Abstract
Nicotinic acetylcholine receptors (nAChRs) play a crucial role in a number of clinically relevant mental and neurological pathways, as well as autonomic and immune functions. The development of subtype-selective ligands for nAChRs therefore is potentially useful for targeted therapeutic management of conditions where nAChRs are involved. We tested if selectivity for a particular nAChR subtype can be achieved through small structural modifications of a lead compound containing the nicotinic pharmacophore by changing the distance between the electronegative elements. For this purpose, analogs of A-84543 were designed, synthesized and characterized as potentially new nAChR subtype-selective ligands. Compounds were tested for their binding properties in rat cerebral cortical tissue homogenates, and subtype-selectivity was determined using stably transfected HEK cells expressing different nAChR subtypes. All compounds synthesized were found to competitively displace [3H]-epibatidine ([3H]EB) from the nAChR binding site. Of all the analogues, H-11MNH showed highest affinity for nAChRs compared to a ~ 5 to10-fold lower affinity of A-84543. All other compounds had affinities > 10,000 nM. Both A-84543 and H-11MNH have highest affinity for α2β2 and α4β2 nAChRs and show moderate affinity for β4- and α7-containing receptors. H-11MNH was found to be a full agonist with high potency at α3β4, while A-84543 is a partial agonist with low potency. Based on their unique pharmacological binding properties we suggest that A-84543 and its desmethylpyrrolidine analog can be useful as pharmacological ligands for studying nAChRs if selective pharmacological and/or genetic tools are used to mask the function of other receptors subtypes.
Keywords: A-85380, cerebral cortex, epibatidine, nicotine, saturation, H-11MNH
Introduction
Over the past two decades a number of CNS disorders (Alzheimer’s, Parkinson’s, Attention deficit hyperactivity disorder (ADHD), schizophrenia, addiction, among others) [1–4] and some ANS dysfunctions (such as autonomic neuropathies and autoimmune autonomic ganglionopathy), as well as neuropathic pain and inflammatory conditions [5–8], have been found to have either a direct or indirect involvement of the neurotransmitter acetylcholine (ACh) and its nicotinic acetylcholine receptors (nAChRs). The nAChRs comprise a large group of ligand-gated ion channels with a hetero- or homo-pentamer configuration that have diverse subunit stoichiometries [9]. Electrophysiological data on in vitro systems expressing individual receptor subtypes, however, has clearly demonstrated that each nAChR subtype is physiologically and pharmacologically distinct in their response to stimulation by ACh, as well as by a variety of natural and synthetic compounds [2,3,10,11,12]. The diversity of the nicotinic acetylcholine receptors (nAChRs) in brain, ganglia and a large group of non-neuronal tissues [1,2,13], presents important opportunities for the development of nAChR subtype-selective compounds to manage many conditions. This realization has prompted a number of studies to find selective drugs for each receptor subtype. Unfortunately, the nAChR subunits share a high (~90%) degree of structural homology in their amino acid sequences [14,15] including a conserved orthosteric binding site [16], the ACh binding site. This close homology has hindered detailed characterization of their individual functional roles and has made it difficult to design nAChR subtype-specific ligands for use as therapeutic agents. One positive outcome of these studies has been the development of ligands for the β2-containing nAChRs, the most abundant in the CNS [17], has been the least challenging and therefore has received the most attention, partly because, with very few exceptions, old and new nicotinic ligands show highest affinity [18–23] and/or higher selectivity for these receptors [2,22–28]. It should be emphasized here, however, that selectivity of all nicotinic drugs so far studied is at best relative, and highly dependent on concentration [24,25,28]. Even at concentrations just slightly higher than their KD or Ki, these drugs become quite promiscuous and may bind all, or almost all, neuronal nAChRs. Nonetheless, one may still find these less than perfectly selective compounds useful in a variety of ways. One can, for example, look at their selectivity ratios, that is, the ratio between their affinity for one receptor subtype vs another (i.e. affinity for α4β2 /affinity of α3β4) [24,25] or their pharmacological footprint (affinity profile, potency profile and efficacy profile) [28]. Understanding their limitations and relative selectivity is important to ascertain their functionality, better design experiments, and interpret the data.
One approach to understanding nAChRs subtypes has been to develop new compounds through the rational modification of well-known active nAChR lead compounds. According to a pre-existing pharmacophore model for nicotine, initially proposed by Beers and Reich (1970) [29] and later modified by others [30], compounds will fit the nAChRs orthosteric site if they meet certain criteria. Thus, minor structural modifications on nicotine has yielded a number of useful ligands including A-85380 [23], A-84543 [22], sazetidine-A [34] and many of their analogues [22,23,31–33], which, although not fully selective, have diverse affinities and high affinity ratios for different nAChR subtypes [22–28,31–33, for review see 2,9], unfortunately, most have not been as successful in the clinic [2,4].
Another lead compound, epibatidine, an alkaloid like nicotine, and its iodinated-analog ([125I]-IPH) exhibit some of the highest affinities (pM–nM) at most nAChR subtypes [19,20,34,35,36,37], however, they lack nAChR selectivity [19,20,36]. Analogues of epibatidine were also synthesized and some were found to have improved binding and functional selectivity for nAChR subtypes when the position of the nitrogen in the pyridine ring was moved to the ortho position [21], but lost efficacy.
Although most drugs will distinguish somewhat between β2-containing vs β4-containing receptors and some have a high selectivity ratio between them, it is crucially important to be able to also differetiate between β2-containing and β4-containing receptor subtypes themselves, since they have very important and distinct functions. For example, with regards to the β2-containing nAChRs, α4β2 nAChRs are primarily cortical and are involved in learning, memory, and attention [1–3], among other functions. In contrast, α6β2*1 nAChRs are expressed at much lower and discrete levels in dopamine terminals and are involved primarily, but not exclusively, in dopamine release [38,39], and thus may be involved in and can affect addiction [40], ADHD, Parkinson’s disease, and Schizophrenia [1–3]. nAChRs containing α3 and β2 subunits, on the other hand, are found prominently in sympathetic nerve terminals [41] and can strongly affect vasodilation of cerebral arteries [41,42]. Therefore, development of ligands that can discriminate between these three subset of receptors would be relevant for the management of subtype-specific nAChR-linked disorder, although other non-β2-containing nAChRs may also be involved.
Using this rationale, A-84543, a 3-pyridyl ether analog of nicotine and with a high affinity for α4β2 receptors [22, 32], was modified by repositioning of the pyridine nitrogen into the ortho and para positions and by desmethylation of the pyrrolidine nitrogen. A-84543, was originally synthesized by Abreo and colleagues from the modification of the linker between the rings in nicotine [22]. This compound was choosen as lead because it fullfills the requirements of the pharmacophore model (see figure 1). Variation in the position of the nitrogen in the pyridine ring of A-84543, we reason, would alter the distance between the electronegative elements (N-N distance), and should yield a series of analogs that can be further characterized for their selectivity ratio and eventually their pharmacological footprint. From these analogs we may also be able to identify potential lead compounds suitable for development into drug candidates for the treatment of a neurological, mental, or autonomic disease involving nAChRs. It should be clear, that such compounds could be full agonists, partial agonists or antagonists, depending on the condition. One also needs to keep in mind that highly efficacious agonists may have more severe adverse effects, and therefore low affinity or low efficacy compounds may have some advantages.
