Abstract
Introduction:
Diagnosing and treating diseases associated with amyloid fibers remain a great challenge despite of intensive research carried out. One important approach in the development of therapeutics is the use of herbal extracts which are rich in aromatic small molecules. Cinnamomum verum extract (CE) contains proanthocyanidin and cinnamaldehyde, which have been suggested to be capable of directly inhibiting amyloid fibril formation in vitro. This study is aimed at characterizing the inhibitory activity of CE against the fibrillation of hen egg white lysozyme (HEWL).
Methods:
Acidic pH and high temperatures were used to drive the protein towards amyloid formation. Lysozyme was dissolved at 2 mg/mL in 50mM glycine buffer (pH 2.5), and then incubated at 57 °C for the specified durations while stirred gently by Teflon magnetic bars. Various techniques including thioflavin T, fluorescence, Congo red absorbance assay and AFM micrography were used to characterize the HEWL fibrillation processes.
Results:
In the absence of CE typical amyloid fibrils (like amyloids formed in Alzheimer disease) became evident after 48 h of incubation. Upon incubation with various extract concentrations in the range of 0.1–1 mg/ml, formation of fibrillar assemblies were significantly inhibited (P<0.05). AFM analysis and MTT assay also confirmed the role of the extract in amyloid inhibition. Our studies showed that the presence of CE did not have any effect on protein stabilization and thus directly interact with amyloid structure and inhibit formation of these structures. Furthermore, a docking experiment showed that a pi-pi interaction may occur between the aromatic component of cinnamaldehyde and W62. Interestingly, W62 is one of the principal aromatic residues that interact with glycine amide, which is an aggregation suppressor of HEWL.
Discussion:
These observations suggest that aromatic small molecules of CE may directly insert into amyloidogenic core of early aggregates and inhibit amyloid fibril formation by disrupting the pi-pi interactions.
Keywords: Hen egg white lysozyme, Cinnamomum verum, Amyloid disaggregation, Docking, Cinnamaldehyde
1. Introduction
Amyloid aggregates are the hallmarks of many neurodegenerative disorders, including Alzheimer’s, Huntington’s and Parkinson’s diseases (Chiti & Dobson, 2006). These age-related brain disorders are increasing in parallel with the increase in life expectancy. It has been estimated that around 81.1 million people in world will suffer from AD (Alzheimer’s disease) by 2040 (Ferri et al., 2006). AD is the most common form of dementia which is associated with neurofibrillary tangles and neuritic plaques in postmortem brain tissues. These plaques contain extracellular aggregates of amyloid beta peptides while tangles are formed by the intraneuronal accumulation of insoluble and hyperphosphorylated tau protein (Haass & De Strooper, 1999; Nordberg, 2004).
Currently, most treatments used in AD are mostly symptomatic therapies, and do not address the underlying causes. Aβ aggregates progressive accumulation is believed to be essential in the development of neurodegenerative pathology. Deposition of these aggregates triggers a cascade of events including neurotoxicity, oxidative damage, and inflammation (Chromy et al., 2003; J. A. Hardy & Higgins, 1992). Some studies have highlighted soluble Aβ oligomers as the primary toxic species in AD (Walsh et al., 2002). Based on the above information, and in order to prevent or treat AD, different strategies have been envisaged to identify inhibitors of β-amyloid protein folding and aggregation. As examples, use of small peptides (as β-sheet blockers) (Gazit, 2002b; Soto et al., 1998), use of agents that reduce Aβ production (γ- and β – secretase inhibitors) (Ghosh, Kumaragurubaran, Hong, Koelsh, & Tang, 2008; Walsh et al., 2002), ways to increase Aβ clearance (amyloid vaccine) (Cao, Lin, Wahi, Jackson, & Potter, 2008; Youm et al., 2008), inhibition of Aβ aggregation (non-steroidal anti-inflammatory agents) (Blanchard et al., 2004; Walsh et al., 2002), use of anti-oxidants (Cheng, Feng, Zhang, & Zhang, 2006; De Felice et al., 2007) and inhibitors of tau protein phosphorylation (Dickey & Petrucelli, 2006) have been studied in this regard. Moreover, there are many reports on small organic compounds, including Congo red, furan (Estrada & Soto, 2007), indole (Pappolla et al., 2002; Ramshini & Ebrahim-Habibi, 2012), curcumin (Liu et al., 2012), and multiple synthetic and natural compounds (Levine III, 2007; Masuda et al., 2006), which have been tested on the fibril formation of different proteins. Before the development of modern medicine, people relied on a large spectrum of natural remedies for the treatment of chronic illnesses, including diseases affecting the central nervous system.
It has been suggested that some traditional herbs may possess anti-aging properties and become potential candidates for the treatment of aging –associated diseases(Ho, So, & Chang). Moreover, successful treatments for dementia have been developed from herbal drugs. Extracts from Ginkgo biloba (J-Gertz & Kiefer, 2004) and Galanthus woronowii (Heinrich & Lee Teoh, 2004), are examples of plants that have been used for treatment of mild to moderate AD. Michael Adams and his co-workers have reviewed plants that were traditionally used in age related brain disorders and have shown the existence of common traditional remedies in different societies to treat age-related cognitive disorders (Adams, Gmünder, & Hamburger, 2007). Cinnamomum verum (Cinnamon) is a herb that has been traditionally used by many ancient cultures. It has been indicated for a variety of ailments, including gastrointestinal problems, urinary infections, symptoms of colds and flu, and possesses remarkable anti-fungal and anti-bacterial properties (Peterson et al., 2009). Some studies have shown that Cinnamon was also used traditionally in age related brain disorders (Peterson et al., 2009). The polyphenolic molecules that are present in the aqueous extract of Cinnamomum, may be responsible for the majority of properties of the plant. In the present study, the effects of Cinnamomum verum extract (CE) were characterized on amyloid disaggregation of hen egg white lysozyme (HEWL) as a model protein, and its possible mechanism of effect was investigated in detail.
