Abstract
Background: This study examined the effects of cardiorespiratory fitness, heart rate recovery, and physical activity on working memory in breast cancer survivors and age-matched controls.
Method: Using a case-control design, 32 women who had received a breast cancer diagnosis and completed primary treatment within the past 36-months (11 radiation only; 21 chemotherapy) and 30 age-matched women with no previous cancer diagnosis completed a n-back continuous performance task commonly used as an assessment of working memory. In addition, cardiorespiratory fitness and heart rate recovery were measured during a submaximal graded exercise test and physical activity was measured using 7-days of accelerometer monitoring.
Results: Breast cancer survivors who had received chemotherapy had poorer heart rate recovery (p = .010) and engaged in less physical activity than women who had received radiation only (p = .004) or non-cancer controls (p = .029). Cancer treatment (radiation; chemotherapy) predicted differences in reaction times on the 1-back working memory task (p = .029). However, more rapid heart rate recovery predicted shorter reaction times on the 1-back task in the age-matched control group (p = .002). All participants with greater cardiorespiratory fitness displayed greater accuracy independent of disease status on the 1-back task (p = .017). No significant group differences in reaction times were observed for 2-back target trials between breast cancer survivors and controls. However, greater total physical activity predicted shorter reaction times in breast cancer survivors (radiation, chemotherapy) on the 2-back task (p = .014). In addition, all participants who exhibited more rapid heart rate recovery demonstrated better greater accuracy regardless of disease status (p = .013).
Conclusion: These findings support differences in physical activty participation, heart rate recovery, and 1- and 2-back working memory reaction times between breast cancer survivors and age-matched controls. Greater cardiorespiratory fitness, heart rate recovery, and physical activity were positively associated with better working memory performance across conditions.
Introduction
Breast cancer is the leading cause of new cancer cases among women in the U.S., and is expected to account for 29% of all new cancers among women.1 This burgeoning population of cancer patients and survivors are living with treatment-related side effects affecting their health and quality of life. A growing body of evidence suggests the consequences of breast cancer and treatment also include cognitive dysfunction.2 Cancer-related cognitive impairment is the loss of mental acuity associated with cancer and cancer treatment. Typical concerns reported by breast cancer survivors include memory lapses, difficulty concentrating, trouble with word retrieval and remembering details, and slower cognitive processing.3 Evidence suggests cancer-related cognitive impairment can be detected in up to 33% of patients prior to treatment, whereas approximately 75% of breast cancer survivors report some impact on cognitive functioning during treatment.2 Additionally, cognitive impairment can persist in approximately 35% of cancer survivors up to 20 years following treatment completion.4,5
An array of biological and psychological mechanisms that contribute to cognitive dysfunction have been proposed. The current literature suggests a complex constellation of mechanisms for cancer-related cognitive impairment that includes neurotoxicity due to treatments (e.g., radiation, chemotherapy, hormone therapy), patient characteristics (e.g., genetic predisposition, age, cognitive reserve), oxidative stress (e.g., telomere shortening, estrogen-mediated effects), psychological factors (e.g., fatigue, anxiety, depression, perceived stress), and immune dysregulation (e.g., irregular cytokine production).6,7 Neuroimaging studies suggest smaller total brain volume and volume of brain structures important for executive function (i.e., frontal and pre-frontal cortex) in chemotherapy-treated individuals compared to healthy controls.8 In resting state neuroimaging, chemotherapy-treated breast cancer survivors displayed altered global and regional network organization compared to healthy controls, including networks implicated in executive control, memory, and emotion regulation.9 However, many breast cancer survivors also report cognitive impairment prior to any adjunctive treatment.3 Given emerging evidence, it is safe to assume the etiology of cancer-related cognitive impairment is multifactorial and should be examined from a variety of perspectives.
One aspect of cancer-related cognitive impairment is working memory, a subset of cognitive processes required for the conscious storage, maintenance, and manipulation of task-related complex information over a short period of time that is essential for higher level cognitive functioning related to learning, decision-making, and problem-solving.10 Findings from studies examining differences in working memory between breast cancer survivors and non-cancer controls using objective cognitive testing have been equivocal. For example, early stage breast cancer survivors pre-chemotherapy have shown slower response times and less accuracy than controls and a non-cancer control group.11 Conversely, in a study investigating women with breast cancer receiving Lupron and healthy women12 no significant differences were found between groups in working memory irrespective of task complexity. However, in another study while reaction times and task performance accuracy in working memory did not differ between breast cancer survivors and non-cancer controls,13 there were significant differences in frontal lobe activation in breast cancer survivors.
