Abstract
Interleukin 10 (IL10) is a key anti-inflammatory cytokine that can inhibit proinflammatory responses of both innate and adaptive immune cells. An association between IL10 and intestinal mucosal homeostasis became clear with the discovery that IL10 and IL10 receptor (IL10R)-deficient mice develop spontaneous intestinal inflammation. Similarly, patients with deleterious mutations in IL10, IL10RA, or IL10RB present with severe enterocolitis within the first months of life. Here, we review recent findings on how IL10- and IL10R-dependent signaling modulates innate and adaptive immune responses in the murine gastrointestinal tract, with implications of their role in the prevention of inflammatory bowel disease (IBD). In addition, we discuss the impact of IL10 and IL10R signaling defects in humans and their relationship to very early-onset IBD (VEO-IBD).
1. INTRODUCTION
Interleukin-10 (IL10) is a key anti-inflammatory cytokine that is produced predominantly by leukocytes including T cells, B cells, monocytes, macrophages (Mϕs), and dendritic cells (DCs), as well as by some epithelial cells (Medzhitov et al., 2011; Moore, de Waal Malefyt, Coffman, & O’Garra, 2001; Saraiva & O’Garra, 2010). In leukocytes, IL10 acts on both innate and adaptive immune cells and has a broad range of immunomodulatory activities that suppress proliferation, cytokine secretion, and costimulatory molecule expression of proinflammatory immune cells (Donnelly, Dickensheets, & Finbloom, 1999; Murray, 2006). A critical role for IL10 signaling in modulating intestinal mucosal homeostasis became evident with the description that IL10-deficient mice develop spontaneous enterocolitis (Kuhn, Lohler, Rennick, Rajewsky, & Muller, 1993). This was subsequently strengthened by the observation that interleukin 10 receptor (IL10R)-deficient mice also develop spontaneous colitis (Spencer et al., 1998). These findings led to an extensive research effort aiming to elucidate the role of IL10-dependent signaling in the regulation of intestinal immune function.
In humans, IL10 and IL10R play critical roles in controlling immune responses in the intestinal mucosa. Single-nucleotide polymorphisms (SNPs) in IL10 have been linked to inflammatory bowel disease (IBD) risk in genome-wide association studies (GWAS) (Franke et al., 2008; Franke, McGovern, et al., 2010; Jostins et al., 2012). In addition, patients with deleterious mutations in either IL10 or its receptor develop severe IBD, usually presenting within the first months of life (Glocker et al., 2009; Kotlarz et al., 2012, Moran et al., 2013). In this chapter, we will review recent findings of how IL10-dependent signaling modulates immune responses, focusing on the role of these signals in the regulation of mucosal homeostasis and prevention of IBD.
2. IL10 AND IL10 RECEPTOR EXPRESSION AND REGULATION
IL10 is the foremost member of the type-II cytokine family, comprising IL19, IL20, IL22, IL24, IL26, IL28, and IL29 (Commins, Steinke, & Borish, 2008). IL10 was first described by Fiorentino et al. as an inhibitor of cytokine synthesis and initially termed “cytokine synthesis inhibitory factor”, as it was released by Th2 cells and inhibited interferon-γ (IFNγ) production by Th1 cells (Fiorentino, Bond, & Mosmann, 1989). Subsequent studies revealed pleiotropic functions of IL10 on various adaptive and innate immune populations (Bhattacharyya et al., 2004; Bogdan, Vodovotz, & Nathan, 1991; de Waal Malefyt et al., 1991; Ding, Linsley, Huang, Germain, & Shevach, 1993; Ding & Shevach, 1992; Fiorentino, Zlotnik, Vieira, et al., 1991; Murphy et al., 1994; Ralph et al., 1992).
Human and mouse IL10 (hIL10 and mIL10, respectively) have roughly 73% sequence homology and are secreted as 178-amino acid proteins (Windsor et al., 1993). While both hIL10 and mIL10 are comprised of noncovalently linked homodimers, (Walter & Nagabhushan, 1995; Zdanov et al., 1995; Zdanov, Schalk-Hihi, Menon, Moore, & Wlodawer, 1997), mIL10 is glycosylated at the N-terminal region and does not activate human cells, whereas hIL10 is not N-glycosylated and can activate both human and mouse cells (Moore et al., 2001; Mosmann et al., 1990). Several innate and adaptive immune cells can secrete IL10 including monocytes, Mϕs, DCs, natural killer (NK) cells, mast cells, neutrophils, CD4 and CD8 T cells, and B cells (Moore et al., 2001; O’Garra & Vieira, 2007; Saraiva & O’Gara 2010). For antigen-presenting cells (e.g., DCs, Mϕs), production of IL10 is triggered by recognition of various bacterial or viral pathogen-associated molecular patterns by cell surface or cytoplasmic pattern recognition receptors (PRR) (Akbari, DeKruyff, & Umetsu, 2001; de Waal Malefyt et al., 1991; Fiorentino, Zlotnik, Vieira, et al., 1991; Siewe et al., 2006). Studies have shown that engagement of transmembrane PRRs, known as Toll-like receptors (TLRs) (i.e., TLR2, TLR3, TLR4 TLR9) lead to production of IL10 by Mϕs and myeloid DCs (Agrawal et al., 2003; Boonstra et al., 2006; Dillon et al., 2004; Netea et al., 2004). Among the cytosolic PRRs, ligation of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) induces IL10 expression (Moreira et al., 2008). Interestingly, a nonfunctional frameshift mutation in NOD2 blocks IL10 transcription and is associated with Crohn’s disease (CD) (Noguchi, Homma, Kang, Netea, & Ma, 2009). Other than TLRs and (NOD)-like receptors, stimulation of C-type lectins, DC-specific ICAM3-grabbing nonintegrin (DC-SIGN), and dectin 1 also induce IL10 production (Geijtenbeek et al., 2003; Rogers et al., 2005). In addition to PRRs, several cytokines such as IL21 produced by Th1 primed cells (Spolski, Kim, Zhu, Levy, & Leonard, 2009) and IL27 produced by Th1, Th2, and Th17 cells can increase IL10 expression via STAT1- and STAT3-dependent mechanisms (Batten et al., 2008; Fitzgerald et al., 2007; Pot et al., 2009; Stumhofer et al., 2007; Xu et al., 2009). In contrast, it has also been reported that IL27 can inhibit TLR-mediated IL10 production in human monocytes (Kalliolias & Ivashkiv, 2008).
The receptor for IL10 is a heterotetramer complex comprising two IL10Rα (also referred to as IL10R1) molecules (encoded by the Il10ra gene) and two IL10Rβ (also referred to as IL10R2) molecules (encoded by the Il10rb gene) (Kotenko et al., 1997; Moore et al., 2001). All IL10-responsive cells express IL10Rα, with antibody-mediated blockade of the surface receptor inhibiting its responsiveness (Ho et al., 1993; Liu et al., 1997; Liu, Wei, Ho, de Waal Malefyt, & Moore, 1994). IL10Rα is expressed on most hematopoietic cells at a basal level but is upregulated by various cells upon activation, suggesting its importance in inhibitory pathways. For example, at steady state, naïve CD4+ T cells have low IL10Rα expression, but in vivo anti-CD3 treatment induces IL10Rα expression on Th17 cells in the small intestine (Huber et al., 2011). In addition, in vitro stimulation of naïve CD45RBhigh T cells, memory/effector T cells, and regulatory T cells (Tregs) leads to upregulation of IL10Rα expression (Kamanaka et al., 2011). Similarly, under basal conditions, human neutrophils express low levels of IL10R1; however, following lipopolysaccharide (LPS) or IL4 stimulation IL10R1 expression is upregulated (Crepaldi et al., 2001). Corinti and colleagues have shown that human DCs become unresponsive to IL10 after maturation by downregulating IL10R1 surface expression enabling them to produce higher levels of proinflammatory mediators and to prime T cells (Corinti, Albanesi, la Sala, Pastore, & Girolomoni, 2001). IL10Rα can also be induced in some nonhematopoietic cells such as fibroblasts upon activation with LPS (Weber-Nordt, Meraz, & Schreiber, 1994) as well as being constitutively expressed in colonic epithelial cells (Bourreille et al., 1999; Denning et al., 2000).
3. DOWN-STREAM SIGNALING THROUGH THE IL10 RECEPTOR
IL10Rα is 90–120 kDa and serves as the ligand binding subunit of the receptor complex (Liu et al., 1997, 1994; Tan, Indelicato, Narula, Zavodny, & Chou, 1993). IL10Rβ is the signaling subunit of the IL10R complex and is constitutively expressed in most cell types (Gibbs & Pennica, 1997; Kotenko et al., 1997; Moore et al., 2001). Earlier studies have suggested that IL10Rβ has almost no role in IL10-binding; its main role is to recruit the downstream signaling kinases (Kotenko et al., 1997; Spencer et al., 1998). More recent studies have found that upon binding to IL10, IL10Rα induces a conformational change in IL10Rβ, permitting IL10Rβ to also bind IL10 (Yoon, Logsdon, Sheikh, Donnelly, & Walter, 2006). Unlike IL10Rα, which is unique to IL10, the IL10Rβ-subunit is shared by receptors for other type-II cytokines including IL22, IL26, and INFλ.