2. Methods
2.1. Materials
HEWL (EC 3.2.1.17), Congo red and ThT were purchased from Sigma (St Louis, MO, USA). ANS, all salts and organic solvents were obtained from Merck (Darmstadt, Germany).
2.2. C. Verum extracts preparation
The plant material was collected in March 2012 from Isfahan Botany herbarium. 30 grams of finely ground C. Verum was added to 300 ml of 50 °C nanopure water in Soxhlet extractor for 4–5 hours. The extract was filtered and the supernatant was concentrated with a rotary evaporator. The extract was then freeze-dried resulting in a powder extract and stored in the dark at room temperature. The resulting powder which was weighed and re-dissolved in water is referred to as cinnamon extract (CE) throughout this study. Aggregation of 2 mg/ml HEWL is initiated by the addition of specific quantity of the extract (1 mg/ml in final concentration) into reaction medium.
2.3. Amyloid preparation
Protein concentration was determined spectrophotometrically at 280 nm, using an extinction coefficient (ε280) of 2.65 L g-1 cm-1 (Sophianopoulos, Rhodes, Holcomb, & Van Holde, 1962). lysozyme was dissolved at 2 mg/mL in 50mM glycine buffer (pH 2.5), and then incubated at 57 °C for the specified durations while stirred gently by Teflon magnetic bars (Morshedi, Rezaei-Ghaleh, Ebrahim-Habibi, Ahmadian, & Nemat-Gorgani, 2007).
2.4. ANS fluorescence spectroscopy
ANS fluorescence experiments were performed on a Cary Eclipse VARIAN fluorescence spectrophotometer. ANS fluorescence studies were measured using a final ANS concentration of 250 μM, and the molar ratio of protein to ANS was 1:50. ANS fluorescence emission was scanned between 400 and 700 nm with an excitation wavelength of 380 nm.
2.5. ThT assay
All fluorescence experiments were carried out on a Cary Eclipse VARIAN fluorescence spectrophotometer (Mul-grave, Australia) at room temperature. Stock solution of ThT was prepared in phosphate buffer (10 mM sodium phosphate, 150 mM NaCl, pH 7) at a concentration of 2.5 mM, passed through a 0.45 μm filter paper and stored at 4°C. At different time intervals, an aliquot (10 μL) of incubated solution was mixed with 490 μl of ThT solution in a quartz cuvette with 1 cm path length. Fluorescence emission spectra were then taken using excitation at 440 nm. The excitation and emission slit widths were set as 5 nm and 10 nm, respectively (Nilsson, 2004).
2.6. CR assay
Samples were prepared as described above. At different time intervals, 60 μL aliquots of each sample were mixed with 440 μL of a solution containing 20 mM CR, 5 mM sodium phosphate buffer, and 150 mM NaCl at pH 7.4. The Congo red solution was filtered using a center-glass N4 filter. Optical absorption spectra were acquired from 400 to 700 nm, after a two to three minute equilibration at 25°C, using a 10 cm path-length cell and a Cecil 7200 UV-visible spectrophotometer (England).
2.7. Atomic force Microscopy (AFM).
In order to show HEWL amyloid fibrils morphologies, atomic force microscopy was used. For this purpose, an aliquot (10 μL) from the incubated solution was placed on freshly cleaved mica at room temperature. After a few minutes mica was slowly washed with 100 μL of deionized water, followed by drying with nitrogen gas. Each image was acquired in a tapping mode at a scan speed of 30 μm/s, loop filter of 3 Hz and force of 200 nN with a Dual Scope Probe Scanner (Solver next, model NTMDT, DME, Russia) with an area of 5×5 μm2. Conical shape silicon tips (mikromasch NSC16) with a resonance frequency of 150 kHz and a nominal constant of 40 N/m were used.
2.8. 3-(4, 5- dimethylthiazol)-2, 5- diphenyltetraazolium bromide (MTT) reduction test
MCF7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) F-12 Ham with 25 mM HEPES and NaHCO3 (1:1) supplemented with 10% fetal calf serum (Sigma-Aldrich), 1.0 mM glutamine and antibiotics (100 unit/mL penicillin and 100 μg/mL streptomycin). The cells were maintained in a 5.0% CO2 humidified atmosphere at 37 °C and grown until 80% confluence for a maximum of 20 passages. HEWL amyloid samples were prepared as described above. The amyloid samples were centrifuged and dried under N2 to remove CE, dissolved in DMEM at a final lysozyme concentration of 2 μ M. Aggregate toxicity was assessed in 96-well plates by the MTT assay. Briefly, the cells were seeded at 5000 cells per well in 96-well cell culture plates. After 24 h incubation, growth media was replaced with fresh growth medium containing lysozyme (2 μM). After the exposure to 2 μM HEWL aggregates for 48 h at 37 °C, the medium was removed and MTT solution (5mg/ml in PBS) was added to the cells. The cells were incubated at 37 °C for 4 hours, then the medium was aspirated, and the formazan product was solubilized with DMSO. The absorption was measured at 570 nm (620nm as a reference) in an ELISA reader. Cell viability was expressed as percent of MTT reduction in treated cells as compared to untreated cells (untreated with lysozyme aggregates).
2.9. Docking studies
Autodock vina was used to do the docking experiment (Trott & Olson, 2010). The 2VB1.pdb file was used as receptor. The protonation state of the protein was adjusted at pH=2.5 with the use of the residue pKa prediction module of MOE 2012.10 (Chemical Computing Group Inc., Montréal, Canada). Grid box of 40 × 40 × 58 points was used with a spacing 1.0 A°, and enclosed the whole molecule. The grid box center location was on x = −1.613, y = 14.597, and z = 24.37. The ligand (cinnamaldehyde) was prepared using MOE 2012.10. Gasteiger charges were assigned to protein and ligand molecules. Exhaustiveness was set on 20 and a computer with four processors was utilized for the computation. 100 poses were generated for the ligand; computing ligand interactions and preparation of the image representing the best pose were performed with MOE 2012.10.
2.10. Statistical analysis
All data are presented as Mean ±SD. The mean values were calculated based on the data from at least 3 independent experiments. The data were analyzed in SPSS 16 using descriptive statistics as well as independent t-test. The statistical significances were achieved when P<0.05.