Cardiorespiratory fitness and physical activity are potentially important moderators of cancer-related cognitive impairment in breast cancer survivors. Cardiorespiratory fitness is the assessment of global cardiovascular function, specifically the ability of the circulatory and respiratory systems to supply oxygen to skeletal muscles during sustained physical activity.14 Clinical research suggests cancer diagnosis and treatment are associated with cardiovascular injury and decreased cardiorespiratory fitness across the survivorship continuum.15 Cardiorespiratory fitness has also been associated with preservation of cognitive function in older adults16 and these effects might even be greater in cognitively impaired populations, such as those with cancer-related cognitive impairment.17 A further related element of cardiorespiratory fitness is heart rate recovery, the rate at which the heart rate decreases following exercise completion. The ability of the heart to recover post-exercise serves as an indicator of parasympathetic nervous system activity,18 impairment of which has been implicated in an increased risk of depression19 and cancer-related fatigue20 in breast cancer survivors. In older adults, those with higher cardiorespiratory fitness had faster heart rate recovery post-exercise and performed significantly better on a memory task than those with poor heart rate recovery.21
Physical activity is any movement of the body resulting in increased energy expenditure and encompasses all bodily movements, from sports to activities of daily living.22,23 Both pre- and post-diagnosis, higher physical activity is associated with better prognosis and reduced breast cancer-specific and all-cause mortality.24 Cancer survivors undergoing or having completed chemotherapy, who increased physical activity, have also reported improved cognitive health over time.25 In older adults, objectively-measured total daily physical activity has been positively associated with global measures of cognition26 and shorter response times during a working memory task in comparison with older adults with lower physical activity levels.27 Unfortunately, the majority of breast cancer survivors do not meet national physical activity recommendations.28 Some findings have suggested breast cancer survivors spend less than 2% of their day engaged in moderate-to-vigorous physical activity and almost 80% of their time engaged in sedentary activity.29
In general, physical activity and cardiorespiratory fitness may improve neurophysiological mechanisms affected by cancer and its treatment, including cell proliferation and survival in the hippocampus, white matter integrity, blood flow and neurotransmitter regulation, neuro- and cardiac-toxicities, bone loss, fatigue, sleep disruption, anxiety and depression as well as improving overall physiological function and body composition.30,31 Higher fitness levels in older adults have been associated with preventing age-related brain tissue loss, more efficient brain processing, and better behavioral performance during cognitive tasks that involve higher-level executive control.32 Higher levels of fitness are also associated with greater hippocampal volume, which is related to better working memory function.33
While the body of research suggesting beneficial effects of exercise in relation to cognitive function in relation to older adults has continued to emerge over the past decade, equivalent research in the area of cancer-related cognitive impairment lags behind. The present study examined whether working memory performance in women who had received a breast cancer diagnosis and completed primary treatment within the past 36-months differed from age-matched women with no previous cancer diagnosis. We hypothesized breast cancer survivors would perform more poorly on a working memory task versus age-matched controls. We further predicted cardiorespiratory fitness, heart rate recovery, and daily physical activity would be positively associated with better working memory performance.
Material and Methods
Participants
Breast cancer survivors were recruited via a local oncology clinic. Oncology clinic research staff assisted with recruitment by screening physician schedules and reviewing patients' electronic medical records to identify those who were within 36 months of completing their primary therapy for breast cancer (i.e., including chemotherapy, radiation therapy, or both) and potentially eligible for the study. In addition, breast cancer survivors and age-matched controls were recruited using print media (newspapers, flyers), websites, and listserve announcements. After expressing initial interest, women were contacted by phone and provided a full study description. During the initial contact, interested individuals completed a demographics questionnaire and a personal medical history questionnaire. Of the 141 total contacts, 73 consented, and 11 women withdrew after consenting owing to schedule conflicts (n = 2), no longer interested (n = 3), or unable to contact (n = 6).
Participants were women (n = 32) who had received a breast cancer diagnosis and completed primary treatment (n = 11 radiation only; n = 21 chemotherapy) within the past 36-months and age-matched controls (n = 30) with no previous cancer diagnosis. Additional inclusion criteria for all participants were female, 18–70 years of age, ability to read, write, and speak English, ability to walk on a treadmill unassisted, normal color vision and normal/corrected visual acuity of at least 20/40. Participants with a score ≤23 on the modified Mini-Mental Status Exam, a history of stroke, transient ischemic attack, or a surgery that involved removal of brain tissue were excluded. In addition, current use of computer-based brain training games (e.g., Lumosity®, BrainHQ®) were excluded due to the similarity of some of these games to the n-back working memory task and to mitigate against practice effects. The protocol was approved by an institutional review board and written informed consent was obtained. All potential study participants also provided written consent from a physician prior to study enrollment indicating clearance to participate in both the cardiorespiratory fitness and cognitive testing. Participants completed an initial visit for cardiorespiratory fitness assessment and 7 days of accelerometer monitoring. During a second visit participants completed cognitive testing in the form of a n-back continuous performance task commonly used as an assessment of working memory.
Demographics
Self-reported medical history, marital status, age, race, ethnicity, occupation, income, and education were collected for all participants.
Breast cancer medical history
Participants who had received a breast cancer diagnosis provided self-report information on breast cancer specific diagnosis and treatment history.
Cardiorespiratory fitness and heart rate recovery
At the initial appointment, height and weight were measured using an electronic stadiometer and scale (model 763, Seca: Chino, CA). Cardiorespiratory fitness was assessed via a submaximal graded exercise treadmill test (Naughton protocol).34 Participants were fitted with a heart rate monitor then walked on a treadmill while speed and/or grade increased in 2-minute stages until the participant achieved 85% of a pre-determined, age-predicted maximum (220–age) heart rate. During each 2-minute stage, participant heart rate, blood pressure, and subjective rating of perceived exertion35 were recorded. Heart rate recovery was measured as an indirect indicator of parasympathetic tone and determined by calculating the difference between peak heart rate during the graded exercise test and heart rate 60 seconds after its' completion while participants remained standing still on the treadmill. This was followed by a 3-minute monitored cool-down period of slower walking and a final 2-minute monitored seated resting period. Cardiorespiratory fitness was then derived from the treadmill test as an estimated V02peak.22
Physical activity
Physical activity was objectively measured by 7-day accelerometer monitoring (model GT3X, Actigraph: Pensacola, FL), given to participants at the end of the cardiorespiratory fitness testing session. Participants wore the accelerometer during waking hours and wear time was recorded on an accelerometer log. Participants were instructed to monitor their accelerometer wear but not to change or monitor their physical activity in any other way. Data were downloaded and digitally converted to “activity counts” per minute (i.e., one epoch), and processed using MeterPlus 4.2 software (Santech Health: San Diego, CA). Only days with at least 10 valid hours of wear time were included in the analyses, and hours with greater than 60 min of consecutive zeros were considered invalid (i.e., non-wearing).36 Activity counts were summed and averaged across the total number of valid days for a total daily activity score.