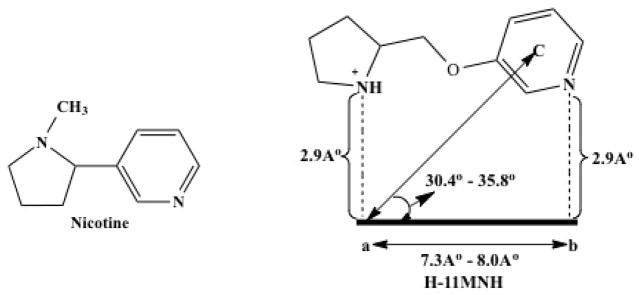
Fig. 1. A-84543 meets the criteria of a nicotinic receptor pharmacophore.
The typical nicotinic structure at left, was modified as seen at right in compound H-11MNH. This new molecule fits the pharmacophore model. The pharmacophore usually consists of a protonated nitrogen and a hydrogen bond donor. The optimum distance between the protonated nitrogen, which acts as a hydrogen bond acceptor and the hydrogen bond donor, is estimated to be 5.5 Å based on measurements obtained from one of the highest affinity nicotinic compounds, epibatidine. a–b, is the distance between the electron acceptor and electron donor; C, center of the electronegative element.
Materials
Unless otherwise noted, all chemicals were purchased from Sigma Aldrich (St. Louis, Mo). [3H]EB was purchased from Perkin Elmer Life and Analytical Sciences (Boston, MA). Adult male rat whole brains were purchased from Pel-Freez Biologicals (Rogers, Arkansas).
Methods
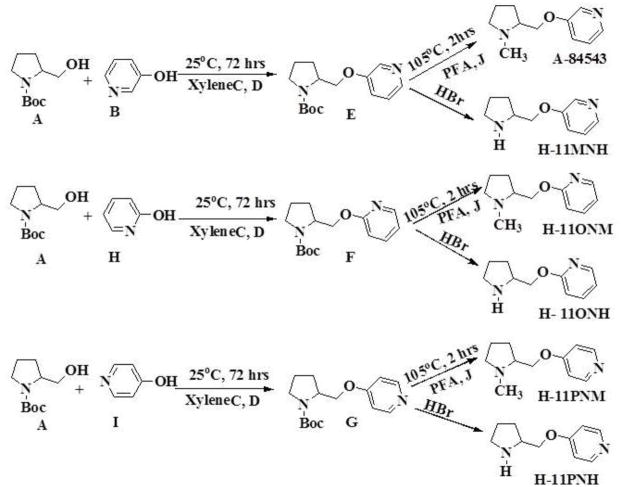
For these studies we designed, synthesized and characterized a set of new nAChR ligands based on A-84543 where we changed the nitrogen in the pyridine ring from the meta position to the ortho and para positions, and further made more subtle structural changes by substituting the methyl group at the pyrrolidine nitrogen to a hydrogen atom. A-84543 and its analogues H-11MNH, H-11ONH, H-11ONM, and H-11PNH (figure 1) were prepared by reacting N-Boc-L-prolinol with the appropriate hydroxy pyridines (B, H, & I) using the method of Mitsunobu reaction as published by O. Mitsunobu [43] and modified by Brown [44]. M-xylene was used as the solvent and the reaction was conducted at room temperature. The progress of the Mitsunobu reaction and other synthetic steps were monitored by thin-layer chromatography (TLC) using precoated Merck silica gel Kiesegel 60 F254 plates and spots were detected under UV light at 254 nm. After 72 hours, excess 100% ethylacetate was added and evaporated under reduced pressure. The resultant crude oil was dissolved on methanol and evaporated before purification via flash chromatography (3% NH4OH; 5% methanol; and 92% ethylacetate) to yield the appropriate intermediate products E, F and G. Flash chromatography was conducted using silica gel 230–400 mesh. Separately, the intermediate products were dissolved in formic acid, mixed and 1.0 mmol of paraformaldehyde was added to each preparation. The mixture was stirred at 100°C for 3hrs. When the intermediate products were completely consumed, as evidence by the disappearance of starting material on the TLC plate, the mixture was evaporated under reduced pressure and the resulting crude oily residue was stirred with 3N HCl solution for 30 min. The aqueous solution was extracted with ethyl acetate (3×50ml) to remove organic impurities and the remaining aqueous layer was basified with 6N NaOH solution and then extracted several times with 100% ethyl acetate. The ethyl acetate layer was concentrated to dryness to obtain A-84543 from E; and H-11ONM from F (see figure 2).
Fig. 2. Schematic representation of the synthesis of A-84543 and its analogs.
Synthesis of compounds H-11MNH, H-11ONH, H-11ONM and H-11PNH was initiated by combining N-Boc-L-prolinol with appropriate hydroxypyridines using the Mitsunobu reaction. The N-Boc protected purified products were then deprotected to give the hydrogen or methyl analogue. A = N-Boc-L-prolinol; B, H, I = 3, 2, and 4-hydroxy pyridines respectively.
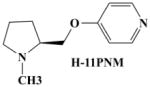
To obtain H-11MNH, H-11ONH and H-11PNH from intermediates E, F, and G respectively, E, F, and G were separately dissolved in ethyl acetate and drops of a solution of HBr in acetic acid were added to each solution until formation of a white precipitate (see figure 1). The mixtures were allowed to settle and the clear supernatant liquid was gently decanted to leave a whitish gel. To remove excess HBr, excess quantities of 100% ethyl acetate were added, warmed, allowed to settle and the supernatant liquid was decanted. This step was repeated until a white insoluble solid was formed in the ethyl acetate. The solid was dried and stored in a desiccator. All compounds were characterized by Elemental analysis (C, H, and N ± 0.4%). 1H, 13C and HETCOR NMR spectroscopy were recorded on a Bruker 400 MHz NMR instrument. The chemical shifts (δ) were measured downfield in ppm relative to an internal standard of tetramethylsilane (TMS). Although H-11PNM was designed, it was not possible to synthesize it for this study.
Design and determination of the internitrogen distance of the analogs
Molecular modeling and comparative molecular field evaluation of the lead compound, A-84543 and five analogs were done using the SYBYL system (version 7.2) running on a Silicon Graphics Tezro (Department of Biochemistry and Molecular Biology, Howard University Washington, DC) to obtain the energy-minimized, conformational structure of the compounds. Energy minimization was performed on the compounds and on the bonds linking the two rings (pyridine and pyrrolidine rings). Because repositioning of the nitrogen in the pyridine ring and/or substitution on the nitrogen of the pyrrolidine ring would alter the distribution of electrons on the molecule, the Gasteiger-Huckel charge was estimated for each compound and the results are presented in Table I.