3. Results
3.1. Characterization of lysozyme aggregation in acidic pH
Both wild-type human lysozyme and HEWL have been shown to undergo amyloid aggregation under specific in vitro conditions (Arnaudov & de Vries, 2005; Morozova-Roche et al., 2000). Since the structure and folding mechanisms of lysozyme have been well studied, the enzyme is considered to be a useful model in amyloid-related research. First, HEWL was driven toward amyloid formation by the use of acidic pH (pH=2.5) and high temperature (57 °C). Thioflavin T and Congo red were used as markers for detection of amyloid fibrillation (Nilsson, 2004). ThT fluorescence emission recorded for 48 hours showed a significant increase in ThT fluorescence emission (from 28.4±5.2 to 646.6±27.3 a.u.) and also the kinetics of amyloid formation was nucleation-dependent. Furthermore, the Congo red absorbance indicated an increase accompanied by gradual higher red shift during the incubation time. Both data are in accordance with previous reports on HEWL amyloid formation (Morshedi et al., 2007). Finally, atomic force microscopy (AFM) image of HEWL samples incubated in this condition confirmed the formation of long, unbranched fibrils, which are characteristic of amyloid structures.
3.2. Effect of C. verum extract (CE) on fibrillation of HEWL
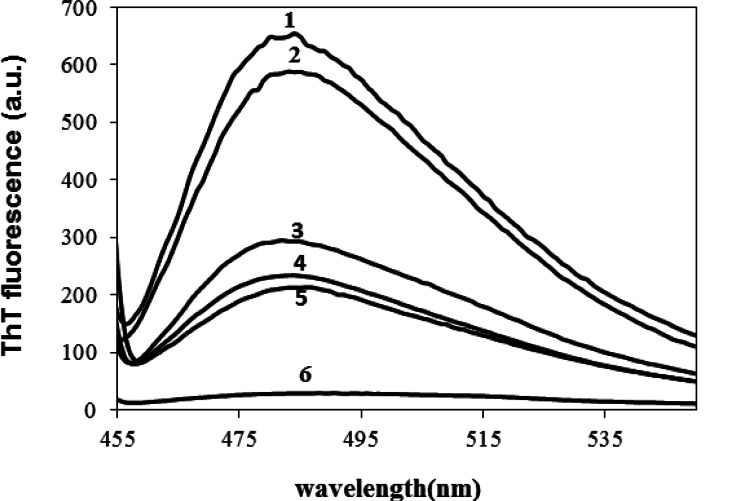
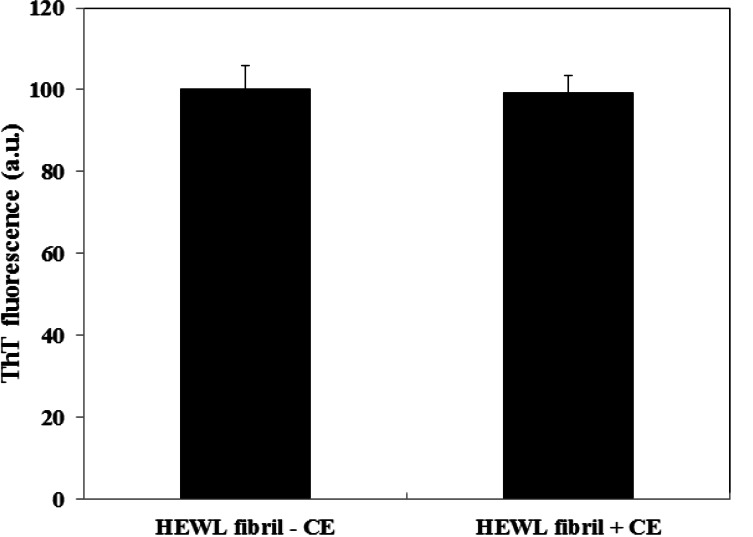
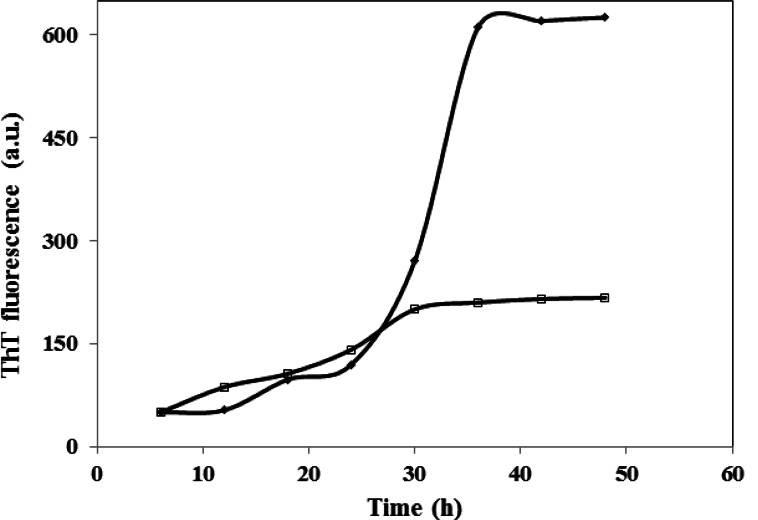
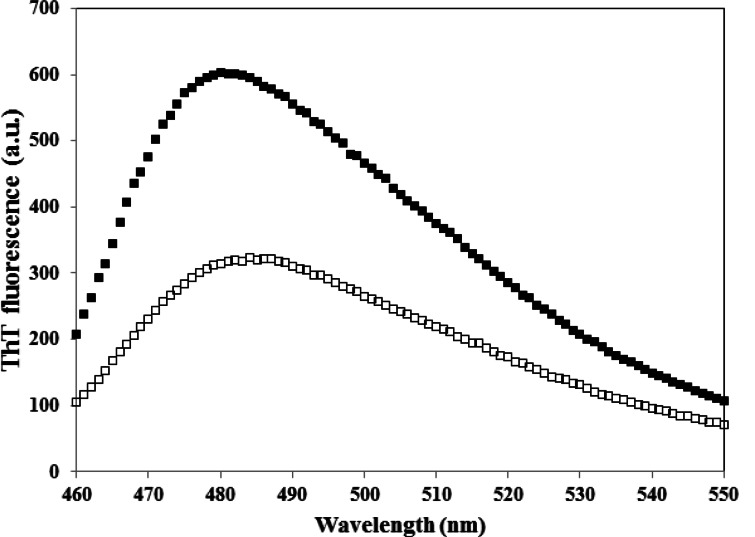
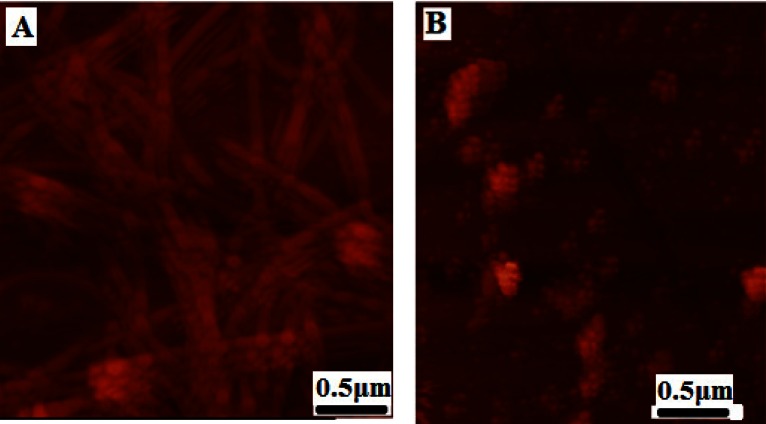
Various concentrations of CE (aqueous extract) ranging from 0.1–1 mg/ml were employed to set the best concentration. A dose dependent effect was observed (reaching a plateau at 0.5 mg/ml) as shown in Figure 1, and concentrations higher than 0.1 mg/ml, significantly inhibited the aggregation of HEWL. The one mg/ml concentration was used for the subsequent experiments. We also designed a control experiment to rule out the possibility that the low ThT intensity in the fluorescence assay might result from a quenching effect of the inhibitor. To this end, 1 mg/ml CE was added to HEWL amyloid sample and fluorescence was immediately measured. No significant quenching effect was observed (Fig. 2). The effect of 1 mg/ml CE on the kinetics of HEWL amyloid formation was also investigated through monitoring maximal ThT emission intensity over the course of 48 hours (Fig. 3). As mentioned before, the amyloid formation process obeys the characteristic nucleation-dependent