Working memory
At a second appointment, participants were tested on an individual basis in a private quiet area kept free of distractions. Cognitive testing was administered using a laptop computer and handheld response pad (model TR-1 × 4-CR, Current Designs Inc.: Philadelphia, PA). Participants completed a n-back task designed to assess variable working memory demands. The n-back task requires subjects to dynamically monitor a continual stream of stimuli, often of uncertain length, while updating mental representations of the target items and dropping non-relevant items from consideration.37 The n-back task has been considered the gold standard task in the assessment of working memory across populations.38,39 In addition, the n-back has been previously used to investigate working memory in breast cancer survivors ranging in age from 42–69 years.11,13,40,41
Each condition required participants to discriminate between 5 distinct colored shapes: blue circles, green triangles, purple stars, red squares, and orange crosses. Each participant completed two conditions of increasing difficulty: the 1-back and 2-back. The difference between the 1-back and 2-back relates to the amount of information that may be successfully recalled by an individual. Specifically, the increasing cognitive load of working memory maintenance and manipulation in higher n-back tasks is thought to reflect greater reallocation of attention and processing capacity to meet these increasing demands.42,43 In each condition, participants were instructed to respond as quickly and accurately as possible with a right button press if the current shape was the same as the previous trial (i.e., hit) or a left button press if the shape was different from one trial prior (i.e., correct reject) during the 1-back condition, and two trials prior for the 2-back condition. All stimuli were 3 cm tall, presented one at a time on a computer screen with a black background for a duration of 2900 ms, with a fixed 3000 ms inter-stimulus interval. In each condition, 80 trials were presented containing 20 (25%) targets and 60 (75%) non-targets. Behavioral data were collected in terms of reaction time (time in milliseconds from stimulus presentation until manual response) and accuracy (percentage of correct responses) for target and non-target trials across each task condition. Participants practiced a version of each n-back task to ensure comprehension of the task. The total time to complete all phases of this task was approximately 10 minutes.
Statistical analysis
All analyses were performed using IBM SPSS version 22 (IBM, 2013). Frequency distributions for the measures were examined to check for missing information and out-of-range values. Variable distributions were inspected, and a 5% winsorization technique was applied to preserve out-of-range rank order values in the distribution while limiting their influence.44 To investigate differences between breast cancer survivors, including those who had received radiation only versus those who had undergone chemotherapy, and age-matched controls, linear mixed models were used. Cohen's d, a distribution-based effect size measure, was calculated between groups (non-cancer controls; radiation only; chemotherapy) for each outcome variable.45 Effect sizes were interpreted using Cohen's criteria of .20 as a small effect, .50 as a moderate effect and .80 as a large effect. Multilevel regression analyses were then conducted to assess the moderating role of demographic, cancer treatment (radiation only; chemotherapy), cancer stage, time since diagnosis, cardiorespiratory fitness, heart rate recovery, and physical activity on working memory. Multilevel models were developed in a stepwise fashion.46 Predictor variables were tested individually for main effects and interaction effects with each time term. Final trimmed models were developed by entering all significant predictors and their interactions to test overall prediction of outcome variables over time (exclusion p > .100). This forward-stepping method of model development has proven robust with smaller sample sizes and ensures these models do not tax the number of parameters a data set can estimate, or the ‘‘carrying-capacity’’ of the dataset.47 Significant 2-way interactions were further decomposed via simple intercepts and slopes analyses.48
Results
Participant characteristics
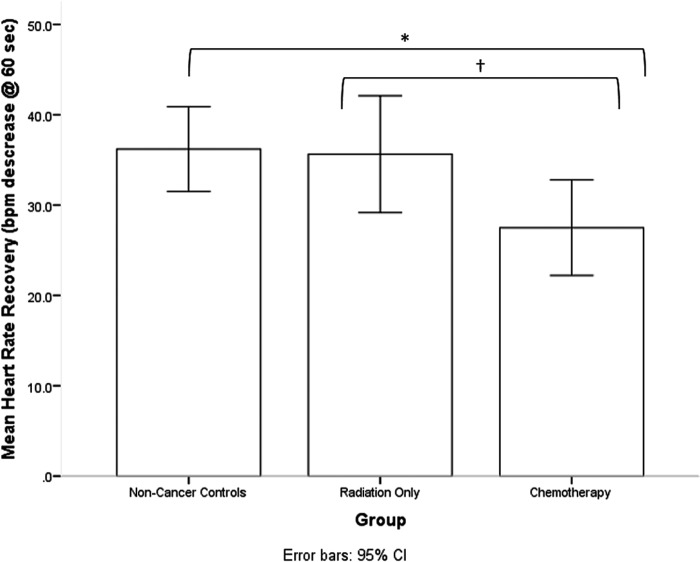
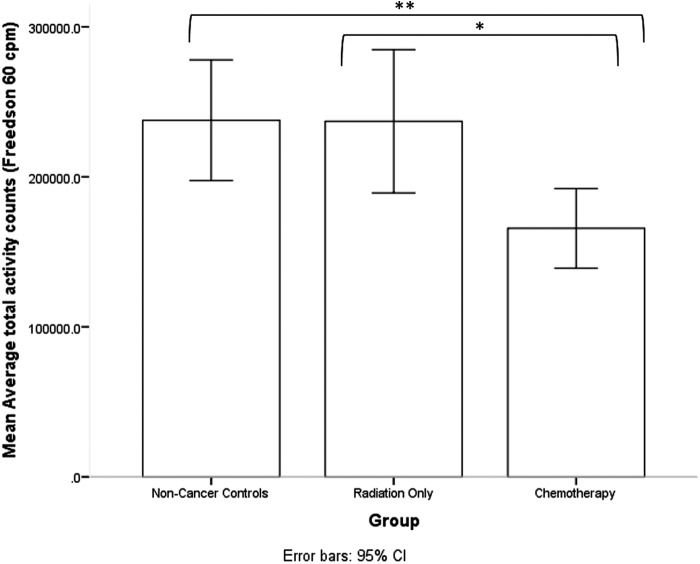
Table 1 summarizes participant characteristics, cardiorespiratory fitness, and physical activity indices. There were no significant differences between groups in age, education, body mass index, or cardiorespiratory fitness. A significant group difference was observed for heart rate recovery [F(1,61) = 3.78, p = .028]. Breast cancer survivors who had received chemotherapy exhibited slower heart rate recovery following completion of a submaximal graded exercise in comparison to age-matched controls (p = .010, d = −0.76) and breast cancer survivors who had received radiation only (p = .062, d = −0.71) (see Fig. 1). A significant group difference was also observed for total daily physical activity [F(1,62) = 4.881, p = .011]. Breast cancer survivors who had received chemotherapy engaged in significantly less total daily physical activity than age-matched controls (p = .004, d = −0.84), as well as breast cancer survivors who had received radiation only (p = .029, d = −0.83) (see Fig. 2).
