Abstract
Gastroparesis leads to inadequate emptying of the stomach resulting in severe negative health impacts. Appropriate long-term treatments for these diseases may require pyloric sphincter tissue replacements that possess functional smooth muscle cell (SMC) and neural components. This study aims to bioengineer, for the first time, innervated human pylorus constructs utilizing autologous human pyloric sphincter SMCs and human neural progenitor cells (NPCs). Autologous SMCs and NPCs were cocultured in dual-layered hydrogels and formed concentrically aligned pylorus constructs. Innervated autologous human pylorus constructs were characterized through biochemical and physiologic assays to assess the phenotype and functionality of SMCs and neurons. SMCs within bioengineered human pylorus constructs displayed a tonic contractile phenotype and maintained circumferential alignment. Neural differentiation within bioengineered constructs was verified by positive expression of βIII-tubulin, neuronal nitric oxide synthase (nNOS), and choline acetyltransferase (ChAT). Autologous bioengineered innervated human pylorus constructs generated a robust spontaneous basal tone and contracted in response to potassium chloride (KCl). Contraction in response to exogenous neurotransmitter acetylcholine (ACh), relaxation in response to vasoactive intestinal peptide (VIP), and electrical field stimulation (EFS) were also observed. Neural network integrity was demonstrated by inhibition of EFS-induced relaxation in the presence of a neurotoxin or nNOS inhibitors. Partial inhibition of ACh-induced contraction and VIP-induced relaxation following neurotoxin treatment was observed. These studies provide a proof of concept for bioengineering functional innervated autologous human pyloric sphincter constructs that generate a robust basal tone and contain circumferentially aligned SMCs, which display a tonic contractile phenotype and functional differentiated neurons. These autologous constructs have the potential to be used as (1) functional replacement organs and (2) physiologically relevant models to investigate human pyloric sphincter disorders.
Introduction
Gastroparesis is a motility disorder that impacts a large number of adults in the United States. Gastroparesis is associated with inadequate emptying of the stomach resulting in severe negative health impacts. Treatments for gastroparesis are rarely curative and primarily assist with managing the condition.1–3 Consequently, newer therapies are being developed and tested, however, these alternative treatments for delayed gastric emptying are producing mixed results.1,4 One promising approach is targeting the function of the pyloric sphincter. Administration of botulinum toxin into the pyloric sphincter leads to significant improvements in gastric emptying, but these effects are only transient and long-term trials show no improvement compared to placebo.5 Patients with gastroparesis caused by damage to the vagus nerve may receive a pyloroplasty to allow for increased passage of food through the pyloric sphincter.6,7 The effectiveness of pyloroplasty is similar to the short-term results after administration of botulinum toxin and the long-term effects are unclear.7 Importantly, surgical interventions in patients with gastroparesis have a high risk of complications.8 These studies provide hope for the treatment of gastroparesis by targeting pyloric function, however, the obvious ineffectiveness of approaches that provides only transient relief indicates the need for strategies that provide sustained modulation of pyloric sphincter function.
Tissue engineering provides a platform for the development of functional organ replacements that can be implanted to reinstate the function of damaged tissues. We have previously bioengineered intrinsically innervated internal anal sphincter (IAS) constructs from human IAS smooth muscle and neural progenitor cells (NPCs).9,10 In addition, we have demonstrated the myogenic and neuronal functionality of bioengineered innervated human IAS constructs.9,10 Currently, bioengineered human pyloric sphincter tissues that possess both smooth muscle cell (SMC) and neural cell components are not available.11 In order for bioengineered pylorus constructs to serve as appropriate tissue replacements they must (1) generate a basal tone, (2) contain SMCs with a contractile phenotype, and (3) possess differentiated motor neurons that can modulate the sphincteric tone.
In this study, for the first time, we bioengineered functionally innervated human pylorus tissues utilizing autologous human pyloric sphincter SMCs and human NPCs. Each bioengineered tissue used in this study contained SMCs and NPCs derived from the same patient. Bioengineered human pylorus constructs align circumferentially around a silicone post and express markers for contractile SMCs (smoothelin) and differentiated neurons (neuronal nitric oxide synthase [nNOS], choline acetyltransferase [ChAT], and βIII-tubulin) after 10 days. Functional studies of bioengineered innervated human pylorus constructs (i.e., myogenic and neuronal components) demonstrate maintenance of functional sphincteric SMCs and motor neurons. This is the first instance of bioengineering an intrinsically innervated human pylorus, where both the myogenic and neuronal components are viable and functional. Since each pylorus construct was bioengineered with cells obtained from the same patient, this study potentially provides a novel platform for the development of an autologous cell therapy.
Materials and Methods
Reagents
Tissue culture reagents were purchased from Invitrogen (Carlsbad, CA) unless otherwise specified. SMC growth media contained Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS), 1× antibiotics–antimycotics, and 2 mM l-glutamine. Neurosphere growth media contained Neurobasal (Life Technologies, Grand Island, NY), 1× N2 supplement (Life Technologies), 20 ng/mL recombinant human epidermal growth factor (EGF; Stemgent, San Diego, CA), 20 ng/mL recombinant basic fibroblast growth factor (bFGF, Stemgent), and 1× antibiotics. Neural differentiation media used for culturing bioengineered constructs contained Neurobasal-A (Life Technologies), 1× B27 supplement (Life Technologies), 2% FBS and 1× antibiotics. Dispase and DNAse were purchased from Roche Applied Science (Indianapolis, IN), collagenase from Worthington Biochemicals (Lakewood, NY), Hank's Balanced Salt Solution (HBSS) from Thermo Scientific HyClone (Logan, UT), and rat tail collagen type I was purchased from BD Biosciences (Bedford, MA).
Isolation of autologous human SMCs and enteric NPCs
Human pylorus and intestinal tissues were provided by Carolina Donor Services and Wake Forest Baptist Medical Center (IRB#: IRB00007586) in strict accordance with The Code of Ethics of the World Medical Association.
