Abstract
Pompe disease is a progressive neuromuscular disorder caused by lysosomal accumulation of glycogen from a deficiency in acid alpha-glucosidase (GAA). Replacement of the missing enzyme is available by repeated protein infusions; however, efficacy is limited by immune response and inability to restore enzymatic function in the central nervous system. An alternative therapeutic option is adeno-associated virus (AAV)-mediated gene therapy, which results in widespread gene transfer and prolonged transgene expression. Both enzyme replacement therapy (ERT) and gene therapy can elicit anti-GAA immune reactions that dampen their effectiveness and pose life-threatening risks to patient safety. To modulate the immune responses related to gene therapy, we show that a human codon-optimized GAA (coGAA) driven by a liver-specific promoter (LSP) using AAV9 is capable of promoting immune tolerance in a Gaa−/− mouse model. Copackaging AAV9-LSP-coGAA with the tissue-restricted desmin promoter (AAV9-DES-coGAA) demonstrates the necessary cell autonomous expression in cardiac muscle, skeletal muscle, peripheral nerve, and the spinal cord. Simultaneous high-level expression in liver led to the expansion of GAA-specific regulatory T-cells (Tregs) and induction of immune tolerance. Transfer of Tregs into naïve recipients prevented pathogenic allergic reactions after repeated ERT challenges. Copackaged AAV9 also attenuated preexisting humoral and cellular immune responses, which enhanced the biochemical correction. Our data present a therapeutic design in which simultaneous administration of two copackaged AAV constructs may provide therapeutic benefit and resolve immune reactions in the treatment of multisystem disorders.
Introduction
Single-vector systems have dominated AAV gene therapy as a successful treatment modality for monogenic diseases. Immune responses against the capsid and transgene product have impinged the longevity and efficiency of AAV gene therapy.1 The use of a dual-vector system in a single intervention addresses three common impediments for successful gene therapy: (1) adequate transduction of target tissues to treat the monogenic disorder, (2) AAV capsid immune responses that would neutralize the potential administration of a second vector, and (3) emergent or preexisting transgene-specific immune responses. Here, we have used the Pompe disease mouse model to illustrate the utility of a dual-vector system to address these issues. Pompe disease is caused by a deficiency of acid alpha-glucosidase (GAA) resulting in the pathologic accumulation of glycogen within the lysosome.2,3 Pompe disease is an autosomal recessive, systemic metabolic disorder with early-onset patients presenting with cardiomegaly, respiratory deficiencies, and failure to reach early motor milestones. The FDA-approved therapy for Pompe disease is enzyme replacement therapy (ERT) using recombinant human GAA (rhGAA). ERT requires a large dose of enzyme (20–40 mg/kg) once every 1–2 weeks to have therapeutic benefit.4–7
Recent works evaluating the genetic and immunological implications relevant for treating Pompe disease have provided insight into when and how therapeutic interventions should be initiated.8–11 High dose and frequent dosing are required for patients who are cross-reactive immunological material (CRIM)-negative.12,13 CRIM status is indicative of the amount of protein expressed because of the patient's GAA mutation. These patients have <1% of normal GAA activity and are the most severely affected with a life expectancy rarely exceeding 2 years without ERT.14 Because of their CRIM status, ERT is recognized by the patient's immune system as foreign and results in high-titer antibody production against the foreign protein and has the potential to elicit life-threatening infusion-associated reactions.15,16 Although CRIM-positive patients may have some degree of endogenous GAA expression, the high-dose rhGAA antigen often leads to an immune response against the treatment.17–19 Therefore, many patients become unable to continue ERT without management of immune response by immunosuppression. Although clinical management of the immune response is feasible with B- and T-cell modulation using immunosuppressive agents (such as rituximab, sirolimus, or methotrexate), long-term immune tolerance induction established through gene therapy stands as a more attractive means to prevent these responses.8,20
All recessive conditions face limitations inherent to gene replacement strategies when there is no residual endogenous protein expressed. Pompe disease is a strong candidate for gene therapy given the response to ERT.6,21,22 Gene therapy using recombinant AAV has demonstrated potential in the treatment of numerous myopathies, hemoglobinopathies, lysosomal diseases, clotting disorders, and neurologic diseases.6,22,23 Many natural and engineered AAV serotypes are available to facilitate long-term transgene expression in a variety of tissues.24,25 As an additional benefit, AAV-mediated gene therapy can trigger antigen-specific immune tolerance after liver-restricted gene expression. The mechanism behind this tolerance induction is based on the generation or expansion of antigen-specific CD4+CD25+FoxP3+ T-cells (Tregs) after hepatic gene transfer that dampen systemic responses through cell contact- and cytokine-dependent pathways.26–30 The systemic pathology associated with Pompe disease may not benefit from long-term liver-restricted expression as any GAA produced into the circulation would mimic ERT and therefore face the same limitations related to inadequate uptake into neuronal tissues.31 Targeting of the liver to induce immune tolerance to GAA has, however, demonstrated some success, but further development of a safe and efficient means to establish systemic correction with accompanying tolerance is warranted.32–35
Significant cardiac muscle, skeletal muscle, neurologic, and neuromuscular pathology has been characterized in a mouse model of Pompe disease (Gaa−/−) that replicates the pathology in human Pompe disease patients.36–41 Gaa−/− mice also display immune toxicities with chronic exposure to ERT.10 Injection of AAV-GAA locally, regionally, or systemically has demonstrated greater potential to attenuate the pathology associated with Pompe disease compared with ERT.42–47 These studies prompted the first clinical trial in Pompe disease patients with intradiaphragm injection of AAV1-GAA.48,49 It is noteworthy that clinical management of the immune response was necessary during the course of this clinical trial to prevent antitransgene immunity as ERT was maintained for ethics considerations.50 Therefore, developing immune tolerance induction strategies to be combined with gene therapies is at the forefront of investigation for Pompe disease and similar diseases that undergo protein replacement therapies.20
In this work, we provide evidence that both disease-related functional deficits and immune tolerance can be simultaneously addressed using AAV9-mediated GAA expression. Gaa−/− mice intravenously treated with a copackaged AAV9 vector, containing a liver-specific expressing vector as well as a vector with tissue-restricted specificity, attenuated skeletal muscle and cardiac pathology while providing antigen-specific tolerance. Furthermore, treatment with the copackaged AAV9 vector showed greater sustained improvements in enzyme activity in mice with preexisting immunity to GAA through attenuation of humoral and cellular responses. This dual-vector modality, provided as a single intervention, is a novel therapeutic design to address immune responses against gene therapies and promotes successful outcomes after gene delivery.
Materials and Methods
AAV vector production
The recombinant AAV vectors and plasmids (pTR-LSP-coGAA and pTR-DES-coGAA) have been described previously.51 Copackaging of AAV vectors was performed by combining the expression plasmids pTR-DES-coGAA and pTR-LSP-coGAA at a molar ratio of 9 DES:1 LSP before transfection with the capsid plasmids rep2/cap9 (courtesy of Dr. James Wilson, University of Pennsylvania) and the adenovirus helper plasmid pXX6-80 (courtesy of Dr. Xiao Xiao, University of North Carolina) in two 6,320 cm2 cell factories of HEK293 cells. AAV was purified via iodixanol step gradients, buffer exchanged in lactated Ringer's solution, and titrated as described.51 AAV9 vector-like particles (vlp; courtesy of Dr. Mavis Agbandje-McKenna, University of Florida) were prepared as previously described.52
Silver staining
Purified virus was denatured at 95°C for 10 min and then electrophoresed on a 10% Tris-HCl gel. After electrophoresis, AAV structural proteins (VP1, VP2, and VP3) were silver stained (Bio-Rad: 161-0443) according to the manufacturer's instruction.
Mice
Male and female 4–6-week-old Gaa−/− 129SVE and wild-type 129SVE mice (Taconic) were handled in accordance with the guidelines set forth by the University of Florida Institutional Animal Care and Use Committee. Mice were weighed before tail vein injection of AAV, rhGAA (Myozyme; Genzyme Corp.), or lactated Ringer's solution (vehicle). Tissues were processed for Treg adoptive transfer, molecular, biochemical, and histological studies 8 weeks post-treatment. Blood was collected from the retro-orbital plexus using heparinized microcapillary tubes. Core body temperatures were measured using a thermocouple thermometer.
GAA activity assay
GAA activity was determined as previously described.53 Liquid nitrogen-frozen tissues were homogenized in water with protease inhibitor (Roche: 04-693-124-001) and subject to three freeze–thaw cycles and centrifugation. Protein was quantified (Bio-Rad: 500-0111) and assayed for activity by measuring fluorescence after 1 hr incubation with 4-methylumbelliferyl-α-D-glucoside (Sigma: M9766) at 37°C.
Enzyme-Linked Immunosorbent Assay
Immulon 4HBX 96-well plates (Thermo: 3855) were coated with rhGAA at 1 μg/ml or 5 × 1013 vlp/ml AAV8 or AAV9 overnight at 4°C. Samples were diluted 1:50 and incubated for 2 hr at 37°C and washed with PBS-T (0.05% Tween-20). Secondary HRP-conjugated antibodies were incubated for 2 hr at 37°C and washed. Plates were developed with Sigmafast OPD tablets (Sigma: P9187) and read using a μQuant microplate spectrophotometer (BioTek Instruments). Standards and antibodies have been previously described.10
Histology
Heart and liver were immediately fixed after extraction in Gendre's fixative (Poly Scientific R&D Corp.: S1808) overnight at 4°C on an orbital shaker and subjected to two washes in 70% ethanol before paraffin embedding. Periodic acid-Schiff (PAS) staining was performed according to manufacturer's protocol (Sigma: 395B-1KT) and counterstained with Richardson's stain.
