Abstract
Cell-based therapy is an emerging paradigm in skeletal regenerative medicine. However, the primary means by which transplanted cells contribute to bone repair and regeneration remain controversial. To gain an insight into the mechanisms of how both transplanted and endogenous cells mediate skeletal healing, we used a transgenic mouse strain expressing both the topaz variant of green fluorescent protein under the control of the collagen, type I, alpha 1 promoter/enhancer sequence (Col1a1GFP) and membrane-bound tomato red fluorescent protein constitutively in all cell types (R26mTmG). A comparison of healing in parietal versus frontal calvarial defects in these mice revealed that frontal osteoblasts express Col1a1 to a greater degree than parietal osteoblasts. Furthermore, the scaffold-based application of adipose-derived stromal cells (ASCs), bone marrow-derived mesenchymal stem cells (BM-MSCs), and osteoblasts derived from these mice to critical-sized calvarial defects allowed for investigation of cell survival and function following transplantation. We found that ASCs led to significantly faster rates of bone healing in comparison to BM-MSCs and osteoblasts. ASCs displayed both increased survival and increased Col1a1 expression compared to BM-MSCs and osteoblasts following calvarial defect transplantation, which may explain their superior regenerative capacity in the context of bone healing. Using this novel reporter system, we were able to elucidate how cell-based therapies impact bone healing and identify ASCs as an attractive candidate for cell-based skeletal regenerative therapy. These insights potentially influence stem cell selection in translational clinical trials evaluating cell-based therapeutics for osseous repair and regeneration.
Introduction
Cell-based approaches are emerging treatment paradigms in skeletal regenerative medicine. However, the mechanisms by which transplanted cells contribute to tissue repair and regeneration continue to be a subject of debate. Stem cell therapies are often focused on healing diseased or damaged tissues, in which inflammatory and apoptotic signals are abundant. Many studies have suggested that stem cells struggle to survive in such environments creating questions about cell fate after transplantation.1,2 Do transplanted cells survive for extended periods and contribute directly to repair? Or do they simply die following transplantation, primarily acting through a paracrine effect by releasing cytokines and signaling molecules into the extracellular environment?
In the field of bone tissue engineering and regeneration, several cell types have been used for cell-based therapy.3–5 Adipose tissue contains an abundant source of multipotent adult stem cells termed “adipose-derived stromal cells” (ASCs), which hold an enormous potential for skeletal regenerative medicine.2,6,7 Bone marrow-derived mesenchymal stem cells (BM-MSCs) have also shown a great promise as a cellular source for therapy despite limitations, such as donor site morbidity following bone marrow harvest.8,9 Additionally, the transplantation and differentiation of osteoblasts from pluripotent stem cells have shown to be a potentially viable clinical strategy for bone regeneration.10
Given the variety of cell types, scaffolds, and signaling molecules that may be used for cell-based bone repair, the utility of a system that allows for rapid detection of cellular functionality and survival after transplantation is apparent. In this study, we have developed such a reporter system by crossing two strains of existing transgenic mice that enables histologic and FACS-based assessment of both collagen expression and viability in situ. Using this system, we gained novel insights into the survival and functionality of ASCs, BM-MSCs, and osteoblasts following transplantation in a previously established and validated calvarial defect model. The system presented here may also be used universally to analyze any cell expressing collagen type 1a1 in the context of physiologic, pathologic, and cell-based processes.
Materials and Methods
Osteoblast harvest
B6.Cg-Tg(Col1a1*3.6-Topaz)2Rowe/J (Col1a1GFP) mice (JAX Stock No. 017466) were first crossed to STOCK Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (R26mTmG) (JAX Stock No. 007576) to create Col1a1GFP; R26mTmG mice heterozygous at both alleles. Osteoblasts were harvested from the long bones of Col1a1GFP; R26mTmG mice. After sacrificing the animals, the long bones were removed and cleaned. The bones were then gently crushed using a mortar and pestle, and the blood and marrow was removed by repeatedly washing with the FACS buffer (2% fetal bovine serum [FBS], 1% penicillin/streptomycin, 1% P188, and phosphate-buffered saline [PBS]). The wash was saved and used to isolate BM-MSCs (see the section BM-MSC harvest).
Fifty milliliters of collagenase I (Sigma‐Aldrich) was prepared (110 mg collagenase, 500 μL 10% bovine serum albumin [BSA], 800 μL 100X DNAse, 50 μL 1 M CaCl2, P188, 500 μL 1 M HEPES, and M199 up to 50 mL). The long bones were placed into a 50-mL conical tube, and 15 mL of collagenase was added. The bones were placed in a 37°C water bath for 10 min. After 10 min, the bones were placed in a 37°C shaker and mechanically shaken for 30 min. After shaking, the liquid was removed and discarded. Fifteen milliliters of fresh collagenase was added to the same tube, and the steps in a water bath and shaker were repeated. After removing from the shaker, the liquid was removed and run through a 70-μm strainer into a fresh 50-mL conical tube.
The FACS buffer was added to the new conical tube at least in a 2:1 volume to dilute the collagenase. The new tube was then centrifuged at 1300 rpm and 4°C for 5 min, and the supernatant was aspirated off and discarded. The cell pellet was resuspended in 5 mL of FACS buffer and placed on ice. A third round of digestion was performed, as previously described, using the remaining 20 mL of collagenase and the long bones. The liquid was filtered through a 70-μm strainer and added to the 5 mL of cells from the second digest. The FACS buffer was again added, and the sample was centrifuged using the same settings.
After aspirating off the supernatant, the cells were resuspended in 7 mL of FACS buffer, and a gradient centrifugation step, to remove any remaining blood cells, was performed using Histopaque. Seven milliliters of room temperature Histopaque was layered on top of the FACS buffer and carefully moved to the centrifuge, where the sample was spun at 1500 rpm for 15 min at room temperature with no brake. After centrifugation, three layers were visible in the sample. A pipette was used to remove the cloudy middle layer, containing the cells, and was again strained through a 70-μm strainer into a fresh 50-mL conical tube. Once the entire cloudy layer was removed, the remaining solution was discarded.
The cells were centrifuged at 1300 rpm and 4°C for 5 min. The supernatant was aspirated and discarded. The cells were resuspended in 6 mL of cell culture media (Dulbecco's modified Eagle's medium [DMEM], 10% FBS, and 1% penicillin/streptomycin). Two milliliters of the cell mixture was added to three 10-cm cell culture dishes. Eight milliliters of fresh media was then added to each dish before incubation. The cells were allowed to settle for 3 days before fresh media were added to the cell culture plates. The cells were maintained using standard cell culture conditions, with media change every 2–3 days.