All tissues were procured directly after receipt from donor services to minimize the amount of ice time which never exceeded 2 h. Human pylori were removed by sharp dissection and SMCs were isolated as described previously.9 Briefly, pylorus tissues were manually cleaned by removing fat and mucosa with a surgical blade. Tissues were washed with HBSS solution containing 2× antibiotics–antimycotics five times and then minced in sterile conditions. After mincing, tissues were washed with sterile HBSS five times. Tissue digestions with HBSS 0.1% collagenase type II (Worthington Biochemicals) were performed twice at 37°C with agitation for 1 h. After digestion, tissue pellets were centrifuged at 600 g for 10 min and washed. Tissue pellets were then resuspended in SMC growth media and plated as a combination of single cells and tissue chunks in tissue culture dishes at 37°C with 5% CO2.
Adult human NPCs were isolated from human small intestinal tissues of the same donor as described previously.9 Briefly, luminal cells were scrapped from tissues using a surgical blade and remaining tissue extensively washed in ice-cold HBSS solution containing 2× antibiotics–antimycotics. Cleaned intestinal tissues were minced in sterile conditions and digested in an HBSS solution containing 0.85% collagenase II, 0.85% dispase, and 30 μg/mL DNAse I on an orbital shaker at 100 rpm for 1 h at 37°C. Supernatant was collected, passed through a 70-μm filter, and placed on ice, and the tissue pellet was digested for an additional hour as before. After second digestion, cell supernatants from both digests were passed through a 70-μm filter, pelleted at 2000 g for 10 min, and washed five times with ice-cold HBSS containing 2× antibiotics. Cell pellets were resuspended in media containing growth factors that induce enteric neural stem cell proliferation and survival (neurosphere growth media detailed above) and passed through 70- and 40-μm meshes. The remaining single-cell suspension (after filtering) was plated on nontissue culture-treated petri dishes and assessed daily for formation of neurosphere-like bodies.
Cell proliferation assay (MTS)
The proliferation of human pyloric SMCs and human enteric NPCs was evaluated by the (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) assay (CellTiter 96® AQueous One Solution; Promega, Madison, WI). Briefly, smooth muscle and NPCs were seeded onto 96-well plates. On the day of the analysis, 20% of MTS reagent was added to each well and incubated at 37°C for 1 h. Absorbance of the wells in the 96-well plates was measured at 490 nm using a spectrophotometric plate reader and cell numbers were determined using a standard curve with respective cell types.
Bioengineering innervated human pyloric sphincter constructs
Bioengineered innervated human pylorus constructs were generated using similar methods to generate bioengineered IAS constructs.10 Briefly, SMCs isolated from human pylori and enteric NPCs isolated from human small intestines were suspended in hydrogels placed in molds made from 35-mm Sylgard-coated culture dishes with a central Sylgard post 8 mm in diameter. Enteric NPCs were dissociated into single cells with Accutase (Invitrogen, Grand Island, NY), counted with a hemocytometer, and 200,000 NPCs were resuspended in 1 mL of 0.8 mg/mL collagen gel containing 10 μg/mL of laminin in a 1× DMEM with 10% FBS media solution. 1 mL of NPC/collagen I gel solution was added to molds and incubated for 15 min at 37°C to allow partial gelation. Next, 500,000 pylorus SMCs were suspended in 1 mL of 0.8 mg/mL collagen I solution in 1× DMEM with 10% FBS. This SMC/collagen I solution was overlaid on plates containing partially gelled NPC solution and incubated at 37°C for 1 h. After gelation, 1 mL of neural differentiation media were added to constructs, which were then incubated at 37°C and 7% CO2. Differentiation media were changed every other day. Bioengineered human pyloric sphincter constructs containing only SMCs were also generated with this technique without the addition of NPC containing gel to serve as a control. NPCs alone do not form tissue rings within hydrogels using this technique since they do not possess contractile properties and will not form rings of tissue. As an additional control, constructs were engineered by mixing both cells in a single layer. Briefly, 500,000 SMCs and 200,000 NPCs were resuspended in 0.8 mg/mL collagen gel containing 10 μg/mL of laminin in a 1× DMEM with 10% FBS media solution. The mixed solution was overlaid around the central post of the Sylgard-coated dishes. The solution was allowed to gel at 37°C. Differentiation media were changed regularly every other day.
RNA isolation, complementary DNA (cDNA) synthesis, and quantitative polymerase chain reaction (qPCR) analysis
Cells were lysed in Trizol and chloroform extraction of RNA was performed. The RNA containing aqueous layer was mixed with equal volumes of 70% ethanol and RNA purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized using the High-Capacity cDNA Reverse Transcription Kit according to manufacturer's instructions (Applied Biosystems, Grand Island, NY). Polymerase chain reaction (PCR) amplification was performed using Power SYBR® Green PCR Master Mix (Applied Biosystems). Quantitative polymerase chain reaction (qPCR) and analysis were performed using standard settings on 7900HT Real-Time PCR System (Applied Biosystems). The specific primer sequences used for real-time qPCR are (1) TUBB3 forward 5′CTCAGGGGCCTTTGGACATC3′ reverse 5′CAGGCAGTCGCAGTTTTCA3′ (160 bp), (2) NOS1 forward 5′CCTCCCGCCCTGCACCATCTT3′ reverse 5′CTTGCCCCATTTCCATTCCTCGTA3′ (175 bp), (3) ChAT forward 5′GGAGGCGTGGAGC TCAGCGACACC3′ reverse 5′CGGGGAGCTCGCTGACG GAGTCTG3′ (256 bp), and (4) the housekeeping gene GAPDH forward 5′CAGGTGGTCTCCTCTGACTTCA AC3′ reverse 5′AGGGTCTCTCTCTTCCTCTTG3′ (223 bp) as an endogenous control and all values presented as dCt.