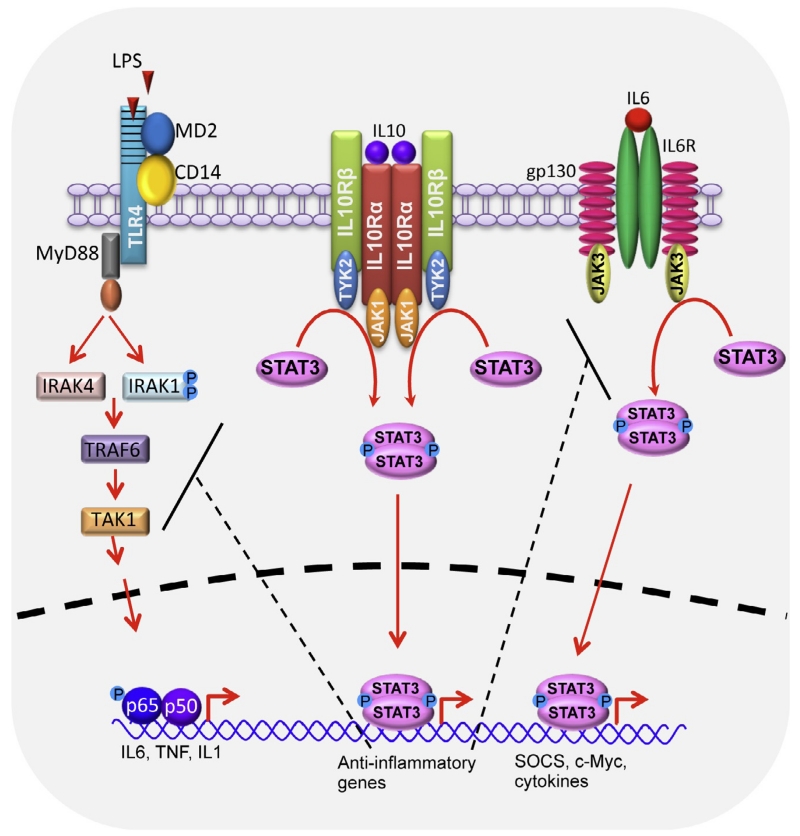
The sequence of receptor assembly is initiated by IL10 binding to IL10Rα (Fig. 5.1). This complex then binds IL10Rβ forming a heterotetramer, permitting the assembly of the signaling complex (Yoon et al., 2006). Once the complex is assembled, tyrosine kinases Jak1 and Tyk2 that are constitutively associated with IL10Rα and IL10Rβ, respectively, are activated and phosphorylate specific tyrosine residues in the intracellular domain of IL10Rα. Phosphorylation of the receptor leads to the recruitment of signal transducer and activator of transcription 3 (STAT3) (Kotenko et al., 1997; Liu et al., 1994; Murray, 2007). Following their recruitment, JAK1 and TYK2 phosphorylate STAT3, leading to its homodimerization and subsequent translocation to the nucleus, where it binds to STAT3-binding elements of IL10-responsive genes (Finbloom & Winestock, 1995; Murray, 2007; Rodig et al., 1998; Williams, Ricchetti, Sarma, Smallie, & Foxwell, 2004). STAT3 also induces the expression of suppressor of cytokine signaling 3 (SOCS3), which inhibits PRR-induced expression of various inflammatory cytokines including TNF, IL6, and IL1β (Berlato et al., 2002; Murray, 2006; Williams, Bradley, Smith, & Foxwell, 2004). While both IL10 and IL6 highly induce STAT3-dependent SOCS3 expression in Mϕs, the inhibitory role of SOCS3 appears to be restricted to IL6 (Croker et al., 2003; Lang et al., 2003; Murray, 2006; Nicholson et al., 2000; Schmitz, Weissenbach, Haan, Heinrich, & Schaper, 2000; Williams, Ricchetti, et al., 2004; Yasukawa et al., 2003). In this scenario, a proinflammatory cytokine (IL6) and an anti-inflammatory cytokine (IL10) signal through a shared transcription factor. While SOCS3 may play a role in driving-specific outputs, a detailed molecular understanding responsible for these differences remains unknown (Murray, 2007). In addition to STAT3, IL10-receptor activation of STAT1 and STAT5 have been reported (Finbloom & Winestock, 1995; Lai et al., 1996; Moore et al., 2001; Weber-Nordt et al., 1996). While much knowledge has been gained in recent years, the broad effects of IL10-mediated activation of STAT1 and STAT5 remain unclear (Miura et al., 2006; Williams, Ricchetti, et al., 2004).
Figure 5.1.
The IL10/IL10R signaling pathway. IL10 signals through heterotetrameric IL10R complex comprising of IL10Rα (IL10R1 in humans) and IL10Rβ (IL10R2 in humans). Binding of IL10 to its receptor leads to JAK1- and TYK2-mediated phosphorylation of STAT3. Following phosphorylation, STAT3 forms a homodimer and undergoes nuclear translocation where it binds to STAT3-binding elements of IL10-responsive genes and drives expression of anti-inflammatory mediators that block various inflammatory pathways. IL10-responsive gene products inhibit TLR4 signaling at the level of IRAK and TRAF6 resulting in reduced NF-κB-mediated expression of IL6, TNF, and IL1 and also inhibit IL6 signaling at the level of gp30 receptor subunit. MyD88, myeloid differentiation primary response gene (88); TLR, toll-like receptor; IRAK, interleukin-1 receptor-associated kinase; TRAF, TNF receptor-associated factor.
4. REGULATION OF INTESTINAL IMMUNE RESPONSES BY IL10 IN MURINE MODELS
Intestinal homeostasis is a highly dynamic process requiring sensitivity to mount appropriate immune responses toward microbial or food antigens, yet necessitating the regulation of these responses in order to prevent chronic inflammation. A critical role for IL10 in maintaining gut homeostasis was first evident in the original description of IL10-deficient mice that developed spontaneous enterocolitis due to immune hyperactivation elicited by intestinal microbial antigens (Kuhn et al., 1993). Similar implications for IL10 and intestinal homeostasis were also demonstrated using a T-cell transfer model of colitis. In this early work by Powrie and colleagues, wild-type (WT) CD4+CD45RBhigh T cells were transferred into lymphopenic severe combined immunodeficiency (SCID) mice to induce colitis, but the disease was abrogated in a cohort of mice-administered exogenous recombinant IL10 (Powrie et al., 1994). This effect was recapitulated in a transfer setting where the CD4+CD45RBhigh T cells containing an IL10 transgene driven by an IL2 promoter prevented the development of colitis (Hagenbaugh et al., 1997). While this shows that IL10 production by an immune cell population is sufficient to prevent colitis, it does not exclude a role for IL10 production in nonleukocyte populations in mucosal tissues. In this regard, transgenic expression of IL10 in intestinal epithelial cells protects mice from colitis induced by either dextran sodium sulfate (DSS) or CD4+CD45RBhigh T cells transfer (De Winter et al., 2002). Intestinal epithelial cells were also reported to produce IL10 following antibody-mediated CD1d cross-linking (Colgan, Hershberg, Furuta, & Blumberg, 1999). A more recent study has shown that depletion of IL10 in mucosal explants leads to downregulation of IL10 inducible genes and upregulation of IFNγ, TNF, and IL17 (Jarry et al., 2008). Finally, intragastric administration of IL10-producing Lactococcus lactis also protects mice from DSS-induced colitis and can prevent development of spontaneous colitis in IL10-deficient mice (Steidler et al., 2000). Together these studies delineate the significance of IL10 in the regulation of intestinal homeostasis. The role of IL10 in regulating innate and adaptive immune response is discussed in the next sections.
4.1. IL10-dependent regulation by innate immune cells
Innate immune responses in the intestine are largely directed toward microbes or virions, which trigger activation of antigen-presenting cells (e.g., DCs, Mϕ) via PRRs. Signaling through PRRs is a critical first line of defense against potential pathogens. However, PPR signaling may result in aberrant activation of antigen presenting cells (APCs) and the development of chronic intestinal inflammation in the setting of specific genetic susceptibilities. PRR-mediated inflammatory responses can be regulated by IL10 (Boonstra et al., 2006; Chang, Guo, Doyle, & Cheng, 2007; Fiorentino, Zlotnik, Mosmann, Howard, & O’Garra, 1991). In the absence of IL10, PRR-induced proinflammatory expression is markedly increased in innate immune cells. One specific IL10-mediated regulatory mechanism occurs through abrogating MyD88-dependent responses by inducing proteosomal degradation of downstream signaling molecules IL1 receptor-associated kinase 4 (IRAK4) and TNF-receptor-associated factor 6 (TRAF6) (Chang, Kunkel, & Chang, 2009). Though microbial communities are required for driving intestinal inflammation in IL10-deficient mice, the essential role for APCs in this process was only recently investigated. The best evidence to date came when deletion of MyD88 in either LysM+ or CD11c+ mononuclear phagocytes in IL10-deficient mice proved to be protective against colitis (Hoshi et al., 2012). These data suggest that upon PRR triggering, defective IL10 signaling in APCs results in the acquisition of a proinflammatory state and/or loss of tolerance. In contrast, deletion of TLR4 in combination with IL10 exaggerates intestinal inflammation due to dysregulation of epithelial turnover leading to accumulation of apoptotic cells in the lamina propria implicating a protective role in TLR4 signaling (Matharu et al., 2009).
A direct role for innate immune cell dependent secretion of IL10 in regulating mucosal homeostasis was first demonstrated by Murai et al. who showed that IL10-deficient Rag1−/− failed to permit suppression of CD4+CD45RBhigh T cells by Foxp3+ Tregs (Murai et al., 2009). Similar studies were reported by Liu et al. who showed that IL10-deficient Rag2−/− mice are more susceptible to CD4+ T-cell transfer-induced colitis than IL10-suficient Rag2−/− mice (Liu, Tonkonogy, & Sartor, 2011). A CD11b+ myeloid cell population in the lamina propria, found to secrete large amounts of IL10, was suggested as a critical cell population in the intestine-regulating immune homeostasis (Murai et al., 2009). Intestinal Mϕs constitutively produce IL10 and are generally hypo-responsive to TLR-mediated stimulation in terms of inflammatory cytokine production (Kamada et al., 2005). The precise role of specific IL10-secreting Mϕs in regulating mucosal homeostasis is not known. Work by Takeda’s group recently showed that intestinal CX3CR1highCD11b+CD11c+ cell populations suppress intestinal inflammation by inhibiting T-cell responses. This process may be mediated by IL10 signaling since protection was abrogated when these cells were isolated from myeloid cell-specific STAT3-deficient mice (Kayama et al., 2012).