In situ force frequency
Force frequency analysis was performed on the tibialis anterior (TA) muscle as described previously.11,37 Under anesthesia the skin and fascia surrounding the distal hind limb were surgically removed exposing the TA. The extensors digitorum and peroneus longus tendons near the ankle were cut to eliminate the influence of additional muscles. Mice were positioned on a heated platform to maintain body temperature at 37°C. A clamp was used to secure the hind limb at 90° at the knee and the paw was secured to the physiology table. The TA tendon was sutured to a 300C-LR-FP muscle lever (Aurora Scientific) and severed at the ankle. Cathode and anode electrodes (Grass Technologies) were inserted proximal to the fibular head to stimulate the peroneal nerve. Optimal length-tension (L0) was determined by performing isometric twitch stimulation at an increasing range of amplitude and varying tensions until maximum twitch amplitude was observed. Three successive tetanic stimulations (200 Hz, 100 pulses per train, 60 s rest between) were performed and the muscle was allowed to rest for 5 min. Single stimulations at 15, 30, 60, 100, 120, 160, and 200 Hz were then performed with 30 s between each stimulation. Data were processed and a torque–frequency curve was derived.
Electrocardiography
Mice were anesthetized (2–2.5% isoflurane, 97.5–98% O2, flow rate 1 liter/min) and then positioned supine on a heating pad maintaining a core temperature of 37°C. Subcutaneous ECG leads (Grass Technologies) were placed in the right shoulder, right and left forelimbs, left hind limb, and tail. ECG tracings were recorded for 5 min per animal using PowerLab and Chart 5 software (ADInstruments). PR intervals were recorded every 100 heartbeats for 5 min, averaged for each animal, and then averaged within each group. PR intervals of 100 heartbeats that exceeded ±2 standard deviations of the raw mean were excluded before averaging the individual mouse recordings.
Treg adoptive transfer
An amount of 1 × 106 CD4+CD25+ splenocytes were magnetically separated (Miltenyi: 130-091-041) from mice that received AAV or vehicle after 8 weeks and adoptively transferred to naïve strain- and age-matched male and female mice by tail vein injection. Recipient mice were challenged with rhGAA (20 mg/kg) or AAV9 (5 × 1011 vlp/kg) 18 hr, 2 weeks, and 4 weeks after adoptive transfer.
Bone marrow ELISpot
Bone marrow ELISpot was performed as described previously.54 ELISpot plates (Millipore: MAHAS4510) were coated with 2 μg rhGAA overnight at 4°C. After blocking with RPMI supplemented with 5% FBS and 0.1% β-mercaptoethanol (cRPMI) for 1 hr at room temperature, bone marrow cells were plated at 2 × 106 cells per well, serially diluted twofold, and incubated overnight (37°C; 5% CO2). Rat antimouse IgG1 HRP (AbD Serotec: MCA336P) was diluted 1:1000 in cRPMI and incubated for 1 hr at room temperature. Spots were developed with AEC substrate (BD Biosciences: 551015) and the reaction was stopped with water. The membrane was dried and scanned using an ImmunoSpot Analyzer (Hightech Instruments).
Statistical analysis
Figures were drawn and statistical analysis was performed using GraphPad Prism v. 5.0 (GraphPad Software). Data were assessed for normality using the D'Agostino–Pearson omnibus test. Data deviating from a normal distribution were analyzed using the Kruskal–Wallis nonparametric test with pairwise comparison via Mann–Whitney U-test as needed. Data within a normal distribution were analyzed using unpaired two-tailed t-test or one- or two-way ANOVA with multiple test comparisons as needed. All results are represented as mean ± SEM. A p < 0.05 was considered statistically significant.
Results
AAV9-LSP-coGAA selectively expresses in the liver after intravenous injection
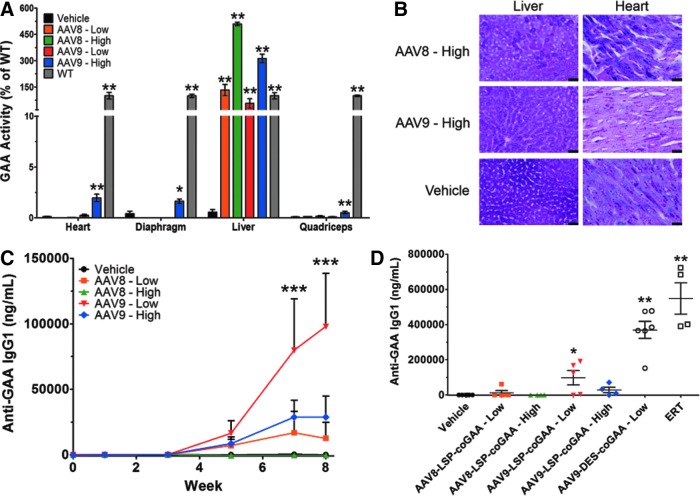
We have previously shown the efficacy of local, regional, and intravenous dosing using AAV1-CMV-, AAV9-CMV-, or AAV9-DES-hGAA to correct skeletal muscle, cardiac, and neuromuscular deficiencies in Pompe disease mice.11,42–47 In addition, we and others have described the capabilities of AAV-mediated expression of GAA to elicit detrimental immune responses against the transgene.1,50,55 To address this complication for improved systemic outcomes, we pursued the use of a liver-specific promoter (apolipoprotein E–hepatocyte locus control region–human alpha-1 antitrypsin promoter [LSP]) to drive expression of human codon-optimized GAA (coGAA) for induction of GAA-specific Tregs capable of dampening a systemic immune response against GAA.56–58 The construct was packaged in two separate serotypes: AAV8 and AAV9. AAV8 was selected because of its natural propensity to target the liver and increase levels of Tregs, and AAV9 was chosen because of its widespread transduction profile.24,30,59,60 Gaa−/− mice (4–6 weeks old; n = 4/group) received AAV-GAA at two IV doses: 0.5 × 1012 (low dose) or 5 × 1012 (high dose) vg/kg of AAV8- or AAV9-LSP-coGAA and compared with lactated Ringer's solution (vehicle). At 8 weeks post–gene transfer, hepatic GAA activity was evaluated and showed a dose-dependent response compared with vehicle-injected mice: 133 ± 32% of wild type in low dose AAV8-LSP-coGAA, 509 ± 10% of wild type in high dose AAV8-LSP-coGAA, 56 ± 26% of wild type in low-dose AAV9-LSP-coGAA, and 312 ± 25% of wild type in high-dose AAV9-LSP-coGAA (p < 0.01; Fig. 1A).
Figure 1.
Liver-directed gene therapy vector comparison in Gaa−/− mice. Results recorded 8 weeks post–gene transfer of 0.5 (low) or 5 × 1012 (high) vg/kg AAV8- or AAV9-LSP-coGAA or vehicle. (A) GAA activity as a percent of wild type within the heart, liver, diaphragm, and quadriceps. Kruskal–Wallis test results with significance levels of pairwise comparisons to vehicle are shown. (B) Periodic acid-Schiff staining for glycogen of the liver and heart in high-dose-treated mice. Images are representative of 4–6 mice per group. Scale bars represent 200 μm. (C) Time-course of anti-GAA IgG1 titers. Two-way ANOVA results with significance levels of Bonferroni multiple comparison posttest compared with vehicle are shown. (D) Anti-GAA IgG1 titers resulting from ERT (20 mg/kg; once every 2 weeks for 4 weeks) or AAV9-DES-coGAA (0.5 × 1012 vg/kg; 1-month post–gene transfer) in comparison to liver gene transfer cohorts at 8 weeks post–gene transfer. Kruskal–Wallis test results with significance levels of pairwise comparisons to vehicle are shown. Results represented as mean ± SEM; n = 4–6/group; *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle. AAV, adeno-associated virus; DES, desmin promoter; GAA, acid alpha-glucosidase; LSP, liver-specific promoter.
Furthermore, the tissue specificity of the gene expression was confirmed by measuring enzymatic activity in other highly targeted tissues with these serotypes and found to be less than 4% of wild-type GAA activity within heart, diaphragm, and quadriceps for both low and high AAV8- or AAV9-LSP-coGAA-treated mice (Fig. 1A). PAS staining for detection of glycogen revealed that glycogen clearance was limited to the liver, as profuse staining was evident in cardiac tissue in the high-dose cohorts (Fig. 1B). Full-length GAA was not detected by Western blot in the heart, diaphragm, or quadriceps, but was in the liver, indicating the small amount of activity within the other tissues was a consequence of cross-correction from hepatic-derived circulating GAA rather than cell-autonomous production from the LSP in nonspecific tissues (Supplementary Fig. S1A–D; Supplementary Data are available online at www.liebertpub.com/hum). Similarly, RT-qPCR confirmed expression of the transgene was limited exclusively to the liver with all treated cohorts displaying a significant increase in transgene mRNA within the liver (p < 0.05; Supplementary Fig. S1E; for further details see Supplementary Methods). Altogether, the LSP restricted GAA expression within the liver at a therapeutic level regardless of serotype of AAV used.