Western blot analysis of bioengineered innervated human pyloric sphincter constructs
The phenotype of SMCs and NPCs within bioengineered constructs was assessed by Western blots and other assays after coculture for 10 days. Protein lysates were collected and prepared for immunodetection as described earlier.12 Briefly, 40 μg of total protein per well was loaded on 8% polyacrylamide gels and separated with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Semidry transfer was performed to immobilize proteins onto nitrocellulose membranes. Ponceau S (0.1%; SigmaAldrich, St Louis, MO) staining was performed to determine success of transfer and even loading. Blocking was performed by incubating membranes in Tris-buffered saline-0.1% Tween 20 (TBS-T) containing 5% nonfat milk for 1 h to minimize nonspecific binding. Primary antibodies specific for smoothelin, βIII-tubulin, nNOS, ChAT (Abcam, Cambridge, MA), or β-actin (SigmaAldrich) were added with agitation for 1 h. Membranes were washed thrice in TBS-T and incubated with a species-specific horseradish peroxidase (HRP)-conjugated secondary antibody at manufacturer-recommended dilutions for 1 h. After three more washes, signal detection was achieved by adding HyGLO chemiluminescent HRP reagent (Denville Scientific, Inc, South Plainfield, NJ) and taking pictures with a chemiluminescent imaging system (FujiFilm LAS-3000, Tokyo, Japan).
Histological and immunofluorescence analysis of bioengineered constructs
The engineered pyloric sphincters were fixed overnight in a neutral-buffered 10% formalin solution, dehydrated, and embedded in paraffin. Five microliters sections were placed on slides, deparaffinized, and rehydrated. Either hematoxylin and eosin (H&E) or immunofluorescent staining was performed on sections. For immunofluorescence, slides were blocked and permeabilized in phosphate-buffered saline (PBS) containing 10% horse serum and 0.15% Triton X-100. Primary antibodies directed against smooth muscle actin (SMA; SigmaAldrich), β-III tubulin (Abcam), and smooth muscle specific heavy caldesmon (Caldesmon; SigmaAldrich) were added in 0.1% bovine serum albumin and 0.075% Triton X-100 for 1 h at room temperature. Unbound antibody was removed by PBS washes before incubation with appropriate fluorescently tagged secondary antibody at room temperature for 1 h. Additional PBS washes were performed followed by mounting in VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Inc., Burlingame, CA). Fluorescence was visualized using a Nikon eclipse Ti inverted microscope (Nikon, Melville, NY).
Physiologic analysis of bioengineered human pyloric sphincter constructs
Bioengineered constructs were hooked in an organ bath between a stationary pin and a measuring arm of an isometric, magnetoresistive force transducer (F10; Harvard Apparatus, Holliston, MA) and force generation data acquired using LabScribe2 software (iWorx, Hanover, NH). Constructs were maintained in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered solution at 37°C throughout all experiments. All force generation studies were performed after the establishment of a stable basal tone. Force generation was evaluated following the addition of potassium chloride (KCl; 60 mM), acetylcholine (ACh; 1 μM), vasoactive intestinal peptide (VIP; 1 μM), or electrical field stimulation (EFS; 5 Hz, 0.5 ms, for 30 s). Constructs were washed thrice between each treatment, incubated in fresh buffer, and allowed to return to baseline for 5–7 min. Pretreatment with the pan-neuronal blocker tetrodotoxin (TTX; 1 μm) was done to evaluate the relative contribution of motor neurons to force generation. Nitric oxide synthase (NOS) inhibitor Nω-Nitro-l-arginine methyl ester hydrochloride (LNAME; 300 μm) was administered for 12 min before EFS stimulation to inhibit nitrergic neurons. GraphPad Prism 5.01 software for Windows (GraphPad Software, San Diego, CA) was used to analyze data. Smoothing was applied (Second-order Savitsky–Golay) and values are expressed as means ± standard error of the mean (SEM) of three experiments (n = 3, and three replicates performed for each force generation test). Quantification of physiologic data was performed relative to basal tone for contraction (Delta Force Max = maximum force [μN] above basal tone) and relaxation (Delta Force Min = minimum force [μN] below basal tone).
Statistical analysis
Data are expressed as mean ± SEM unless noted otherwise. Significant differences (p-values below 0.05) were determined using t-tests on at least three independent experiments unless specified otherwise (GraphPad Software). Human cells used in these experiments were obtained from three different donors and the smooth muscle and NPCs in each construct were derived from the same donor (n = 3).
Results
Cell viability assay MTS
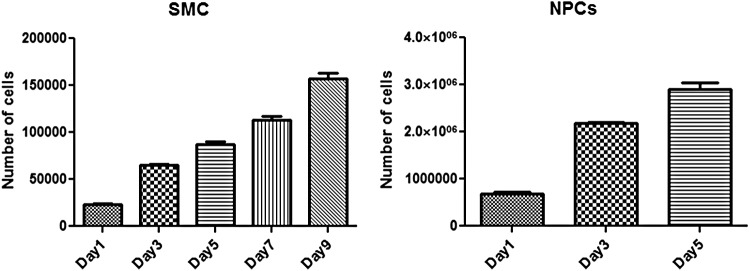
Proliferation of SMCs and enteric NPCs was assessed over time using MTS assays. All cells were isolated from a 5 cm2 piece of tissue from each individual donor. As shown in Figure 1, the number of smooth muscle and NPCs increased over time in culture. Over a period of 9 days, the number of SMCs increased eight times from the initial seeding number producing more than 150,000 total cells. For enteric NPCs, the number of cells increased almost four times over a period of 5 days producing roughly 3,000,000 cells during that time period. The results indicated the feasibility of expanding the cells obtained from the human donor tissues to engineer the innervated smooth muscle constructs.
FIG. 1.
Cell proliferation assay. Isolated human smooth muscle cells (SMCs) and enteric neural progenitor cells (NPCs) were followed over a period of 9 and 5 days, respectively. Results have shown increase in cell number.