BM-MSC harvest
BM-MSCs were harvested from the long bones of Col1a1GFP; R26mTmG mice. After sacrificing the animals, the long bones were removed and cleaned. The bones were then gently crushed using a mortar and pestle, and the blood and marrow was removed by repeatedly washing with the FACS buffer (2% FBS, 1% penicillin/streptomycin, 1% P188, and PBS). The FACS solution was strained through a 70-μm strainer into a 50-mL conical tube. The long bones were gently crushed and washed until the FACS buffer visually became clear. The residual bone chips were used for the osteoblast harvest (see the section Osteoblast harvest). After a clear wash, the conical tube was filled with the FACS buffer, and the cell suspension was centrifuged at 1300 rpm and 4°C for 5 min.
After aspirating off the supernatant, the cells were resuspended in 7 mL of FACS buffer, and a gradient centrifugation step, to remove red blood cells, was performed using Histopaque. Seven milliliters of room temperature Histopaque was layered on top of the FACS buffer and carefully moved to the centrifuge, where the sample was spun at 1500 rpm for 15 min at room temperature with no brake. After centrifugation, three layers were visible in the sample. A pipette was used to remove the cloudy middle layer, containing the cells, and was again strained through a 70-μm strainer into a fresh 50-mL conical tube. Once the entire cloudy layer was removed, the remaining solution was discarded. The cells were centrifuged at 1300 rpm and 4°C for 5 min. The supernatant was aspirated and discarded.
The cells were resuspended in 6 mL of cell culture media (DMEM, 10% FBS, and 1% penicillin/streptomycin). Two milliliters of the cell mixture was added to three 10-cm cell culture dishes. Eight milliliters of fresh media was then added to each dish before incubation. The cells were allowed to settle for 5 days before fresh media were added to the cell culture plates. The cells were maintained using standard cell culture conditions, with media change every 2–3 days.
ASC harvest
ASCs were harvested from the abdominal fat pads of Col1a1GFP; R26mTmG mice. After sacrificing the animals, the mice were placed supine and sprayed with 70% ethanol. The abdominal fat pads were exposed, and the two fat pads near the hind legs were collected and placed on ice. The fat pads were then washed in serial dilutions of Betadine. The fat pads were then finely minced using scissors and placed into a 50-mL conical tube. Fifteen milliliters of collagenase (11.25 mg type 2 collagenase and 15 mL of DMEM) was added to the minced fat. The fat was then placed in a 37°C water bath with agitation for 30 min. If fat is not completely digested, continue to digest for 10 additional minutes.
After the digest, 35 mL of cell culture media (DMEM, 10% FBS, and 1% penicillin/streptomycin) was added to the solution to inactivate the collagenase. The cells were then centrifuged at 1000 rpm and 4°C for 5 min. Following centrifugation, the cream layer, adipocytes, at the top was discarded. The supernatant was aspirated and discarded, leaving about 1 mL of media on the cell pellet. The cells were then resuspended in 15 mL of culture media. The cell suspension was strained through a 100-μm cell strainer into a fresh 50-mL conical tube. The old tube was rinsed with 5 mL of fresh media, and the media were then added to the new conical tube after passing through the cell strainer.
The solution was again centrifuged at 1000 rpm and 4°C for 5 min. Following centrifugation, the supernatant was aspirated and discarded. The cells were resuspended in 10 mL of culture media, and all 10 mL was plated onto a 10-cm cell culture dish. The following morning, the culture dish was washed twice with fresh culture media to remove red blood cells and unattached cells. Ten milliliters of fresh media was then added to the culture dish. The cells were maintained using standard cell culture conditions, with media change every 2–3 days.
Cell seeding on scaffolds
ASCs, BM-MSCs, and long-bone osteoblasts derived from Col1a1GFP; R26mTmG mice were trypsinized, washed with FBS, and counted. The cells (250,000) were suspended in 100 μL fetal calf serum and seeded on 4-mm-diameter apatite-coated poly(lactic-co-glycolic acid) (PLGA) scaffolds, which were fabricated from 85:15 poly(lactic-co-glycolic acid) by solvent casting and a particulate leaching process as previously described.11 Scaffolds were implanted in calvarial defects 2 h after in vitro seeding.
Animal surgery
All animal experiments were performed in accordance with the Stanford University Animal Care and Use Committee guidelines.
In vitro healing capacity was assessed in 21-day-old male Crl:CD1-Foxn1nu mice (Charles River Laboratories) that underwent 4-mm calvarial defect procedures as previously described.12 For experiments involving a comparison of frontal and parietal healing in Col1a1GFP; R26mTmG mice, 2-mm calvarial defects were created in frontal and parietal bones. After anesthesia with an intraperitoneal injection of ketamine 100 mg/kg + xylazine 20 mg/kg + acepromazine 3 mg/kg and disinfection of the surgical site of the mice, nonhealing, critical-sized 4-mm calvarial defects were created with a trephine drill bit in left parietal bones as previously described.13 Care was taken to protect the underlying dura mater or neighboring cranial sutures. After seeding, the scaffolds were placed in the defects; the wound was closed, and the animals were allowed to recover.
FACS analysis of endogenous cells in 2-mm frontal and parietal defects
Defects were harvested at 2 and 4 weeks posttransplant. Defects were gently crushed using a mortar and pestle, and 25 mL of collagenase digestion mixture was added (110 mg collagenase I (Sigma‐Aldrich), 500 μL 10% BSA, 800 μL 100X DNase, 50 μL 1 M CaCl2, P188, 500 μL 1 M HEPES, and M199 up to 50 mL). This mixture was then placed in a 37°C water bath for 10 min. After 10 min, the bones were placed in a 37°C shaker and mechanically shaken for 30 min. After shaking, the liquid was run through a 70-μm strainer into a fresh 50-mL conical tube, and 25 mL of FACS buffer was added and then centrifuged at 1300 rpm and 4°C for 5 min. The supernatant was aspirated off and discarded.
The cell pellet was resuspended in 1 mL of FACS buffer containing CD45 (1:100), Ter-119 (1:100), Tie2 (1:50), CD90 (1:100), and CD105 (1:100) antibodies conjugated to Pacific Blue and incubated on ice for 20 min. The cells were then washed for three cycles with the FACS buffer. Following centrifugation in the last wash, cell pellets were resuspended in 2 mL of FACS buffer containing 10 μg/mL DNase and 1X DAPI viability dye on ice. Analysis was performed using flow cytometer BD LSR Fortessa.