Table 1.
Participant Characteristics and Physical Fitness Indices for Breast Cancer Survivors and Age-Matched Controls
| Age-Matched Controls (n = 30) | Breast Cancer Survivors – Radiation Only (n = 11) | Breast Cancer Survivors – w/ Chemotherapy (n = 21) | |
|---|---|---|---|
| Age (Mean, SD) | 55.2 (10.6) | 55.1 (5.2) | 56.1 (9.8) |
| Education (n, %) | |||
| <4-year college degree | 7 (23.3) | 3 (27.3) | 8 (38.1) |
| ≥4-year college degree | 23 (76.7) | 8 (72.7) | 13 (61.9) |
| Ethnicity (n, %) | |||
| Hispanic | 2 (6.7) | 0 | 0 |
| Non-Hispanic | 28 (93.3) | 11 (100.0) | 21 (100.0) |
| Stage (n, %) | |||
| 0 (DCIS) | — | 4 (36.4) | 1 (4.8) |
| I | — | 5 (45.5) | 4 (19.0) |
| II | — | 1 (9.1) | 10 (47.6) |
| III | — | 0 | 4 (19.0) |
| Unknown | — | 1 (9.1) | 2 (9.6) |
| Time Since Primary Diagnosis – Months (Mean, SD) | — | 18.0 (11.9) | 25.7 (11.8) |
| Treatment | |||
| Surgery (n, %) | — | 11 (100.0) | 21 (100.0) |
| Radiation only (n, %) | — | 11 (100.0) | 0 |
| Chemotherapy only (n, %) | — | 0 | 7 (33.3) |
| Radiation + chemotherapy (n, %) | — | 0 | 14 (66.7) |
| Endocrine therapy (n, %) | — | 7 (63.6) | 14 (66.6) |
| Body Mass Index (kg/m2) (Mean, SD) | 27.9 (5.7) | 25.6 (2.0) | 28.8 (6.2) |
| Cardiorespiratory Fitness (Age-predicted VO2Peak, ml. kg. min; Mean, SD) | 30.4 (6.5) | 28.8 (4.9) | 28.6 (8.4) |
| Heart Rate Recovery (bpm decrease @ 60 seconds; Mean, SD) | 36.2 (12.6) | 35.6 (9.6) | 27.5 (11.3) |
| Accelerometer Average Daily Total Counts – (Freedson 60 cpm; Mean, SD) | 237718.3 (107841.0) | 237079.4 (71073.9) | 165679.3 (58208.4) |
FIG. 1.
Breast cancer survivors who had received chemotherapy exhibited slower heart rate recovery following completion of a submaximal graded exercise in comparison to age-matched controls (p = .010, d = −0.76) and breast cancer survivors who had received radiation only (p = .062, d = −0.71). * < .05, †< .10.
FIG. 2.
Breast cancer survivors who had received chemotherapy engaged in significantly less total daily physical activity than age-matched controls (p = .004, d = −0.84), as well as breast cancer survivors who had received radiation only (p = .029, d = −0.83). ** < .01, * < .05.
1-Back condition
For the 1-back condition, group means and multilevel regression analyses for working memory performance are summarized in Tables 2 and 3, respectively.
Table 2.
Mean (SE) for N-Back (1,2) Reaction Time, Response Accuracy for Breast Cancer Survivors and Age-Matched Controls
| Age-Matched Controls (n = 30) | Breast Cancer Survivors – Radiation Only (n = 11) | Breast Cancer Survivors – w/ Chemotherapy (n = 21) | |
|---|---|---|---|
| 1-back | |||
| Target RT | 613.1 (21.9) | 702.1 (35.4) | 672.8 (25.6) |
| Non-Target RT | 628.2 (22.8) | 712.8 (37.6) | 736.6 (27.2) |
| Target Accuracy | 93.3 (1.2) | 95.5 (2.0) | 93.3 (1.5) |
| Non-Target Accuracy | 99.0 (0.2) | 98.8 (0.4) | 99.6 (0.3) |
| 2-back | |||
| Target RT | 922.3 (43.6) | 893.9 (72.0) | 1001.4 (52.1) |
| Non-Target RT | 1134.0 (51.8) | 1126.0 (85.5) | 1290.3 (61.9) |
| Target Accuracy | 86.4 (1.9) | 90.5 (3.2) | 83.3 (2.3) |
| Non-Target Accuracy | 90.46 (1.3) | 92.5 (2.1) | 89.2 (1.5) |
RT, reaction time in milliseconds; Accuracy presented as %.