Even when IL10 is absent from innate immune cells, T cells are required for colitis induction (Liu et al., 2011; Murai et al., 2009). Increasing data suggest that innate immune cell-derived IL10 is required for the maintenance and/or function of Tregs (Liu et al., 2011; Murai et al., 2009). One study demonstrated that the CD11b+ myeloid cell-derived IL10 can regulate Foxp3 expression in Tregs in vitro (Murai et al., 2009). These authors also showed that myeloid cell-specific deletion of IL10 leads to reduced T reg numbers after Citrobacter rodentium infection compared to controls (Murai et al., 2013). These data point to a critical role for myeloid cell-derived IL10 in the generation and/or maintenance of Tregs and bridge a critical gap in the cross talk between the innate and adaptive immune systems in mucosal homeostasis. Several groups have suggested that intestinal CX3CR1highF4/80+ myeloid cell population secretes IL10 at steady state (Bain et al., 2013; Rivollier, He, Kole, Valatas, & Kelsall, 2012; Zigmond et al., 2012). These cells continue to produce high levels of IL10 in the presence of inflammation, both in vivo during DSS challenge (Zigmond et al., 2012) and in vitro following LPS stimulation (Bain et al., 2013). CX3CR1high cells have also been shown to express high levels of several IL10-inducible genes, such as CD163 and CD209, and Kayama et al. have recently reported that their transfer to SCID recipient can prevent CD45RBhigh-associated colitis (Kayama et al., 2012).
4.2. IL10-dependent regulation by adaptive immune cells
Chronic inflammation of the gut is often associated with expansion of Th1, Th2, or Th17 cells. IL10 signaling can inhibit the expansion of these pathological helper-T-cell populations to promote immune homeostasis. Many effector helper-T-cells can also produce IL10 under specific stimulating conditions. For instance, Th1 cells can produce IL10 in the presence of a strong T-cell receptor signal and APC-derived IL12 (Saraiva et al., 2009). Th2 cells produce IL10 in the presence of IL4-dependent STAT6 activation, and Th17 cells produce IL10 downstream of STAT3 activation (Fitzgerald et al., 2007; McGeachy et al., 2007; Stumhofer et al., 2007). Although effector T cells can produce IL10 in certain conditions, CD4+ T-cell activation and proliferation are typically globally inhibited by IL10-dependent suppression of APCs (de Waal Malefyt et al., 1991; Fiorentino, Zlotnik, Vieira, et al., 1991). While this seems to be redundant, IL10 expression by activated T cells may ensure another negative feedback loop to avoid pathogenic T-cell expansion.
Treg-derived IL10 is critical for the maintenance of immunological tolerance in the intestinal mucosa (Rubtsov et al., 2008). Under steady state, Tregs in the spleen and mesenteric lymph nodes (MLNs) express very little IL10. However, lamina propria Tregs express high amounts of IL10 accounting for nearly 30% of the IL10-producing CD4+ T cells in the small intestine (Maloy et al., 2003; Tiittanen, Westerholm-Ormio, Verkasalo, Savilahti, & Vaarala, 2008; Uhlig et al., 2006). Loss of IL10 in Foxp3+ Tregs is sufficient to promote the development of colitis in mice, albeit to a lesser extent than global IL10 deficiency when mice are colonized with Helicobacter spp. (Roers et al., 2004; Rubtsov et al., 2008). However, these mice do not develop systemic autoimmunity, which further indicates the regulatory specificity of Treg-derived IL10 in the intestinal mucosa (Rubtsov et al., 2008). In contrast, some, but not all studies, have suggested that IL10-deficient CD4+CD45RBlow Tregs can function normally in the suppression of CD4+CD45RBhigh T-cell-induced colitis in SCID mice (Asseman, Mauze, Leach, Coffman, & Powrie, 1999; Murai et al., 2009). These studies suggest unique characteristics of Foxp3+ and CD4+CD45RBlow regulatory T-cell subsets.
Finally, although much of the attention regarding IL10 secretion has focused primarily on myeloid cells and T lymphocytes, B cells can also produce IL10 and possess regulatory functions. A special subset of B cells, termed B10 regulatory B cells (B10 cells), secrete IL10 and upregulate CD1d under chronic inflammatory conditions, which serves to suppress intestinal inflammation (DiLillo, Matsushita, & Tedder, 2010; Mauri & Bosma, 2012; Mizoguchi, Mizoguchi, Takedatsu, Blumberg, & Bhan, 2002; Yanaba et al., 2008).
5. REGULATION OF MUCOSAL HOMEOSTASIS BY IL10 RECEPTOR SIGNALING IN MICE
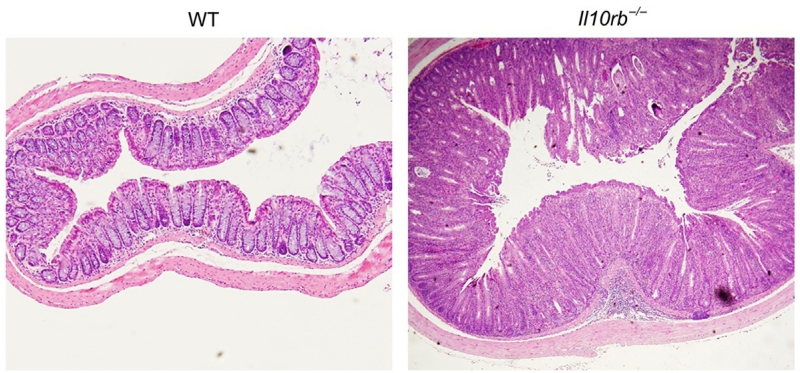
A critical role for IL10R signaling in the regulation of mucosal homeostasis was demonstrated over 15 years ago with the report that mice on a mixed 129SvEv/C57BL/6 background devoid of Il10rb develop colonic, but not small bowel, inflammation (Spencer et al., 1998). More recent studies have shown that IL10Rβ-deficient mice on the C57BL/6 background develop mild disease at 3–4 months of age, which becomes pronounced when TGFβ signaling in T cells is also impaired (Kang et al., 2008). Our own unpublished studies indicate that Il10rb−/− mice on the 129SvEv background develop moderate to severe colitis by 2–4 months of age, characterized by marked crypt hyperplasia, lamina propria infiltration, presence of crypt abscesses (Fig. 5.2). Since IL10Rβ is shared by several other important cytokine receptors (IL22R, IL26R, IFNλR) (Pestka et al., 2004), the mucosal inflammation observed in these aforementioned studies may result, at least in part, by loss or impairment of cytokine signaling by these additional members. In support of this hypothesis, IL10Rα-deficient mice generated on the C57BL/6 background do not develop colitis spontaneously (Pils et al., 2010), similar to IL10-deficient mice which are also resistant to colitis on this strain (Bristol et al., 2000; Farmer et al., 2001). Somewhat paradoxically, IL10Rα deletion limited to Tregs was associated with the development of severe spontaneous colitis (Chaudhry et al., 2011). While these results are rather perplexing, the intestinal flora can differ among animal facilities, altering microbial exposure and the development of intestinal inflammation in mice lacking IL10 or IL10R (see discussion below). Thus, phenotypic disparities between strains and studies may be impacted most from variations in microbial communities across institutions. Taken together, these data, complemented by similar clinical presentation and sequelae in IL10RA- or IL10RB-deficient patients, solidify the integral role IL10R-signaling plays in maintaining immunological tolerance in the intestinal mucosa (Begue et al., 2011; Engelhardt et al., 2013; Glocker et al., 2009; Kotlarz et al., 2012; Moran et al., 2013). Studies investigating the specific cell types requiring IL10R signaling are addressed in the following sections.
Figure 5.2.
Il10rb−/− mice develop spontaneous colitis. Histology images (4×) of distal colonic tissue obtained from 6-month-old wild type and Il10rb−/− mice.
5.1. IL10R signaling regulates T-cell function
IL10R signaling on Tregs has been shown by several groups to be critical for the regulation of intestinal immune responses. Murai and colleagues cotransferred Il10rb−/− Tregs with WT CD4+CD45RBhigh T cells and showed that these Tregs failed to suppress colitis (Murai et al., 2009). In these experiments, Tregs lost Foxp3 expression over time but not when transferred in the absence of CD4+CD45RBhigh cells, suggesting that IL10 signaling in Tregs is important for maintenance of Foxp3 expression under inflammatory conditions. Similarly, Chaudhry et al. demonstrated that mice with a targeted deletion of Il10ra in Foxp3+ Tregs develop severe spontaneous colitis, associated with a marked increase in memory T cells and a selective impairment in suppression of Th17+ cells (Chaudhry et al., 2011). Interestingly, Foxp3 expression was maintained in these Il10ra-deficient Tregs, even in the presence of inflammation. These differences may be explained by inherent limitations associated with the CD45RB transfer model and/or the effect of different microbiota on the intestinal immune system. Likewise, we reported the presence of Foxp3+ Tregs in the blood of a patient harboring an IL10RA null mutation, suggesting that IL10R signaling is dispensable for the development of Foxp3+ Tregs (Moran et al., 2013). However, further studies are needed to assess the function of Tregs in the blood and tissue of IL10R-deficient patients.