Table I. Measurement of the N-N bond distance for A-84543 and its Analogs.
These data were computed using the SYBYL system (version 7.2) running on Silicon Graphics Tezro. The Gasteiger-Huckel charge (shown for the 2 nitrogens in each of the compounds) is a measure of the partial charge on a given atom.
| Structure | N-N Bond distance | Gasteiger-Huckel Charge (eV) | |
|---|---|---|---|
| Pyridine Nitrogen | Pyrrolidine Nitrogen | ||
|
7.225 Å | −0.307 | −0.360 |
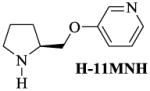
|
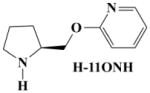
7.111 Å | −0.307 | −0.371 |
|
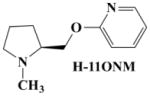
6.496 Å | −0.274 | −0.360 |
|
6.486 Å | −0.274 | −0.371 |
|
8.241 Å | −0.313 | −0.360 |
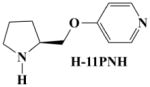
|
8.298 Å | −0.313 | −0.371 |
Binding Assays
Tissue Preparation
The tissue preparation and dissection of rat brain has been described elsewhere [20]. In brief, whole brains were obtained from adult male Sprague-Dawley rats (Pel-Freez Biologicals, Rogers AR). The brains were dissected and the cerebral cortices were removed and stripped of olfactory bulbs, hippocampus, caudate and septum. The brain was allowed to slightly thaw on a glass petri dish over ice, sliced and dissected to remove the cerebral cortex (CTX). Tissues were stored at −80°C until needed for binding assays. For all binding assays, the tissues were suspended and homogenized in ice cold 50mM Tris-HCl buffer, pH 7.4 @ 4°C with a Brinkmann Polytron homogenizer. The homogenate was washed by centrifugation at 36,000 × g for 20 min at 4°C. The supernatant was discarded and the resultant pellet was suspended in fresh buffer to give a final tissue concentration of 10mg/100μL, kept on ice, and vortexed again just before aliquoting it into the tubes.
Saturation Binding Assays
Saturation binding curves were used to determine the equilibrium dissociation constant and the Bmax values for 10 mg of tissue were obtained using a previously described method [19,20]. Briefly, for saturation binding assays, 10mg of CTX tissue were added to tubes containing 50mM Tris-HCl buffer pH 7.4 at ambient temperature and [3H]EB (56.2Ci/mmol; NEN, Boston, MA) at concentrations ranging from ~0.1pM to 3nM. The total assay volume was maintained at 1.0ml for saturation studies to ensure that the binding was achieved even at the lowest concentration of [3H]EB. Preliminary time course studies indicated that under these conditions the binding reaction in rat CTX reaches equilibrium within 4 hours, even at low [3H]EB concentrations, and this observation correlated with previously published data [19]. Therefore, tubes containing CTX tissues, buffer and the radioligand were incubated for 4 hours at 24°C. Non-specific binding was determined in parallel tubes incubated in the presence of 300μM nicotine hydrogen tartrate (NIC) (Sigma Chemical Co. St. Louis, MO). In all assays, reactions were started by the addition of the CTX tissue homogenates. Specific binding was determined as the difference between total binding and non-specific binding. Incubations were terminated by vacuum filtration through Whatman GF/C filter papers, which were mounted on a Brandel cell harvester pre-wetted with 0.5% polyethylenimine to reduce binding of free [3H]EB to the filter [19]. The [3H]EB - tissue complex was collected onto the filter paper. The filter papers were washed three times with 1.5ml aliquots of 50mM Tris-HCl buffer pH 7.4 at 24°C (@ RT). Radioactivity was measured in 500μl of scintillation fluid using a beta liquid scintillation counter (1450 Microbeta Jet, Wallac Perkin Elmer, MD). The KD and Bmax values were determined by non-linear regression analysis using a computer assisted graph and statistical package (GraphPad prism 5 software Inc., San Diego, CA).
Competition Binding Assays in CTX
Competition assays were run with the synthesized compounds against 150pM [3H]EB to obtain the IC50 and determine the affinity (Ki). Competition assays were done in parallel with well characterized nicotinic compounds for comparison. For all studies, nicotinic drugs and new synthesized compounds were diluted in buffer to obtain twelve serial dilutions ranging from ~0.1pM–30mM, depending on the compound (see figure 2). For each assay, the reaction was initiated by the addition of CTX tissue homogenate (10mg) to give a final assay volume of 1.0ml. Non-specific binding was determined in parallel preparations incubated with 300μM nicotine hydrogen tartrate. Specific drug binding was defined as the difference between total binding and binding in the presence of drug minus non-specific binding. Incubation time was 4 hrs @ RT and was terminated by vacuum filtration through Whatman GF/C filter papers as described above [19]. The filter papers were washed three times with 1ml aliquots of 50mM Tris-HCl buffer and the radioactivity bound to the filter papers was measured in a liquid scintillation counter (1450 Microbeta Jet, Wallac Perkin Elmer, MD). The IC50 values for each experiment were determined by non-linear regression analysis (GraphPad Prism 5 software Inc., San Diego, CA). The Kis in CTX tissue for all compounds were calculated using the Cheng-Prusoff equation (Ki = IC50/1+([L]/KD)) [45] where the KD was 35 nM, as per the saturation curve.
Competition Binding Assays in Transfected Cell Lines
To determine the selectivity of the ligands for different nAChR subtypes, competition binding was done using transfected HEK 293 cells previously characterized [24,25,35,37]. This was done because all native tissues may contain several nAChR subtypes, including the CTX, which also contains large numbers of the low affinity α7 nAChR. Briefly, cultured cells at >70% confluence were harvested in 50 mM Tris-HCl, pH 7.4, and homogenized with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY). Homogenates were centrifuged at 36,000 × g for 10 min, and pellets were washed twice with fresh buffer. Membrane pellets were resuspended in fresh buffer and aliquots equivalent to 60–200 μg of protein were used for binding assays. Binding of [3H]EB to cell membrane homogenates was measured as described previously [35], with minor modifications. Membrane preparations were incubated with 500 pM [3H]EB for 4 h at 24°C. The incubation volume for competition binding assays was 0.5 ml/tube. Non-specific binding was assessed in parallel cell membrane homogenates in the presence of 300 μM NIC. Cell membrane homogenates were treated and harvested as described above. Additional statistical analysis was performed by one way ANOVA using SigmaSTAT (Aspire Software International, Ashburn, VA).