pattern, with the three distinct phases of initial nucleation, elongation and equilibration clearly observed. To assess which phase was inhibited in presence of CE, the extract was added to HEWL sample after nucleation phase was completed and fibrillogenesis had already started (after 36 hours of incubation). The result showed that CE inhibited the polymerization and equilibrium phases (Fig. 4.). In order to determine the CE effect on the ultra-structural properties of the HEWL assemblies, AFM images in absence (Fig. 5A) or presence (Fig. 5B) of the extract were analyzed. While mature fibrils were observed in the absence of CE (Fig. 5A), samples containing lysozyme and 1mg/ml CE were devoid of fibril and contained only a very small amount of small oligomers (Fig. 5B).
Figure 1.
Effect of various concentrations of CE on HEWL amyloid fibrillation. This result was obtained at pH 2.5 and 57 °C by monitoring the change in ThT emission enhancement after 48 hours. CE was added at different concentrations at 0.1(2), 0.25(3), 0.5(4) and 1(5) mg/ml. Protein concentration was 2 mg/ml. ThT enhancement of HEWL without CE(1) and ThT alone (6) are also shown for comparison.
Figure 2.
Study of the quenching activity of CE. HEWL amyloid were acquired after 48 hours incubation at pH 2.5 and 57 °C and immediately monitored by ThT fluorescence assay before and after adding CE in final concentration of 1 mg/ml to the cell.
Figure 3.
Kinetics of HEWL fibrillation in the presence of 1 mg/ml CE. Kinetics was followed by monitoring maximal ThT emission intensity at various times of incubation at pH 2.5 and 57 °C (□). HEWL in the absence of CE is indicated by (▪). The protein concentration was 2 mg/ml.
Figure 4.
Effect of CE on HEWL amyloid fibrils when added after the nucleation step. CE (with 1 mg/ml final concentration) was added to HEWL samples incubated for 36 h, at pH 2.5 and 57 °C. ThT fluorescence changes were then monitored over 48 h incubation in the same condition (□). HEWL in the absence of CE is indicated by (▪). Protein concentration was 2 mg/ml.
Figure 5.
AFM images of HEWL aggregates deposited on mica following inhibition by CE. AFM samples were taken when ThT fluorescence indicated that control samples containing HEWL alone had reached the equilibration phase. Control samples showed long and mature fibrils (A). HEWL in presence of 1 mg/ml CE after 48 hour incubation at 57 °C revealed the presence of small oligomers and disappearance of fibrils (B).