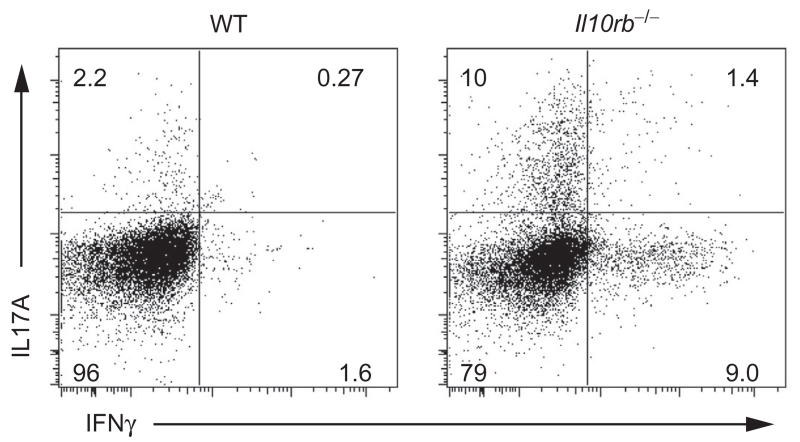
IL10 sensing by effector T cells is also important for intestinal mucosal homeostasis. Asseman and colleagues were the first to show that IL10R-blocking antibodies administered to SCID mice previously transferred with CD4+CD45RBlow cells results in intestinal inflammation (Asseman et al., 1999), suggesting that within this fraction of T cells, now known to contain Foxp3+ regulatory cells, are pathogenic cells responsive to IL10. This finding was corroborated when Kamanaka et al. transferred IL10R-deficient CD4+CD45RBlowFoxp3-delpeted T cells, which escaped control by Tregs and elicited a Th17-mediated colitis in lymphopenic hosts (Kamanaka et al., 2011). Furthermore, colitis induction by the transfer of Th17 cells Rag1−/− recipient mice is prevented by the cotransfer of Foxp3+ Tregs or IL10+Foxp3− Tr1 cells (Huber et al., 2011). However, such suppression requires functional expression of IL10R on Th17 cells, since abrogation of IL10R function on T cells is associated with expansion of both IL17A+IFNγ− and IL17A+IFNγ+ subsets (Huber et al., 2011). These studies underscore the importance of IL10R-dependent signals on restricting Th17 responses. The role of IL10R signaling on Th1 cells is less clear. Kamanaka and colleagues showed that among CD45RBhigh cells, which are known to trigger a Th1 response when adoptively transferred into lymphopenic hosts, IL10R signaling was not required for the T-effector cells to receive suppressive signals by WT Tregs (Kamanaka et al., 2011). However, we have observed in our l10rb−/− mouse colony expansion of both Th1+ and Th17+ CD4+ T cells in the lamina propria (Fig. 5.3), suggesting that IL10R might regulate proliferation of both Th1 and Th17 subsets.
Figure 5.3.
Increase in IL17A+ and INFγ+ CD4+ T cells in the lamina propria of Il10rb−/− mice. Lamina propria cells of 4-month-old WT and Il10rb−/− mice were obtained, and stimulated with phorbol myristate acetate (PMA) and ionomycin for 4 h, in the presence of GolgiStop. Plots gated on CD4+ T cells.
5.2. IL10R signaling regulates innate immune cell function
Recent data presented above describe an important role of IL10R signaling in maintaining intestinal mucosal homeostasis in various T-cell subsets. For many years, it has been known that IL10 decreases the expression of MHCII and costimulatory molecules on DCs and Mϕ, as well as their proinflammatory cytokine secretion (Bhattacharyya et al., 2004; Corinti et al., 2001; Fiorentino, Zlotnik, Mosmann, et al., 1991; Steinbrink, Wolfl, Jonuleit, Knop, & Enk, 1997). In addition, pretreating immature DCs in vitro with IL10 decreases their capacity to stimulate CD4+ T cells in a dose-dependent manner. However, the role of IL10R-dependent signaling in innate immune cells at mucosal surfaces such as the gastrointestinal tract has not been well defined. Mellilo and colleagues reported that DC-specific loss of STAT3 signaling, which is downstream of the IL10R, leads to mild small- and large-intestine inflammation (Melillo et al., 2010). However, STAT3 is also a component of the signaling cascade of other cytokines, such as IL6, and therefore the phenotype observed in these mice might not be solely due to loss of IL10R signaling. Nevertheless, evidence from other immune compartments suggests that IL10R signaling plays an important role in differentiation and function of various innate immune cells. Il10ra-targeted deletion in monocytes/Mϕs (LysM-Cre+-Il10raflox/flox mice) leads to enhanced susceptibility to an LPS-induced model of endotoxemia, with elevated serum levels of IL17, TNF, IL1, and IL12 (Pils et al., 2010). In addition, employing CD11c-Cre+-Il10raflox/flox mice, Girard-Madoux et al. assessed the role of IL10Rα in DCs (Girard-Madoux, Kel, Reizis, & Clausen, 2012). At steady state, IL10Rα-deficient DCs retain an immature phenotype and express similar levels of costimulatory molecules compared to nontransgenic mice. However, LPS or sCD40L stimulation results in enhanced DC secretion of IL6 and TNF. In both transgenic mouse models, secretion of IL10 by stimulated DCs or Mϕs was enhanced, implying that there might be an autocrine feedback regulating IL10 production in these cells. These investigators also assessed the role of IL10R signaling on DCs in different phases of a contact hypersensitivity model. They demonstrate that priming of T cells by DCs in the skin draining lymph node does not require IL10R signaling in DCs, as CD11c-Cre+-Il10raflox/flox mice have similar ear swelling 24 h following hapten challenge. However, T-effector cell responses in the skin of mice 48 h after challenge were enhanced with exaggerated ear swelling. These data suggest that autocrine or paracrine sensing of IL10 by DCs is critical to limiting T-effector-induced inflammation.
IL10R signaling has also been shown to orchestrate the development of monocytes into different subsets of peritoneal Mϕs (Nguyen, Tran, Muller, & Jack, 2012). During early phases of peritonitis, IL10 expression is increased, and monocytes that migrate into the peritoneum develop into MHCIIlow Mϕs, while in the later stages when IL10 levels are decreased, monocytes differentiate into MHCIIhigh Mϕ. WT monocytes transferred into Il10ra-deficient mice develop into both MHClow and MHChigh Mϕ, while host monocytes develop almost exclusively into MHChigh Mϕ. This is supported by lack of MHCIIlow monocytes in the peritoneum of IL10-deficient mice. It remains to be determined whether IL10R-dependent signals regulate myeloid cell differentiation in other immune compartments, especially at mucosal surfaces.
IL10 can also modulate responses of neutrophils and NK cells. In non-inflammatory environments, neutrophils do not express IL10Rα and are therefore unresponsive to IL10 (Crepaldi et al., 2001). However, following in vitro stimulation of human neutrophils with LPS or IL4, or in neutrophils isolated from septic patients (Crepaldi et al., 2001; Tamassia et al., 2008), IL10Rα is synthesized, allowing for modulation of cytokine production by IL10 and enhanced expression of IL1RA, a soluble factor that competes with cognate receptors for proinflammatory mediators IL1α and IL1β (Crepaldi et al., 2001). In NK cells, IL10 has a unique role in which it delivers a stimulatory signal rather than a suppressive one. IL10, possibly in combination with IL18, has been shown to enhance cell proliferation and cytotoxic activity (Cai, Kastelein, & Hunter, 1999; Mocellin et al., 2004). The role of IL10 in modulating intestinal neutrophils and NK cell function at baseline and in the setting of inflammation at the present time remains unknown.
6. IL10-DEPENDENT SIGNALING SHAPES THE INTESTINAL MICROBIOME
The initial characterization of IL10-deficient mice pointed to a critical role for the intestinal flora in regulating disease severity and location (Kuhn et al., 1993). Conventionally, housed IL10-deficient mice develop severe enterocolitis while disease in specific pathogen free (SPF) conditions is restricted to the colon. Interestingly, SPF IL10-deficient mice free of Helicobacter species do not develop colitis (Burich et al., 2001). Moreover, germfree IL10-deficient mice are also free of intestinal inflammation (Sellon et al., 1998). Subsequently, considerable effort has been focused on identifying single bacterial strains sufficient to drive disease initiation in IL10-deficient mice. Extensive work from the Sartor laboratory characterized germ-free IL10-deficient mice mono-colonized with various bacterial strains. While most individual strains failed to induce intestinal inflammation including Helicobacter hepaticus, Pseudomonas fluorescens, Candida albicans, or L. lactis, monocolonization with Enterococcus faecalis, E. coli, or Bifidobacterium animalis is each sufficient to induce intestinal inflammation, albeit with different kinetics and anatomical locations (Dieleman et al., 2000; Kim, Tonkonogy, Karrasch, Jobin, & Sartor, 2007; Moran, Walter, Tannock, Tonkonogy, & Sartor, 2009). This is not an artifact of mono-association since colonization of germfree WT mice with any of these strains does not induce intestinal inflammation. Further support for microbial stimulation influencing IL10-dependent disease initiation comes from more recent work by Stappenbeck and colleagues using a non-germ-free approach which demonstrated that IL10rb−/− mice defective in T-cell TGFβ signaling (CD4-dnTgfbr2 Il10rb−/−), following a course of antibiotic treatment, developed severe colitis upon colonization with commensal Bacteroides species (B. thetaiotaomicron and B. vulgatus) (Bloom et al., 2011). It is noteworthy that although Helicobacter spp. is required for colitis development in IL10−/− mice, Helicobacter spp. alone is insufficient to cause disease (Dieleman et al., 2000). This suggests that the role of Helicobacter spp. in colitis associated with IL10 deficiency may be attributed to alterations in the microbial community or changes in bacterial metabolism that, in turn, impact immunological tolerance. Interestingly, although Enterobacteriaceae species are elevated in colitis, colonization of CD4-dnTgfbr2.Il10rb−/− mice with these organisms following antibiotic treatment does not induce disease, highlighting a possible disconnect between abundance and consequence in IBD (Bloom et al., 2011).