An expression threshold within the liver is necessary for immune nonresponsiveness
Serum was drawn before and once every two weeks after gene transfer from all animals at every dose to assess for anti-GAA IgG1 (TH2-type response) and anticapsid IgG2a (TH1-type response) to understand the dynamics of the immune response after vector delivery. A significant immune response directed against GAA was not observed until weeks 7 (79,808 ± 39,347 ng/ml) and 8 (97,930 ± 40,620 ng/ml) in the 0.5 × 1012 vg/kg AAV9-LSP-coGAA-treated cohort compared with vehicle-treated mice (p < 0.001; Fig. 1C). AAV8-treated groups and the 5 × 1012 vg/kg AAV9-treated group did not produce a significant antibody titer against GAA throughout the end of the study compared with the vehicle group (AAV8-LSP-coGAA—Low, p = 0.46; AAV8-LSP-coGAA—High, p = 0.98; AAV9-LSP-coGAA—High, p = 0.13; Fig. 1C). In comparison to Gaa−/− mice injected with rhGAA (n = 4) at 20 mg/kg every 2 weeks, simulating ERT, or mice systemically injected with AAV9-DES-coGAA (n = 6) at a dose of 0.5 × 1012 vg/kg, anti-GAA IgG1 generation was negligible across either AAV8- or 5 × 1012 vg/kg AAV9-LSP-coGAA-treated groups by the 8-week time point compared with vehicle-injected controls (p < 0.01; Fig. 1D). Anti-AAV8 or AAV9 IgG2a was consistently produced and showed no evidence of cross-reactivity between the serotypes (Supplementary Fig. S2A and B). Hematoxylin and eosin staining of the liver did not reveal evidence of lymphocyte infiltration of the parenchyma, indicating that gene transfer and the subsequent high gene expression were well tolerated (Supplementary Fig. S2C), consistent with results previously reported in preclinical safety and toxicology studies for AAV1-GAA.61 FACS analysis of the spleen was performed upon sacrifice to measure the degree of T-cell activation. The percentages of CD4+CD25+FoxP3+ regulatory T-cells (Tregs) were not significantly increased in comparison to vehicle controls, nor was the early activation marker CD69 increased in CD4+ or CD8+ T-cells (Supplementary Fig. S2D–F). These results revealed that AAV9 was able to promote nonresponsiveness to a similar degree as AAV8-mediated gene transfer when the dose allowed for greater than wild-type expression of GAA.
Tolerance induction using dual AAV9 is vector dose dependent
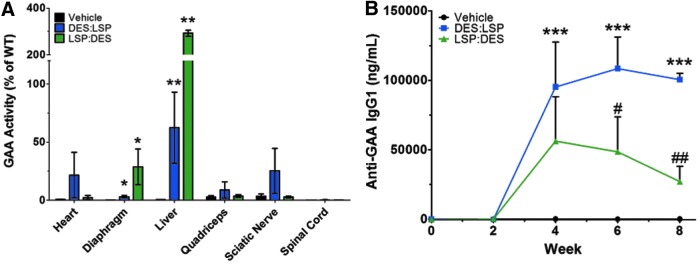
The ability of AAV9-LSP-coGAA to confer hyporesponsiveness toward GAA and restrict gene expression to the liver led us to test the hypothesis that systemic codelivery of AAV9-LSP-coGAA with AAV9-DES-coGAA can promote simultaneous immune tolerance and prevent the onset of Pompe disease-related pathology. AAV9-DES-coGAA and AAV9-LSP-coGAA were combined before dosing wherein the vector delivered as the major component was in excess 9 parts to 1 part minor component. For example, DES:LSP indicates that AAV9-DES-coGAA constitutes 9/10 of the total preparation with AAV9-LSP-coGAA at 1/10. Gaa−/− mice were systemically injected with DES:LSP or LSP:DES at a dose of either 0.5 (low dose) or 5 × 1013 (high dose) vg/kg and after 8 weeks the response to the vectors was analyzed. These doses were selected primarily because nonresponsiveness in the AAV9-LSP-coGAA-treated cohort was observed at a dose of 0.5 × 1013 vg/kg (Fig. 1C). Delivery of the combination vectors at the low dose provided evidence of tolerance induction but was not physiologically therapeutic, which prompted us to consider a 10-fold higher dose.
Eight weeks post–gene transfer in the cohorts that received the combination DES:LSP or LSP:DES at the low dose (0.5 × 1013 vg/kg; n = 4/group), the heart, liver, diaphragm, quadriceps, sciatic nerve, and spinal cord were removed and assessed for GAA activity (Fig. 2A). The DES:LSP group had the highest enzyme activity in the heart (22 ± 20% of wild type), quadriceps (9 ± 7% of wild type), and sciatic nerve (25 ± 20% of wild type), whereas the LSP:DES group had the greatest enzyme activity in the diaphragm (29 ± 15% of wild type), liver (292 ± 13% of wild type), and spinal cord (0.55 ± 0.06% of wild type). GAA activity was observed to be of a therapeutically relevant level in the liver (DES:LSP: 62 ± 31% of wild type; LSP:DES: 292 ± 13% of wild type; p < 0.001; Fig. 2A). This degree of systemic expression was not corrective of the biochemical phenotype because PAS staining revealed that the clearance of glycogen was negligible in both treated groups, particularly in the diaphragm (Supplementary Fig. S3). Although there was no increase in total Tregs (Supplementary Fig. S4A), the anti-GAA antibody response indicated some degree of tolerance. Anti-AAV9 IgG2a titers remained high in both cohorts (Supplementary Fig. S4B) but the anti-GAA IgG1 titer in the LSP:DES cohort revealed a peak titer of 56,234 ± 31,895 ng/ml at week 4 and a significant 2-fold reduction in titer at week 8 (27,124 ± 10,953 ng/ml) compared with DES:LSP (p < 0.01; Fig. 2B), indicating that GAA-specific Tregs may have developed to dampen the systemic immune response although a total increase in Tregs was not observed.
Figure 2.
Subtherapeutic dose of coadministered AAV9 vectors leads to immune tolerance in Gaa−/− mice. Results recorded 8 weeks post–gene transfer of 0.5 × 1013 vg/kg of combined AAV9-DES-coGAA (DES) and AAV9-LSP-coGAA (LSP) vectors. (A) GAA activity as a percent of wild type observed in the heart, diaphragm, liver, quadriceps, sciatic nerve, and spinal cord. Kruskal–Wallis test results with significance levels of pairwise comparisons to vehicle are shown. (B) Anti-GAA IgG1 titers observed during gene transfer. Two-way ANOVA results with significance levels of Bonferroni multiple comparison posttest compared with vehicle are shown. Results represented as mean ± SEM; n = 4/group; *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle; #p < 0.05, ##p < 0.01 LSP:DES versus DES:LSP. DES, desmin promoter; GAA, acid alpha-glucosidase; LSP, liver-specific promoter; DES:LSP (9 DES: 1 LSP of total dose); LSP:DES (9 LSP: 1 DES of total dose).
Copackaged vectors provide efficacy in a single intervention
We hypothesized that the favorable immune response observed in the LSP:DES low-dose group (0.5 × 1013 vg/kg; Fig. 2B) would be observed in the DES:LSP group at a 10-fold higher dose (5 × 1013 vg/kg) and emphasize that a preponderance of liver-derived GAA (LSP:DES) at this dose would not be sufficient to correct CNS pathology. Additionally, we have previously described a method of AAV production that allows for the manufacturing of multiple vectors in a single preparation.51 With the hypothesis that the DES:LSP ratio would confer adequate cardiac and neuromuscular expression based on previous publications, we copackaged these constructs at that ratio to produce a single gene therapy product (copackaged DES:LSP).11,46,47 We hypothesized that this would have the same benefits as producing these vectors separately and combining them but would provide a quicker and more cost-effective method of production.
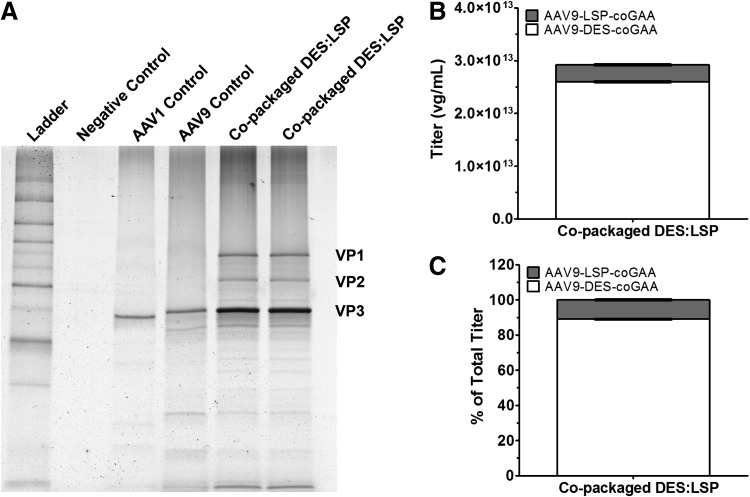
After iodixanol step gradient purification and concentration, the copackaged DES:LSP vector titer was 2.92 × 1013 vg/ml. The vector preparation was highly pure with no detectable contaminants (Fig. 3A). Titration of the individual vectors within the copackaged preparation revealed a titer for AAV9-DES-coGAA of 2.60 × 1013 ± 5.77 × 1010 vg/ml (Fig. 3B) comprising 89 ± 0.21% of the total titer (Fig. 3C) and a titer for AAV9-LSP-coGAA of 3.20 × 1012 ± 6.25 × 1010 vg/ml (Fig. 3B) comprising 11 ± 0.21% of the total titer (Fig. 3C), corroborating previous observations that AAV can be faithfully copackaged at predetermined ratios and directly compared with admixed preparations within margins of pipetting or titration error.51
Figure 3.
Characterization of copackaged DES:LSP. (A) Silver-stained gel image of AAV vector preparations visualizing structural proteins VP1 (87 kDa), VP2 (73 kDa), and VP3 (62 kDa). The amount of viral particles, as a measure of vector genomes, loaded in each lane were 1.05 × 1011 vg for AAV1 control, 6.85 × 1010 vg for AAV9 control, and 1.46 × 1011 vg for copackaged DES:LSP. Copackaged DES:LSP lanes represent aliquots from individually transfected cell factories. (B) Titration of individual vectors after copackaging determined by qPCR. (C) Percent contribution of the vectors to the total vector titer. Data represent the average of two separate experiments titrated in triplicate. AAV, adeno-associated virus; DES, desmin promoter; LSP, liver-specific promoter; DES:LSP (9 DES: 1 LSP of total dose); vg, vector genome.