86Rb+ Efflux Assay
Because binding data does not correlate with the ability of the drug to initiate a biological response, functional assays are used to determine the ability of the ligands to activate or in some cases antagonize the nAChR’s function. 86Rb+ is a cation that mimics both Na+ and K+ and uses the Na+ channel to go inside the cell. Upon stimulation of the nAChRs 86Rb+ replaces K+ and leaves the cell. Thus, activation of nAChRs in 86Rb+-loaded cells allows for 86Rb+ efflux and therefore measurements of receptor function [46]. The agonist/antagonist function of A-84543 and H-11MNH was therefore determined using a 86Rb+ efflux assay as described by Lukas and Cullen [46], and modified by Xiao et al. [35] at α3β4 nAChRs expressed in transfected cells. In brief, cells were plated onto 24-well plates coated with poly-d-lysine. The plated cells were grown at 37°C for 18–24 hr to reach 70–95% confluence. The cells were then incubated in growth medium (0.5–1 ml/well) containing 86RbCl (2 μCi/ml) for 4 hr at 37°C. HEK 293 cells expressing the nAChRs were washed three times with buffer (15 mM HEPES, 140 mM NaCl, 2 mM KCl, 1 mM MgSO4, 1.8 mM CaCl2, 11 mM glucose, pH 7.4) for 30 sec, 5 min, and 30 sec, respectively. One milliliter of buffer, with or without A-84543 or H-11MNH, was added to each well. After an additional incubation for 2 min, the assay buffer was collected, and the amount of 86Rb+ in the buffer was determined. Cells were then lysed by the addition of 1 ml of 0.1M NaOH and the lysate was collected for determination of the amount of 86Rb+ left in the cells Radioactivity of the assay samples and lysates was measured by liquid scintillation counting. Total loading (cpm) radioactivity was calculated as the sum of the counts per assay volume removed from the sample before lysis and the counts found in the lysate from each well. Values of total loading were 100,000–200,000 cpm/well. The amount of 86Rb+ efflux was expressed as a percentage of total 86Rb+ loaded. Stimulated 86Rb+ efflux was defined as the difference between efflux in the presence or absence of compounds. The EC50 values were estimated by linear regression analysis.
Results
N-N Distance
The distance between the two nitrogen atoms (N-N distance) from A-84543, and all designed A-84543 analogs were calculated to be between 6 to 8 Ǻ. An intermediate distance of ~7 Ǻ was found in A-84543 and H-11MNH (3-pyridyl ether compounds) earlier [22]. H-11MNH differs from A-84543 by a substitution of the methyl group at the pyrrolidine nitrogen to a hydrogen atom, a proton (see Table I). As a result of this small structural difference, the Gasteiger-Huckel charges for A-84543 and H-11MNH were −360eV and −371eV at the pyrrolidine nitrogens respectively; indicating a small difference of 11eV in the distribution of electrons at this nitrogen atom. These 3-pyridyl ethers showed the highest affinity at nAChRs [32]. The distribution pattern of electrons on the pyrrolidine nitrogen was maintained in the 2-pyridyl ether analogs (H-11ONH and H-11ONM) and 4-pyridyl ether analogs (H-11PNH and H-11PNM). However, the electron distribution on the pyridine nitrogen was different for each group of analogs. The Gasteiger-Huckel charge on the pyridine nitrogen for the 2-pyridyl ethers was found to be −274eV, and −313eV for the 4-pyridyl ether compounds compared to Gasteiger-Huckel charge of −307eV on the pyridine ring of the 3-pyridyl ether compounds.
Saturation Curves
Under the present conditions specific binding of [3H]EB in the CTX tissue homogenates was saturable (Fig. 2). The dissociation constant (KD) for rat brain cortex was found to be 35pM with the Bmax of 45.9fmol/mg tissue (Fig. 3).
Fig. 3. Representative saturation binding curve for [3H]EB in membrane homogenates of rat brain cortex.
Plot shows representative curves for three independent binding experiments. Total binding was done in the presence of increasing concentrations (0.1pM–3nM) of [3H]EB. Non-specific binding was determined in parallel preparations under identical conditions in the presence of 300μM (−)-nicotine. Specific binding was then calculated as the difference between total and non-specific binding. Each point represents the average of triplicate determinations for each concentration. Data was analyzed by nonlinear least square regression with GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). The Mean Bmax value was 84 fmol/mg protein and the KD = 35pM for 10 mg tissue.
Binding in CTX
Competition binding assays were used to determine the affinity (Ki) of the compounds using 150pM [3H]EB. A concentration of 150pM was chosen to be above the experimentally determined KD for high affinity CTX receptors (KD ~25–100pM) and close to the KD for lower affinity CTX receptors (KD ~100–500pM) [23–24]. Thus, at this concentration most high affinity sites and at least 30% of the low affinity sites would be labeled. All synthesized compounds were found to be competitive ligands at nAChRs despite the different position of their nitrogen atom in the pyridine ring, or whether they had a methyl or a hydrogen in the pyrrolidine nitrogen, however, they had hugely different affinities.
Nicotine, the standard drug at nAChRs, DHBE, a selective β2-antagonist, and A-85380, another well characterized nicotine-derived analog previously described by Sullivan et al [22], were used for comparison. Thus, the affinity of H-11MNH was found to be highest for CTX nAChRs, and about the same as that of A-85380 (Ki values of 0.46 ± 0.21nM vs 0.37 ± 0.11nM, respectively) but 10 fold greater than the affinity of the lead compound A-84543 (Ki = 3.44 ± 0.79 nM). In general, the affinity of the compounds was stronger in those compounds containing the proton attached to the pyrrolidine nitrogen compared to the compounds with a methyl group in the same position. Since compound H-11PNM was not easily synthesized, it was not characterized further.
Binding in Cell Lines
Under the established conditions, H-11MNH was by far the competitive analog with highest affinity for most nAChRs examined followed by A-84543. All other compounds, although competitive for the nAChRs were of extremely low affinity (>10,000 nM) (see Table III).
Table III. In vitro binding affinities of A-84543, A-84543 analogues, and nicotine (NIC) at nAChR subtypes expressed in stably transfected HEK cells or on α4β2* nAChRs from rat forebrain tissue (FB).
Binding was done against ~500 pM [3H]EB. Ki values presented are the Mean ± SEM of three independent measurements and were calculated from competition graphs using the Cheng-Prusoff equation where the rat forebrain KD value of 50 pM was used.
| Ki (nM) vs 500pM [3H]EB | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analogues | α2β2 | α2β4 | α3β2 | α3β4 | α4β2 | α4β2*-FB | α4β4 | α7 |
| A-84543 | 2.0±0.3 | 370±40 | 12±1 | 1,900±20 | 1.4±0.2 | 5.4±0.6 | 150±10 | 340±50 |
| H-11MNH | 0.35±0.09 | 76±12 | 1.3±0.3 | 210±40 | 0.39±0.05 | 0.71±0.10 | 31±6 | 65±5 |
| H-11ONM | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 |
| H-11ONH | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 |
| H-11PNH | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 |
| Nicotine | 21±14 | 91±13 | 52±3 | 370±40 | 7.4±0.9 | 9.8±0.8 | 35±3 | 550±10 |
| A-853803 | 0.073±0.01 | 18±4 | 0.21±0.04 | 78±10 | 0.014±0.003 | 0.25±0.05 | 8.1±1.1 | 420±50 |
Values for A-85380 have been added for the purpose of comparison from ref. [24]
For comparison purposes, the affinities of the drugs were examined in parallel with membrane homogenates of adult male forebrain (FB). FB tissue (mostly cerebral cortex) is thought to contain predominantly the α4β2 nAChR subtype [17]. A-84543 and H-11NMH showed a similar order of binding affinities in both adult rat FB compared to cultured transfected cells. Both compounds were better at competing for the α4β2 than for the α3β4 nAChRs. Although it should also be noted that the affinities of A-84543 and H-11NMH are at least 2 times higher or more at α4β2 nAChRs expressed in transfected cells than at α4β2 nAChRs from rat FB homogenates. As with CTX tissue homogenate, all other analogs had very low affinity for the α4β2 FB nAChRs (Table III).