Table 3.
Summary of Multilevel Regression Analyses Predicting 1-Back Task Performance
| Target | Non-Target | |||||||
|---|---|---|---|---|---|---|---|---|
| RT | Accuracy | RT | Accuracy | |||||
| Est. (SE) | Sig. | Est. (SE) | Sig. | Est. (SE) | Sig. | Est. (SE) | Sig. | |
| Intercept | 669.46 (15.53) | .000 | 93.87 (0.83) | .000 | 687.90 (16.13) | .000 | 98.78 (0.17) | .000 |
| Main Effects | ||||||||
| Group | 33.37 (17.30) | .058 | 0.19 (0.93) | .841 | 55.16 (17.79) | .003 | −0.23 (0.19) | .226 |
| Cardiorespiratory Fitness (CRF) | — | — | 0.30 (0.12) | .017 | — | — | — | — |
| Heart Rate Recovery (HRR) | −1.53 (1.30) | .241 | — | — | — | — | — | — |
| 2-way Interactions | — | — | — | — | — | — | ||
| Group * HRR | 3.62 (1.38) | .011 | — | — | — | — | — | — |
| Pseudo R2 | 0.10 | 0.10 | 0.13 | 0.02 | ||||
Dashes in cells indicate excluded variables not entered into the model.
Excluded (p > .1): age, education, cancer stage, time since cancer diagnosis, body mass index, total daily physical activity.
Reaction time
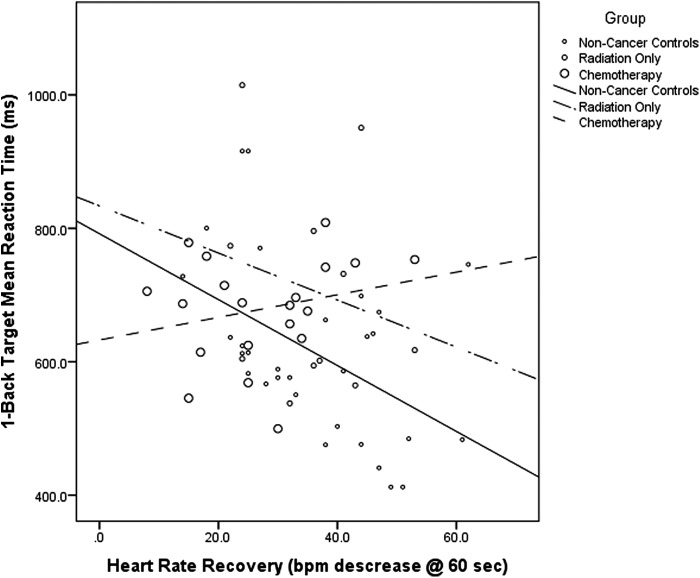
A group difference that approached statistical significance was observed for 1-back target trials [F(1,62) = 3.08, p = .053], indicating longer reaction times in breast cancer survivors who had received radiation only versus non-cancer controls (p = .029, d = 0.79). A significant interaction effect between disease status and heart rate recovery (p = .011) further suggested those in the age-matched control group who had faster heart rate recovery also had shorter reaction times for 1-back target trials (Est. = −5.17, p = .002, R2 = 0.23). No such effect was evident in either breast cancer survivor group (radiation only: p = 0.241; chemotherapy: p = .329) (see Fig. 3). A significant group difference was also observed for 1-back non-target trials [F(1,62) = 5.12, p = .009], indicating breast cancer survivors who had received chemotherapy had significantly longer reaction times than controls (p = .003, d = 0.87), but this was not moderated by cardiorespiratory fitness or physical activity (p > .100).
FIG. 3.
A significant interaction effect between disease status and heart rate recovery (p = .011) suggested those in the age-matched control group who had faster heart rate recovery also had shorter reaction times for 1-back target trials (Est. = −5.17, p = .002, R2 = 0.23). No such effect was evident in either breast cancer survivor group (radiation only: p = .241; chemotherapy: p = .329).
Accuracy
No significant group differences in accuracy were observed for 1-back target trials [F(1,62) = 0.45, p = .640]. However, higher cardiorespiratory fitness was associated with greater accuracy on 1-back target trials regardless of disease status (p = .017). For 1-back non-target trials, again no group differences in accuracy were evident [F(1,60) = 0.75, p = .476] and no further indices of cardiorespiratory fitness or physical activity moderated these effects (p > .100).
2-Back condition
For the 2-back condition, group means and multilevel regression analyses for working memory performance are summarized in Tables 2 and 4, respectively.
Table 4.
Summary of Multilevel Regression Analyses Predicting 2-Back Task Performance
| Target | Non-Target | |||||||
|---|---|---|---|---|---|---|---|---|
| RT | Accuracy | RT | Accuracy | |||||
| Est. (SE) | Sig. | Est. (SE) | Sig. | Est. (SE) | Sig. | Est. (SE) | Sig. | |
| Intercept | 901.68 (33.76) | .000 | 86.79 (1.29) | .000 | 1199.46 (33.17) | .000 | 90.39 (0.87) | .000 |
| Group | −41.06 (39.27) | .300 | 0.46 (2.37) | .503 | 44.14 (38.46) | .256 | −0.07 (0.97) | .947 |
| Education | — | — | 0.16 (0.46) | .013 | — | — | 1.75 (0.78) | .028 |
| Heart Rate Recovery @ 60 Seconds (HRR) | −4.32 (2.44) | .081 | 0.02 (0.05) | .013 | −10.27 (2.83) | .001 | — | — |
| Total Daily Physical Activity (TDPA) | −0.13 (0.05) | .007 | — | — | — | — | — | — |
| Group * TDPA | −0.12 (0.05) | .014 | — | — | — | — | — | — |
| Pseudo R2 | 0.20 | 0.19 | 0.25 | 0.17 | ||||
Dashes in cells indicate excluded variables not entered into the model.