3.3. Cell damage induced by lysozyme oligomers and protective effect of CE
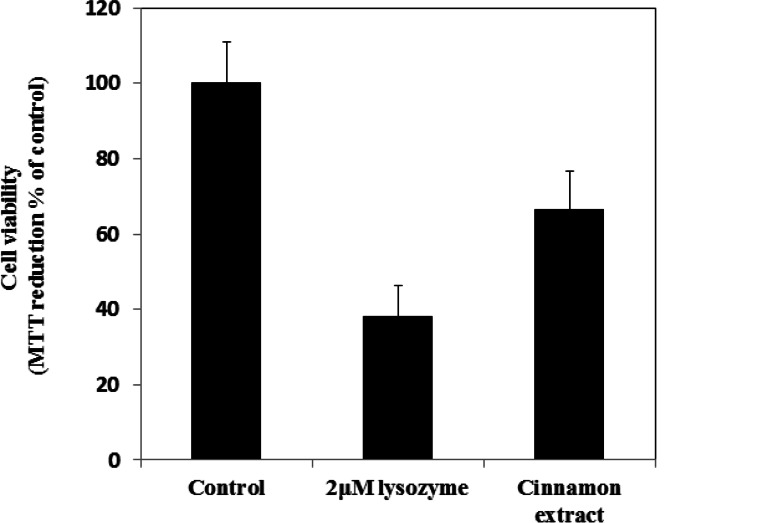
To further evaluate whether CE can inhibit oligomers toxicity, lysozyme oligomers which were prepared after 48 h incubation in glycine buffer pH 2.5, 57 °C with and without CE (1 mg/ml) were transferred to a physiological medium and the resulting suspensions were added to the cell culture media of MCF7 cells at a concentration of 2 μM (all concentrations refer to monomer concentration). The cell viability was evaluated by MTT assay (Mosmann, 1983). Untreated cells did not cause any detectable decrease in the ability of cells to reduce MTT (Fig. 6). By contrast, the cell viability of MCF7 cells were significantly decreased to 38±8.3 % after a 48 h exposure to HEWL aggregates. In presence of CE, the ability of cells to reduce MTT were increased to 66.5±10%, thus the extract could significantly prevent MCF7 (P< 0.05) from the cytotoxicity of lysozyme oligomers and improved cell viability.
Figure 6.
Cytotoxicity of HEWL aggregates. Cell viability was determined by the MTT reduction test in MCF7 cells exposed to 2 μM native HEWL aggregates incubated for 48 hours at 57.5 °C, pH 2.5 (bar 2). HEWL aggregates incubated for 48 hours at 57.5 °C, pH 2.5 and in presence of 1 mg/ml CE (bar 3). The reported values are representative of two independent experiments, each carried out four times. Untreated cells were assumed to be viable as a control (bar 1).
3.4. CE cannot stabilize native state of HEWL.
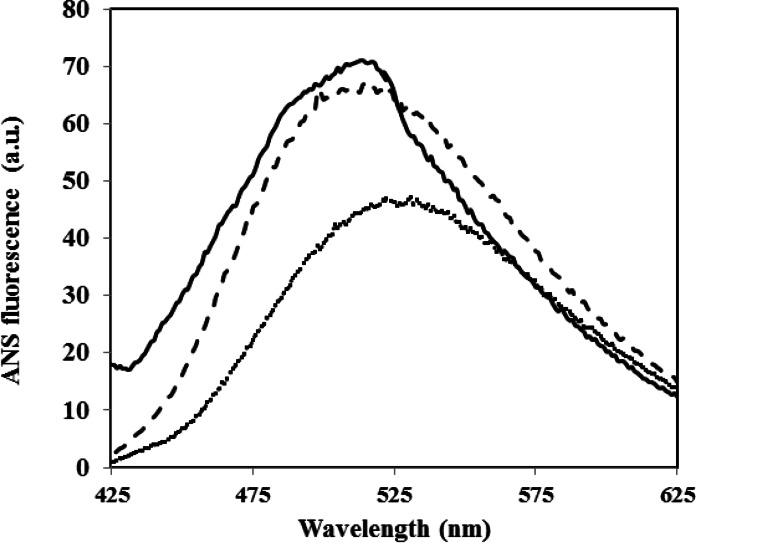
Small molecules can inhibit amyloid aggregation with various mechanisms including stabilization of native state, destabilization of nucleus and inhibition of elongation and lateral accumulation of fibrils; some small molecules may influence more than one of the steps (Li, Zhu, Rajamani, Uversky, & Fink, 2004; Morshedi et al., 2007). It is suggested that induction of aggregation generally requires the presence of partially unfolded state (Dobson, 1999). Indeed, low pH induces formation of molten globule-like structures in HEWL, in which exposure of relatively mobile hydrophobic patches occurs on the protein surface (Cardamone & Puri, 1992). Here too, fluorescence measurements involving 8-anilinonaphthalenesulfonate (ANS) a hydrophobic reporter probe, revealed exposure of large hydrophobic patches on the surface of the protein molecule in the amyloid aggregation-prone state (Fig. 7). As shown in Figure 7, the addition of CE into incubation medium did not prevent exposure of hydrophobic patches; therefore, it seems that the extract couldn’t inhibit HEWL amyloid fibril formation by protecting the native protein from structural changes.
Figure 7.
Effect of CE on the surface hydrophobicity of HEWL. Changes in ANS emission spectra obtained in the presence of HEWL when incubated at pH 2.5 and 57 °C with 1 mg/ml with (—) and without CE (---). Background ANS fluorescence recorded in the absence of the protein is also presented. For more details please see materials and methods.
3.5. Docking studies
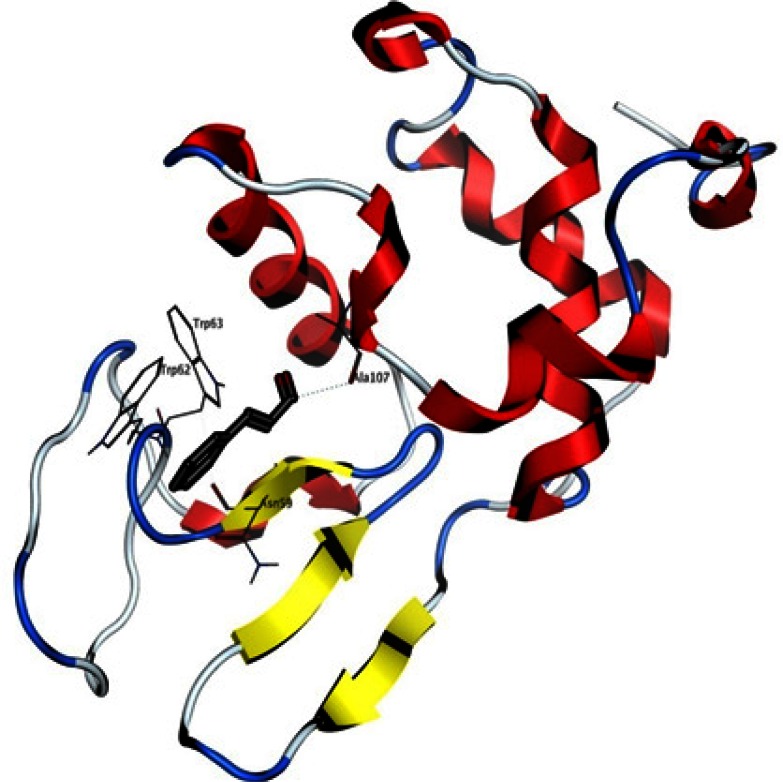
Most of cinnamon biological effects are attributed to its cinnamaldehyde component. A docking experiment was done as a theoretical attempt to further explain the positive anti-amyloid effect of cinnamon extract with regard to cinnamaldehyde. The best pose of the ligand with a scoring of − 7.3 kcal/mol is shown in the HEWL structure in Figure 8. This interaction site is relatively far from the catalytic residues, but still located in the binding site of lysozyme substrates. A putative hydrogen bond was detected by the software between A107 backbone and the carbonyl group of the ligand, as well as a pi-pi interaction between the aromatic component of the ligand and W62.