What is the mechanism by which certain bacterial species drive intestinal inflammation? IL10−/− in hematopoietic lineages is linked to colitis development since transplantation of WT bone marrow into IL10−/− mice prevents diseases (Bamba et al., 2006). Using a more focused approach, experiments from the Rennick laboratory dissected the role of CD4+ T cells in colitis induction in IL10−/− mice. CD4+ T cells isolated from the lamina propria of colitic IL10−/− mice that were injected into Rag2−/− mice transferred colitis (Davidson et al., 1996). Later, it was shown that H. hepaticus Ag-specific IL10-deficient CD4+ T-cell clones elicit colitis in Rag2−/− mice only when they are colonized with Helicobacter spp. (Kullberg et al., 2002). Since in the transfer studies with IL10-deficient colitogenic T cells from the lamina propria, the CD4+ cell population was unfractionated and also contained regulatory CD4+ T cells (Davidson et al., 1996); it is possible that the phenotype may have been driven by microbial-induced defects in the development or function Tregs. Evidence supporting this hypothesis came from additional transfer experiments in which cotransfer of WT CD4+CD45RBlow Tregs prevented induction of colitis by IL10−/− CD4+ T cells into Rag1−/− mice, only when the WT Tregs were obtained from Helicobacter-infected donor mice (Kullberg et al., 2002).
One of the primary functions of Tregs in the intestine is to induce/maintain immunological tolerance to luminal antigens by suppressing helper-T-cell responses, which requires interactions with APCs in the draining MLNs. Altered sensing of microbial signals by APCs in IL10−/− animals may be critical to the disruption of mucosal homeostasis. The Medzhitov laboratory recently explored this hypothesis employing IL10−/− mice also deficient in MyD88, an essential signaling adapter molecule downstream of many bacterial sensing TLRs, in selective myeloid cell subsets. They demonstrated that MyD88-dependent microbial sensing in CD11c+ or LysM+ cells, but not epithelial cells, was required to cause colitis in IL10−/− mice (Hoshi et al., 2012). What is striking about these results is that earlier work showed that Il10−/− mice deficient for TLR4 developed worse disease (Matharu et al., 2009). Thus, it seems that microbial sensing through TLRs can promote mucosal immune homeostasis, but these regulatory mechanisms are compromised in a setting of defective IL10 signaling.
Finally, a link between chronic inflammation and cancer development has been appreciated for more than a century by initial observation made by Rudolf Virchow in 1863 (Balkwill & Mantovani, 2001). However, the contribution of the microbiota in colitis-associated cancer has only recently been explored. Jobin and colleagues demonstrated that monoassociating germ-free IL10−/− mice with E. coli, but not E. coli lacking the polyketide synthases (pks) pathogenicity island, in combination with the mutagen azoxymethane, possess the capacity to drive dysplastic lesions at sites of inflammation (Arthur et al., 2012). This highlights the need to consider microbial metagenomics when investigating the role of inflammation and intestinal cancer development in genetically susceptible hosts, such as those with IL10 deficiency.
Collectively, these data suggest that enteric microbial antigens drive intestinal inflammation in genetically susceptible IL10- and IL10R-deficient mice. In the absence of immunomodulatory IL10-dependent signals, upon recognition of commensal bacteria, myeloid cells adopt a proinflammatory phenotype driving antigenic Th1/Th17 CD4+ T-cell responses. Regulatory circuits may be further compromised in the setting of IL10 deficiency due to the decreased suppressive capacity of induced Tregs (Schmitt et al., 2012). The resulting crescendo is a breakdown in immunological tolerance to specific enteric commensal bacteria leading to chronic intestinal inflammation.
7. IMPACT OF IL10 AND IL10R SIGNALING DEFECTS IN HUMANS
Recent studies have additionally confirmed a role for IL10-dependent signals regulating mucosal homeostasis in humans, with particular relevance to the development of IBD. Large-scale GWAS that have focused on adult-onset IBD identified IL10 as an IBD risk allele, along with several down-stream IL10/IL10R signaling components, including TYK2 and STAT3 (Franke et al., 2008; Franke, McGovern, et al., 2010; Jostins et al., 2012). However, while large-scale adolescent and adult GWAS have not identified IL10RA or IL10RB to be associated with IBD risk (Imielinski et al., 2009; Kugathasan et al., 2008), numerous studies focusing on infantile-onset IBD (IO-IBD) have captured the importance of IL10R variants in this regard (Begue et al., 2011; Engelhardt et al., 2013; Glocker et al., 2009, 2010; Glocker, Kotlarz, Klein, Shah, & Grimbacher, 2011; Kotlarz et al., 2012; Mao et al., 2012). Furthermore, a recent study focusing on very early-onset IBD (VEO-IBD), defined as disease onset before 6 years of age, has further expanded the importance of IL10RA variants in IBD risk in infants and young children with ulcerative colitis (UC) (Moran et al., 2013). This section will review the current data of the various mutations identified in IL10 and the IL10R genes and their relationship to the development of IO- and VEO-IBD.
The study of infants presenting with IBD has led to monumental advances in our understanding of the pivotal role of IL10/IL10R signaling in maintaining human gut homeostasis. In 2009, Klein, Grimbacher, and colleagues were the first to identify causative loss of function variants in IL10RA and IL10RB in patients with severe infantile-IBD (Glocker et al., 2009). These patients typically present within the first few months of life with severe enterocolitis, perianal abscesses, enterocutaneous fistulas, and chronic folliculitis (Begue et al., 2011; Glocker et al., 2009, 2011; Kotlarz et al., 2012). One of the first illustrative examples includes a family with two affected infants, harboring a causal homozygous nonsense mutation in IL10RB (p.W159Stop) that led to loss of surface expression of IL10R2 and aberrant IL10R-dependent signaling characterized by defective IL10-induced STAT3 phosphorylation (Glocker et al., 2009). This group concurrently described two distinct homozygous missense mutations in IL10RA identified in two unrelated patients presenting with infantile colitis: (p.T84I and p.G141R), each leading to abrogated IL10-mediated signaling (Glocker et al., 2009).
While some reported cases of infantile colitis with variant IL10R result in loss of IL10R surface expression, a novel compound heterozygote mutation in IL10RA (p.T84I; p.R101W) was recently identified, resulting in normal IL10R1 expression, normal binding to IL10, but lack of IL10R1 phosphorylation following IL10 binding, ultimately resulting in defective IL10-mediated signaling (Mao et al., 2012). In many cases, the functional significance of the identified variant was confirmed by demonstrating that the patient cell lines (or the expressed variant in cell lines) could not induce IL10-mediated STAT3 phosphorylation or inhibition of TNF-induced proinflammatory cytokine secretion in mononuclear cells (Glocker et al., 2009; Kotlarz et al., 2012).
Subsequently, numerous additional causative mutations have been identified in patients with infantile-IBD in IL10RA, IL10RB as well as in IL10 itself (Table 5.1). These include homozygous mutations including nonsense mutations, missense mutations, large deletions of exons, non-protein coding single nucleotide mutations (splice site and 3′UTR mutations), as well as compound heterozygote mutations (Begue et al., 2011; Engelhardt et al., 2013; Glocker et al., 2009; Kotlarz et al., 2012; Mao et al., 2012; Moran et al., 2013). Our review of the literature to date indicates that there are at least 24 distinct variants identified in IL10R in patients presenting with IO- and VEO-IBD. Of interest, all but one of these variants was identified in IO-IBD patients (Engelhardt et al., 2013).
Table 5.1.