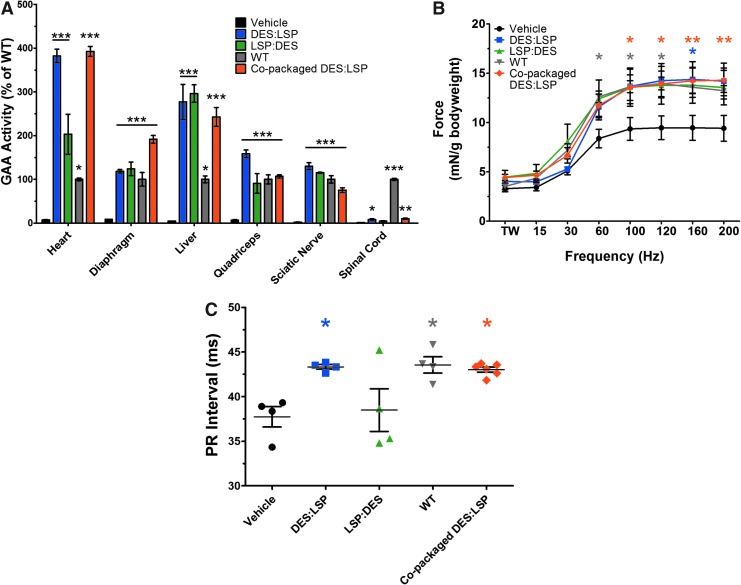
Eight weeks post–gene transfer (5 × 1013 vg/kg; n = 4–6/group), GAA activity was significantly increased in all treated mice in the heart, diaphragm, liver, quadriceps, and sciatic nerve compared with vehicle-treated mice (Fig. 4A). Activity within the spinal cord did not reach wild-type levels but significantly improved in the admixed DES:LSP (8 ± 2% of wild type; p < 0.05) and copackaged DES:LSP (10 ± 1% of wild type; p < 0.01) cohorts compared with vehicle-injected knockouts. The physiologic effects of enzyme activity were apparent based on in situ force frequency and electrocardiograms (ECG). In all treated groups there was an observed benefit in force production in the TA. The force generated by the TA in the admixed DES:LSP (100 Hz: 14 ± 2 mN/g bodyweight; 120 Hz: 14 ± 2 mN/g bodyweight; 160 Hz: 14 ± 2 mN/g bodyweight; 200 Hz: 14 ± 2 mN/g bodyweight) and copackaged DES:LSP (100 Hz: 14 ± 1 mN/g bodyweight; 120 Hz: 14 ± 1 mN/g bodyweight; 160 Hz: 14 ± 1 mN/g bodyweight; 200 Hz: 14 ± 1 mN/g bodyweight) cohorts was significantly greater than vehicle-treated mice (100 Hz: 9 ± 1 mN/g bodyweight; 120 Hz: 9 ± 1 mN/g bodyweight; 160 Hz: 9 ± 1 mN/g bodyweight; 200 Hz: 9 ± 1 mN/g bodyweight; p < 0.05) and was comparable to wild-type levels (100 Hz: 14 ± 0.1 mN/g bodyweight; 120 Hz: 14 ± 1 mN/g bodyweight; 160 Hz: 14 ± 1 mN/g bodyweight; 200 Hz: 13 ± 1 mN/g bodyweight; p = 0.59, p = 0.47, respectively; Fig. 4B).
Figure 4.
Dual AAV9 vectors correct biochemical and physiologic pathology in Gaa−/− mice. Results recorded 8 weeks post–gene transfer of 5 × 1013 vg/kg of admixed DES:LSP, LSP:DES, or copackaged DES:LSP. (A) GAA activity as a percent of wild type in the heart, diaphragm, liver, quadriceps, sciatic nerve, and spinal cord. One-way ANOVA results with significance levels of Tukey multiple comparison posttest compared with vehicle are shown. (B) In situ force frequency analysis of the tibialis anterior muscle. Two-way ANOVA results with significance levels of Bonferroni multiple comparison posttest compared with vehicle are shown. (C) PR interval recordings. One-way ANOVA results with significance levels of Tukey multiple comparison posttest compared with vehicle are shown. Results represented as mean ± SEM; n = 3–6/group; *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle. DES, desmin promoter; GAA, acid alpha-glucosidase; LSP, liver-specific promoter; DES:LSP (9 DES: 1 LSP of total dose); LSP:DES (9 LSP: 1 DES of total dose).
One of the primary clinical manifestations of Pompe disease is cardiomegaly and a shortened PR interval.7 After 8 weeks, there was evidence of successful gene transfer by change in the PR interval in the admixed DES:LSP (43 ± 0.25 ms) and copackaged DES:LSP (43 ± 0.28 ms) groups with PR intervals equivalent to age-matched wild-type mice (44 ± 1 ms; p = 0.82 and p = 0.53, respectively) compared with vehicle-treated mice (38 ± 1 ms; p < 0.05; Fig. 4C). The LSP:DES group did not demonstrate a statistically significant improvement in force generation (100 Hz: 14 ± 2 mN/g bodyweight; 120 Hz: 14 ± 2 mN/g bodyweight; 160 Hz: 14 ± 2 mN/g bodyweight; 200 Hz: 14 ± 2 mN/g bodyweight; p = 0.06) or PR interval (38 ± 2 ms; p = 0.79), possibly indicating insufficient transduction of the peripheral nervous system. Substantiating the GAA activity, strength, and ECG analyses, GAA and PAS staining revealed GAA staining in cardiac, diaphragm, and liver tissue with punctate staining in the longitudinal and cross sections of the sciatic nerve with clearance of glycogen (Supplementary Fig. S5).
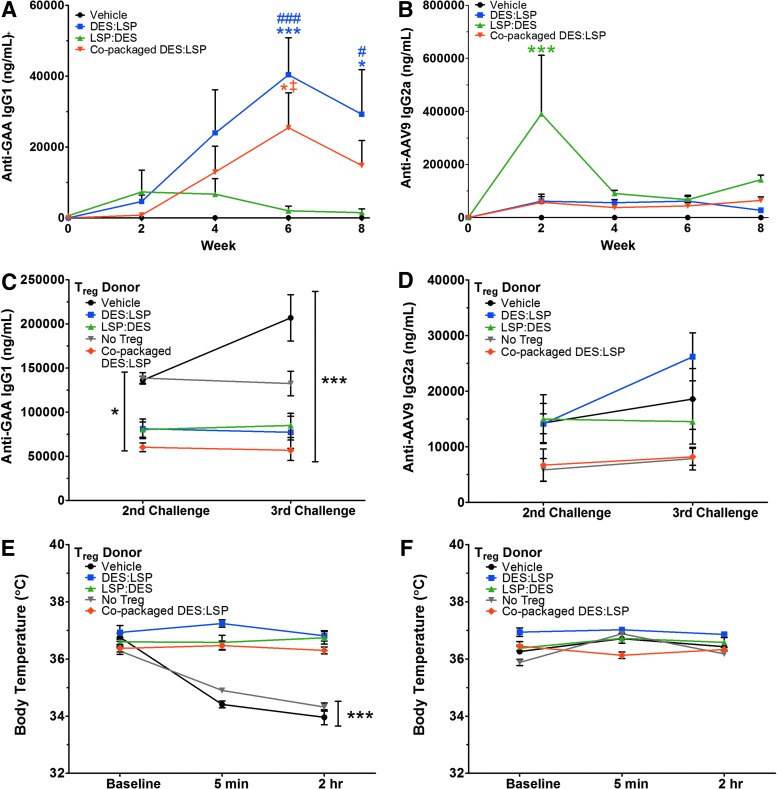
Antibodies generated against GAA in the LSP:DES-treated mice were significantly lower than the DES:LSP-treated group after 8 weeks (p < 0.05; Fig. 5A). Anti-GAA IgG1 titers in the LSP:DES cohort began to decline from 7,334 ± 6,162 ng/ml at week 2 to 1,495 ± 1,024 ng/ml by week 8 (Fig. 5A). Between weeks 6 and 8, anti-GAA titers dropped approximately 1.4-fold in the DES:LSP group (week 6 titer: 40,419 ± 10,403 ng/ml; p < 0.001 vs. vehicle; week 8 titer: 29,215 ± 12,606 ng/ml; p < 0.05 vs. vehicle), which may indicate that it had taken longer for enough antigen-specific Tregs to generate to dampen the immune response. Anti-GAA IgG1 titers in the copackaged DES:LSP cohort also emulated the kinetics of admixed DES:LSP (week 6 titer: 24,437 ± 9,889 ng/ml; p < 0.05 vs. vehicle and LSP:DES; week 8 titer: 14,771 ± 7,093 ng/ml; p = 0.12 vs. vehicle and p = 0.15 vs. LSP:DES). Anti-AAV9 titers were not different throughout the 8 weeks with exception of an acute response in the LSP:DES group at 2 weeks postinjection because of unknown circumstances (p < 0.001; Fig. 5B). The response resolved by the end of the study to a comparable titer in the other treated cohorts (p = 0.08). Taken together, immune tolerance may have been induced even in the context of systemic transgene expression after combination AAV9 delivery, and copackaged AAV9 vectors provide identical efficacy as vectors combined before dosing.
Figure 5.