Biochemical characterization of the bioengineered innervated human pylorus constructs
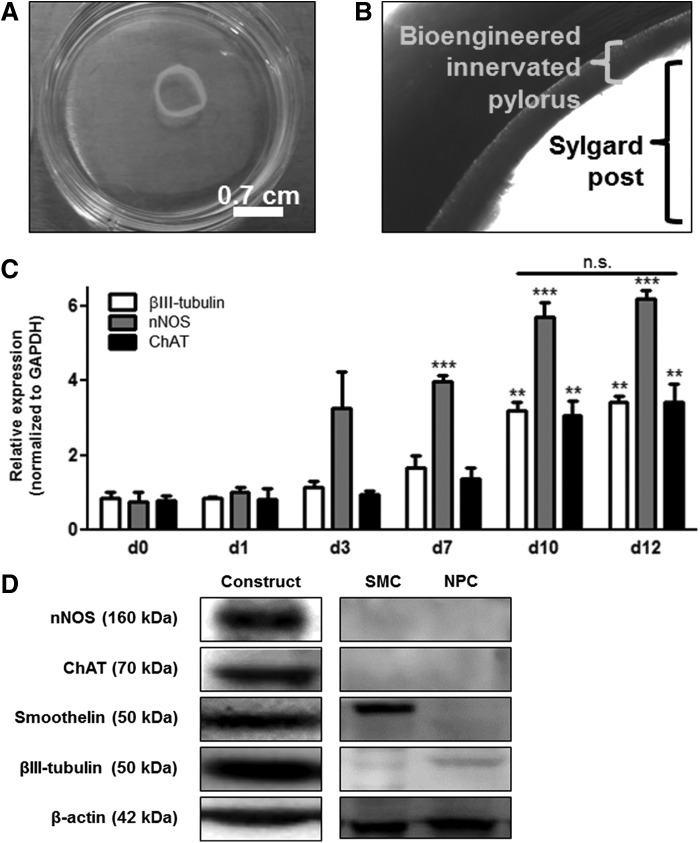
SMCs within bioengineered innervated human pylorus constructs contract around 0.7-cm-diameter Sylgard posts to form rings of tissue (Fig. 2A, B). Expression of neural differentiation markers over time was assessed using qPCR. A significant increase in nNOS transcript levels was observed after 7 days of coculture with SMCs in bioengineered constructs (Fig. 2C, ***p < 0.001). Transcript expression of βIII-tubulin, nNOS, and ChAT were all significantly increased by day 10 (Fig. 2C, **p < 0.01, ***p < 0.001) and remained at similar levels by day 12 (n.s.p > 0.05). After 10 days, protein lysates collected from bioengineered innervated constructs were analyzed for contractile SMC markers and neural differentiation markers by Western blot analysis. Expression of SMC marker smoothelin was observed (Fig. 2D) indicating that SMC within bioengineered constructs maintained a contractile phenotype. Pan-neuronal marker βIII-tubulin was also expressed in constructs after 10 days (Fig. 2D) demonstrating the presence of differentiated neurons. nNOS and ChAT expression were also observed indicating the presence of nitrergic and cholinergic neural subset populations after coculture of NPCs and SMCs within bioengineered constructs. No expression of nNOS or ChAT was observed in cultures of SMCs or NPCs alone (Fig. 2D). Smoothelin expression was observed in cultures of SMCs, but not NPCs, and low levels of βIII-tubulin were observed in NPC cultures, but not SMC only cultures (Fig. 2D).
FIG. 2.
Biochemical characterization of the bioengineered human pylorus constructs. Bioengineered innervated human pylorus SMC constructs form (A) rings of tissue around (B) 0.7-cm-diameter Sylgard posts (40× magnification). Levels of neural differentiation markers over time were measured in RNA isolated from lysates of bioengineered autologous human pylorus constructs using quantitative polymerase chain reaction (qPCR). (C) Significant increases in transcript levels of βIII-tubulin, neuronal nitric oxide synthase (nNOS), and choline acetyltransferase (ChAT) within bioengineered constructs were observed by day 10, and no further increase was observed at day 12, indicating that peak neural gene expression occurred at 10 days of coculture. Protein lysates collected from bioengineered constructs were analyzed for contractile SMC markers and neural differentiation markers by Western blot analysis. **p < 0.01, ***p < 0.001. (D) SMC marker smoothelin and pan-neuronal marker βIII-tubulin were expressed in bioengineered innervated human pylorus constructs demonstrating the presence of contractile SMCs and differentiated neurons. Neural subset markers, nNOS and ChAT, were observed after 10 days of in vitro coculture of SMCs and NPCs within bioengineered constructs. Smoothelin expression was detected in SMCs only cultures, however, no expression of neural differentiation markers was observed in SMC monocultures. NPCs cultured alone did not express smoothelin, nNOS, or ChAT, but did express low levels of βIII-tubulin (Fig. 2D). n.s., non significant.
Histological and immunohistochemical characterization of the bioengineered constructs
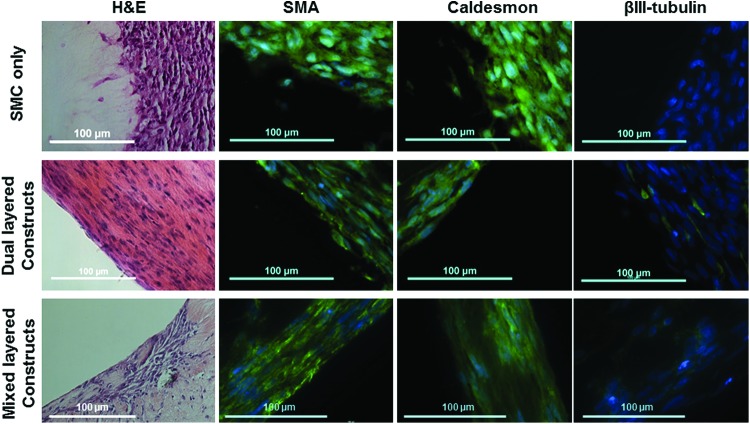
Bioengineered constructs were generated with cocultures of SMCs or SMCs alone using two different methods. Circular alignment of cells within bioengineered constructs was demonstrated by H&E staining (Fig. 3). Immunofluorescence studies demonstrated positive staining for SMA and smooth muscle specific heavy caldesmon (Caldesmon), in both bioengineered innervated constructs and SMC only constructs, indicating the maintenance of a smooth muscle phenotype (Fig. 3). These SMC stains further demonstrate the circumferential alignment of SMCs within these constructs. To verify the presence of differentiated neurons within bioengineered constructs, immunofluorescence staining for pan-neuronal marker, βIII-tubulin, was performed. βIII-tubulin-positive cells were observed in bioengineered constructs containing SMC and NPC cocultures, but not SMCs alone, demonstrating the presence of differentiated neurons innervating the SMCs within these bioengineered human pylorus constructs (Fig. 3).