FACS analysis of transplanted cells in 4-mm parietal defects
Defects were harvested at 72 h and 2 weeks posttransplant. Defects were gently crushed using a mortar and pestle, and 25 mL of collagenase was added (110 mg collagenase, 500 μL 10% BSA, 800 μL 100X DNase, 50 μL 1 M CaCl2, P188, 500 μL 1 M HEPES, and M199 up to 50 mL). This mixture was then placed in a 37°C water bath for 10 min. After 10 min, the bones were placed in a 37°C shaker and mechanically shaken for 30 min. After shaking, the liquid was run through a 70-μm strainer into a fresh 50-mL conical tube, and 25 mL of FACS buffer was added and then centrifuged at 1300 rpm and 4°C for 5 min. The supernatant was aspirated off and discarded, and the cell pellet was resuspended in 5 mL of FACS buffer containing 10 μg/mL DNase and 1X DAPI viability dye on ice. Analysis was performed using flow cytometer BD LSR Fortessa.
μCT scanning
μCT scanning was performed as previously described.6 Mice were scanned with a high-resolution MicroCAT IITM scanner (ImTek, Inc.), with an X-ray voltage of 80 kVP and an anode current of 450 μA. A resolution of 80 μm was obtained with 144 steps over 360° rotation.
X-ray data reconstruction was performed with Cobra EXXIM (EXXIM Computing Corp.) and Micro View Software (GE Healthcare). Each mouse was scanned with a CT phantom, which was used to calibrate each scan. The precise threshold for regenerating calvarial bone was previously determined as equivalent to 510 Hounsfield Units. The rest-defect area was then determined with the Magic Wand Tool in Photoshop (Adobe Systems). Percentage of healing was determined by dividing the rest-defect area by the mean of the defect size 1 day postoperatively. Crl:CD1-Foxn1nu mice were scanned 24 h postsurgery and at weeks 2, 4, and 8. The Student's t-test was used for statistical analysis. A *p < 0.05 was considered statistically significant.
Reverse transcription and quantitative real-time polymerase chain reaction
RNA from cultured cells was extracted using TRIzol (Life Technologies) and an RNeasy MinElute Cleanup Kit (QIAGEN) as per the manufacturer's protocol. Isolated RNA was quantified using the NanoDrop 2000 (ThermoFisher Scientific) and then reverse transcribed using the TaqMan Reverse Transcription Reagents (Invitrogen). Expression of Col1a1 was determined by quantitative real-time polymerase chain reaction (qRT-PCR) using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) and SYBR Green PCR Master Mix (Applied Biosystems). Target quantities were normalized to endogenous β-actin quantities using the standard curve method. Primers for Col1a1 and β-actin were constructed based on their PrimerBank (Massachusetts General Hospital, Boston, MA) sequences (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tea).
Results
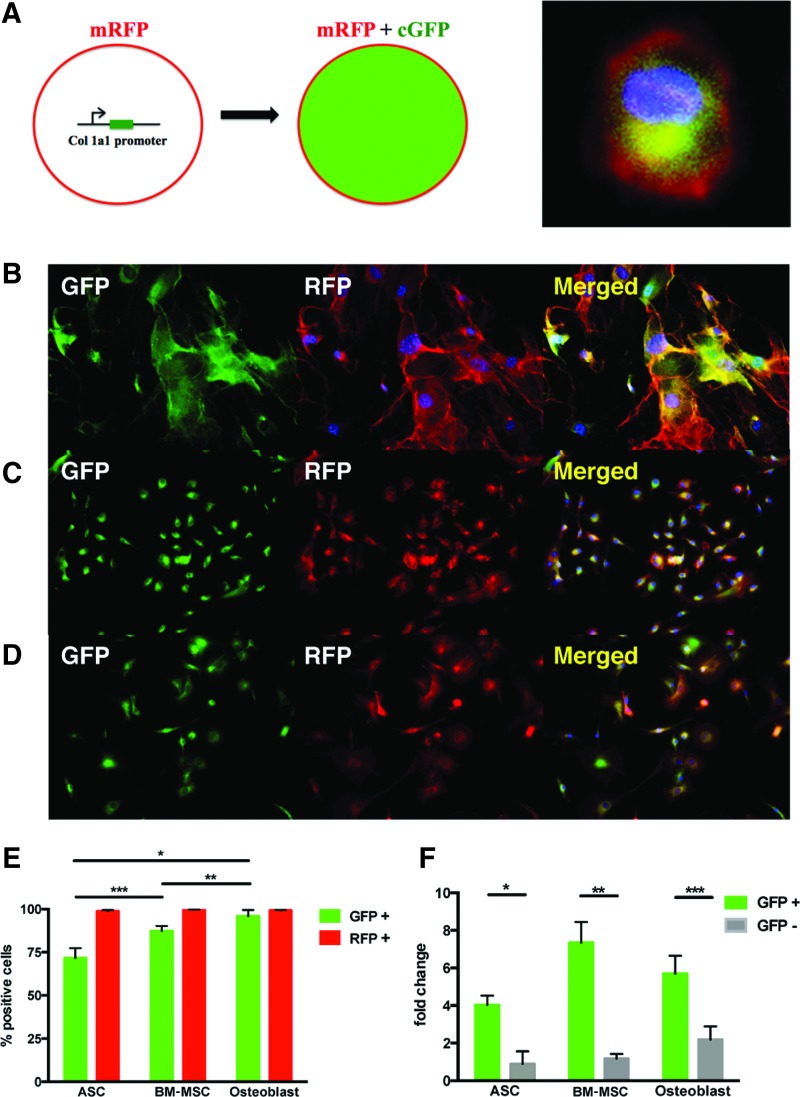
Transgenic pOBCol3.6GFPtpz mice (Col1a1GFP) expressing the topaz variant of green fluorescent protein (GFP) under the control of the collagen, type I, alpha 1 (Col1a1) promoter/enhancer sequence were crossed to ROSA26mTmG (R26mTmG) mice, which express membrane-bound tomato red (RFP) constitutively in all cells. The resulting Col1a1GFP; R26mTmG offspring was positive for cytoplasmic GFP in cells with Col1a1 promoter activity and expressed membrane-bound RFP in all cell types (Fig. 1A).
FIG. 1.
Green fluorescent protein (GFP) expression is an accurate reporter of Col1a1 mRNA expression in adipose-derived stromal cells (ASCs), bone marrow-derived mesenchymal stem cells (BM-MSCs), and osteoblasts isolated from Col1a1GFP; R26mTmG mice. (A) Schematic demonstrating that cells in the Col1a1GFP; R26mTmG system express membrane-bound RFP (mRFP), but only those that also express Col1a1 will express cytoplasmic GFP (cGFP). A representative high-magnification image of a cultured BM-MSC (DAPI, blue; mRFP, red; and cGFP, green) is also shown (right). Microscopic images of cultured (B) ASCs, (C) BM-MSCs, and (D) osteoblasts isolated from adult Col1a1GFP; R26mTmG mice with GFP (green), RFP (red), and merged images with DAPI (blue). (E) Flow cytometry analysis of GFP and RFP expression in cultured ASCs, BM-MSCs, and osteoblasts (*p < 0.05, **p < 0.05, and ***p < 0.05). (F) qRT-PCR analysis of Col1a1 mRNA expression in GFP-positive versus GFP-negative cultured ASCs, BM-MSCs, and osteoblasts (*p < 0.01, **p < 0.01, and ***p < 0.01). Color images available online at www.liebertpub.com/tea
We first cultured ASCs derived from the abdominal fat pads of adult 6-week-old Col1a1GFP; R26mTmG mice. BM-MSCs and osteoblasts were derived from the long bones of adult 6-week-old Col1a1GFP; R26mTmG mice and cultured. ASCs, BM-MSCs, and osteoblasts were analyzed for both RFP and GFP expression using gross microscopic and flow cytometry analysis. As expected, all cultured ASCs expressed RFP, and a large subset expressed cytoplasmic GFP by gross microscopic analysis (Fig. 1B).