Dual AAV9 vector treatment establishes immune tolerance and prevents hypersensitivity reactions in Gaa−/− mice. (A) Anti-GAA IgG1 titers observed during gene transfer. (B) Anti-AAV9 IgG2a titers observed during gene transfer. (C) Anti-GAA IgG1 titers observed in Gaa−/− mice that received 1 × 106 Tregs originating from AAV9-treated or vehicle donors (or no Tregs) after the 2nd and 3rd challenge with rhGAA (20 mg/kg). (D) Anti-AAV9 IgG2a titers observed in Gaa−/− mice that received 1 × 106 Tregs originating from AAV9-treated or vehicle donors (or no Tregs) after the 2nd and 3rd challenge with empty AAV9 capsids (5 × 1011 vlp/kg). (E) Core body temperature recordings observed during the 3rd challenge of rhGAA after Treg adoptive transfer. (F) Core body temperature recordings observed during the 3rd challenge of AAV9 after Treg adoptive transfer. Two-way ANOVA with significance levels of Bonferroni multiple comparison posttest compared with vehicle are shown. Results represented as mean ± SEM; n = 4–6/group; *p < 0.05, ***p < 0.001 versus vehicle; #p < 0.05, ###p < 0.001 LSP:DES versus DES:LSP; ‡p < 0.05 LSP:DES versus copackaged DES:LSP. DES, desmin promoter; GAA, acid alpha-glucosidase; LSP, liver-specific promoter; Treg, regulatory T-cell; DES:LSP (9 DES: 1 LSP of total dose); LSP:DES (9 LSP: 1 DES of total dose); vlp, vector-like particles.
Treg transfer ameliorates humoral responses against GAA and anaphylaxis
To confirm antigen specificity associated with declining anti-GAA IgG1 titers, we attempted Treg adoptive transfers. An amount of 1 × 106 Tregs were isolated from the spleens of mice that received AAV9 vectors (DES:LSP [n = 8], LSP:DES [n = 5], copackaged DES:LSP [n = 6]) or from vehicle-treated animals (n = 8) and transferred to naïve recipients. One group of mice did not receive any Tregs as a control for nonspecific suppression (n = 5). Mice were challenged with rhGAA at the clinically prescribed dose of 20 mg/kg at 18 hr posttransfer and once every 2 weeks for 4 weeks, simulating ERT. After the second challenge, anti-GAA IgG1 titers were 81,166 ± 11,146 ng/ml in mice that received Tregs from the DES:LSP group, 80,200 ± 8,671 ng/ml in the mice given Tregs from LSP:DES donors, 60,196 ± 4,890 ng/ml in mice that received Tregs from copackaged DES:LSP donors, 136,266 ± 4,345 ng/ml in mice that received Tregs from vehicle-treated mice, and 138,625 ± 6,034 ng/ml in those mice that did not receive Tregs, indicating a significant difference between groups of mice that received Tregs from AAV9-treated donors compared with those that did not (p < 0.05; Fig. 5C).
An additional challenge with 20 mg/kg rhGAA 2 weeks later resulted in modest decreases in anti-GAA IgG1 titer in mice that received Tregs from DES:LSP mice to 77,190 ± 18,218 ng/ml, to 56,754 ± 11,586 ng/ml in copackaged DES:LSP Treg recipients, and a marginal increase in titer in those mice that received Tregs from LSP:DES mice to 84,890 ± 13,673 ng/ml, but titers in mice that received vehicle Tregs developed titers of 206,929 ± 26,238 ng/ml and mice that did not receive Tregs developed titers of 132,444 ± 13,894 ng/ml, maintaining a significant difference between recipients of AAV9-treated donor-derived Tregs compared with vehicle Treg recipients and mice that did not receive Tregs (p < 0.001; Fig. 5C). Tregs from gene therapy-treated or untreated mice were also injected into naïve recipients and similarly challenged with empty AAV9 vector-like particles (vlp) at a dose of 5 × 1011 vlp/kg on the identical schedule as rhGAA-challenged mice. The resulting anti-AAV9 IgG2a titers from the DES:LSP (n = 6), LSP:DES (n = 6), copackaged DES:LSP (n = 4), vehicle (n = 8)-treated mice, or no Tregs (n = 4) were not different, indicating that tolerance was GAA specific (p = 0.60; Fig. 5D).
To measure the degree of pathogenicity related to these antibody titers, we measured differences in core body temperature upon the final rhGAA injection. Before injection, all mice had a baseline temperature of 36–37°C. Within 5 min, and up to 2 hr later, the body temperature of those mice that received Tregs from vehicle-treated mice dropped to 34 ± 0.1°C and 34 ± 0.3°C, respectively, and in mice that did not receive Tregs dropped to 35 ± 0.04°C and 34 ± 0.1°C, respectively, whereas those mice that received Tregs from gene therapy-treated mice maintained normal baseline core temperature between 36°C and 37°C (p < 0.001; Fig. 5E). The drop in temperature in the first two groups was also accompanied by outward signs of anaphylaxis: prostration, piloerection, and hyperventilation leading to moribund body conditions (not shown). Conversely, body temperatures recorded in mice that were challenged with empty AAV9 capsid did not result in any temperature differences even at 2 hr and did not show any signs of outward distress (p = 0.54; Fig. 5F). In conclusion, although antibodies were generated against GAA, they were not pathogenic to a sufficient degree to result in anaphylaxis. In total, these data suggest that a single gene therapy product that coordinates gene expression in discrete tissues is capable of the simultaneous induction of antigen-specific tolerance with prevention of Pompe disease pathology.
Copackaged AAV9 treatment suppresses preexisting immunity against rhGAA and enhances efficacy of gene therapy
Preexisting immunity toward GAA is a substantial roadblock to successful ERT and gene therapy. Representing a clinical situation where anti-GAA antibody titers are present, we hypothesized that the copackaged AAV9 product (copackaged DES:LSP) would efficiently target the liver to establish immune tolerance capable of overcoming ongoing immune responses directed against GAA. In a mouse model of hemophilia B, factor IX-immunized mice treated with hepatic AAV8 gene transfer reversed antibody formation and cellular responses directed against factor IX.58
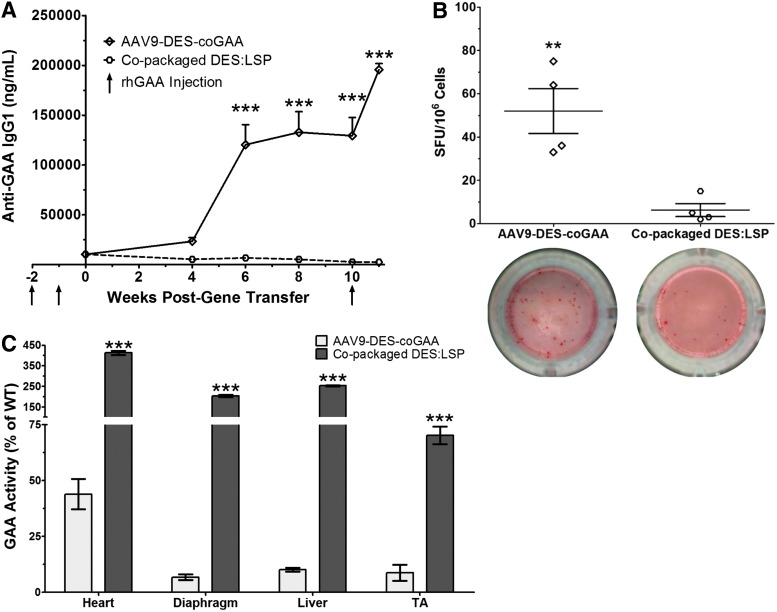
An immune response toward GAA was established in Gaa−/− mice after two doses of rhGAA (20 mg/kg) one week apart. The mice were divided into two cohorts (n = 4–5) and treated with AAV9-DES-coGAA or copackaged DES:LSP at a dose of 5 × 1013 vg/kg one week after the second injection of rhGAA. The AAV9-DES-coGAA cohort was included to emphasize the necessity of liver-directed gene transfer to induce tolerance. Before gene transfer, the AAV9-DES-coGAA cohort had an anti-GAA IgG1 titer of 10,349 ± 294 ng/ml and the copackaged DES:LSP cohort had a titer of 10,461 ± 970 ng/ml (p = 0.99; Fig. 6A). At 6 weeks posttransfer, mice treated with AAV9-DES-coGAA developed titers of 120,331 ± 20,158 ng/ml, which was significantly greater than the copackaged DES:LSP cohort, which had titers of 6,818 ± 2,728 ng/ml (p < 0.001; Fig. 6A). By week 10, the titers in the AAV9-DES-coGAA cohort had stabilized at 129,398 ± 18,347 ng/ml but the titers in the copackaged DESL:LSP cohort continued to decline to 2,694 ± 850 ng/ml (p < 0.001; Fig. 6A). An additional injection of rhGAA was administered at week 10 to evaluate long-term immune tolerance. One week later, AAV9-DES-coGAA-treated mice had an anti-GAA IgG1 titer of 195,732 ± 6035 ng/ml, a 1.5-fold increase from week 10, while the copackaged DES:LSP cohort displayed titers of 2,540 ± 561 ng/ml, a 1.1-fold decrease from week 10 (p < 0.001; Fig. 6A), thus indicating that the liver-specific expression conferred through copackaged DES:LSP established robust, sustained suppression of anti-GAA antibody production.
Figure 6.
Copackaged AAV9 vectors attenuate preexisting immunity and demonstrate greater efficacy in Gaa−/− mice. (A) Anti-GAA IgG1 titers in rhGAA-immunized mice observed after gene transfer of 5 × 1013 vg/kg AAV9-DES-coGAA or copackaged DES:LSP. Two-way ANOVA results with significance levels of Bonferroni multiple comparison posttest are shown. (B) ELISpots for anti-GAA IgG1-secreting bone marrow cells in rhGAA-immunized mice observed after gene transfer of 5 × 1013 vg/kg AAV9-DES-coGAA or copackaged DES:LSP. (C) GAA activity as a percent of wild type in the heart, diaphragm, liver, and tibialis anterior (TA). Significance levels of unpaired two-tailed t-test are shown. Results represented as mean ± SEM; n = 4–5/group; **p < 0.01, ***p < 0.001. DES, desmin promoter; GAA, acid alpha-glucosidase; LSP, liver-specific promoter; DES:LSP (9 DES: 1 LSP of total dose); rhGAA, recombinant human GAA; SFU, spot-forming unit.