FIG. 3.
Histological and immunohistochemical analysis of the bioengineered human pylorus constructs. Alignment of SMCs within bioengineered innervated human pylorus constructs was assessed by hematoxylin and eosin (H&E) staining (200× magnification). H&Es demonstrated compacted circularly aligned SMCs within pylorus constructs after 10 days. Circumferential SMC alignment was further verified by SMA (600× magnification) and smooth muscle specific caldesmon (600× magnification) staining. The presence of differentiated neurons innervating these bioengineered constructs was verified through positive staining for pan-neuronal marker, βIII-tubulin, after 10 days (600× magnification). All scale bars = 100 μm. Color images available online at www.liebertpub.com/tea
Physiological assessment of the bioengineered human constructs
Basal tone and potassium chloride response
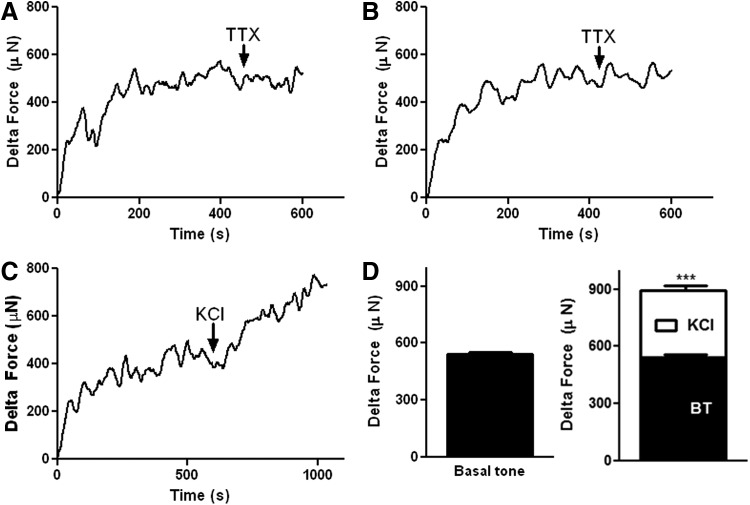
To investigate the myogenic functionality of bioengineered constructs, force generation studies were performed. The generation of a spontaneous basal tone was measured by applying 20% stretch to tissues hooked to a force transducer in an organ bath with no external stimuli. Bioengineered innervated human pylorus constructs (Fig. 4A, black traces) and noninnervated human pylorus constructs (Fig. 4B, black traces) generated robust spontaneous basal tones indicating this tone was myogenic in nature. Contraction of SMCs in response to KCl has been shown previously to be primarily myogenic.10 Constructs generated myogenic contractions above the basal tone in response to KCl (Fig. 4C, black traces). Quantification of basal tone and KCl-induced contraction of bioengineered innervated constructs were calculated (Fig. 4D) and the basal tone was 544 ± 7 and KCl-induced contraction was 350 ± 30 above the basal tone (***p < 0.001).
FIG. 4.
Physiologic assessment of basal tone and contraction in response to potassium chloride (KCl) in bioengineered human pylorus constructs. Force generation studies were performed to investigate the myogenic functionality of bioengineered human pylorus constructs after 10 days. Spontaneous basal tone of bioengineered (A) innervated and (B) noninnervated (SMC only) human pylorus constructs was measured after hooking constructs to a force transducer in an organ bath with no external stimuli (black traces). (C) Myogenic contraction above basal tone of bioengineered innervated human pylorus constructs in response to KCl was assessed (black traces). (D) Quantification of basal tone and KCl-induced contraction demonstrated the generation of a robust basal tone and a significant contraction in response to KCl above basal tone (***p < 0.001).
Cholinergic response
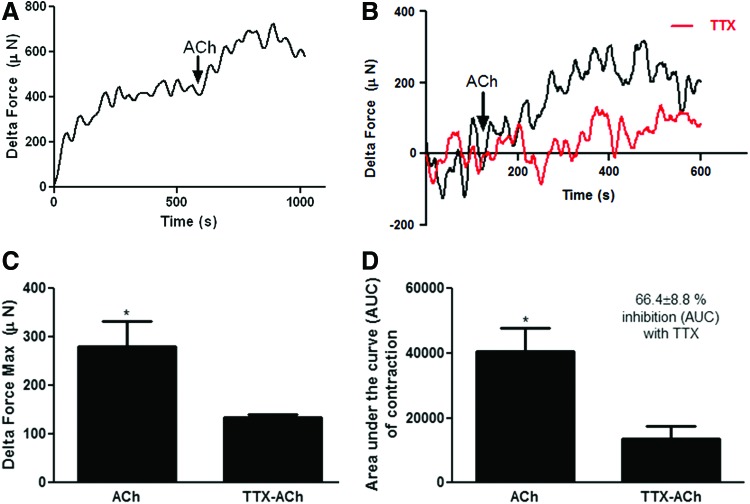
Bioengineered innervated human pylorus constructs were evaluated in the presence of exogenous contractile neurotransmitter ACh with or without pretreatment with neurotoxin (TTX). Constructs demonstrated robust contractions above the basal tone upon addition of 1 μM ACh (Fig. 5A, B; black traces) indicating SMCs express functional ACh receptors. As expected, TTX alone had no effect on the basal tone. ACh-induced contraction above the basal tone was partially diminished when constructs were pretreated with TTX (Fig. 5B, red traces), demonstrating that neurons contributed to ACh-induced contraction of constructs. Quantification of maximum force of contraction above the basal tone (Fig. 5C) and area under the curve (AUC) of contraction (Fig. 5D) demonstrated a significant decrease in ACh-induced contraction of bioengineered constructs upon pretreatment with TTX (%inhibition of AUC with TTX = 66.4 ± 8.8, *p < 0.05), indicating that the contraction of these constructs stimulated by exogenous ACh involved both myogenic and neurogenic components.