In order to assess the selectivity of the compounds between nAChR subtypes; the selectivity ratio was calculated using the binding affinity constants (Ki). The values are shown in Table IV. In transfected cell lines, the lead compound A-84543 was found to be ~ 1,350 times more selective for α4β2 nAChRs than for the α3β4 subtype. In contrast, H-11NMH was ~ 530 times more selective for α4β2 than for the α3β4 nAChR subtypes. On the other hand nicotine only showed a 50 fold selectivity between these receptors. Interestingly, when the selectivity ratio for α4β2 was compared using adult rat FB tissue, the selectivity ratio for both A-84543 and H-11NMH were similar at ~300. Comparing the affinity of the drugs between α7 and α4β2 also shows H-11MNH to have a high affinity ratio of 622, although, it falls short of A-85380’s affinity ratio of ~3,000 between these two subtypes. Finally, the affinity ratio for most drugs between α3β4 and α7 is poor (< than 6), thus one needs to be cautious on using adequate pharmacological tools to differentiate between these two subtypes.
Table IV. Selectivity ratios of A-84543 and its analogues compared to nicotine and A-85380.
Data indicate that A-84543 has the greatest selectivity ratio for α4β2 compared to α3β4 nAChR expressed in transfected cell lines (Only I-A-85380 (not shown) has a higher selectivity ratio of ~ 4,000 [24]. H-11MNH showed moderate selectivity ratios between α3β4 compared to α4β2 or α4β2*FB, on the other hand it has the highest affinity for α7 compared to A-84543 or A-85380 yet still a good ratio between α7 and α4β2. Interestingly, A-84543 and H-11MNH as well as nicotine and A-85380, have low selectivity ratios between α3β4 and α7 nAChRs with great implications on the interpretation of their pharmacological and physiological functions.
| Ligands | α3β4/α4β2 | Ratio4 | α3β4/ α4β2*FB | Ratio5 | α7/α4β2 | Ratio | α3β4/α7 | Ratio |
|---|---|---|---|---|---|---|---|---|
| A-84543 | 1,900/1.4 | 1,357 | 1,900/5.4 | 351.9 | 340/1.4 | 242 | 1,900/340 | 5.58 |
| H-11MNH | 210/0.39 | 538.5 | 210/0.71 | 295.8 | 65/0.39 | 622 | 210/65 | 3.23 |
| Nicotine | 370/7.4 | 50.0 | 370/9.8 | 37.8 | 550/74 | 7.43 | 370/550 | 0.67 |
| A-853806 | 78/0.14 | 557.14 | 78/0.25 | 312 | 420/0.14 | 3,000 | 78/420 | 0.18 |
The selectivity ratios were determined from the Ki of the compounds in α4β2, α3β4, and α7 stably transfected cells.
Selectivity ratio was determined using rat forebrain (FB) as the natural α4β2 receptor vs receptor expressed in stably transfected cells.
Values for A-85380 have been added only for the purpose of comparison [24].
86Rb+ Efflux Assays
Abreo et al., 1986 [22] have used nicotine (100μM)-stimulated 86Rb+ efflux to measure potency and efficacy of four A-85380 analogs (including some of our compounds) in A-84543 (2a) and 2b, and H-11MNH (3a) and 3b in Human α4β2 nAChRs expressed in K177 cells. A-84543, H-11MNH and 3b were found to be full agonists with EC50 values of 0.75, 4 and 4.3μM, respectively, while analog 2b was found to be an antagonist (IC50 = 110μM). They also measure 86Rb+ efflux in neuroblastoma IMR-32 cells, and A-84543, H-11MNH and 3b were also found to be full agonists, while analog 2b was an antagonist. IMR-32 cells are thought to possess predominantly ganglionic-like α3β4 nAChRs, however, they express α3, α5, α7, β2, and β4 AChR subunits and thus potentially several other nAChRs. Therefore, A-84543 and H-11MNH were further tested here for their function at α3β4 nAChRs expressed in HEK 293 cells. In this assay, the α3β4 transfected cells were loaded with 86Rb+ and then stimulated with various concentrations (0.1 nM–10mM) of either A-84543, H-11MNH, or nicotine to allow for 86Rb+ efflux to occur. During this assay, a separate group of cells was treated with 100 μM nicotine to establish the Emax. Nicotine’s EC50 was determined to be ~ 32 μM with maximal efficacy. Efficacy for H-11MNH was found to be 83.33% with an EC50 of 24±1 μM, thus it is a full agonist with high efficacy at these nAChRs. In contrast, A-84543 was determined to be a partial agonist (37.5% of Emax) with an EC50 of 160±4 μM, a potency 6 fold lower than that of H-11MNH (Table V).
Table V. Determination of agonist activity of A-84543, H-11MNH and nicotine at rat α3β4 nAChRs by measuring stimulation of 86Rb+ efflux from transfected cells.
The concentration-dependent activation of nAChR function of α3β4 transfected cells by the compounds was measured as described previously [35]. The EC50 values and Relative Emax are the mean ± SEM of 3 independent experiments.
| Agonist Activity | ||
|---|---|---|
| Compound | EC50 (μM) | * Relative Emax |
| A-84543 | 160 ± 40 | 37.5 ± 6 |
| H-11MNH | 24 ± 1 | 83.3 ± 3 |
| Nicotine | 32 ± 2 | 100 ± 8 |
% of the stimulation by 100 μM nicotine
Discussion
Clinical and preclinical data suggests that nAChRs are involved in many neurophysiological functions in both the central and peripheral nervous systems, as well as many non-neuronal tissues [13], including immune cells [47]. The diversity of native nAChRs and their selective involvement in so many physiological functions and potential neuropathologies makes them great candidates as clinical therapeutic targets. Encouraged by the high affinity of 3-pyridyl analogs, many researchers have attempted to improve the selectivity of this class of compounds for specific nAChR subtypes. In line with this reasoning, Wei and colleagues [48] demonstrated the importance of attachment of a hydrophobic or hydrogen bonding alkynyl group to the C5 position of the pyridyl ring of EB and A-84543 in order to improve selectivity for neuronal nAChR subtypes. It was therefore postulated that attachment of electron withdrawing or electron donating substituents to the ortho and para positions would generate A-84543 analogs that differ in their affinity for nAChRs. Therefore, several analogs of A-84543 were synthesized that vary in the distance between their nitrogens, to test if this would confer greater binding ability or selectivity for the orthosteric site in the nAChRs. Differential positioning of the nitrogen and exchanging the methyl group for a hydrogen in the lead molecule changed the selectivity of the analogs for nAChRs. The charge on the pyridine or pyrrolidine nitrogens was not very different between the compounds. However, the N-N distance for both A-84543 and H-11MNH was found to be intermediate (~7 Å) between that for the other two pairs of compounds (~6 or 8Å), and this may be important in conferring these ligands their high affinity (in the nanomolar range for A-84543 and picomolar range for H11MNH) for nAChRs, vs 10,000 for other analogs (H-11ONH and H-11ONM, or H-11PNH).