Excluded (p > .1): age, cancer stage, time since cancer diagnosis, body mass index, cardiorespiratory fitness.
Reaction time
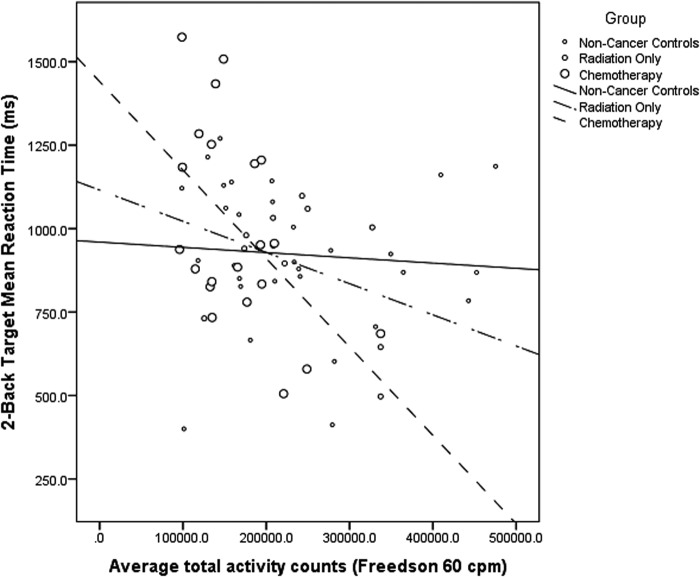
No significant group differences in reaction times were observed for 2-back target trials [F(1,62) = 0.97, p = .384]. However, a significant group by average total daily physical activity interaction (p = .014) suggested all breast cancer survivors, independent of chemotherapy treatment, who engaged in more total physical activity had shorter reaction times for 2-back target trials (radiation only: Est. = −0.13, SE = 0.04, p = .002, R2 = 0.12; chemotherapy: Est. = −0.24, SE = 0.07, p = 0.002, R2 = 0.27) (see Fig. 4). In contrast, no such effect was evident in the age-matched control group (p = .727). In addition, a main effect for heart rate recovery was observed that approached statistical significance (p = .081), suggesting those who had faster heart rate recovery also had shorter reaction times for 2-back target trials, regardless of group status. For 2-back non-target trials, no significant group differences in reaction time were observed [F(1,62) = 2.17, p = .123]. However, a main effect for heart rate recovery (p = .001) indicated those with faster cardiovascular recovery times had shorter reaction times independent of disease status.
FIG. 4.
A significant group by average total daily physical activity interaction (p = .014) suggested all breast cancer survivors, independent of chemotherapy treatment, who engaged in more total physical activity had shorter reaction times for 2-back target trials (radiation only: Est. = −0.13, SE = 0.04, P = .002, R2 = 0.12; chemotherapy: Est. = −0.24, SE = 0.07, p = .002, R2 = 0.27).
Accuracy
No significant group differences in accuracy were observed for 2-back target trials [F(1,60) = 1.72, p = .189]. However, those with higher education (p = .013) and faster heart rate recovery (p = .013) were more accurate regardless of group status. For 2-back non-target trials, no group differences in accuracy were observed [F(1,62) = 0.79, p = .460]. However, those with higher education (p = .028) were more accurate independent of disease status. No additional indices of cardiorespiratory fitness or physical activity further moderated accuracy for 2-back non-target trials (p > .100).
Discussion
The present study examined whether differences in working memory existed between breast cancer survivors, including those who had received chemotherapy or radiation therapy, and non-cancer controls and whether cardiorespiratory fitness and physical activity indices moderated these effects. Findings suggest women who had received chemotherapy exhibited slower heart rate recovery in comparison to women who had received radiation only and age-matched controls. These women also engaged in significantly less physical activity than women who had received radiation only or non-cancer controls. There were no differences between groups in cardiorespiratory fitness. The lack of conventional statistical between-group differences in cardiorespiratory fitness may have been related to our use of a submaximal graded exercise test, which could have overestimated peak cardiorespiratory fitness in both groups.49 Overall, these findings suggest that while breast cancer survivors may have had similar age-predicted cardiorespiratory fitness levels, they differed in their cardiovascular recovery ability from a submaximal graded exercise test and overall physical activity levels.
For the 1-back condition of the working memory task, breast cancer survivors had significantly longer reaction times across trials. Fitness-related between-group differences in reaction times in target trials favoring the non-cancer control group may have been related to slower heart rate recovery in both breast cancer survivor groups. These findings parallel research in the aging literature that suggest older adults with faster heart rate recovery perform significantly better on cognitive tasks than older adults with poor posttest heart rate recovery.21 While there were no significant group differences in response accuracy for the 1-back condition, there was an overall positive effect for cardiorespiratory fitness across all groups independent of disease status. These findings suggest cardiorespiratory fitness may exert a protective influence on working memory performance in both breast cancer survivors and women without a history of cancer.