FIG. 5.
Contraction of bioengineered human pylorus constructs in response to acetylcholine (ACh) with or without tetrodotoxin pretreatment. Contraction of bioengineered constructs above basal tone in response to exogenous addition of ACh was assessed. (A) After establishment of basal tone, constructs further contracted upon addition of 1 μM ACh (black traces). (B) ACh-induced contraction was significantly diminished when the constructs were pretreated with tetrodotoxin (TTX; red traces). (C) Quantification of maximum force of contraction above basal tone by constructs demonstrated a significant decrease in ACh-induced contraction following pretreatment with TTX compared to ACh alone (*p < 0.05). (D) Pretreatment with TTX led to a significant decrease in area under the curve (AUC) of contraction in response to ACh (%inhibition of AUC with TTX = 66.4% ± 8.8%, *p < 0.05). Color images available online at www.liebertpub.com/tea
Relaxation in response to VIP
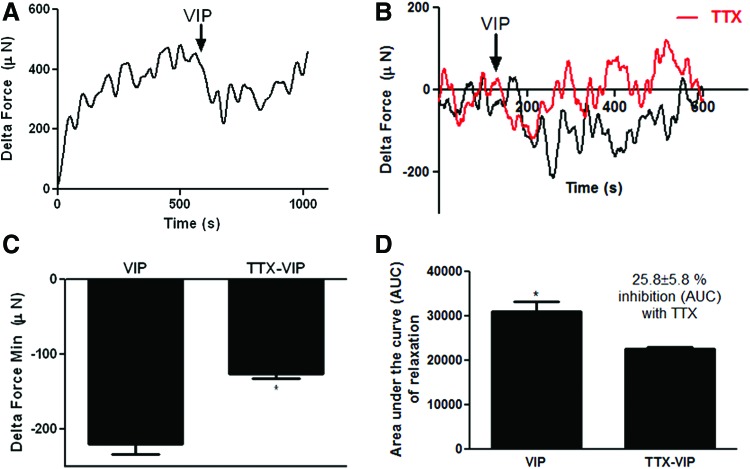
Relaxation of basal tone was evaluated following the addition of VIP with or without TTX pretreatment. Constructs were treated with 1 μM VIP either in the absence of TTX (black traces) or in the presence of TTX (red traces). Relaxation of basal tone in response to VIP (Fig. 6A and B, black traces) was observed. TTX alone had no effect of basal tone, however, VIP-induced relaxation of constructs was significantly diminished in the presence of TTX (Fig. 6B, red traces). Quantification of maximum force of relaxation of basal tone (Fig. 6C) and AUC of relaxation (Fig. 6D) in response to VIP demonstrated a significant decrease in VIP-induced relaxation of bioengineered constructs following pretreatment with TTX (%inhibition of AUC of relaxation with TTX = 25.8% ± 5.8%, *p < 0.05), indicating that VIP-induced relaxation of basal tone was mediated by both direct action on SMCs and through activation of relaxant neurons.
FIG. 6.
Assessing the relaxation of basal tone of bioengineered human pylorus constructs in response to vasoactive intestinal peptide (VIP). Relaxation of basal tone was evaluated through force generation experiments after addition of VIP. (A) After establishing basal tone, constructs were treated with 1 μM VIP alone (black traces) or (B) after pretreatment with TTX (red traces). Relaxation of constructs in response to VIP was diminished upon pretreatment with TTX. VIP-induced relaxation with or without TTX was quantified by (C) minimum delta force below the basal tone and (D) AUC of relaxation. VIP-induced maximum relaxation of constructs was significantly diminished after pretreatment with TTX (*p < 0.05). AUC of relaxation or constructs was also significantly decreased following pretreatment with TTX (%inhibition of AUC of relaxation with TTX = 25.8% ± 5.8%, *p < 0.05). Color images available online at www.liebertpub.com/tea
Relaxation in response to EFS
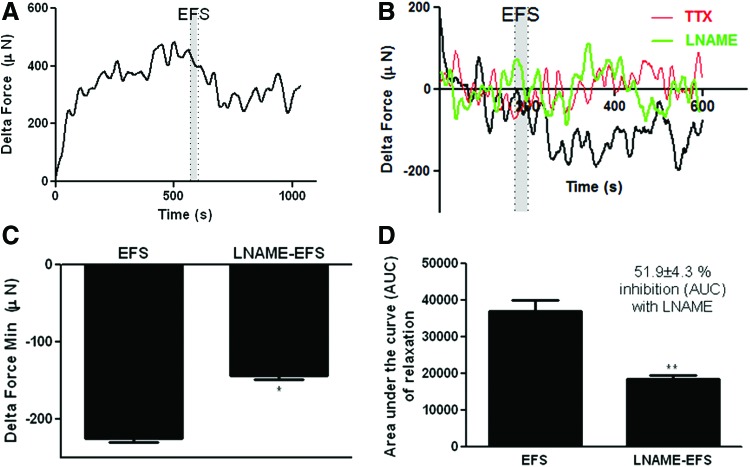
The presence of differentiated neurons was further evaluated by stimulating the neurons in the constructs with an electrical field (EFS; 5 Hz, 0.5 ms; grey area). Constructs' basal tone was diminished in response to EFS (Fig. 7A, B; black traces). EFS-induced relaxation of bioengineered human pylorus constructs was abrogated by TTX (Fig. 7B, red traces). Nitrergic neurotransmission was evaluated by pretreating the constructs with nNOS inhibitor (LNAME; 300 μM). EFS-induced relaxation was partially inhibited in the presence of LNAME, indicating that the presence of nitrergic neurons is partially responsible for the observed relaxation in response to EFS (Fig. 7B, green traces). Quantification of maximum force of relaxation of basal tone (Fig. 7C) and AUC of relaxation (Fig. 7D) demonstrated a significant decrease in EFS-induced relaxation of bioengineered constructs following pretreatment with LNAME (*p < 0.05, **p < 0.01), indicating the presence of functional nNOS-expressing neurons in these constructs (%inhibition of AUC or relaxation with LNAME = 51.9 ± 4.3, **p < 0.01).