We next quantitatively assessed RFP and GFP expression using flow cytometry analysis and found that 71.5% of ASCs expressed GFP and 98.6% expressed RFP (Fig. 1E). Similarly, all cultured BM-MSCs expressed RFP, and a large subset expressed cytoplasmic GFP (Fig. 1C). Quantification of fluorescence by flow cytometry revealed that 87.3% of BM-MSCs expressed GFP and 99.6% expressed RFP (Fig. 1E). Finally, all cultured osteoblasts expressed RFP, and the vast majority expressed cytoplasmic GFP by gross microscopic analysis (Fig. 1D). Flow cytometry analysis showed that 95.9% of osteoblasts expressed GFP and 99.1% expressed RFP (Fig. 1E).
To confirm that GFP fluorescence correlated with Col1a1 promoter activity, we FACS sorted both GFP+ and GFP− populations from cultured ASCs, BM-MSCs, and osteoblasts and assessed Col1a1 mRNA expression by qRT-PCR analysis. As expected, qRT-PCR analysis revealed that Col1a1 mRNA was increased 4.55-fold in GFP+ ASCs compared to GFP− ASCs (*p < 0.01) (Fig. 1F). Similarly, Col1a1 mRNA was increased 6.28-fold in GFP+ BM-MSCs compared to GFP− BM-MSCs (**p < 0.01) (Fig. 1F). Finally, Col1a1 mRNA was increased 2.6-fold in GFP+ osteoblasts compared to GFP− osteoblasts (***p < 0.01) (Fig. 1F).
Having confirmed that ASCs, BM-MSCs, and osteoblasts isolated from adult Col1a1GFP; R26mTmG mice were robustly RFP+ and expressed cytoplasmic GFP appropriately based on Col1a1 promoter activity, we next assessed the fidelity of the Col1a1GFP; R26mTmG system for tracking both cells and Col1a1 expression in vitro using histologic fluorescent analysis. Our group has previously demonstrated that the different embryonic origins of the bones, which constitute the mammalian calvarium, influence their regenerative and osteogenic capacity using in vitro and in vitro studies.14–17
It has been demonstrated that neural crest-derived frontal bone osteoblasts are bestowed with enhanced endogenous activation of pro-osteogenic signaling pathways relative to parietal bone osteoblasts, such as Wnt, FGF, and BMP signaling, whereas the paraxial mesoderm-derived parietal bone osteoblasts have enhanced TGFβ signaling and increased apoptotic activity.14–18 It was therefore hypothesized that temporospatial differences in collagen expression during bone healing may exist in these two regions.
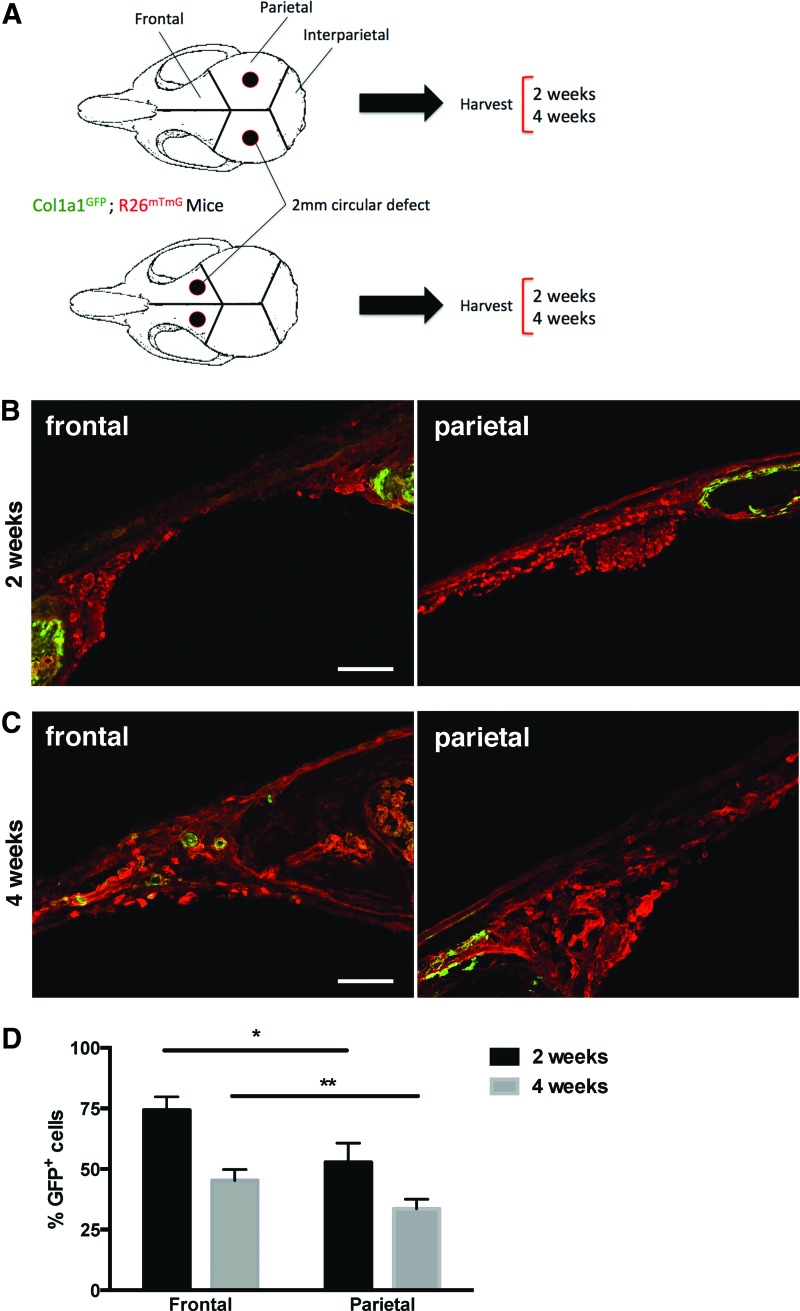
First, 2-mm calvarial defects were induced in both parietal and frontal bones in adult Col1a1GFP; R26mTmG mice (Fig. 2A). At 2 and 4 weeks after defect surgery, skulls were harvested and processed for histologic analysis. Microscopic analysis revealed that cells at the edges of the healing defect robustly expressed GFP and therefore had a high Col1a1 promoter activity at 2 weeks postsurgery (Fig. 2B) in both frontal and parietal bones, although subjectively more in frontal bone than in parietal bone. As early as 4 weeks postsurgery, GFP expression had begun to extend more centrally across the dura mater, spanning the defect in frontal bone and less so in parietal bone (Fig. 2C).