Identified mutations in IL10RA, IL10RB, and IL10 leading to infantile and very early onset IBD
| Mutation | Clinical manifestation | Age of onset |
References |
|---|---|---|---|
| IL10R1: p.G141R (homozygous)a |
Severe enterocolitis, enterocutaneous fistulas, Perianal abscesses, chronic folliculitis |
<1 year | Glocker et al. (2009) |
| IL10R1: p.T84I (homozygous)a |
Severe pancolitis | 3 months | Glocker et al. (2009) |
| IL10R1: p.R101W (homozygous)a |
Severe and progressive colitis |
< 3 months | Kotlarz et al. (2012) |
| IL10R1: p.I169T | Severe and progressive colitis, folliculitis, atopic dermatitis |
< 3 months | Kotlarz et al. (2012) |
| IL10R1: p.I169T (homozygous) |
Severe and progressive colitis, folliculitis, atopic dermatitis |
< 3 months | Kotlarz et al. (2012) |
| IL10R1: p.R262C (homozygous) |
Pancolitis, perianal disease, no small intestinal inflammation, but granulomas present |
1 month | Begue et al. (2011) |
| IL10R1: p.P206X (homozygous mutation in 5′ splice donor site, leading to premature stop codon) |
Fevers, skin folliculitis, bloody diarrhea and colonic erosions, perianal disease with fistula, joint effusions |
< 5 months | Moran et al. (2013) |
| IL10R1: p.G125R (homozygous)a,b |
Severe colitis. Both patients had family members with fatal infantile colitis |
1 month | Engelhardt et al. (2013) |
| IL10R1: p.L125R (homozygous)a |
Severe colitis, recurrent infections |
1 month | Engelhardt et al. (2013) |
|
IL10RA: Ex1_3del (homozygous deletions) |
Severe colitis, enteritis, otitis media, urinary tract infections. Cousin with neonatal onset diarrhea, died in infancy |
2 months | Engelhardt et al. (2013) |
| IL10R1: p.Y57Y; p.V23fsX31 (compound heterozygote) |
Colitis, sinusitis | 3 months | Engelhardt et al. (2013) |
| IL10R1: p.T84I; p.R101W (compound heterozygote) |
Neonatal onset CD with severe growth failure and nonspecific inflammation from rectum to descending colon |
<2nd week of life |
Mao et al. (2012) |
| IL10R1: pY57C; R117C (compound heterozygote) |
Severe and progressive colitis, arthralgia, arthritis, Kawasaki disease |
<3 months | Kotlarz et al. (2012) |
| IL10R2: p.W159X (homozygous)c,d |
Proctitis, folliculitis, perianal abscesses, recurrent infections, impaired wound healing, multiple enterocutaneous fistulas. |
3 months |
Glocker et al. (2009) and Kotlarz et al. (2012) |
| IL10R2: p.C66Y | Severe and progressive colitis, folliculitis |
<3 months | Kotlarz et al. (2012) |
| IL10R2: p.R117H (homozygous) |
Severe colitis, recurrent ear infections, oral ulcers, toxic megacolon, perforation, peritonitis. Brother died of severe enteropathy |
3½ years old |
Engelhardt et al. (2013) |
| IL10R2: p.G193R (homozygous) |
Intestinal adhesions, colitis, fatal septicemia. Had two siblings with fatal colitis |
10 days | Engelhardt et al. (2013) |
|
IL10RB: c.C52T (in 3′ UTR) (homozygous) |
Severe progressive colitis, folliculitis |
<3 months | Kotlarz et al. (2012) |
| IL10R2: p.W204X; p.S230X (compound heterozygote) |
Severe and progressive colitis, dermatitis |
<3 months | Kotlarz et al. (2012) |
| IL10R2: p.W204X (homozygous) |
Severe and progressive colitis, folliculitis |
< 3 months | Kotlarz et al. (2012) |
| IL10R2: p.E141X (homozygous) |
Pancolitis, perianal lesions, no small intestinal inflammation, but granulomas |
3 months | Begue et al. (2011) |
|
IL10RB c.331+907_574del (homozygous deletion) |
Severe and progressive colitis, folliculitis |
< 3 months | Kotlarz et al. (2012) |
| IL10R2 deletion p. W18fsX29 (homozygous) |
Colitis, recurrent otitis media |
1 month | Engelhardt et al. (2013) |
| IL10R2: p.L59fsX72 (homozygous) |
Severe colitis, bronchitis, oral ulcers, gingivitis. Two siblings and an uncle with fatal colitis |
1 month | Engelhardt et al. (2013) |
| IL10: p.G153N (homozygous)c |
Severe and progressive colitis |
< 3 months | Kotlarz et al. (2012) |
| IL10: p.G113R (homozygous)b,c,d |
Bloody diarrhea. Female afflicted with perianal, rectovaginal fistulas |
3 and 11 months |
Glocker et al. (2010) |
Underwent HSCT with clinical remission.
Identified in two patients.
Identified in three patients.
Two patients underwent HSCT, both with clinical remission.
We have yet to determine the significance of more subtle deficits in the IL10/IL10R pathway on patients with less extreme presentations of IBD, including older children and adults. It is possible that altered but not complete loss of function of IL10/IL10R signaling results in less severe presentations of IBD, either in childhood or later in life. There is some evidence that such a scenario might hold true. As noted earlier, GWAS have identified SNPs in IL10 and are associated with increased risk of both UC and CD (Franke et al., 2008; Franke, Balschun, et al., 2010; Jostins et al., 2012). Moreover, Sanchez et al. linked SNPs within IL10 to disease location of childhood IBD (Sanchez, Levy, Costea, & Sinnett, 2009). While large-scale GWAS have not identified IL10R as a risk allele in adolescent- and adult-onset IBD, we have identified two IL10RA polymorphisms (rs2228054 and rs2228055) associated with increased risk of UC presenting in young children prior to 6 years of age (Moran et al., 2013). Even so, missense mutations in IL10RA have been studied for their role in predisposition to adult-onset IBD. Within IL10RA, a polymorphism resulting in a single-amino acid change, S138G, results in loss of function with defective IL10-mediated STAT1- or STAT3-dependent signaling (Grundtner et al., 2008). However, the relevance of this mutation with respect to overall IBD predisposition remains unclear as, rather paradoxically, it was found to be protective for the risk of UC in some, but not all, populations (Grundtner et al., 2008). In addition, another SNP in the IL10RA gene, G330R, in strong linkage disequilibrium with the S138G variant appears to affect the kinetics of STAT-phosphorylation (Finsterbusch, Khare, Campregher, Evstatiev, & Gasche, 2011). Finally, a variant identified in the IL10 gene in the proximal end of the 3′UTR has been found to be associated with predisposition to both UC and CD in several populations due to its impact on IL10 expression (Andersen et al., 2010; Doecke et al., 2013). Further studies are needed to define the functional significance of these IL10 and IL10R SNPs in regulating anti-inflammatory circuits in IBD.
Most patients with deleterious mutations in IL10 and/or IL10R exhibit severe and extensive intestinal inflammation that is resistant to immunosuppressive medications including steroids, anti-TNF antibodies, methotrexate, and thalidomide (Begue et al., 2011; Engelhardt et al., 2013; Glocker et al., 2010, 2009; Kotlarz et al., 2012). The understanding, that loss of IL10R signaling on hematopoietic cells is a key factor driving the inflammation, has put forth an alternate means of treatment: allogeneic hematopoietic stem cell transplantation (HSCT). Glocker and colleagues described the first case of HSCT in their patient with a nonsense mutation in IL10RB (W159X) (Glocker et al., 2009). While grade III graft versus host disease involving the skin developed, this was treated with prednisone and no further complications ensued. Most remarkable is that within a year this patient experienced resolution of cutaneous folliculitis, perianal fistulas, and intestinal inflammation (Glocker et al., 2009). To date, HSCT has been reported successfully in at least seven patients with mutations in IL10RA and IL10RB (five for mutations in IL10RA; two for IL10RB) (Engelhardt et al., 2013; Glocker et al., 2009; Kotlarz et al., 2012; Mao et al., 2012; Dinwiddie et al., 2013). In addition, another patient underwent successful HSCT for a functional variant in IL10 (Glocker et al., 2010). Strikingly, many of these patients have since been able to discontinue immunosuppressive therapy and continue to do well clinically. Interestingly, no obvious differences in the outcome of transplantation have been reported for patients transplanted for either IL10R1 or IL10R2 deficiency (Engelhardt et al., 2013; Glocker et al., 2009; Kotlarz et al., 2012; Moran et al., 2013). This is noteworthy since IL10R2 is also expressed on nonhematopoietic cells and is utilized as the signaling component in concert with other cytokine receptors (IL22R, IFNλR) known to regulate intestinal epithelial function. To date, HSCT is the only available curative intervention for patients with such severe clinical phenotype resulting from IL10/IL10R mutations with aberrant signaling. These patients should be strongly considered for transplant, as they tend to remain refractory to the most optimized medical and surgical interventions.
Given the critical role of IL10/IL10R signaling in dampening the inflammatory response and its strong implication in IBD, this avenue has been explored for innovative therapeutic benefits. Several human clinical trials have examined the role of IL10 for the treatment of adult CD, with disappointing results (Braat et al., 2006; Colombel et al., 2001; Fedorak et al., 2000; Schreiber et al., 2000; van Deventer, Elson, & Fedorak, 1997). The Cochrane review concluded that recombinant human IL10 did not show any benefit in the treatment of active CD, as compared to placebo (Buruiana, Sola, & Alonso-Coello, 2010). Several explanations have been proposed for lack of therapeutic benefit, including insufficient intestinal concentrations of IL10 to yield consistent therapeutic response, interpatient variability in response, short half-life of IL10, and concern that elevated levels of circulating IL10 may result in a proinflammatory state with associated increased IFNγ production (Marlow, van Gent, & Ferguson, 2013; Paul, Khare, & Gasche, 2012). Despite these results, novel approaches of delivering IL10 that permit increased stability or target to the intestinal mucosa are warranted. In addition, identifying patient cohorts predicted based on genetic and/or functional findings, to respond to IL10 merits further study.
8. CONCLUSIONS
In this chapter, we have reviewed recent data of how IL10 regulates different cell populations and modulates immune responses, especially in the intestine. Evidence from murine models lacking IL10/IL10R signaling as well as from patients harboring functional mutations in either IL10 or IL10R genes suggest that this cytokine and its receptor are key regulators of mucosal homeostasis. Despite tremendous progress in our understanding of the biology related to IL10-associated responses, several important questions remain unanswered: How do cells that express cytokine receptors that both signal through STAT3 (e.g., IL6R and IL10R) result in either a proinflammatory or anti-inflammatory signature? What is the precise role of innate immune IL10R signaling in the gut? What are the sources of IL10 in the intestine that mediate tolerance? What are the functional consequences of different variants within IL10 and IL10RA/IL10RB in humans that do not lead to complete loss of function? Further exploration of these processes will aid in developing new therapies for IBD and other inflammatory disorders—especially those that entail targeted IL10 delivery to the intestinal mucosa.
ACKNOWLEDGMENTS
S.B.S is supported by NIH Grants HL59561, DK034854, and AI50950 and the Wolpow Family Chair in IBD Treatment and Research. This work was also funded in part by a grant from the Helmsley Charitable Trust to C.K., A.M.M., and S.B.S.