After synthesizing A-84543 and its analogs, competition assays were performed to determine if they would bind at the nAChRs. All the analogs were found to be competitive, although, as stated before, with very diverse affinities. H-11MNH had the highest affinity (~0.5nM) for CTX nAChRs, while H-11ONM had the lowest (~60,000nM). The rank order for the analogs at displacing 150pM [3H]EB was: A85380 > H-11MNH > A-84543 > nicotine > DHBE >H-11ONH > H-11PNH > H-11ONM. The compounds were then tested for selectivity in stably transfected HEK cells expressing different nAChRs subtypes, here again, A-84543 and its analog H-11MNH, showed high affinity for most nAChRs. Of all the analogues, H-11MNH showed highest affinity for all nAChRs while A-84543 had a ~ 5 to10-fold lower affinity. All other compounds had very low affinities (> 10,000 nM). Interestingly, other than having high affinity, A-84543 and H-11MNH had very distinct pharmacological profiles, and both differ from that of nicotine and A-85380. Both A-84543 and H-11MNH had highest affinity for α2β2 and α4β2 nAChRs and showed moderate affinity for β4- and α7-containing receptors, which makes them interesting for further functional characterization. Interestingly, A-84543 had the greatest selectivity ratio for α4β2 vs α3β4 nAChR but moderate ratio between α4β2 from FB and α3β4. This also calls for further studies to determine what element in the receptors determines this large difference in binding affinity. The differences may be due to the presence of small differences in assembly between the subunits, or perhaps a different stoichiometry of the subunits assembled. One must always take into account that the machinery between the human embryonic kidney cells, HEK cells, and the neurons in rat FB or CTX may be slightly different and potentially affect the final form of the receptor, for example a slight change in the glycosylation of the receptor may be enough to cause a small change in drug affinity. The moderate ratio between α4β2 and α7, also requires further investigation especially of drugs thought to be exclusively β2-selective (non-classical α7 nicotinic drugs) on α7 nAChRs, or α7-selective (non-classical β2 nicotinic drugs) on β2 nAChRs to determine if indeed they are without functional effects on the other nAChRs. H-11MNH only has moderate selectivity ratios between α4β2 vs α3β4, and α4β2 vs α7 nAChRs, thus, this observation may be important to understand the properties of the orthosteric site, since the natural ACh molecule also has moderate selectivity ratios between many nAChRs [24,25,28]. Both A-84543 and H-11MNH have a very low selectivity ratio between α3β4 and α7 nAChRs and this should also be further warning that compounds are not as clean as we would like to assume, and that they can interact with multiple nAChRs and sometimes even with unexpected receptors, and that they may act differentially as full agonists in some nAChR subtypes, partial agonists in others, or antagonists in yet other subtypes. For example, previously published data [22] showed that both the A-84543 (2a) and other A-85380 analogs (2b, H-11MNH (3a) and 3b bind [3H]cytisine-sensitive sites (α4β2 and, α3β4*) with high affinity. Back then it was thought that [3H]cytisine was a selective β2-containing receptor ligand. However, we now know it is a partial agonist at α4β2 nAChRs (with high potency) and a full agonist at α3β4 nAChRs (with low potency). It is important to also mention that other drugs, such as veranicline, have also been found to be full agonists at these receptors, although they seem to have low potency for them as well, and are partial agonists at α4β2-containing nAChRs (with high potency) [49]. A-84543 and H-11MNH, nevertheless, were found to both be full agonists at α4β2 human receptors expressed in K177 cells and in α3β* nAChRs naturally found in the neuroblastoma derived IMR-32 cells using 86Rb efflux assays [22]. Although IMR-32 cells have more than one nAChR subtype [50]. Noteworthy is Abreo’s study [22], where the isomers for A-85380 (A84543 (2a) and 2b) were quite different in their potency and efficacy, while H-11MNH (3a) and 3b had similar potency and efficacy.
Altogether, we have not shown a direct correlation between affinity and nitrogen distance, nor a correlation with the Gasteiger-Huckel charge in the pyridyl nitrogen or pyrrolidine nitrogen, nevertheless, repositioning of the pyridyl nitrogen does not alter the competitive nature of the pyridyl ethers but does result in changes in the affinities of the ligands for the nAChR subtypes. Out of five analogs only A-84543 and H-11MNH have high affinity for the CTX α4β2 nAChRs, as shown previously against [3H]cytisine [22]. Here we also demonstrate they have reasonable affinity for α3β4 and α7 nAChRs. In contrast, the 2- and 4-pyridyl ether analogs have negligible affinity. We also demonstrated that H-11MNH has higher potency and higher efficacy compared to A-84543 at α3β4 nAChRs. It is possible that further modifications of A-84543 and H-11MNH may still hold potential for enhanced affinity, potency and efficacy at select nAChRs.
Continued development and detailed characterization of new ligands with different nAChR subtype profiles may ultimately allow for the design of more subtype-specific compounds that may shed light on the biophysical story of how these receptors ultimately function. It is important to note that positive and negative allosteric modulators also have a role to play and should make important contributions, in conjunction with orthosteric ligands, in the functional characterization of nAChR subtypes since their selectivity does not dependent on the orthosteric binding site [51–54]. This should allow for some interesting and innovative modulation of the nAChRs.
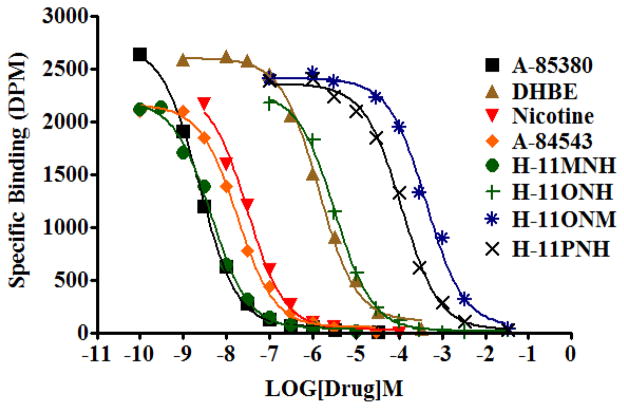
Fig. 4. Competition curves of nicotinic ligands for binding sites labeled with 150pM [3H]EB.