FIG. 2.
Frontal osteoblasts xpress higher levels of Col1a1 mRNA than parietal osteoblasts during calvarial healing. (A) Schematic experimental plan with 2-mm parietal versus frontal defects in the skulls of adult Col1a1GFP; R26mTmG mice. (B) Histologic images of frontal and parietal bones at 2 weeks following induction of 2-mm calvarial defects. Scale bar = 200 μm. (C) Histologic images of frontal and parietal bones at 4 weeks following induction of 2-mm calvarial defects. Scale bar = 100 μm. (D) Flow cytometry analysis of GFP expression in osteoblasts isolated from parietal and frontal defects at 2 and 4 weeks postsurgery (*p < 0.01 and **p < 0.01). Color images available online at www.liebertpub.com/tea
FACS analysis of GFP expression in osteoblasts isolated from healing frontal defects compared to those isolated from healing parietal defects revealed that a significantly greater percentage of frontal osteoblasts express GFP, and therefore Col1a1, than do parietal osteoblasts at both 2 (*p < 0.01) and 4 (**p < 0.01) weeks after defect surgery (Fig. 2D). Osteoblasts were defined as CD45−, Ter-119−, Tie2−, CD90−, and CD105−.19 These findings are consistent with more robust collagen expression seen at the advancing osteogenic fronts of frontal bone defects in comparison to parietal bone defects (Fig. 2B, C) and with existing literature.14–18
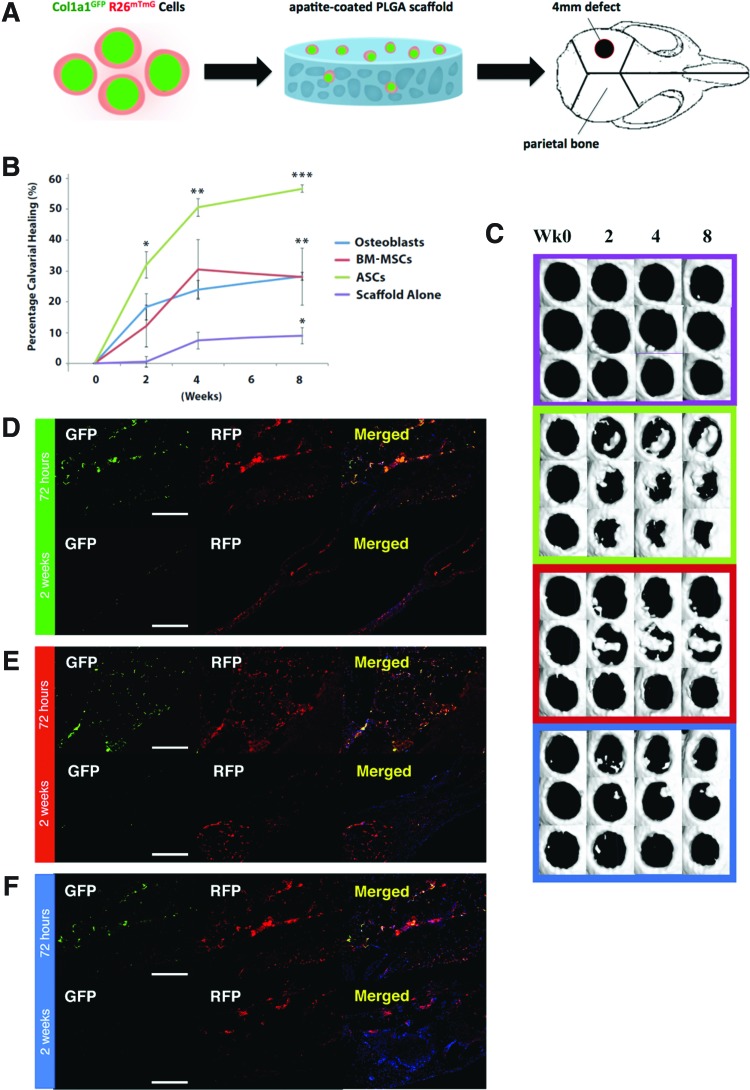
We next assessed the survival and collagen-producing activity of ASCs, BM-MSCs, and osteoblasts following transplantation into 4-mm critical-sized calvarial defects that do not heal spontaneously (Fig. 3A). ASCs, BM-MSCs, and osteoblasts were isolated from the long bones of adult Col1a1GFP; R26mTmG mice and seeded on apatite-coated poly(lactic-co-glycolic acid) (PLGA) scaffolds overnight (106 cells per scaffold). The seeded scaffolds were transplanted into 4-mm critical-sized calvarial defects created in the parietal bone of immunodeficient Crl:CD1-Foxn1nu mice on day 0 of surgery using an established model.13,20 Healing was allowed to proceed for up to 8 weeks with periodic μCT scanning to track the healing progress. Small cohorts of mice were sacrificed at 72 h and 14 days postsurgery for histologic analysis.
FIG. 3.
ASCs, BM-MSCs, and osteoblasts potentiate bone repair in vivo. ASCs accelerated bone repair more than other treatment groups. (A) Schematic representation of parietal calvarial defect made in parietal bone of 21-day-old nude mice skulls. Four millimeter nonhealing defects were made, and poly(lactic-co-glycolic acid) (PLGA) scaffold inserted with or without cells. (B) Quantification of defect repair according to μCT scan results. Statistical analysis was performed using one-way ANOVA testing and multiple comparison analysis (***p < 0.0001 for ASCs vs. scaffold alone, **p < 0.01 for ASCs vs. osteoblasts and BM-MSCs, and *p < 0.05 for BM-MSCs and osteoblasts vs. scaffold alone). (C) Healing kinetics of three representative skull defect μCT scans from each treatment group. Histologic images of (D) ASC-treated, (E) BM-MSC-treated, and (F) osteoblast-treated 4-mm parietal defects at 72 h and 2 weeks postsurgery. Scale bar = 200 μm. Color images available online at www.liebertpub.com/tea
ASC-, BM-MSC-, osteoblast, and control-treated calvarial defects in Crl:CD1-Foxn1nu mice were scanned by μCT at 24 h and 2, 4, and 8 weeks postsurgery. Percentage of healing compared across the groups revealed that the calvarial defects treated with ASCs healed significantly faster than those treated with BM-MSCs or osteoblasts (Fig. 3B, C). At 2 weeks postsurgery, the highest healing rates were seen among ASC-treated defects, with ASCs achieving a mean 32% healing, BM-MSCs 12% healing, osteoblasts 18% healing, and scaffold alone 0.5% healing. At 2 weeks, the only statistically significant difference (*p < 0.05) was between ASCs and scaffold alone (Fig. 3B).