Figure 8.
Interaction site of cinnamaldehyde with HEWL, as detected by docking. The ligand is represented with sticks, HEWL secondary structure is shown with ribbons and some HEWL residues located in the 3.5 A° vicinity of the ligand are visible and labelled. A putative hydrogen bond may be formed between A107 and the ligand (shown with dots).
4. Discussion
Since Aβ aggregation and accumulation to amyloid plaques is considered to be the initiator of events leading to neurotoxicity and the clinical symptoms of Alzheimer’s disease, thus the search for anti-amyloid therapies has become an important strategy in AD-related research, (Chromy et al., 2003; J. Hardy & Selkoe, 2002; J. A. Hardy & Higgins, 1992). Reducing the production of the amyloidogenic form of proteins, increasing the clearance rate of misfolded or aggregated proteins, or stabilizing the native state of amyloidogenic proteins, as well as the direct inhibition of the self-assembly process (Cohen & Kelly, 2003) have been so far proposed as potential therapeutic approaches. Emphasis is currently put onto controlling the prefibrillar conformers (soluble oligomers) of amyloidogenic proteins, since it has been shown that these are usually the most toxic aggregates both in vitro and in vivo (Campioni et al.,; Chiti & Dobson, 2006; Glabe, 2008). Instead of targeting all possible intermolecular contacts, a better way to alter the nucleation pathway and prevent formation of fibrils could be the use of small molecules such as sugars, polyols, amino acids, amines, salts, polymers and surfactants which disrupt specific intermolecular contacts. Nowadays, increasing interest is observed toward herbal medicine, since they are often positively perceived as more natural treatments compared to synthetic drugs. Specifically, successful treatments for dementia have been developed from herbal drugs (Adams et al., 2007), including CE that has been reported to inhibit the tau aggregation process (Peterson et al., 2009). Analysis of the aqueous extract of Cinnamon has showed that it contains proanthocyanidin trimer species and cinnamaldehyde and its anti-aggregation activity was attributed to these compounds (Peterson et al., 2009). Herein we have confirmed that an aqueous extract of cinnamon can inhibit the aggregation of HEWL in vitro. In the present study, we first prepared a HEWL amyloid aggregation by using acidic pH and high temperature and formation of amyloid fibrils was verified by various techniques, including specific dyes (ThT and Congo red). AFM images of HEWL samples confirmed the formation of HEWL long, unbranched fibrils. Then we showed the anti-aggregation activity of the extract on HEWL by ThT fluorimetric assay (Figure 1). After examining the inhibitory effect of the extract on the HEWL fibrillogenesis at the end point, we studied its effect on the kinetics of fibrillogenesis. The results showed that the compound could significantly inhibit both polymerization and equilibrium phase and the slope of the kinetic curve changed too (Fig. 2). The suppression of ThT intensity was not due to intensity quenching of CE (Fig. 3). On the other hand, our study showed that the extract could also destabilize mature fibrils if added to the medium after the nucleation step (Fig. 4). We performed an AFM assay to qualitatively validate the inhibitory potency of CE on HEWL amyloidogenesis. Figures 5 A and B clearly demonstrated that CE could inhibit formation of mature fibrils. AFM images supported the suggestion that CE blocks lysozyme fibrillation in the early stages of elongation, and that formation of mature fibrils from oligomers was inhibited. It is widely believed that brain amyloidosis begins with the production of a soluble native protein that is misfolded to yield the precursor for fibril (Chiti, Taddei, Stefani, Dobson, & Ramponi, 2001; Dobson, 1999) formations. Our studies showed that the presence of CE did not prevent exposure of hydrophobic patches in misfolded monomers but instead accentuated it (Fig. 6). As demonstrated by Figures 2, 4 and 6, nucleation, elongation and equilibrium phases seem to be affected by the presence of CE.
In order to evaluate whether CE, which inhibited HEWL amyloid production, could prevent the lysozyme-induced cell death, the samples prepared in amyloidogenic condition (2 mg lysozyme, pH 2.5 after 24 hour) in presence or absence of 1 mg/ml CE were assessed by performing the MTT reduction test. Prefibrillar samples without pretreatment with CE were found to cause a significant decrease in MTT reduction but aggregates pre-incubated with CE were less toxic (Fig. 7). Taking these finding together, it seems that CE is not capable to inhibit HEWL amyloid formation by protecting the native protein from structural change (Fig. 6) but the extract exert its inhibitory effect on other phases of the fibrillation process. Several studies have suggested that interactions between aromatic units have an important role in molecular recognition and self – assembly (Bemporad, Taddei, Stefani, & Chiti, 2006; Gazit, 2002a; Reches & Gazit, 2006), especially with regard to the attractive non-bonded interactions between planar aromatic ring (π- π interactions or π-stacking). In the formation of supramolecular structures, one important factor is the steric constrains resulting from π-stacking (Hunter, Lawson, Perkins, & Urch, 2001). Based on this potential role of π-stacking interactions, which are also present in amyloid structures, aromatic small molecules that may be capable of blocking π-stacking could be potential candidates to control amyloid diseases (Hunter et al., 2001). Cinnamaldehyde and proanthocyanidin are aromatic small molecules that are contained in CE, with cinnamaldehyde as the major compound of the effective reagents. The theoretical docking experiment that was done showed a putative π- π interaction between the aromatic component of the ligand and W62, in addition to the putative hydrogen bond with A107 backbone. Interestingly, W62 is one of the principal aromatic residues that interact with glycine amide, which is an aggregation suppressor of HEWL (Ito, Shiraki, Makino, Hasegawa, & Kumasaka, 2011). In a study which involved indole derivatives as anti-amyloid compounds, potential interaction of indole-3-carbinol was detected (by a docking method) with W63 and W108 (Morshedi et al., 2007), in a location close to what we have found here. In conclusion, the results presented demonstrated that CE acts on HEWL fibril formation by disrupting initial intermediate structures that are formed in the beginning of the process and may achieve this effect, at least partly, via disruption of vital π- π interaction.