FIG. 7.
Relaxation of basal tone of bioengineered human pylorus constructs in response to electrical field stimulation (EFS). The presence of inhibitory motor neurons was evaluated by force generation studies after stimulating constructs with EFS (5 Hz, 0.5 ms; grey area). (A) Constructs relaxed below basal tone in response to EFS (black traces). (B) EFS-induced relaxation was completely abolished upon pretreatment with TTX (red traces). Pretreatment of constructs with nNOS inhibitor, LNAME, was performed before EFS to determine nitrergic neurotransmission (green traces). EFS-induced relaxation was partially inhibited in the presence of LNAME, indicating the presence of nitrergic neurons (green traces). (C) Quantification maximum relaxation in response to EFS with or without LNAME pretreatment demonstrated a significant inhibition of maximum relaxation of basal tone of constructs by LNAME (*p < 0.05). (D) AUC of relaxation of constructs in response to EFS was significantly diminished by LNAME (%inhibition of AUC of relaxation with LNAME = 51.9% ± 4.3%, **p < 0.01). Color images available online at www.liebertpub.com/tea
Discussion
Inadequate emptying of the stomach associated with gastroparesis results in severe negative health impacts. Gastroparesis is associated with decreased levels of nitrergic neurons in the pylorus and stomach of affected patients.7,13 Treatments for gastroparesis are rarely curative and primarily help manage the condition.1–4 Alternative treatments for gastroparesis have produced mixed results.3,4 Regenerative medicine approaches may provide long-term treatments for these diseases through the development of functional engineered pyloric sphincters that possess the appropriate smooth muscle and neural components to modulate relaxation of sphincter tone. Evidence of the postnatal development of enteric nervous system indicates that SMCs in the gut mesenchyme release factors that stimulate NPC differentiation.14 We have previously bioengineered functional innervated human IAS constructs,9,10,15 which maintained functionality of both myogenic and neurogenic components and contained markers for differentiated excitatory and inhibitory motor neurons.4,10
In this study, for the first time, we successfully bioengineered intrinsically innervated autologous human pylorus tissues. Constructs were allowed to mature in neural differentiation media for 10 days in vitro. After 10 days, constructs were analyzed biochemically and physiologically. Constructs exhibited concentric smooth muscle alignment and expressed contractile smooth muscle markers. Constructs also expressed excitatory and inhibitory motor neurons necessary for regulating smooth muscle contraction and relaxation of basal tone, respectively.
Alignment of SMCs was maintained after 10 days in culture as demonstrated by H&E, SMA, and caldesmon staining. Culturing SMCs that possess a contractile phenotype is an extensive area of research.16–20 Smooth muscle phenotype was maintained in these innervated bioengineered constructs, which is essential for appropriate smooth muscle function. Previous studies have failed to maintain the contractile phenotype of dissociated and cultured SMCs.21,22 In sphincters, the generation of tone is extremely important for these tissues to perform their function of maintaining tonic closure. These bioengineered innervated human pylorus constructs generated a robust myogenic basal tone recapitulating the tonic nature of this tissue in its native state. Basal tone in constructs was insensitive to TTX, indicating that the tone was primarily myogenic, which is consistent with previous studies.9,15,23 Furthermore, the generation of a spontaneous myogenic basal tone by bioengineered innervated human pylorus constructs indicates these tissues behave similar to native tissues, which also maintain tonic closure through myogenic mechanisms.24,25 Previous studies conducted by our laboratory and others using bioengineered human IAS constructs have shown that the engineered sphincter tissues generated lower force than the native tissue. In vitro force generation measurements of human pyloric tissues are not available, however, our method for bioengineering functional innervated sphincters is scalable,9,26 therefore, it is possible to optimize and tailor the basal tone generated by these constructs in future studies.
Myogenic physiological studies were designed to assess (1) the integrity of the smooth muscle membrane by depolarizing the membrane using potassium chloride and (2) the cholinergic contraction in response to ACh. Our results, which are summarized in Table 1, demonstrate that the electromechanical coupling integrity of smooth muscle as well as neurotransmitter receptors on smooth muscle were preserved in bioengineered human pylorus constructs. The levels of physiologic myogenic functionality of the bioengineered human pylorus constructs developed here and the bioengineered IAS constructs developed previously in our laboratory are similar, demonstrating that bioengineered sphincteric tissues possess similar characteristics.10,15
Table 1.
Outline of Physiological Data
| Basal tone | KCl | ACh | TTX-ACh | VIP | TTX-VIP | EFS | LNAME-EFS | |
|---|---|---|---|---|---|---|---|---|
| Max force (μN) | 544 ± 7 | 350 ± 30 | 280 ± 52 | 134 ± 5 | −220 ± 14 | −126 ± 6 | −225 ± 6 | −144 ± 4 |
| AUC | 191841 ± 39739 | 51497 ± 9207 | 40493 ± 12519 | 13610 ± 6483 | 31112 ± 3671 | 22676 ± 782 | 37004 ± 5085 | 18588 ± 1591 |
AUC, area under the curve; EFS, electrical field stimulation; VIP, vasoactive intestinal peptide.