By week 4, the trend extended the differences, with ASCs achieving 51% healing, BM-MSCs 30% healing, osteoblasts 23% healing, and scaffold alone 7% healing. Again, ASCs achieved statistically significant healing compared to scaffold alone at week 4 (**p < 0.01). By week 8, ASCs achieved 57% healing, BM-MSCs and osteoblasts 28% healing, and scaffold alone 9% healing. Therefore, by week 8, ASCs demonstrated significantly more healing than both osteoblasts and BM-MSCs (**p < 0.01) and more than scaffold alone (***p < 0.0001). Both BM-MSCs and osteoblasts resulted in significantly more healing than scaffold alone (*p < 0.05). There was no statistically significant difference between the healing rates of defects treated with osteoblasts and those treated with BM-MSCs (Fig. 3B, C).
Histologic analysis of defects receiving ASCs revealed that significant numbers of transplanted cells survived (RFP+) and still expressed Col1a1 (GFP+) at 72 h postsurgery (Fig. 3D). However, at 2 weeks postsurgery, although many ASCs survived, as demonstrated by persisting RFP signal, only scattered cells expressed GFP.
Histologic analysis of defects receiving BM-MSCs revealed that large numbers of transplanted BM-MSCs survived and robustly expressed GFP at 72 h postsurgery (Fig. 3E). By 2 weeks postsurgery, BM-MSCs were primarily localized to regions of bone marrow rather than compact bone. Relatively few cells expressed GFP at 2 weeks postsurgery (Fig. 3E). Last, histologic analysis of defects receiving osteoblasts revealed that large numbers of transplanted osteoblasts survived and robustly expressed GFP at 72 h postsurgery (Fig. 3F). By 2 weeks postsurgery, relatively few osteoblasts survived, and there was no expression of GFP (Fig. 3F).
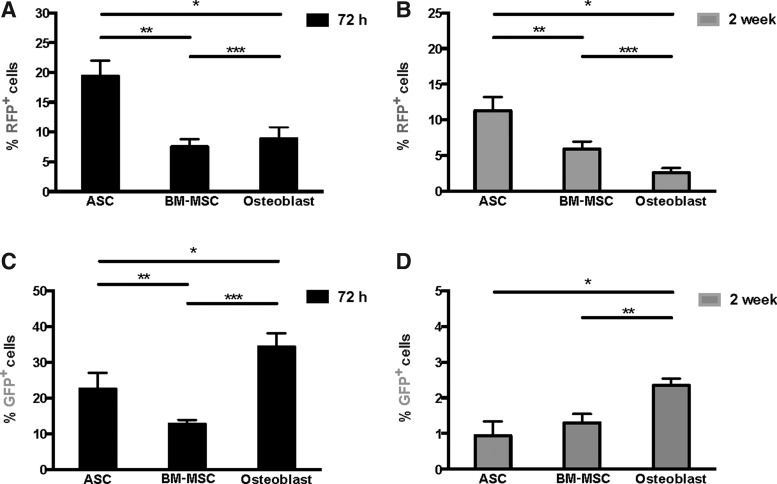
To quantitatively assess the degree of active collagen expression and survival of transplanted (RFP+) cells, we used flow cytometry to analyze all cells isolated from calvarial defects at 72 h and 2 weeks postsurgery. At 72 h posttransplant, RFP+ cells represented 19.4%, 7.5%, and 8.9% of total viable cells in defects that had received ASCs, BM-MSCs, and osteoblasts, respectively (Fig. 4A). However, at 2 weeks posttransplant, RFP+ cells represented 11.3%, 5.9%, and 2.6% of total cells in defects that had received ASCs, BM-MSCs, and osteoblasts, respectively (Fig. 4B).
FIG. 4.
ASCs demonstrate increased survival over BM-MSCs and osteoblasts following transplantation into calvarial defects. Flow cytometry analyses of RFP expression among all viable cells isolated from 4-mm parietal defects at (A) 72 h and (B) 2 weeks postsurgery that received ASCs, BM-MSCs, or osteoblasts on PLGA scaffolds at the time of defect surgery. *p < 0.01, **p < 0.01, and ***p < 0.01. (C) Flow cytometry analysis of GFP expression among all viable RFP+ cells isolated from 4-mm parietal defects at 72 h postsurgery that received ASCs, BM-MSCs, or osteoblasts on PLGA scaffolds at the time of defect surgery. *p < 0.05, **p < 0.01, and ***p < 0.01. (D) Flow cytometry analysis of GFP expression among all viable RFP+ cells isolated from 4-mm parietal defects at 2 weeks postsurgery that received ASCs, BM-MSCs, or osteoblasts on PLGA scaffolds at the time of defect surgery. *p < 0.01 and **p < 0.05.
This observation indicated that in terms of both initial and longer term survival posttransplant, ASCs persisted to a significantly greater degree than BM-MSCs and osteoblasts. Interestingly, ASC-treated defects healed significantly faster than either BM-MSC- or osteoblast-treated defects. Enhanced survival among ASCs may explain this difference.
We next assessed the level of Col1a1 expression among transplanted cells at both 72 h and 2 weeks posttransplant using flow cytometry analysis. The percentage of GFP+ cells of the total transplanted cells (defined by RFP positivity) differed markedly among the three groups at 72 h posttransplant. In defects receiving ASCs, 22.5% of RFP+ cells were also GFP+ at 72 h (Fig. 4C). In defects receiving BM-MSCs, 12.7% of RFP+ cells were also GFP+ at 72 h posttransplant (Fig. 4C). In defects receiving osteoblasts, 34.3% of RFP+ cells were also GFP+ at 72 h (Fig. 4C). As healing progressed, relatively fewer transplanted cells expressed GFP. At 2 weeks posttransplant, only 0.94%, 1.3%, and 2.35% of RFP+ cells were also GFP+ in defects receiving ASCs, BM-MSCs, and osteoblasts, respectively (Fig. 4D).
Discussion
Deciphering the mechanisms by which transplanted cells contribute to tissue repair and regeneration is critical to improving cell-based therapies.21 Certain cell types, particularly when transplanted into hostile recipient sites, may benefit considerably from interventions that improve cell survival. Indeed, a recent study by Hyun et al. showed that minicircle-mediated overexpression of Bcl-2 not only enhanced survival of adipose-derived stem cells in two clinical tissue repair models but also increased tissue regeneration.22
More robust cell types may be able to tolerate and survive the transplantation process but lack the capability to contribute directly to repair. In these cases, enhancement of repair capacity through targeted genetic manipulation may therefore be the most effective means to increase therapeutic efficacy. However, our ability to select the appropriate cellular modification for a given cell type first requires detailed knowledge concerning both the survival and functionality of that cell type following transplantation.