In the 2-back condition, breast cancer survivors who engaged in more total daily physical activity had shorter reaction times on target trials. This effect also corresponds to findings in the aging literature that suggest greater total daily physical activity is positively associated with shorter reaction times in older adults during a working memory task.27 That this total daily physical activity effect was evident only in the breast cancer survivor groups may suggest that greater physical activity provides unique protection to breast cancer survivors for more cognitively complex working memory tasks and further research is warranted. In addition, participants with faster heart rate recovery demonstrated shorter reaction times across trials and greater accuracy in target trials regardless of group status. These findings suggest the associaction of heart rate recovery on working memory processes in both cancer survivors and age-matched controls and parallel research suggesting improving heart rate recovery may also improve not only general wellbeing and health but cognitive function.21
We are conscious that the comparatively small sample size could have limited the potential for observing significant findings among predictor variables in the multilevel regression models. However, research suggests samples of 50 study participants or more can be sufficient, showing little bias in the regression coefficients.50 In addition, no corrections were made for multiple comparisons, as it has been suggested these corrections unnecessarily inflate the Type II error rate.51 Instead, effect sizes for the estimated marginal means models (Cohen's d) to quantify the magnitude of these changes over time and the pseudo R2 statistic were calculated for each multilevel growth curve model to account for variance explained.
While the breast cancer sample in the present study was heterogeneous, chemotherapy as part of cancer treatment was considered in these analyses. Cancer stage and time since diagnosis were also assessed and not found to be significant predictors of working memory in any of the 1- or 2-back models. Future research should utilize larger sample sizes and/or more targeted research based on age, cancer diagnosis and treatment, to further examine the independent effects of these factors.11 If these inter-related mechanisms are shown to lead to cognitive impairment, then interventions can be targeted towards vulnerable breast cancer survivors with the aim of preventing or even reversing further physical and cognitive decline.
For the present study we chose to focus exclusively on the associations between fitness indices and working memory in cancer survivors and age-matched controls. Other research suggests a more overarching neuropsychological assessment of cognitive function in the following domains: attention, processing speed, memory, and executive function.52,53 This breadth of neuropsychological testing and corresponding neuroimaging is essential for further evaluating the presence, severity, and locus of cognitive impairments in cancer survivors, and to deconstruct which elements of cognitive function are most affected in breast cancer survivors and how physical activity and exercise training may differentially affect these constituent elements.13,54
In summary, we report differences in working memory between breast cancer survivors and age-matched controls. Greater cardiorespiratory fitness, heart rate recovery, and physical activity were positively associated with better working memory performance. Future research should use appropriately-powered longitudinal observational and intervention-based designs to further investigate the moderating role of cardiorespiratory fitness, heart rate recovery, and physical activity in cancer-related cognitive impairment. Research of this nature will help to better identify women with breast cancer at greater risk for cognitive decline and the development of exercise training interventions for the prevention and treatment of cancer-related cognitive impairment.
Acknowledgments
The authors acknowledge the assistance of Ruth M. Sosnoff, PhD, Eric S. Drollette, BS, and Kendrith M. Rowland, MD and his research team. We would also like to thank the three anonymous reviewers for their constructive comments. The investigators are supported by the National Institute on Aging at the National Institutes of Health (grant numbers R01AG020118 and R37AG025667).
Author Disclosure Statement
No competing conflicts of interest to declare.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A: Cancer statistics, 2014. CA: Cancer J Clin 2014;64:9–29 [DOI] [PubMed] [Google Scholar]
- 2.Janelsins MC, Kesler SR, Ahles TA, Morrow GR: Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 2014;26:102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher A: Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil 2011;90:S16–S26 [DOI] [PubMed] [Google Scholar]
- 4.Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB: Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol 2012;30:1080–1086 [DOI] [PubMed] [Google Scholar]
- 5.Yamada T, Denburg N, Beglinger L, Schultz S: Neuropsychological outcomes of older breast cancer survivors: Cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci 2010;22:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahles TA: Brain vulnerability to chemotherapy toxicities. Psycho‐Oncology 2012;21:1141–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker CH, Drew BA, Antoon JW, Kalueff AV, Beckman BS: Neurocognitive effects of chemotherapy and endocrine therapies in the treatment of breast cancer: Recent perspectives. Cancer Invest 2012;30:135–148 [DOI] [PubMed] [Google Scholar]
- 8.Koppelmans V, Groot Md, de Ruiter MB, et al. : Global and focal white matter integrity in breast cancer survivors 20 years after adjuvant chemotherapy. Hum Brain Mapp 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno J, Hosseini S, Kesler S: Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis 2012;48:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baddeley A: Working memory: Looking back and looking forward. Nature reviews neuroscience 2003;4:829–839 [DOI] [PubMed] [Google Scholar]
- 11.Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A: Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: An FMRI study. Front Hum Neurosci 2011;5:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreano JM, Waisman J, Donley L, Cahill L: Effects of breast cancer treatment on the hormonal and cognitive consequences of acute stress. Psycho‐Oncology 2012;21:1091–1098 [DOI] [PubMed] [Google Scholar]
- 13.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ: Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional magnetic resonance imaging study. J Clin Oncol 2012;30:2500–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones LW: Cardiorespiratory exercise testing in adult cancer patients. In: Exercise and cancer survivorship. Springer: New York, 2010, pp 223–236. [Google Scholar]