Competition assays were performed in adult rat brain cortex homogenates labeled with 150pM [3H]EB. Binding of A-84543 (◆), H-11ONM (*), (●) H-11MNH, (+) H-11ONH and H-11PNH (X), were compared to that of A-85380 (■), a high affinity nAChR agonist, DHβE (▲) a high affinity nAChR antagonist, and Nicotine (▼). The data were analyzed by nonlinear least square regression method using GraphPad Prism 5 software (GraphPad Software Inc. San Diego, CA). IC50 and Ki values are presented in Table II. The data shown is representative of n = 3–6 independent experiments using triplicates.
Table II. Binding Characteristics of nAChRs ligands against 150pM [3H]Epibatidine.
Binding of A-84543, its analogs, and A-85380 was compared to that of nicotine and the nicotinic β2 receptor antagonist DHβE in cerebral cortical rat membrane homogenates (10 mg) in a total volume of 1 ml for 4 hr at 24°C. The inhibition constants (Ki) were calculated using the Cheng-Prusoff equation from measured IC50s and a KD value of 35pM for [3H]EB from the saturation experiments. The values presented are the average of n = 3–6 independent experiments per ligand run in triplicates. Ligand binding was determined for each new compound as the hydrobromide (HBr) salt (H-11MNH, H-11ONH, and H-11PNH) or as the synthesized base (A-84543, and H-11ONM); while nicotine was used as the hydrogen tartrate salt.
| IC50 | Ki | ||||
|---|---|---|---|---|---|
| nM | nM | ||||
| DRUGS | AVG | SEM | AVG | SEM | n |
| A-85380 | 2.09 | 0.38 | 0.37 | 0.06 | 4 |
| H-11MNH2 | 2.51 | 0.59 | 0.46 | 0.11 | 5 |
| A-84543 | 17.90 | 1.95 | 3.44 | 0.40 | 5 |
| Nicotine | 32.30 | 9.85 | 5.99 | 1.44 | 5 |
| DHβE | 344.67 | 42.22 | 61.33 | 5.89 | 3 |
| H-11ONH | 115,000 | 41,845 | 21,800 | 7,971 | 6 |
| H-11PNH | 303,000 | 75,850 | 59,200 | 16,722 | 5 |
| H-11ONM | 322,700 | 98,342 | 60,300 | 18,880 | 6 |
This work represents new syntheses and new data of the A-85380 2a analog A84543 and the 3a analog, here named H-11MNH, that complements work originally done by Abreo et al. [22].
Acknowledgments
This work was partially supported by the National Institutes of Health grants 5R24MH067627 and NIGMS-NIH S06 GM08016-34 to MDG.
Abbreviations
- CTX
cerebral cortex
- DHβE
Dihydro-β-erythroidine
- [3H]EB
[3H]epibatidine
- FB
rat forebrain
- nAChRs
nicotinic acetylcholine receptors
Footnotes
For nAChRs the *, as per the International Union of Basic and Clinical Pharmacology (IUPHAR), denotes that there may be additional subunits which are presently unknown.
Conflict of Interest: The authors declare that there is no conflict of interest.
Ethical Approval: This article does not contain any studies with animals performed by any of the authors.
References
- 1.Jensen AA, Bente F, Liljefors T, Krogsgard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48(15):4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- 2.Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137(1):22–54. doi: 10.1016/j.pharmthera.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36(2):96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becchetti A, Aracri P, Meneghini S, Brusco S, Amadeo A. The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front Physiol. 2015;6:22. doi: 10.3389/fphys.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–9. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Winston N, Vernino S. Recent advances in autoimmune autonomic ganglionopathy. Curr Opin Neurol. 2010;23(5):514–8. doi: 10.1097/WCO.0b013e32833d4c7f. [DOI] [PubMed] [Google Scholar]
- 7.Muppidi S, Vernino S. Autoimmune autonomic failure. Handb Clin Neurol. 2013;117:321–7. doi: 10.1016/B978-0-444-53491-0.00025-0. [DOI] [PubMed] [Google Scholar]
- 8.Bagdas D, Al-Sharari SD, Freitas K, Tracy M, Damaj MI. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem Pharmacol. 2015 doi: 10.1016/j.bcp.2015.04.013. pii: S0006-2952(15)00218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2014;96(PtB):302–11. doi: 10.1016/j.neuropharm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol. 2014;89(1):1–11. doi: 10.1016/j.bcp.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Changeux JP. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J Biol Chem. 2012;287(48):40207–15. doi: 10.1074/jbc.R112.407668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertrand S, Bertrand D. Overview of electrophysiological characterization of neuronal nicotinic acetylcholine receptors. Curr Protoc Pharmacol. 2004;Chapter 11(Unit11.7) doi: 10.1002/0471141755.ph1107s23. [DOI] [PubMed] [Google Scholar]
- 13.Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS J. 2006;7(4):E885–94. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popot JL, Changeux JP. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev. 1984;64(4):1162–239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- 15.Lindstrom J, Anand R, Peng X, Gerzanich V, Wang F, Li Y. Neuronal nicotinic receptor subtypes. Ann N Y Acad Sci. 1995;757:100–16. doi: 10.1111/j.1749-6632.1995.tb17467.x. [DOI] [PubMed] [Google Scholar]
- 16.Le Novère N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. JNeurobiol. 2002;53(4):447–56. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- 17.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41(1):31–7. [PubMed] [Google Scholar]
- 18.Pabreza LA, Dhawan S, Kellar KJ. [3H]cytisine binding to nicotinic cholinergic receptors in brain. Mol Pharmacol. 1991;39(1):9–12. [PubMed] [Google Scholar]
- 19.Houghtling RA, Davila-Garcia MI, Kellar KJ. Characterization of (6)-[3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Mol Pharmacol. 1995;48:280–287. [PubMed] [Google Scholar]
- 20.Dávila-García MI, Musachio JL, Perry DC, Xiao Y, Horti A, London ED, Dannals RF, Kellar KJ. [125I]IPH, an epibatidine analog, binds with high affinity to neuronal nicotinic cholinergic receptors. J Pharmacol Exp Ther. 1997;282:445–451. [PubMed] [Google Scholar]