To our knowledge, this is the first demonstration of bioengineering a human pylorus construct that is innervated with functional differentiated neurons. One of the main regulators of sphincter contraction and relaxation are neurotransmitters released by neurons.24,27–30 In this study, we provide functional innervation to the engineered human pylorus constructs by incorporating NPCs derived from the small intestine of the same donor as the SMCs. The NPCs within bioengineered constructs differentiated into mature functional neurons expressing pan-neuronal marker βIII-tubulin. The presence of nitrergic and cholinergic neural subset populations within bioengineered constructs was observed after 10 days of culture. Cholinergic innervations were shown to be responsible for 66.4% ± 8.8% of the ACh-induced AUC of contraction. AUC of relaxation in response to VIP was inhibited by 25.8% ± 5.8% by pretreatment with TTX. VIP not only acts directly on SMCs but also by inducing enteric neurons to increase NO production.31 Neuronally mediated AUC of relaxation stimulated by EFS was abolished by pretreatment with TTX and reduced by 51.9% ± 4.3% by specifically inhibiting nitrergic neurons with LNAME. The presence of functional nitrergic innervations in bioengineered human pylorus constructs is essential for modulating the tone, which is crucial for developing appropriate sphincteric tissue replacements. Importantly, for patients with gastroparesis, these constructs provide inhibitory motor neurons (nNOS and VIP) that may reduce the tone of the native pylorus leading to increased gastric emptying and providing sustained treatment options for these diseases.
The results in this study provide the first demonstration of bioengineering autologous innervated human pyloric sphincter constructs. These constructs (1) maintained smooth muscle alignment and phenotype, (2) expressed mature excitatory and inhibitory neurons, and (3) exhibited spontaneous basal tone and responded to physiological stimuli. Bioengineered innervated human pyloric sphincter constructs have the potential for use as (1) functional replacement organs for patients with gastroparesis and (2) physiologically relevant models to investigate human pyloric sphincter disorders.
Acknowledgments
This work was supported by the Army, Navy, National Institute of Health, Air Force, Veterans Affairs, and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-13-2-0052; GU 7, Wake Forest School of Medicine Institutional Funds and the National Institute of Diabetes and Digestive and Kidney Diseases NIH/NIDDK Award No. R01DK071614.
Disclosure Statement
No competing financial interests exist.
References
- 1.Parkman H.P., Fass R., and Foxx-Orenstein A.E. Treatment of patients with diabetic gastroparesis. Gastroenterol Hepatol (N Y) 6, 1, 2010 [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson D., et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol 94, 1230, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Koch K.L., et al. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol 84, 1069, 1989 [PubMed] [Google Scholar]
- 4.Bielefeldt K. Gastroparesis: concepts, controversies, and challenges. Scientifica (Cairo) 2012, 424802, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arts J., et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther 26, 1251, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Sarosiek I., et al. Surgical approaches to treatment of gastroparesis: gastric electrical stimulation, pyloroplasty, total gastrectomy and enteral feeding tubes. Gastroenterol Clin North Am 44, 151, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Hibbard M.L., Dunst C.M., and Swanstrom L.L. Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg 15, 1513, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Dudekula A., O'Connell M., and Bielefeldt K. Hospitalizations and testing in gastroparesis. J Gastroenterol Hepatol 26, 1275, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Raghavan S., et al. Perianal implantation of bioengineered human internal anal sphincter constructs intrinsically innervated with human neural progenitor cells. Surgery 155, 668, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmont R.R., et al. Bioengineering of physiologically functional intrinsically innervated human internal anal sphincter constructs. Tissue Eng Part A 20, 1603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rego S.L., et al. Bioengineering functional human sphincteric and non-sphincteric gastrointestinal smooth muscle constructs. Methods 2015. [Epub ahead of print]; DOI: 10.1016/j.ymeth.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rego S.L., Helms R.S., and Dreau D. Breast tumor cell TACE-shed MCSF promotes pro-angiogenic macrophages through NF-kappaB signaling. Angiogenesis 17, 573, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Zandecki M., et al. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil 20, 818, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Rego S.L., et al. Enteric neural differentiation in innervated, physiologically functional, smooth muscle constructs is modulated by bone morphogenic protein 2 secreted by sphincteric smooth muscle cells. J Tissue Eng Regen Med 2015. [Epub ahead of print]; DOI: 10.1002/term.2027 [DOI] [PubMed] [Google Scholar]
- 15.Raghavan S., et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology 141, 310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beamish J.A., et al. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev 16, 467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christen T., et al. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation 103, 882, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Thyberg J., et al. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem 45, 837, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Niklason L.E., et al. Functional arteries grown in vitro. Science 284, 489, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Han T.Y., et al. Intestinal smooth muscle phenotype determines enteric neuronal survival via GDNF expression. Neuroscience 290, 357, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Davies M.G., and Hagen P.O. Structural and functional consequences of bypass grafting with autologous vein. Cryobiology 31, 63, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Walthers C.M., et al. Smooth muscle strips for intestinal tissue engineering. PLoS One 9, e114850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou Y., et al. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil 14, 477, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Farre R., and Sifrim D. Regulation of basal tone, relaxation and contraction of the lower oesophageal sphincter. Relevance to drug discovery for oesophageal disorders. Br J Pharmacol 153, 858, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Godoy M.A., and Rattan S. Autocrine regulation of internal anal sphincter tone by renin-angiotensin system: comparison with phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 289, G1164, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Somara S., et al. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 137, 53, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Sivarao D.V., Mashimo H., and Goyal R.K. Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology 135, 1258, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behar J., Kerstein M., and Biancani P. Neural control of the lower esophageal sphincter in the cat: studies on the excitatory pathways to the lower esophageal sphincter. Gastroenterology 82, 680, 1982 [PubMed] [Google Scholar]
- 29.Rattan S., and Goyal R.K. Neural control of the lower esophageal sphincter: influence of the vagus nerves. J Clin Invest 54, 899, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim C.D., Goyal R.K., and Mashimo H. Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower esophageal sphincter. Am J Physiol 277, G280, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Chakder S., and Rattan S. Evidence for VIP-induced increase in NO production in myenteric neurons of opossum internal anal sphincter. Am J Physiol 270, G492, 1996 [DOI] [PubMed] [Google Scholar]