To demonstrate active suppression of cellular responses, bone marrow was harvested to detect suppression of anti-GAA IgG1-producing bone marrow cells using an ELISpot assay. Before the conclusion of the study period, one mouse in the AAV9-DES-coGAA cohort spontaneously died because of unknown circumstances. Mice immunized with rhGAA that received 5 × 1013 vg/kg AAV9-DES-coGAA developed 52 ± 10 spot-forming units (SFU)/106 bone marrow cells, but immunized mice that received 5 × 1013 vg/kg copackaged DES:LSP developed 6 ± 3 SFU/106 bone marrow cells (p < 0.01; Fig. 6B). Therefore, hepatic expression attenuated pathogenic antibody production from antigen-specific bone marrow cells.
High-titer antibodies can profoundly inhibit the effectiveness of ERT or gene therapy. To illustrate enhanced therapeutic efficacy to gene therapy when immune tolerance is induced, GAA activity was assessed in the heart, diaphragm, liver, and TA. Mice immunized with rhGAA treated with AAV9-DES-coGAA displayed significantly reduced levels of GAA activity compared with the copackaged DES:LSP cohort. In AAV9-DES-coGAA-treated mice, GAA activity was 44 ± 7% of wild type in the heart, 7 ± 1% of wild type in the diaphragm, 10 ± 0.8 in the liver, and 9 ± 4 in the TA compared with the copackaged DES:LSP-treated mice demonstrating activity levels of 413 ± 10% of wild type in the heart (p < 0.001), 204 ± 5% of wild type in the diaphragm (p < 0.001), 253 ± 2% of wild type in the liver (p < 0.001), and 70 ± 4% of wild type in the TA (p < 0.001; Fig. 6C). These data suggest that greater therapeutic efficacy can be achieved when immune tolerance is established despite preexisting immunity.
Discussion
Therapeutic proteins sequestered by antibodies can pose a significant risk to patient safety by eliciting infusion-associated reactions.13,17 Recently, it has been shown that up to 83.5% of rhGAA in circulation can be bound by anti-GAA antibodies.62 Hence, immune responses can not only be life threatening but also limit therapeutic efficacy. The purpose of this study was to devise a single gene therapy product capable of inducing immune tolerance while simultaneously preventing the onset of Pompe disease pathology. Observations from other systemic diseases indicate that induction of immune tolerance will improve the efficacy and safety of gene and protein replacement therapies.1,20,55 The liver-specific construct we evaluated, packaged in AAV8 or AAV9, to express GAA was capable of achieving persistent transgene expression exclusively in the liver. Despite previous reports, the two different doses tested within this study did not demonstrate therapeutic benefit resulting from liver-derived GAA in striated or cardiac muscle using biochemical and histologic parameters compared with untreated Gaa−/− mice.63,64 The use of the liver as a depot for enzyme production for systemic efficacy faces the same limitations as ERT as it relies on the inefficiencies of mannose-6-phosphate receptor-mediated uptake, the receptor density on target tissues, and intracellular GAA trafficking to the lysosome. Consistent with those previous reports, however, achieving wild-type equivalent or greater enzyme activity within the tissue provided evidence for immune tolerance induction.32–35 The elicited immune response against GAA from the LSP-coGAA vectors was marginal compared with other systemic therapies we tested (ERT or AAV9-DES-coGAA alone), and no apparent hepatotoxicity accompanied transgene expression at any vector dose. These findings emphasize the utility of liver-directed gene therapy to attenuate immune responses to therapeutic proteins.
AAV8 has typically been the serotype of choice in promoting tolerance to transgenes by relying on its propensity to target to the liver and the naturally immune tolerant environment within the organ.20,65 Although AAV8 has been the most widely used vector for immune tolerance induction, we report expanding evidence that AAV9 is as capable of establishing immune tolerance when provided at a sufficient dose and the transgene is under the control of a liver-specific promoter.66 As it is a human-derived serotype, AAV9 may therefore have improved liver tropism compared with AAV8 in humans, thus providing an indication that alternative serotypes may achieve tolerance in the context of clinical translation.
Given that liver-derived GAA may not be sufficient for full-body correction of pathology, providing an additional vector simultaneously under immune tolerant conditions may give way to greater therapeutic efficacy. The tropism of AAV9 to transduce cardiac muscle, skeletal muscle, and neuronal tissue, with the presented evidence to target the liver to a sufficient degree to induce tolerance, reasoned us to maintain with AAV9.31,37,38,46,47 Providing an additional AAV9 vector (under the control of a desmin promoter) prevented the shortening of PR interval and limb neuromuscular dysfunction. Evidence of marked cardiac tropism for AAV9 in mice may represent a species-specific bias, however, as transduction profiles between small- and large-animal models vary.67 Additionally, ubiquitous and tissue-restricted promoters used for systemic expression, such as desmin, CMV, or CBA, have a propensity to express in antigen presenting cells, resulting in deleterious innate and adaptive responses that eliminate transduced cells.68–71 Therefore, inclusion of an immune tolerance-inducing construct may attenuate the immunogenicity of these vectors. A systemic dose of 5 × 1013 vg/kg using our dual-vector approach resulted in supraphysiologic levels of enzyme activity in most tissues tested.
Although the GAA activity in the spinal cord did not reach wild-type levels, there was a significant increase compared with the untreated knockout group when the desmin vector was provided as the major component (DES:LSP and copackaged DES:LSP). Minor but significant increases in activity observed within the spinal cord may have clinical significance as well. Haploinsufficiency of GAA does not result in Pompe disease, the onset of disease symptoms inversely correlates with GAA quality and quantity, and even 2–3% of normal activity has shown to be clinically relevant in outcome measures for similar protein deficiencies, such as hemophilia B.48,72,73 Considering also that the duration of these studies was 8 weeks and the ability for AAV9 to remain in circulation for prolonged periods, transduction may increase over time.24 Based on evidence of central and peripheral nervous system pathology, adequate transgene expression within these tissues will be necessary to achieve long-term therapeutic benefit, regardless of the onset of disease progression.37–39
Early restoration of enzyme activity to both neural and skeletal muscle tissue is likely to have the greatest effect on physiological outcomes related to treatment strategies as our studies and those of others have demonstrated time-dependent arrests in functional decline after gene therapy.11,74,75 Reliance upon liver-derived GAA to target neural tissues is dependent upon cross-correction of neighboring cells through secretion and reuptake of GAA, yet the protein is too large to cross the blood–brain barrier and therefore does not address the neuropathology related to Pompe disease.6,31 For these reasons, ERT or exclusively hepatic gene transfer would not address glycogen accumulation particularly in the motor neurons involved with ambulation or respiration as evidenced by patients continuing to be wheelchair- and ventilator-dependent after long-term treatment of ERT.16,41 Although it is recognized that GAA expressed from the desmin promoter would similarly be unable to cross the blood–brain barrier, evidence of crossing the blood–brain barrier by AAV9 would confer cell autonomous correction of the CNS.59,76 Building on success with AAV9-DES-GAA to attenuate functional decline of cardiac, respiratory, and skeletal muscle pathology in Pompe disease, we have applied a new paradigm to address both the immunological and neuromuscular complications using a copackaged, dual-AAV9 vector system that addresses the multisystem disease progression simultaneous with overcoming immune-related complications, which is likely to have the most persistent therapeutic benefit and increase survival rates.11,37,46,47
The induction of immune tolerance enhances the success of gene therapy. As ERT cannot be ethically withheld from patients undergoing gene therapy, the induction of tolerance to the transgene may provide greater therapeutic efficacy. The inclusion of the liver-directed vector lessened the severity of the immune response in vector-treated mice, which resulted in a protective effect after adoptive transfer of Tregs. Indeed we showed that the codelivered vectors were able to induce GAA-specific Tregs capable of attenuating immune responses to ERT, which minimized the risk of immunotoxicities as evidenced by the lack of accompanying anaphylaxis even without prophylactic diphenhydramine. Tregs derived from mice that received the copackaged AAV9 preparation were also capable of mitigating the response. Transferred Tregs had limited suppression in the recipient hosts, which was evident in the modestly reduced titers yet titers were sufficiently lessened to prevent anaphylaxis. To confer greater suppressive capabilities, ex vivo expansion is necessary.77 Transfer of Tregs from AAV9-DES-coGAA-treated mice would not be expected to protect from anaphylaxis as we show here and in previous studies the similar anti-GAA antibody titers that arise from this vector compared with mice treated with ERT alone indicating no evidence of tolerance induction.47
Despite these limitations, the requirement of Tregs in immune tolerance induction is widely supported in Treg depletion studies. The clearest evidence was obtained in a mouse model of hemophilia B treated with AAV8-hF.IX, where immune tolerance was broken after FoxP3+ cells were effectively depleted with diphtheria toxin.58 We additionally show the capacity to overcome preexisting humoral and cellular immune responses using copackaged AAV9, which was not observed when AAV9-DES-coGAA was delivered alone. Significant improvement of GAA activity in tissues primarily affected in Pompe disease was observed in the copackaged DES:LSP cohort compared with the AAV9-DES-coGAA cohort and lasting suppression of antibody production was achieved. Although not tested here, the AAV9-DES-coGAA cohort would have been expected to improve to some degree in cardiac and skeletal muscle function based on GAA activity in the present study and compared with previous observations.11,47 Mouse models are poor predictors of potential antibody-dependent cytotoxic responses, however, and the persistent, high-titer anti-GAA antibodies in the AAV9-DES-coGAA cohort may produce deleterious cytotoxic responses similar to those observed in ERT-treated Pompe disease patients and gene therapy clinical trials for hemophilia B.73,78–81 Supporting previous studies, these data highlight the role of Tregs induced after hepatic gene transfer in amelioration of immune responses after gene transfer and how immune tolerance induction benefits systemic therapies.34,35,58,82
A limitation of this study is the incomplete initiation of immune tolerance. The impact of anti-GAA antibodies on infused recombinant protein certainly affects efficacy of ERT. Incomplete immune tolerance induction brought on by insufficient expression of the transgene has been shown in models of hemophilia B.58 This may be an explanation for the higher titers observed at the 0.5 × 1012 vg/kg dose of AAV9-LSP-coGAA compared with the higher doses of AAV9 rather than promiscuous expression of the liver-specific promoter in other tissues. Although anti-GAA titers gradually decreased after gene transfer of the combined vectors, lower-titer antibodies were still produced, likely because of widespread transgene expression rather than inadequate expression. Despite the lack of anaphylaxis after multiple injections of rhGAA, the titers generated may have clinical significance. However, antibodies are less likely to influence the efficacy of GAA that remains intracellularly and is efficiently trafficked to the lysosome, which is a distinct advantage of gene therapy compared with ERT. The antibody titers may also be less of an issue in patients if AAV9 efficacy is greater for gene transfer to human liver. Conversely, growing clinical experience with pharmacological immune modulation in Pompe patients may facilitate development of combination therapies to induce tolerance and prevent antibody production.8,54,83,84
The cost of manufacturing and release testing of an AAV vector is not inconsequential.85 In the case of large transgenes that exceed the carrying capacity of AAV, methods have been developed to facilitate packaging and delivery of these longer genes as demonstrated by emerging therapies for Duchenne's muscular dystrophy, dysferlinopathies, hemophilia A, Usher 1B, and Tay–Sachs disease.86–91 In most cases these vectors are manufactured separately and combined before dosing. In the present study, two vectors are similarly necessary to achieve efficacy. To expedite manufacturing and improve cost-effectiveness of the process, we copackaged the vectors at the desired ratio simultaneously. The resulting effect of delivering the copackaged preparation recapitulated observations in the admixed DES:LSP cohort with increased benefits in elevation of CNS GAA activity above knockout animals, force output and PR interval similar to wild-type mice, lower anti-GAA titer, as well as induced immune tolerance. The clinical translation of such a modality would be essentially as described for any AAV-centric application. Once the appropriate dose and ratio of the two (or potentially more) vectors has been empirically determined in small- and large-animal models, that preparation would stand as the clinical candidate vector.