- 21.Spang JE, Bertrand S, Westera G, Patt JT, Schubiger PA, Bertrand D. Chemical modification of epibatidine causes a switch from agonist to antagonist and modifies its selectivity for neuronal nicotinic acetylcholine receptors. Chem Biol. 2000;7(7):545. doi: 10.1016/s1074-5521(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 22.Abreo MA, Lin NH, Garvey DS, Gunn DE, Hettinger AM, Wasicak JT, Pavlik PA, Martin YC, Donnelly-Roberts DL, Anderson DJ, Sullivan JP, Williams M, Arneric SP, Holladay MW. Novel 3-pyridyl ethers with subnanomolar affinity for central neuronal nicotinic acetylcholine receptors. J Med Chem. 1996;39:817–825. doi: 10.1021/jm9506884. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Piattoni-Kaplan M, Campbell JE, McKenna DG, Molinari E, Hettinger AM, Garvey DS, Wasicak JT, Holladay MW, Williams M, Arneric SP. A-85380 [3-(2(S)-azetidinylmethoxy) pyridine]: in vitro pharmacological properties of a novel, high affinity alpha 4 beta 2 nicotinic acetylcholine receptor ligand. Neuropharmacology. 1996;35(6):725–34. doi: 10.1016/0028-3908(96)84644-2. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Y, Baydyuk M, Wang HP, Davis HE, Kellar KJ. Pharmacology of the agonist binding sites of rat neuronal nicotinic receptor subtypes expressed in HEK 293 cells. Bioorg Med Chem Lett. 2004;14(8):1845–8. doi: 10.1016/j.bmcl.2003.09.105. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- 26.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach R, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 27.Chellappan SK, Xiao Y, Tueckmantel W, Kellar KJ, Kozikowski AP. Synthesis and pharmacological evaluation of novel 9- and 10-substituted cytisine derivatives. Nicotinic ligands of enhanced subtype selectivity. J Med Chem. 2006;49(9):2673–6. doi: 10.1021/jm051196m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, Marks MJ. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58(7):1054–66. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beers WH, Reich E. Structure and activity of acetylcholine. Nature. 1970;228:917–922. doi: 10.1038/228917a0. [DOI] [PubMed] [Google Scholar]
- 30.Glennon RA, Dukat M. Central nicotinic receptor ligands and pharmacophores. Pharmaceutica Acta Helvetiae. 2000;74:103–114. doi: 10.1016/s0031-6865(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 31.Koren AO, Horti AG, Mukhin AG, Gündisch D, Kimes AS, Dannals RF, London ED. 2-, 5-, and 6-Halo-3-(2(S)-azetidinylmethoxy)pyridines: synthesis, affinity for nicotinic acetylcholine receptors, and molecular modeling. J Med Chem. 1998;41(19):3690–8. doi: 10.1021/jm980170a. [DOI] [PubMed] [Google Scholar]
- 32.Lin NH, Gunn DE, Li Y, He Y, Bai H, Ryther KB, Kuntzweiler T, Donnelly-Roberts DL, Anderson DJ, Campbell JE, Sullivan JP, Arneric SP, Holladay MW. Synthesis and structure-activity relationships of pyridine-modified analogs of 3-[2-((S)-pyrrolidinyl)methoxy]pyridine, A-84543, a potent nicotinic acetylcholine receptor agonist. Bioorg Med Chem Lett. 1998;8(3):249–54. doi: 10.1016/s0960-894x(98)00019-5. [DOI] [PubMed] [Google Scholar]
- 33.Fan H, Scheffel UA, Rauseo P, Xiao Y, Dogan AS, Yokoi F, Hilton J, Kellar KJ, Wong DF, Musachio JL. [125/123I] 5-Iodo-3-pyridyl ethers. Syntheses and binding to neuronal nicotinic acetylcholine receptors. Nucl Med Biol. 2001;28(8):911–21. doi: 10.1016/s0969-8051(01)00258-x. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70(4):1454–60. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol. 1998;54(2):322–33. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- 36.Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ. Measuring nicotinic receptors with characteristics of α4β2, α3β2, and α3β4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y, Abdrakhmanova GR, Baydyuk M, Hernandez S, Kellar KJ. Rat neuronal nicotinic acetylcholine receptors containing alpha7 subunit: pharmacological properties of ligand binding and function. Acta Pharmacol Sin. 2009;30(6):842–50. doi: 10.1038/aps.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoli M, Moretti M, Zanardi A, McIntosh MJ, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22(20):8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 40.Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 2013;3(1):a012112. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Edvinsson L, Lee TJ. Mechanism of nicotine-induced relaxation in the porcine basilar artery. J Pharmacol Exp Ther. 1998;284(2):790–7. [PubMed] [Google Scholar]
- 42.Lee RH, Liu YQ, Chen PY, Liu CH, Chen MF, Lin HW, Kuo JS, Premkumar LS, Lee TJ. Sympathetic α3β2-nAChRs mediate cerebral neurogenic nitrergic vasodilation in the swine. Am J Physiol Heart Circ Physiol. 2011;301(2):H344–54. doi: 10.1152/ajpheart.00172.2011. [DOI] [PubMed] [Google Scholar]
- 43.Mitsunobu O, Wada M, Sano T. Stereospecific and stereoselective reactions. I. Preparation of amines from alcohols. J Am Chem Soc. 1972;94(2):679–680. [Google Scholar]
- 44.Brown LL, Kulkarni S, Pavlova AO, Koren AO, Mukhin AG, Newman AH, Horti AG. Synthesis and Biological Evaluation of Novel Carbon-11 Labeled Pyridyl Ethers: Candidate Ligands for In Vivo Imaging of α4β2 Nicotinic Acetylcholine Receptors (α4β2-nAChRs) in the brain with Positron Emission Tomography. J Med Chem. 2002;45:2841–2849. [Google Scholar]
- 45.Cheng YC, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 46.Lukas RJ, Cullen MJ. An isotopic rubidium ion efflux assay for the functional characterization of nicotinic acetylcholine receptors on clonal cell lines. Anal Biochem. 1988;175:212–218. doi: 10.1016/0003-2697(88)90380-6. [DOI] [PubMed] [Google Scholar]
- 47.Razani-Boroujerdi S, Boyd R, Dávila-García MI, Nandi JS, Mishra N, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and Leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response to increase intracellular. J Immunol. 2007;179:2889–2898. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- 48.Wei ZL, Xiao Y, Yuan H, Baydyuk M, Petukhov PA, Musachio JL, Kellar KJ, Kozikowski AP. Novel pyridyl ring C5 substituted analogues of epibatidine and 3-(1-methyl-2(S)-pyrrolidinylmethoxy)pyridine (A-84543) as highly selective agents for neuronal nicotinic acetylcholine receptors containing beta2 subunits. J Med Chem. 2005;48(6):1721–4. doi: 10.1021/jm0492406. [DOI] [PubMed] [Google Scholar]
- 49.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 50.Dávila-García MI, Houghtling RA, Kellar KJ. [3H]Epibatidine binds two non-α4β2 high affinity sites in IMR-32 cells. Soc Neurosci Abstr 527.8. 1995;21(2):1333. [Google Scholar]
- 51.Pandya AA, Yakel JL. Effects of neuronal nicotinic acetylcholine receptor allosteric modulators in animal behavior studies. Biochem Pharmacol. 2013;86(8):1054–62. doi: 10.1016/j.bcp.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Changeux JP. The concept of allosteric modulation: an overview. Drug Discov Today Technol. 2013;10(2):e223–8. doi: 10.1016/j.ddtec.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Nirogi R, Goura V, Abraham R, Jayarajan P. α4β2* neuronal nicotinic receptor ligands (agonist, partial agonist and positive allosteric modulators) as therapeutic prospects for pain. Eur J Pharmacol. 2013;712(1–3):22–9. doi: 10.1016/j.ejphar.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 54.Uteshev VV. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol. 2014;727:181–5. doi: 10.1016/j.ejphar.2014.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]