- 15.Jones LW, Courneya KS, Mackey JR, et al. : Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 2012;30:2530–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colcombe S, Kramer AF: Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci 2003;14:125–130 [DOI] [PubMed] [Google Scholar]
- 17.Erickson KI, Miller DL, Weinstein AM, Akl SL, Banducci S: Physical activity and brain plasticity in late adulthood: A conceptual and comprehensive review. Ageing Research 2012;3:e6 [Google Scholar]
- 18.Peçanha T, Daniel Silva‐Júnior N, Lúcia de Moraes Forjaz, Cláudia: Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin Physiol Funct Imaging 2014;34:327–329 [DOI] [PubMed] [Google Scholar]
- 19.Giese-Davis J, Wilhelm FH, Conrad A, et al. : Depression and stress reactivity in metastatic breast cancer. Psychosom Med 2006;68:675–683 [DOI] [PubMed] [Google Scholar]
- 20.Fagundes CP, Murray DM, Hwang BS, et al. : Sympathetic and parasympathetic activity in cancer-related fatigue: More evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology 2011;36:1137–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearman A, Lachman ME: Heart rate recovery predicts memory performance in older adults. Appl Psychophysiol Biofeedback 2010;35:107–114 [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins: Philadelphia, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Caspersen CJ, Powell KE, Christenson GM: Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep 1985;100:126–131 [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong S, Jiang T, Ma T, et al. : Association between physical activity and mortality in breast cancer: A meta-analysis of cohort studies. Eur J Epidemiol 2014:1–14 [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick TR, Edgar L, Holcroft C: Assessing the relationship between physical fitness activities, cognitive health, and quality of life among older cancer survivors. J Psychosoc Oncol 2012;30:556–572 [DOI] [PubMed] [Google Scholar]
- 26.Buchman AS, Wilson RS, Bennett DA: Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry 2008;16:697–701 [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Huang C, Chen K, Hung T: Physical activity and working memory in healthy older adults: An ERP study. Psychophysiology 2013;50:1174–1182 [DOI] [PubMed] [Google Scholar]
- 28.Lynch BM, Friedenreich CM, Winkler EA, et al. : Associations of objectively assessed physical activity and sedentary time with biomarkers of breast cancer risk in postmenopausal women: Findings from NHANES (2003–2006). Breast Cancer Res Treat 2011;130:183–194 [DOI] [PubMed] [Google Scholar]
- 29.Sabiston CM, Brunet J, Vallance JK, Meterissian S: Prospective examination of objectively assessed physical activity and sedentary time after breast cancer treatment: Sitting on the crest of the teachable moment. Cancer Epidemiol Biomarkers Prev 2014;23:1324–1330 [DOI] [PubMed] [Google Scholar]
- 30.Gehring K, Roukema JA, Sitskoorn MM: Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev of Anticancer Ther 2012;255–269 [DOI] [PubMed] [Google Scholar]
- 31.Janelsins MC, Mustian KM, Peppone LJ, et al. : Interventions to alleviate symptoms related to breast cancer treatments and areas of needed research. J Cancer Sci Ther 2011;S2:S2–001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez‐Pinilla F, Hillman C: The influence of exercise on cognitive abilities. Comprehensive Physiology 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson KI, Voss MW, Prakash RS, et al. : Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108:3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ: Principles of exercise testing and interpretation: Including pathophysiology and clinical applications. Lippincott Williams & Wilkins: Philadelphia, 2005 [Google Scholar]
- 35.Borg G: Borg's Perceived Exertion and Pain Scales. Human Kinetics; 1998 [Google Scholar]
- 36.Mailey EL, Gothe NP, Wojcicki TR, et al. : Influence of allowable interruption period on estimates of accelerometer wear time and sedentary time in older adults. J Aging Phys Act 2014;22:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW: Working memory span tasks: A methodological review and user's guide. Psychon Bull Rev 2005;12:769–786 [DOI] [PubMed] [Google Scholar]
- 38.Owen AM, McMillan KM, Laird AR, Bullmore E: N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redick TS, Lindsey DR: Complex span and n-back measures of working memory: A meta-analysis. Psychon Bull Rev 2013;20:1102–1113 [DOI] [PubMed] [Google Scholar]
- 40.Conroy SK, McDonald BC, Smith DJ, et al. : Alterations in brain structure and function in breast cancer survivors: Effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat 2013;137:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumas JA, Makarewicz J, Schaubhut GJ, et al. : Chemotherapy altered brain functional connectivity in women with breast cancer: A pilot study. Brain Imaging Behav 2013;7:524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watter S, Geffen GM, Geffen LB: The n-back as a dual-task: P300 morphology under divided attention. Psychophysiology 2001;38:998–1003 [DOI] [PubMed] [Google Scholar]
- 43.Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B: The concurrent validity of the n-back task as a working memory measure. Memory 2010;18:394–412 [DOI] [PubMed] [Google Scholar]
- 44.Tabachnick BG, Fidell LS: Using multivariate statistics. Allyn and Bacon: Boston, MA, 2001 [Google Scholar]
- 45.Cohen J: Statistical power analysis for the behavioral sciences. Academic Press: Waltham, MA, 2013 [Google Scholar]
- 46.Hox J: Multilevel analysis: Techniques and applications. Routledge: London, 2010 [Google Scholar]
- 47.Nezlek JB: An introduction to multilevel modeling for social and personality psychology. Soc Personal Psychol Compass 2008;2:842–860 [Google Scholar]
- 48.Preacher KJ, Curran PJ, Bauer DJ: Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat 2006;31:437–448 [Google Scholar]
- 49.Lakoski SG, Barlow CE, Koelwyn GJ, et al. : The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: The Cooper Center Longitudinal Study. Breast Cancer Res Treat 2013;138:909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maas CJ, Hox JJ: Sufficient sample sizes for multilevel modeling. Methodology 2005;1:86 [Google Scholar]
- 51.Nakagawa S: A farewell to bonferroni: The problems of low statistical power and publication bias. Behav Ecol 2004;15:1044–1045 [Google Scholar]
- 52.Cheung YT, Tan EH, Chan A: An evaluation on the neuropsychological tests used in the assessment of postchemotherapy cognitive changes in breast cancer survivors. Support Care Canc 2012;20:1361–1375 [DOI] [PubMed] [Google Scholar]
- 53.Jean-Pierre P: Management of cancer-related cognitive dysfunction-conceptualization challenges and implications for clinical research and practice. US Oncology 2010;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szabo AN, McAuley E, Erickson KI, et al. : Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology 2011;25:545. [DOI] [PMC free article] [PubMed] [Google Scholar]