To gain an insight into this question, we developed and validated a transgenic mouse model that permits rapid evaluation of both the functional capacity and survival of transplanted cells in the context of bone healing. In general, we found that survival of transplanted cells correlated with increased rates of calvarial healing. With equal numbers of cells used per transplant across treatment groups, ASCs showed the highest overall rates of 72-h and 2-week posttransplant survival. However, differential rates of cellular attachment when seeding ASCs, BM-MSCs, and osteoblasts on PLGA scaffolds have been observed.23 This represents a potential limitation of the study as we control cell number only and do not assess the rate of nonadherence to the scaffold.
BM-MSCs and osteoblasts in comparison did not heal defects as rapidly and showed lower overall rates of survival posttransplant at both 72 h and 2 weeks. Interestingly, BM-MSCs, although showing poor survival at 72 h, demonstrated marked resilience at 2 weeks posttransplant and ultimately had the highest relative survival during this period. Osteoblasts, in comparison, died rapidly and by 2 weeks posttransplant represented less than 3% of total viable cells in the defect.
However, analysis of Col1a1 expression (GFP+) showed that osteoblasts expressed Col1a1 to a significantly greater degree than the other cell types at 72 h and 2 weeks posttransplant. These data suggest that if osteoblast survival could be enhanced, the benefits of their inherently superior functionality (i.e., Col1a1 production) could be realized following transplantation. Indeed, prior studies have shown that Bcl-2 can be used to enhance stem cell survival following transplantation into calvarial defects and stented full-thickness skin wounds.22 Osteoblasts may represent an ideal target for such an intervention.
Our results lend support to the notion that certain cell types are capable of surviving and functionally contributing to bone regeneration to a greater extent than other cell types posttransplantation. In the context of previous literature, this observation is not surprising. Indeed, prior literature has demonstrated that certain stem cell types do not survive for long periods in situ after transplantation.24–26 Rather than making a direct contribution to repair, these transplanted cells facilitate regeneration primarily through paracrine-mediated recruitment of autologous cells.27–29
However, it has also been shown that certain scaffolds and signaling molecules can significantly increase survival of transplanted cells.21,30,31 Levi et al. observed that induced pluripotent stem cells exhibited a high degree of survival and acquired a fully differentiated osteogenic state when transplanted on a biomimetic scaffold into calvarial defects.10 In general, we found that increased survival time of transplanted cells correlated with increased rates of healing in the calvarial defects.
The mouse model used here may also be used to gain an insight into the role of endogenous cells during normal calvarial bone healing. It has been demonstrated that neural crest-derived frontal bone osteoblasts possess enhanced endogenous activation of pro-osteogenic signaling pathways relative to parietal bone osteoblasts. In contrast, the paraxial mesoderm-derived parietal bone osteoblasts have enhanced TGFβ signaling and increased apoptotic activity.14–18 Using the Col1a1GFP; R26mTmG system presented here, we verified that temporospatial differences in collagen expression during bone healing exist between frontal and parietal bones, with frontal osteoblasts exhibiting higher expression of Col1a1 than parietal osteoblasts.
Although critical to the formation of bone, Col1a1 is not the only gene involved in bone healing. A host of growth factors and signaling factors critically impact the behavior of both endogenous and transplanted cells. It is this extracellular milieu coupled with heterotypic cellular interactions that ultimately determine the rate and quality of bone regeneration.32,33 Although the Col1a1GFP; R26mTmG system does provide an informative look into the behavior of endogenous and transplanted cells during bone healing, it does so through the lens of Col1a1 expression.
In addition to the studies and concepts discussed above, cell-based therapies stand to benefit greatly from the identification of markers that correlate with a specific functional characteristic (i.e., collagen production and secretion). This is a principle that has already proven fruitful for the purposes of bone tissue engineering with markers, such as CD90 and CD105.34
The Col1a1GFP; R26mTmG system presented here could be used as a means to rapidly screen and identify surface markers that correlate with Col1a1 expression by flow cytometry analysis. Many of the cell types (i.e., ASCs) currently used for cellular transplantation represent heterogeneous populations of cells. Flow cytometry is capable of differentiating not only among GFP+ and GFP− cells but also among varying degrees of GFP expression. In this manner, surface markers could be identified that enrich cells with enhanced functional capacity on the basis of correlating surface protein expression with a high GFP expression.
Conclusion
In conclusion, the novel reporter system presented and validated here represents a useful tool for improving cell-based regenerative therapies in the context of bone repair by allowing for rapid assessment of both the survival and function of transplanted cells in vitro. Using this system, we have gained an insight into how cell-based therapies impact bone healing. ASCs displayed both increased survival and increased Col1a1 expression compared to BM-MSCs and osteoblasts following calvarial defect transplantation. These insights have the potential to inform translational clinical trials aimed at evaluating cell-based therapeutics for enhanced skeletal healing.
Supplementary Material
Acknowledgment
We thank Siny Shailendra for her assistance with microscopic imaging.
Author Contributions
G.G.W. and K.S.-Y.: conception and design, collection, and/or assembly of data, data analysis and interpretation, and article writing. T.L.W.: conception and design, collection, and/or assembly of data. S.M., M.S.H., D.D., Z.N.M., J.M.T., and E.R.Z.: collection and/or assembly of data. I.L.W., G.C.G., and H.P.L.: financial support and article writing. M.T.L.: conception and design, financial support, article writing, and final approval of article.