We and others have shown that copackaging is a reproducible and scalable method of vector production.51,88 The present study included, consistent reproducibility of the predicted packaging ratios in AAV2, AAV8, and AAV9 shows that the process is serotype-independent; production levels at surface areas ranging from 148 to 6,320 cm2 using roller bottles or flasks highlight the scalability and flexibility in manufacturing; purification of copackaged vectors are not affected whether performing cesium-chloride or iodixanol centrifugation; and careful titration of the expression plasmids results in consistent ratios across preparations.51,88,92 Rearrangement of the plasmid genome brought on by homologous recombination during packaging is a concern but seems to occur at a negligible rate as the output ratios are highly consistent of the predicted ratios.51 Once the manufacturing process is defined, the copackaged candidate vector can therefore be treated as a single product for toxicology, stability, sterility, and release testing as performed for any current AAV clinical trial rather than as two independent products. Dose assessment and identity studies will be necessary for clinical protocol validation but inclusion of next-generation sequencing would confirm if the ratio has been maintained and indicate if homologous recombination occurred. With these considerations, the similarity of results between copackaged and admixed vectors is encouraging that this production method would effectively reduce the cost and time of clinical application for multiple AAV vectors. This method stands as a more efficient means to advance translational application when high titers of multiple vectors are required for preclinical and clinical trials.
In conclusion, our results show that copackaged AAV9 vectors impart therapeutic benefit in Pompe disease and simultaneously induce immune tolerance in a mouse model of the disease. The dual-vector strategy prevented disease-related functional deficits and produced negligible antitransgene antibody titers likely because of the induction of antigen-specific Tregs, which, after adoptive transfer, protected recipient hosts from ERT-induced anaphylaxis. Importantly, the dual-vector strategy also dampened preexisting immunity that facilitated greater biochemical correction. Described at doses compatible with clinical application, with further substantiation of copackaging as an improved method of multivector production, these results pave the way to use multiple vectors to address the immune complications related to systemic gene delivery for Pompe disease.
Supplementary Material
Acknowledgments
We thank Marda Jorgensen (University of Florida) for performing GAA IHC, H&E, and PAS staining; Mavis Agbandje-McKenna (University of Florida) for providing AAV capsids; Brad Hoffman (University of Florida) for assistance in FACS analysis; Martha Campbell-Thompson (University of Florida) for reviewing histological sections; the Powell Gene Therapy Center Vector Core (University of Florida) for assistance with vector production and characterization; and Jonathan Shuster (University of Florida) for advice on statistical analysis.
This work was supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 (to B.J.B. and P.A.D.), NIH/NICHD traineeship T32 HD043730 (to P.A.D.), American Heart Association fellowship 15POST22820004 (to A.G.T.), NIH/NIAMS mentored career award K01 AR066077 (to D.J.F.), fellowship from Wenner-Gren Foundation Fellowship-Stockholm and Kronprinsessan Lovisas Förening and Swedish Society of Medicine grants (to S.N.), and NIH/NHLBI research awards P01 HL59412 and R01 HD052682 (to B.J.B.). The content is solely the responsibility of the authors and the National Institutes of Health; National Center for Advancing Translational Sciences; National Institute of Child Health & Human Development; National Institute of Arthritis and Musculoskeletal and Skin Diseases; National Heart, Lung and Blood Institute; American Heart Association; Wenner-Gren Foundation-Stockholm; Kronprinsessan Lovisas Förening; or Swedish Society of Medicine were not involved in the study design; collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Author Disclosure
B.J.B. is a founder and owner of founder equity of Applied Genetic Technologies Corporation and an unpaid member of the Scientific Advisory Board of Audentes Therapeutics, Solid GT, LLC, and Bristol-Meyers Squibb. P.A.D., N.C., D.J.F., B.J.B., Johns Hopkins University, and University of Florida could be entitled to patent royalties for inventions described concerning AAV copackaging and AAV9-GAA technology. The other authors declare that they have no competing interests.
References
- 1.Mingozzi F, High KA. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013;122:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet 2008;372:1342–1353 [DOI] [PubMed] [Google Scholar]
- 3.Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: Design, methodology, and early findings from the Pompe Registry. Mol Genet Metab 2011;103:1–11 [DOI] [PubMed] [Google Scholar]
- 4.Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: Results of a phase I/II clinical trial. Genet Med 2001;3:132–138 [PubMed] [Google Scholar]
- 5.Koeberl DD, Kishnani PS, Chen YT. Glycogen storage disease types I and II: Treatment updates. J Inherit Metab Dis 2007;30:159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne BJ, Falk DJ, Clément N, Mah CS. Gene therapy approaches for lysosomal storage disease: Next-generation treatment. Hum Gene Ther 2012;23:808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishnani PS, Steiner RD, Bali D, et al. Pompe disease diagnosis and management guideline. Genet Med 2006;8:267–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elder ME, Nayak S, Collins SW, et al. B-cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 2013;163:847–854.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayak S, Sivakumar R, Cao O, et al. Mapping the T helper cell response to acid α-glucosidase in Pompe mice. Mol Genet Metab 2012;106:189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayak S, Doerfler PA, Porvasnik SL, et al. Immune responses and hypercoagulation in ERT for Pompe disease are mutation and rhGAA dose dependent. PLoS One 2014;9:e98336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd AG, McElroy JA, Grange RW, et al. Correcting neuromuscular deficits with gene therapy in Pompe disease. Ann Neurol 2015;78:222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010;99:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banugaria SG, Prater SN, Ng Y-K, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: Lessons learned from infantile Pompe disease. Genet Med 2011;13:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishnani PS, Hwu W-L, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 2006;148:671–676.e2 [DOI] [PubMed] [Google Scholar]
- 15.Brooks DA, Kakavanos R, Hopwood JJ. Significance of immune response to enzyme-replacement therapy for patients with a lysosomal storage disorder. Trends Mol Med 2003;9:450–453 [DOI] [PubMed] [Google Scholar]
- 16.Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med 2009;11:210–219 [DOI] [PubMed] [Google Scholar]
- 17.De Vries JM, van der Beek NAME, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab 2010;101:338–345 [DOI] [PubMed] [Google Scholar]
- 18.Patel TT, Banugaria SG, Case LE, et al. The impact of antibodies in late-onset Pompe disease: A case series and literature review. Mol Genet Metab 2012;106:301–309 [DOI] [PubMed] [Google Scholar]
- 19.Park J-S, Kim H-G, Shin J-H, et al. Effect of enzyme replacement therapy in late onset Pompe disease: Open pilot study of 48 weeks follow-up. Neurol Sci 2015;36:599–605 [DOI] [PubMed] [Google Scholar]
- 20.Sack BK, Herzog RW, Terhorst C, Markusic DM. Development of gene transfer for induction of antigen-specific tolerance. Mol Ther Methods Clin Dev 2014;1:14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne BJ, Falk DJ, Pacak CA, et al. Pompe disease gene therapy. Hum Mol Genet 2011;20:R61–R68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mah CS, Soustek MS, Todd AG, et al. Adeno-associated virus-mediated gene therapy for metabolic myopathy. Hum Gene Ther 2013;24:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: Poised at the clinical crossroads. Mol Ther 2012;20:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008;16:1073–1080 [DOI] [PubMed] [Google Scholar]
- 25.Marsic D, Govindasamy L, Currlin S, et al. Vector design tour de force: Integrating combinatorial and rational approaches to derive novel adeno-associated virus variants. Mol Ther 2014;22:1900–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palomares O, Martín-Fontecha M, Lauener R, et al. Regulatory T cells and immune regulation of allergic diseases: Roles of IL-10 and TGF-β. Genes Immun 2014;15:511–520 [DOI] [PubMed] [Google Scholar]
- 27.Wisniewski J, Agrawal R, Woodfolk JA. Mechanisms of tolerance induction in allergic disease: Integrating current and emerging concepts. Clin Exp Allergy 2013;43:164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 2012;30:733–758 [DOI] [PubMed] [Google Scholar]
- 29.Dobrzynski E, Mingozzi F, Liu Y-L, et al. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood 2004;104:969–977 [DOI] [PubMed] [Google Scholar]
- 30.Cao O, Dobrzynski E, Wang L, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 2007;110:1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boustany R-MN. Lysosomal storage diseases—the horizon expands. Nat Rev Neurol 2013;9:583–598 [DOI] [PubMed] [Google Scholar]
- 32.Franco LM, Sun B, Yang X, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005;12:876–884 [DOI] [PubMed] [Google Scholar]
- 33.Sun B, Bird A, Young SP, et al. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet 2007;81:1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun B, Kulis MD, Young SP, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine Pompe disease. Mol Ther 2010;18:353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P, Sun B, Osada T, et al. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine Pompe disease. Hum Gene Ther 2012;23:460–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raben N, Nagaraju K, Lee E, et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 1998;273:19086–19092 [DOI] [PubMed] [Google Scholar]
