
- Yang, J. K., Yoon, H.-J., Ahn, H. J., Lee, B. I., Cho, S. H., Waldo, G. S., Park, M. S. & Suh, S. W. (2002). Acta Cryst. D58, 303–305. [DOI] [PubMed]