Funding
This work was supported in part by a grant from the NIH grant R01 GM087609 (H.P.L.), a gift from Ingrid Lai and Bill Shu in honor of Anthony Shu (H.P.L.), the Hagey Laboratory for Pediatric Regenerative Medicine and the Oak Foundation (M.T.L., H.P.L., and G.C.G.), the NIH grant U01 HL099776 (M.T.L.), and the Gunn/Olivier fund (M.T.L.). G.G.W. was supported by the Stanford School of Medicine, the Stanford Medical Scientist Training Program, and NIGMS training grant GM07365. M.S.H. was supported by the California Institute for Regenerative Medicine Clinical Fellow training grant TG2-01159. Z.N.M. was supported by the Plastic Surgery Foundation Research Fellowship grant 114288.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hyun J.S., Tran M.C., Wong V.W., Chung M.T., Lo D.D., Montoro D.T., Wan D.C., and Longaker M.T. Enhancing stem cell survival in vivo for tissue repair. Biotechnol Adv 31, 736, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Levi B., and Longaker M.T. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 29, 576, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson L., Elliman S.J., and Coleman C.M. From isolation to implantation: a concise review of mesenchymal stem cell therapy in bone fracture repair. Stem Cell Res Therapy 5, 51, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stegemann J.P., Verrier S., Gebhard F., Laschke M.W., Martin I., Simpson H., and Miclau T. Cell therapy for bone repair: narrowing the gap between vision and practice. Eur Cells Mater 27, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosset P., Deschaseaux F., and Layrolle P. Cell therapy for bone repair. Orthop Traumatol Surg Res 100, S107, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Barba M., Cicione C., Bernardini C., Michetti F., and Lattanzi W. Adipose-derived mesenchymal cells for bone regereneration: state of the art. Biomed Res Int 2013, 416391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodle J.C., Hanson A.D., and Loboa E.G. Adipose-derived stem cells in functional bone tissue engineering: lessons from bone mechanobiology. Tissue Eng Part B Rev 17, 195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight M.N., and Hankenson K.D. Mesenchymal stem cells in bone regeneration. Adv Wound Care (New Rochelle) 2, 306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panetta N.J., Gupta D.M., Quarto N., and Longaker M.T. Mesenchymal cells for skeletal tissue engineering. Panminerva Med 51, 25, 2009 [PubMed] [Google Scholar]
- 10.Levi B., Hyun J.S., Montoro D.T., Lo D.D., Chan C.K., Hu S., Sun N., Lee M., Grova M., Connolly A.J., Wu J.C., Gurtner G.C., Weissman I.L., Wan D.C., and Longaker M.T. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc Natl Acad Sci U S A 109, 20379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan C.M., Shi Y.Y., Aalami O.O., Chou Y.F., Mari C., Thomas R., Quarto N., Contag C.H., Wu B., and Longaker M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22, 560, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Behr B., Sorkin M., Lehnhardt M., Renda A., Longaker M.T., and Quarto N. A comparative analysis of the osteogenic effects of BMP-2, FGF-2, and VEGFA in a calvarial defect model. Tissue Eng Part A 18, 1079, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behr B., Tang C., Germann G., Longaker M.T., and Quarto N. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells 29, 286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quarto N., Wan D.C., Kwan M.D., Panetta N.J., Li S., and Longaker M.T. Origin matters: differences in embryonic tissue origin and Wnt signaling determine the osteogenic potential and healing capacity of frontal and parietal calvarial bones. J Bone Miner Res 25, 1680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S., Quarto N., and Longaker M.T. Activation of FGF signaling mediates proliferative and osteogenic differences between neural crest derived frontal and mesoderm parietal derived bone. PLoS One 5, e14033, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quarto N., Behr B., Li S., and Longaker M.T. Differential FGF ligands and FGF receptors expression pattern in frontal and parietal calvarial bones. Cells Tissues Organs 190, 158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behr B., Panetta N.J., Longaker M.T., and Quarto N. Different endogenous threshold levels of fibroblast growth factor-ligands determine the healing potential of frontal and parietal bones. Bone 47, 281, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Li S., Meyer N.P., Quarto N., and Longaker M.T. Integration of multiple signaling regulates through apoptosis the differential osteogenic potential of neural crest-derived and mesoderm-derived osteoblasts. PLoS One 8, e58610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan C.K., Lindau P., Jiang W., Chen J.Y., Zhang L.F., Chen C.C., Seita J., Sahoo D., Kim J.B., Lee A., Park S., Nag D., Gong Y., Kulkarni S., Luppen C.A., Theologis A.A., Wan D.C., DeBoer A., Seo E.Y., Vincent-Tompkins J.D., Loh K., Walmsley G.G., Kraft D.L., Wu J.C., Longaker M.T., and Weissman I.L. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc Natl Acad Sci U S A 110, 12643, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levi B., James A.W., Nelson E.R., Vistnes D., Wu B., Lee M., Gupta A., and Longaker M.T. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 5, e11177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walmsley G.G., Hyun J., McArdle A., Senarath-Yapa K., Hu M.S., Chung M.T., Wong V.W., Longaker M.T., and Wan D.C. Induced pluripotent stem cells in regenerative medicine and disease modeling. Curr Stem Cell Res Ther 9, 73, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Hyun J., Grova M., Nejadnik H., Lo D., Morrison S., Montoro D., Chung M., Zimmermann A., Walmsley G.G., Lee M., Daldrup-Link H., Wan D.C., and Longaker M.T. Enhancing in vivo survival of adipose-derived stromal cells through Bcl-2 overexpression using a minicircle vector. Stem Cells Transl Med 2, 690, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanatta G., Rudisile M., Camassola M., Wendorff J., Nardi N., Gottfried C., Pranke P., and Netto C.A. Mesenchymal stem cell adherence on poly(d, l-lactide-co-glycolide) nanofibers scaffold is integrin-beta 1 receptor dependent. J Biomed Nanotechnol 8, 211, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Lin Y., and Hogan W.J. Clinical application of mesenchymal stem cells in the treatment and prevention of graft-versus-host disease. Adv Hematol 2011, 427863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minteer D.M., Marra K.G., and Rubin J.P. Adipose stem cells: biology, safety, regulation, and regenerative potential. Clin Plast Surg 42, 169, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Kapur S.K., Dos-Anjos Vilaboa S., Llull R., and Katz A.J. Adipose tissue and stem/progenitor cells: discovery and development. Clin Plast Surg 42, 155, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Wingard J.R., Hsu J., and Hiemenz J.W. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am 24, 257, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Cutler C., and Antin J.H. An overview of hematopoietic stem cell transplantation. Clin Chest Med 26, 517, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Isakson M., de Blacam C., Whelan D., McArdle A., and Clover A.J. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cells Int 2015, 831095, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walmsley G.G., McArdle A., Tevlin R., Momeni A., Atashroo D., Hu M.S., Feroze A.H., Wong V.W., Lorenz P.H., Longaker M.T., and Wan D.C. Nanotechnology in bone tissue engineering. Nanomedicine 11, 1253, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tevlin R., McArdle A., Atashroo D., Walmsley G.G., Senarath-Yapa K., Zielins E.R., Paik K.J., Longaker M.T., and Wan D.C. Biomaterials for craniofacial bone engineering. J Dent Research 93, 1187, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zigdon-Giladi H., Rudich U., Michaeli Geller G., and Evron A. Recent advances in bone regeneration using adult stem cells. World J Stem Cells 7, 630, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soucacos P.N., Johnson E.O., and Babis G. An update on recent advances in bone regeneration. Injury 39 Suppl 2, S1, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Chung M.T., Liu C., Hyun J.S., Lo D.D., Montoro D.T., Hasegawa M., Li S., Sorkin M., Rennert R., Keeney M., Yang F., Quarto N., Longaker M.T., and Wan D.C. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19, 989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.