- 37.Falk DJ, Todd AG, Lee S, et al. Peripheral nerve and neuromuscular junction pathology in Pompe disease. Hum Mol Genet 2014;24:625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeRuisseau LR, Fuller DD, Qiu K, et al. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci USA 2009;106:9419–9424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KZ, Qiu K, Sandhu MS, et al. Hypoglossal neuropathology and respiratory activity in Pompe mice. Front Physiol 2011;2:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corti M, Smith BK, Falk DJ, et al. Altered activation of the tibialis anterior in individuals with Pompe disease: Implications for motor unit dysfunction. Muscle Nerve 2015;51:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuller DD, ElMallah MK, Smith BK, et al. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol 2013;189:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rucker M, Fraites TJ, Porvasnik SL, et al. Rescue of enzyme deficiency in embryonic diaphragm in a mouse model of metabolic myopathy: Pompe disease. Development 2004;131:3007–3019 [DOI] [PubMed] [Google Scholar]
- 43.Mah C, Cresawn KO, Fraites TJ, et al. Sustained correction of glycogen storage disease type II using adeno-associated virus serotype 1 vectors. Gene Ther 2005;12:1405–1409 [DOI] [PubMed] [Google Scholar]
- 44.Mah CS, Falk DJ, Germain SA, et al. Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol Ther 2010;18:502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ElMallah MK, Falk DJ, Nayak S, et al. Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in Pompe mice. Mol Ther 2014;22:702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falk DJ, Mah CS, Soustek MS, et al. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther 2013;21:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falk DJ, Soustek MS, Todd AG, et al. Comparative impact of AAV and enzyme replacement therapy on respiratory and cardiac function in adult Pompe mice. Mol Ther Methods Clin Dev 2015;2:15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith BK, Collins SW, Conlon TJ, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: Initial safety and ventilatory outcomes. Hum Gene Ther 2013;24:630–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byrne BJ, Collins S, Mah C, et al. Phase I/II trial of diaphragm delivery of recombinant adeno-associated virus acid alpha-glucosidase (rAAV1-CMV-GAA) gene vector in patients with Pompe disease. Hum Gene Ther Clin Dev 2014;25:134–163 [DOI] [PubMed] [Google Scholar]
- 50.Corti M, Elder M, Falk D, et al. B-cell depletion is protective against anti-AAV capsid immune response: A human subject case study. Mol Ther Methods Clin Dev 2014;1:14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doerfler PA, Byrne BJ, Clément N. Copackaging of multiple adeno-associated viral vectors in a single production step. Hum Gene Ther Methods 2014;25:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiMattia MA, Nam H-J, Van Vliet K, et al. Structural insight into the unique properties of adeno-associated virus serotype 9. J Virol 2012;86:6947–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraites TJ, Schleissing MR, Shanely RA, et al. Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors. Mol Ther 2002;5:571–578 [DOI] [PubMed] [Google Scholar]
- 54.Doerfler PA, Nayak S, Herzog RW, et al. BAFF blockade prevents anti-drug antibody formation in a mouse model of Pompe disease. Clin Immunol 2015;158:140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cresawn KO, Fraites TJ, Wasserfall C, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther 2005;16:68–80 [DOI] [PubMed] [Google Scholar]
- 56.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 57.Mingozzi F, Liu Y-L, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003;111:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markusic DM, Hoffman BE, Perrin GQ, et al. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med 2013;5:1698–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuster DJ, Dykstra JA, Riedl MS, et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat 2014;8:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruzo A, Marcó S, García M, et al. Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Hum Gene Ther 2012;23:1237–1246 [DOI] [PubMed] [Google Scholar]
- 61.Conlon TJ, Erger K, Porvasnik S, et al. Preclinical toxicology and biodistribution studies of recombinant adeno-associated virus 1 human acid α-glucosidase. Hum Gene Ther Clin Dev 2013;24:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bronsema KJ, Bischoff R, Pijnappel WWMP, et al. Absolute quantification of the total and anti-drug antibody-bound concentrations of recombinant human α-glucosidase in human plasma using protein-G extraction and LC-MS/MS. Anal Chem 2015;87:4394–4401 [DOI] [PubMed] [Google Scholar]
- 63.Sun B, Zhang H, Franco LM, et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005;11:57–65 [DOI] [PubMed] [Google Scholar]
- 64.Ziegler RJ, Bercury SD, Fidler J, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther 2008;19:609–621 [DOI] [PubMed] [Google Scholar]
- 65.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147–163 [DOI] [PubMed] [Google Scholar]
- 66.Vandendriessche T, Thorrez L, Acosta-Sanchez A, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost 2007;5:16–24 [DOI] [PubMed] [Google Scholar]
- 67.Asokan A, Samulski RJ. An emerging adeno-associated viral vector pipeline for cardiac gene therapy. Hum Gene Ther 2013;24:906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jayandharan GR, Aslanidi G, Martino AT, et al. Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc Natl Acad Sci USA 2011;108:3743–3748 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Rogers GL, Martino AT, Aslanidi G V, et al. Innate immune responses to AAV vectors. Front Microbiol 2011;2:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogers GL, Suzuki M, Zolotukhin I, et al. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J Innate Immun 2015;7:302–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki M, Bertin TK, Rogers GL, et al. Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo. Mol Ther 2013;21:796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroos M, Hoogeveen-Westerveld M, Michelakakis H, et al. Update of the Pompe disease mutation database with 60 novel GAA sequence variants and additional studies on the functional effect of 34 previously reported variants. Hum Mutat 2012;33:1161–1165 [DOI] [PubMed] [Google Scholar]
- 73.Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 2010;28:271–274 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Duque SI, Arnold WD, Odermatt P, et al. A large animal model of spinal muscular atrophy and correction of phenotype. Ann Neurol 2015;77:399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gray SJ, Matagne V, Bachaboina L, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: A comparative study of adult mice and nonhuman primates. Mol Ther 2011;19:1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarkar D, Biswas M, Liao G, et al. Ex vivo expanded autologous polyclonal regulatory T cells suppress inhibitor formation in hemophilia. Mol Ther Methods Clin Dev 2014;1:14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basner-Tschakarjan E, Bijjiga E, Martino AT. Pre-clinical assessment of immune responses to adeno-associated virus (AAV) vectors. Front Immunol 2014;5:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jawa V, Cousens LP, Awwad M, et al. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin Immunol 2013;149:534–555 [DOI] [PubMed] [Google Scholar]
- 80.Banati M, Hosszu Z, Trauninger A, et al. Enzyme replacement therapy induces T-cell responses in late-onset Pompe disease. Muscle Nerve 2011;44:720–726 [DOI] [PubMed] [Google Scholar]
- 81.Nathwani AC, Tuddenham EGD, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mueller C, Chulay JD, Trapnell BC, et al. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J Clin Invest 2013;123:5310–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sack BK, Merchant S, Markusic DM, et al. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One 2012;7:e37671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moghimi B, Sack BK, Nayak S, et al. Induction of tolerance to factor VIII by transient co-administration with rapamycin. J Thromb Haemost 2011;9:1524–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ylä-Herttuala S. Glybera's second act: The curtain rises on the high cost of therapy. Mol Ther 2015;23:217–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lostal W, Kodippili K, Yue Y, Duan D. Full-length dystrophin reconstitution with adeno-associated viral vectors. Hum Gene Ther 2014;562:552–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mah C, Sarkar R, Zolotukhin I, et al. Dual vectors expressing murine factor VIII result in sustained correction of hemophilia A mice. Hum Gene Ther 2003;14:143–152 [DOI] [PubMed] [Google Scholar]
- 88.Wang Q, Dong B, Firrman J, et al. Efficient production of dual recombinant adeno-associated viral vectors for factor VIII delivery. Hum Gene Ther Methods 2014;25:261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dyka FM, Boye SL, Chiodo VA, et al. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods 2014;25:166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cachón-González MB, Wang SZ, McNair R, et al. Gene transfer corrects acute GM2 gangliosidosis—potential therapeutic contribution of perivascular enzyme flow. Mol Ther 2012;20:1489–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pryadkina M, Lostal W, Bourg N, et al. A comparison of AAV strategies distinguishes overlapping vectors for efficient systemic delivery of the 6.2 kb Dysferlin coding sequence. Mol Ther Methods Clin Dev 2015;2:15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Strobel B, Miller FD, Rist W, Lamla T. Comparative analysis of cesium chloride- and iodixanol-based purification of recombinant adeno-associated virus (AAV) vectors for preclinical applications. Hum Gene Ther Methods 2015;112:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.