REFERENCES
- Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. Journal of Immunology. 2003;171:4984. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature Immunology. 2001;2:725. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Andersen V, Ernst A, Christensen J, Ostergaard M, Jacobsen BA, Tjonneland A, et al. The polymorphism rs3024505 proximal to IL-10 is associated with risk of ulcerative colitis and Crohns disease in a Danish case-control study. BMC Medical Genetics. 2010;11:82. doi: 10.1186/1471-2350-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. The Journal of Experimental Medicine. 1999;190:995. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6C(hi) monocyte precursors. Mucosal Immunology. 2013;6:498. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bamba S, Lee CY, Brittan M, Preston SL, Direkze NC, Poulsom R, et al. Bone marrow transplantation ameliorates pathology in interleukin-10 knockout colitic mice. The Journal of Pathology. 2006;209:265. doi: 10.1002/path.1967. [DOI] [PubMed] [Google Scholar]
- Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. Journal of Immunology. 2008;180:2752. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. The American Journal of Gastroenterology. 2011;106:1544. doi: 10.1038/ajg.2011.112. [DOI] [PubMed] [Google Scholar]
- Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effectsof IL-10 on lipopolysaccharide-induced macrophage activation. Journal of Immunology. 2002;168:6404. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS, Jr., Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IkappaB kinase activity. Blood. 2004;104:1100. doi: 10.1182/blood-2003-12-4302. [DOI] [PubMed] [Google Scholar]
- Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, et al. Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host & Microbe. 2011;9:390. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. The Journal of Experimental Medicine. 1991;174:1549. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. Journal of Immunology. 2006;177:7551. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- Bourreille A, Segain JP, Raingeard de la Bletiere D, Siavoshian S, Vallette G, Galmiche JP, et al. Lack of interleukin 10 regulation of antigen presentation-associated molecules expressed on colonic epithelial cells. European Journal of Clinical Investigation. 1999;29:48. doi: 10.1046/j.1365-2362.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clinical Gastroenterology and Hepatology. 2006;4:754. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Bristol IJ, Farmer MA, Cong Y, Zheng XX, Strom TB, Elson CO, et al. Heritable susceptibility for colitis in mice induced by IL-10 deficiency. Inflammatory Bowel Diseases. 2000;6:290. doi: 10.1002/ibd.3780060407. [DOI] [PubMed] [Google Scholar]
- Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2001;281:G764. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- Buruiana FE, Sola I, Alonso-Coello P. Recombinant human interleukin 10 for induction of remission in Crohn’s disease. Cochrane Database of Systematic Reviews. 2010;11:CD005109. doi: 10.1002/14651858.CD005109.pub3. http://dx.doi.org/10.1002/14651858.CD005109.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. European Journal of Immunology. 1999;29:2658. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chang EY, Guo B, Doyle SE, Cheng G. Cutting edge: Involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. Journal of Immunology. 2007;178:6705. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
- Chang J, Kunkel SL, Chang CH. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18327. doi: 10.1073/pnas.0905815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Hershberg RM, Furuta GT, Blumberg RS. Ligation of intestinal epithelial CD1d induces bioactive IL-10: Critical role of the cytoplasmic tail in autocrine signaling. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13938. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut. 2001;49:42. doi: 10.1136/gut.49.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. The Journal of Allergy and Clinical Immunology. 2008;121:1108. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. Journal of Immunology. 2001;166:4312. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- Crepaldi L, Gasperini S, Lapinet JA, Calzetti F, Pinardi C, Liu Y, et al. Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. Journal of Immunology. 2001;167:2312. doi: 10.4049/jimmunol.167.4.2312. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nature Immunology. 2003;4:540. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. The Journal of Experimental Medicine. 1996;184:241. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. The Journal of Experimental Medicine. 1991;174:915. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter H, Elewaut D, Turovskaya O, Huflejt M, Shimeld C, Hagenbaugh A, et al. Regulation of mucosal immune responses by recombinant interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterology. 2002;122:1829. doi: 10.1053/gast.2002.33655. [DOI] [PubMed] [Google Scholar]
- Denning TL, Campbell NA, Song F, Garofalo RP, Klimpel GR, Reyes VE, et al. Expression of IL-10 receptors on epithelial cells from the murine small and large intestine. International Immunology. 2000;12:133. doi: 10.1093/intimm/12.2.133. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Arends A, Tonkonogy SL, Goerres MS, Craft DW, Grenther W, et al. Helicobacter hepaticus does not induce or potentiate colitis in interleukin-10-deficient mice. Infection and Immunity. 2000;68:5107. doi: 10.1128/iai.68.9.5107-5113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Annals of the New York Academy of Sciences. 2010;1183:38. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. Journal of Immunology. 2004;172:4733. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. Journal of Immunology. 1993;151:1224. [PubMed] [Google Scholar]
- Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. Journal of Immunology. 1992;148:3133. [PubMed] [Google Scholar]
- Dinwiddie DL, Bracken JM, Bass JA, Christenson K, Soden SE, Saunders CJ, et al. Molecular diagnosis of infantile onset inflammatory bowel disease by exome sequencing. Genomics. 2013;102(5-6):442. doi: 10.1016/j.ygeno.2013.08.008. http://dx.doi.org/10.1016/j.ygeno.2013.08.008. Epub 2013 Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doecke JD, Simms LA, Zhao ZZ, Huang N, Hanigan K, Krishnaprasad K, et al. Genetic susceptibility in IBD: Overlap between ulcerative colitis and Crohn’s disease. Inflammatory Bowel Diseases. 2013;19:240. doi: 10.1097/MIB.0b013e3182810041. [DOI] [PubMed] [Google Scholar]
- Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. Journal of Interferon & Cytokine Research. 1999;19:563. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- Engelhardt KR, Shah N, Faizura-Yeop I, Kocacik Uygun DF, Frede N, Muise AM, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. The Journal of Allergy and Clinical Immunology. 2013;131:825. doi: 10.1016/j.jaci.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Farmer MA, Sundberg JP, Bristol IJ, Churchill GA, Li R, Elson CO, et al. A major quantitative trait locus on chromosome 3 controls colitis severity in IL-10-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13820. doi: 10.1073/pnas.241258698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, et al. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. Gastroenterology. 2000;119:1473. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. Journal of Immunology. 1995;155:1079. [PubMed] [Google Scholar]
- Finsterbusch M, Khare V, Campregher C, Evstatiev R, Gasche C. An intracytoplasmic IL-10 receptor variant permits rapid reduction in STAT3 activation. Genes and Immunity. 2011;12:575. doi: 10.1038/gene.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. The Journal of Experimental Medicine. 1989;170:2081. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. Journal of Immunology. 1991;147:3815. [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. Journal of Immunology. 1991;146:3444. [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature Immunology. 2007;8:1372. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nature Genetics. 2008;40:1319. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- Franke A, Balschun T, Sina C, Ellinghaus D, Hasler R, Mayr G, et al. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL) Nature Genetics. 2010;42:292. doi: 10.1038/ng.553. [DOI] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nature Genetics. 2010;42:1118. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. The Journal of Experimental Medicine. 2003;197:7. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs VC, Pennica D. CRF2-4: Isolation of cDNA clones encoding the human and mouse proteins. Gene. 1997;186:97. doi: 10.1016/s0378-1119(96)00690-7. [DOI] [PubMed] [Google Scholar]
- Girard-Madoux MJ, Kel JM, Reizis B, Clausen BE. IL-10 controls dendritic cell-induced T-cell reactivation in the skin to limit contact hypersensitivity. The Journal of Allergy and Clinical Immunology. 2012;129:143. doi: 10.1016/j.jaci.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, et al. Infant colitis—It’s in the genes. Lancet. 2010;376:1272. doi: 10.1016/S0140-6736(10)61008-2. [DOI] [PubMed] [Google Scholar]
- Glocker E-O, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England Journal of Medicine. 2009;361:2033. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B. IL-10 and IL-10 receptor defects in humans. Annals of the New York Academy of Sciences. 2011;1246:102. doi: 10.1111/j.1749-6632.2011.06339.x. [DOI] [PubMed] [Google Scholar]
- Grundtner P, Gruber S, Murray SS, Vermeire S, Rutgeerts P, Decker T, et al. The IL-10R1 S138G loss-of-function allele and ulcerative colitis. Genes and Immunity. 2008;10:84. doi: 10.1038/gene.2008.72. [DOI] [PubMed] [Google Scholar]
- Hagenbaugh A, Sharma S, Dubinett SM, Wei SH, Aranda R, Cheroutre H, et al. Altered immune responses in interleukin 10 transgenic mice. The Journal of Experimental Medicine. 1997;185:2101. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW. A receptor for interleukin 10 is related to interferon receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11267. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N, Schenten D, Nish SA, Walther Z, Gagliani N, Flavell RA, et al. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nature Communications. 2012;3:1120. doi: 10.1038/ncomms2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O’Connor W, Jr., Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nature Genetics. 2009;41:1335. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. The Journal of Clinical Investigation. 2008;118:1132. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. Journal of Immunology. 2008;180:6325. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. Journal of Immunology. 2005;175:6900. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O’Connor W, Jr., et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. The Journal of Experimental Medicine. 2011;208:1027. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Bloom SM, Norian LA, Geske MJ, Flavell RA, Stappenbeck TS, et al. An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Medicine. 2008;5:e41. doi: 10.1371/journal.pmed.0050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, et al. Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5010. doi: 10.1073/pnas.1114931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Tonkonogy SL, Karrasch T, Jobin C, Sartor RB. Dual-association of gnotobiotic IL-10−/− mice with 2 nonpathogenic commensal bacteria induces aggressive pancolitis. Inflammatory Bowel Diseases. 2007;13:1457. doi: 10.1002/ibd.20246. [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO Journal. 1997;16:5894. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: Implications for diagnosis and therapy. Gastroenterology. 2012;143:347. doi: 10.1053/j.gastro.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Kugathasan S, Baldassano RN, Bradfield JP, Sleiman PM, Imielinski M, Guthery SL, et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nature Genetics. 2008;40:1211. doi: 10.1038/ng.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. The Journal of Experimental Medicine. 2006;203:2485. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, et al. T regulatory cells suppress Helicobacter hepaticus-induced colitis. The Journal of Experimental Medicine. 2002;196:505. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CF, Ripperger J, Morella KK, Jurlander J, Hawley TS, Carson WE, et al. Receptors for interleukin (IL)-10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements. The Journal of Biological Chemistry. 1996;271:13968. doi: 10.1074/jbc.271.24.13968. [DOI] [PubMed] [Google Scholar]
- Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, et al. SOCS3 regulates the plasticity of gp130 signaling. Nature Immunology. 2003;4:546. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- Liu Y, de Waal Malefyt R, Briere F, Parham C, Bridon JM, Banchereau J, et al. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. Journal of Immunology. 1997;158:604. [PubMed] [Google Scholar]
- Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141:653. doi: 10.1053/j.gastro.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. Journal of Immunology. 1994;152:1821. [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. The Journal of Experimental Medicine. 2003;197:111. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Yang W, Lee PPW, Ho MH-K, Yang J, Zeng S, et al. Exome sequencing identifies novel compound heterozygous mutations of IL-10 receptor 1 in neonatal-onset Crohn’s disease. Genes and Immunity. 2012;13:437. doi: 10.1038/gene.2012.8. [DOI] [PubMed] [Google Scholar]
- Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World Journal of Gastroenterology. 2013;19:3931. doi: 10.3748/wjg.v19.i25.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu KS, Mizoguchi E, Cotoner CA, Nguyen DD, Mingle B, Iweala OI, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology. 2009;137:1380. doi: 10.1053/j.gastro.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annual Review of Immunology. 2012;30:221. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature Immunology. 2007;8:1390. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, Gordon S, et al. Highlights of 10 years of immunology in Nature Reviews Immunology. Nature Reviews. Immunology. 2011;11:693. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. The Journal of Immunology. 2010;184:2638. doi: 10.4049/jimmunol.0902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Nishimura Y, Katsuyama H, Maeda M, Hayashi H, Dong M, et al. Involvement of IL-10 and Bcl-2 in resistance against an asbestos-induced apoptosis of T cells. Apoptosis. 2006;11:1825. doi: 10.1007/s10495-006-9235-4. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Panelli M, Wang E, Rossi CR, Pilati P, Nitti D, et al. IL-10 stimulatory effects on human NK cells explored by gene profile analysis. Genes and Immunity. 2004;5:621. doi: 10.1038/sj.gene.6364135. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moran JP, Walter J, Tannock GW, Tonkonogy SL, Sartor RB. Bifidobacterium animalis causes extensive duodenitis and mild colonic inflammation in monoassociated interleukin-10-deficient mice. Inflammatory Bowel Diseases. 2009;15:1022. doi: 10.1002/ibd.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran CJ, Walters TD, Guo CH, Kugathasan S, Klein C, Turner D, et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflammatory Bowel Diseases. 2013;19:115. doi: 10.1002/ibd.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LO, El Kasmi KC, Smith AM, Finkelstein D, Fillon S, Kim YG, et al. The TLR2-MyD88-NOD2-RIPK2 signalling axis regulates a balanced pro-inflammatory and IL-10-mediated anti-inflammatory cytokine response to Gram-positive cell walls. Cellular Microbiology. 2008;10:2067. doi: 10.1111/j.1462-5822.2008.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Schumacher JH, Fiorentino DF, Leverah J, Moore KW, Bond MW. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. Journal of Immunology. 1990;145:2938. [PubMed] [Google Scholar]
- Murai M, Krause P, Shui J-W, Madan R, Karp C, Kronenberg M. Immune regulation by CD11b+ myeloid cells in the intestine (P3195) The Journal of Immunology. 2013;190:171.10. [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature Immunology. 2009;10:1178. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, et al. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. The Journal of Experimental Medicine. 1994;180:223. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Current Opinion in Pharmacology. 2006;6:379. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: Input and output integration. Journal of Immunology. 2007;178:2623. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. Journal of Immunology. 2004;172:3712. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- Nguyen HH, Tran BT, Muller W, Jack RS. IL-10 acts as a developmental switch guiding monocyte differentiation to macrophages during a murine peritoneal infection. Journal of Immunology. 2012;189:3112. doi: 10.4049/jimmunol.1200360. [DOI] [PubMed] [Google Scholar]
- Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6493. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi E, Homma Y, Kang X, Netea MG, Ma X. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nature Immunology. 2009;10:471. doi: 10.1038/ni.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nature Reviews. Immunology. 2007;7:425. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- Paul G, Khare V, Gasche C. Inflamed gut mucosa: Downstream of interleukin-10. European Journal of Clinical Investigation. 2012;42:95. doi: 10.1111/j.1365-2362.2011.02552.x. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annual Review of Immunology. 2004;22:929. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, et al. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. European Journal of Immunology. 2010;40:443. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. Journal of Immunology. 2009;183:797. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Ralph P, Nakoinz I, Sampson-Johannes A, Fong S, Lowe D, Min HY, et al. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. Journal of Immunology. 1992;148:808. [PubMed] [Google Scholar]
- Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. The Journal of Experimental Medicine. 2012;209:139. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. The Journal of Experimental Medicine. 2004;200:1289. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Sanchez R, Levy E, Costea F, Sinnett D. IL-10 and TNF-alpha promoter haplotypes are associated with childhood Crohn’s disease location. World Journal of Gastroenterology. 2009;15:3776. doi: 10.3748/wjg.15.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature Reviews. Immunology. 2010;10:170. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Schmitt EG, Haribhai D, Williams JB, Aggarwal P, Jia S, Charbonnier L-M, et al. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. The Journal of Immunology. 2012;189:5638. doi: 10.4049/jimmunol.1200936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. The Journal of Biological Chemistry. 2000;275:12848. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, et al. Crohn’s Disease IL-10 Cooperative Study Group Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Gastroenterology. 2000;119:1461. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and Immunity. 1998;66:5224. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Muller W, Roers A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. European Journal of Immunology. 2006;36:3248. doi: 10.1002/eji.200636012. [DOI] [PubMed] [Google Scholar]
- Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. The Journal of Experimental Medicine. 1998;187:571. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. Journal of Immunology. 2009;182:2859. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. Journal of Immunology. 1997;159:4772. [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature Immunology. 2007;8:1363. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Tamassia N, Calzetti F, Menestrina N, Rossato M, Bazzoni F, Gottin L, et al. Circulating neutrophils of septic patients constitutively express IL-10R1 and are promptly responsive to IL-10. International Immunology. 2008;20:535. doi: 10.1093/intimm/dxn015. [DOI] [PubMed] [Google Scholar]
- Tan JC, Indelicato SR, Narula SK, Zavodny PJ, Chou CC. Characterization of interleukin-10 receptors on human and mouse cells. The Journal of Biological Chemistry. 1993;268:21053. [PubMed] [Google Scholar]
- Tiittanen M, Westerholm-Ormio M, Verkasalo M, Savilahti E, Vaarala O. Infiltration of forkhead box P3-expressing cells in small intestinal mucosa in coeliac disease but not in type 1 diabetes. Clinical and Experimental Immunology. 2008;152:498. doi: 10.1111/j.1365-2249.2008.03662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. Journal of Immunology. 2006;177:5852. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer SJ, Elson CO, Fedorak RN, Crohn’s Disease Study Group Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Gastroenterology. 1997;113:383. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- Walter MR, Nagabhushan TL. Crystal structure of interleukin 10 reveals an interferon gamma-like fold. Biochemistry. 1995;34:12118. doi: 10.1021/bi00038a004. [DOI] [PubMed] [Google Scholar]
- Weber-Nordt RM, Meraz MA, Schreiber RD. Lipopolysaccharide-dependent induction of IL-10 receptor expression on murine fibroblasts. Journal of Immunology. 1994;153:3734. [PubMed] [Google Scholar]
- Weber-Nordt RM, Riley JK, Greenlund AC, Moore KW, Darnell JE, Schreiber RD. Stat3 recruitment by two distinct ligand-induced, tyrosine-phosphorylated docking sites in the interleukin-10 receptor intracellular domain. The Journal of Biological Chemistry. 1996;271:27954. doi: 10.1074/jbc.271.44.27954. [DOI] [PubMed] [Google Scholar]
- Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. Journal of Immunology. 2004;172:567. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation—A continuing puzzle. Immunology. 2004;113:281. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor WT, Syto R, Tsarbopoulos A, Zhang R, Durkin J, Baldwin S, et al. Disulfide bond assignments and secondary structure analysis of human and murine interleukin 10. Biochemistry. 1993;32:8807. doi: 10.1021/bi00085a011. [DOI] [PubMed] [Google Scholar]
- Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, et al. c-Maf regulates IL-10 expression during Th17 polarization. Journal of Immunology. 2009;182:6226. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nature Immunology. 2003;4:551. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Yoon SI, Logsdon NJ, Sheikh F, Donnelly RP, Walter MR. Conformational changes mediate interleukin-10 receptor 2 (IL-10R2) binding to IL-10 and assembly of the signaling complex. The Journal of Biological Chemistry. 2006;281:35088. doi: 10.1074/jbc.M606791200. [DOI] [PubMed] [Google Scholar]
- Zdanov A, Schalk-Hihi C, Gustchina A, Tsang M, Weatherbee J, Wlodawer A. Crystal structure of interleukin-10 reveals the functional dimer with an unexpected topological similarity to interferon gamma. Structure. 1995;3:591. doi: 10.1016/s0969-2126(01)00193-9. [DOI] [PubMed] [Google Scholar]
- Zdanov A, Schalk-Hihi C, Menon S, Moore KW, Wlodawer A. Crystal structure of Epstein-Barr virus protein BCRF1, a homolog of cellular interleukin-10. Journal of Molecular Biology. 1997;268:460. doi: 10.1006/jmbi.1997.0990. [DOI] [PubMed] [Google Scholar]
- Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C(hi) monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]