Acknowledgements
This work was supported by a grant from the research council of the University of Payam Noor.
Abbreviations:
- CE:
Cinnamomum verum extract,
- HEWL:
hen egg white lysozyme,
- ThT:
thioflavin T,
- CR:
Congo red,
- AFM:
atomic force microscopy,
- MTT:
3-(4, 5-dimethylthiazol)-2, 5-diphenyltetraazolium bromide,
- AD:
Alzheimer’s disease,
- ANS:
8-anilinonaphthalene-sulfonate,
- DMEM:
Dulbecco’s Modified Eagle’s Medium
References
- Adams M., Gmünder F., Hamburger M. (2007). Plants traditionally used in age related brain disorders—a survey of ethnobotanical literature. Journal of Ethnopharmacology, 113 (3), 363– 381. [DOI] [PubMed] [Google Scholar]
- Arnaudov L. N., de Vries R. (2005). Thermally induced fibrillar aggregation of hen egg white lysozyme. Biophysical Journal, 88 (1), 515– 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemporad F., Taddei N., Stefani M., Chiti F. (2006). Assessing the role of aromatic residues in the amyloid aggregation of human muscle acylphosphatase. Protein Science, 15 (4), 862– 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard B. J., Chen A., Rozeboom L. M., Stafford K. A., Weigele P., Ingram V. M. (2004). Efficient reversal of Alzheimer’s disease fibril formation and elimination of neurotoxicity by a small molecule. Proceedings of the National Academy of Sciences of the United States of America, 101 (40), 14326– 14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni S., Mannini B., Zampagni M., Pensalfini A., Parrini C., Evangelisti E., et al. A causative link between the structure of aberrant protein oligomers and their toxicity. Nature Chemical Biology, 6(2), 140–147. [DOI] [PubMed] [Google Scholar]
- Cao C., Lin X., Wahi M. M., Jackson E. A., Potter H. (2008). Successful adjuvant-free vaccination of BALB/c mice with mutated amyloid β peptides. BMC Neuroscience, 9 (1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardamone M., Puri N. K. (1992). Spectrofluorimetric assessment of the surface hydrophobicity of proteins. Biochemical Journal, 282 (Pt 2), 589– 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Feng Z., Zhang Q.-Z. z., Zhang J.-T. t. (2006). Beneficial effects of melatonin in experimental models of Alzheimer disease1. Acta Pharmacologica Sinica, 27 (2), 129– 139. [DOI] [PubMed] [Google Scholar]
- Chiti F., Dobson C. M. (2006). Protein misfolding, functional amyloid, and human disease. Annual Review of Biochemistry, 75, 333– 366. [DOI] [PubMed] [Google Scholar]
- Chiti F., Taddei N., Stefani M., Dobson C. M., Ramponi G. (2001). Reduction of the amyloidogenicity of a protein by specific binding of ligands to the native conformation. Protein Science, 10 (4), 879– 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromy B. A., Nowak R. J., Lambert M. P., Viola K. L., Chang L., Velasco P. T., et al. (2003). Self-assembly of Aβ1-42 into globular neurotoxins. Biochemistry, 42 (44), 12749– 12760. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Kelly J. W. (2003). Therapeutic approaches to protein-misfolding diseases. Nature, 426 (6968), 905– 909. [DOI] [PubMed] [Google Scholar]
- De Felice F. G., Velasco P. T., Lambert M. P., Viola K., Fernandez S. J., Ferreira S. T., et al. (2007). Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. Journal of Biological Chemistry, 282 (15), 11590– 11601. [DOI] [PubMed] [Google Scholar]
- Dickey C. A., Petrucelli L. (2006). Current strategies for the treatment of Alzheimer’s disease and other tauopathies. Expert Opinion on Therapeutic Targets, 10 (5), 665– 76. [DOI] [PubMed] [Google Scholar]
- Dobson C. M. (1999). Protein misfolding, evolution and disease. Trends in Biochemical Sciences, 24 (9), 329– 332. [DOI] [PubMed] [Google Scholar]
- Estrada L. D., Soto C. (2007). Disrupting-amyloid aggregation for alzheimer disease treatment. Current Topics in Medicinal Chemistry, 7 (1), 115– 126. [DOI] [PubMed] [Google Scholar]
- Ferri C. P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M., et al. (2006). Global prevalence of dementia: a Delphi consensus study. The Lancet, 366 (9503), 2112– 2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit E. (2002a). Global analysis of tandem aromatic octapeptide repeats: The significance of the aromatic-glycine motif. Bioinformatics, 18 (6), 880– 883. [DOI] [PubMed] [Google Scholar]
- Gazit E. (2002b). A possible role for π--stacking in the self-assembly of amyloid fibrils. The FASEB Journal, 16 (1), 77– 83. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Kumaragurubaran N., Hong L., Koelsh G., Tang J. (2008). Memapsin 2 (beta-secretase) inhibitors: drug development. Current Alzheimer Research, 5 (2), 121– 131. [DOI] [PubMed] [Google Scholar]
- Glabe C. G. (2008). Structural classification of toxic amyloid oligomers. Journal of Biological Chemistry, 283 (44), 29639– 29643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., De Strooper B. (1999). The presenilins in Alzheimer’s disease--proteolysis holds the key. Science, 286 (5441), 916– 919. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science, 297 (5580), 353– 356. [DOI] [PubMed] [Google Scholar]
- Hardy J. A., Higgins G. A. (1992). Alzheimer’s disease: the amyloid cascade hypothesis. Science, 256 (5054), 184– 5. [DOI] [PubMed] [Google Scholar]
- Heinrich M., Lee Teoh H. (2004). Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. Journal of Ethnopharmacology, 92 (2), 147– 162. [DOI] [PubMed] [Google Scholar]
- Ho Y.-S., So K.-F., Chang R. C.-C. Anti-aging herbal medicine—How and why can they be used in aging-associated neurodegenerative diseases? Ageing Research Reviews, 9(3), 354–362. [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Lawson K. R., Perkins J., Urch C. J. (2001). Aromatic interactions. Journal of the Chemical Society, Perkin Transactions, 2 (5), 651– 669. [Google Scholar]
- Ito L., Shiraki K., Makino M., Hasegawa K., Kumasaka T. (2011). Glycine amide shielding on the aromatic surfaces of lysozyme: Implication for suppression of protein aggregation. FEBS Letters, 585 (3), 555– 560. [DOI] [PubMed] [Google Scholar]
- J-Gertz H., Kiefer M. (2004). Review about Ginkgo biloba special extract EGb 761 (Ginkgo). Current Pharmaceutical Design, 10 (3), 261– 264. [DOI] [PubMed] [Google Scholar]
- Levine H., III (2007). Small molecule inhibitors of A beta assembly. Amyloid, 14 (3), 185– 197. [DOI] [PubMed] [Google Scholar]
- Li J., Zhu M., Rajamani S., Uversky V. N., Fink A. L. (2004). Rifampicin inhibits α-synuclein fibrillation and disaggregates fibrils. Chemistry & Biology, 11 (11), 1513– 1521. [DOI] [PubMed] [Google Scholar]
- Liu K.-N., Lai C.-M., Lee Y.-T., Wang S.-N., Chen R. P. Y., Jan J.-S., et al. (2012). Curcumin’s pre-incubation temperature affects its inhibitory potency toward amyloid fibrillation and fibril-induced cytotoxicity of lysozyme. Biochimica et Biophysica Acta (BBA)-General Subjects. 1820(11), 1774–86. [DOI] [PubMed] [Google Scholar]
- Masuda M., Suzuki N., Taniguchi S., Oikawa T., Nonaka T., Iwatsubo T., et al. (2006). Small molecule inhibitors of α-synuclein filament assembly. Biochemistry, 45 (19), 6085– 6094. [DOI] [PubMed] [Google Scholar]
- Morozova-Roche L. A., Zurdo J., Spencer A., Noppe W., Receveur V., Archer D. B., et al. (2000). Amyloid fibril formation and seeding by wild-type human lysozyme and its disease-related mutational variants. Journal of Structural Biology, 130 (2), 339– 351. [DOI] [PubMed] [Google Scholar]
- Morshedi D., Rezaei-Ghaleh N., Ebrahim-Habibi A., Ahmadian S., Nemat-Gorgani M. (2007). Inhibition of amyloid fibrillation of lysozyme by indole derivatives- possible mechanism of action. Febs Journal, 274 (24), 6415–6425. [DOI] [PubMed] [Google Scholar]
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65 (1), 55– 63. [DOI] [PubMed] [Google Scholar]
- Nilsson M. R. (2004). Techniques to study amyloid fibril formation in vitro. Methods, 34 (1), 151– 160. [DOI] [PubMed] [Google Scholar]
- Nordberg A. (2004). PET imaging of amyloid in Alzheimer’s disease. The Lancet Neurology, 3 (9), 519– 527. [DOI] [PubMed] [Google Scholar]
- Pappolla M. A., Simovich M. J., Bryant Thomas T., Chyan Y. J., Poeggeler B., Dubocovich M., et al. (2002). The neuroprotective activities of melatonin against the Alzheimer β protein are not mediated by melatonin membrane receptors. Journal of Pineal Research, 32 (3), 135– 142. [DOI] [PubMed] [Google Scholar]
- Peterson D. W., George R. C., Scaramozzino F., La Pointe N. E., Anderson R. A., Graves D. J., et al. (2009). Cinnamon extract inhibits tau aggregation associated with Alzheimer’s disease in vitro. Journal of Alzheimer’s Disease, 17 (3), 585– 597. [DOI] [PubMed] [Google Scholar]
- Ramshini H., Ebrahim-Habibi A. (2012). Thermal disaggregation of type B yeast hexokinase by indole derivatives: A mechanistic study. International Journal of Biological Macromolecules, 50 (5), 1260– 1266. [DOI] [PubMed] [Google Scholar]
- Reches M., Gazit E. (2006). Designed aromatic homo-dipeptides: formation of ordered nanostructures and potential nanotechnological applications. Physical Biology, 3(1), S10. [DOI] [PubMed] [Google Scholar]
- Sophianopoulos A. J., Rhodes C. K., Holcomb D. N., Van Holde K. E. (1962). Physical studies of lysozyme I. Characterization. Journal of Biological Chemistry, 237 (4), 1107– 1112. [PubMed] [Google Scholar]
- Soto C., Sigurdsson E. M., Morelli L., Kumar R. A., Castaño E. M., Frangione B. (1998). βsheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer’s therapy. Nature Medicine, 4 (7), 822– 826. [DOI] [PubMed] [Google Scholar]
- Trott O., Olson A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31 (2), 455– 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., et al. (2002). Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. Nature, 416 (6880), 535– 539. [DOI] [PubMed] [Google Scholar]
- Youm J. W., Jeon J. H., Kim H., Kim Y. H., Ko K., Joung H., et al. (2008). Transgenic tomatoes expressing human beta-amyloid for use as a vaccine against Alzheimer’s disease. Biotechnology Letters, 30 (10), 1839– 1845. [DOI] [PMC free article] [PubMed] [Google Scholar]