Summary
Recent advances have defined a role for abnormally short telomeres in a broad spectrum of genetic disorders. They include rare conditions such as dyskeratosis congenita as well pulmonary fibrosis and emphysema. Now, there is new evidence that some familial cancers, such as melanoma, are caused by mutations that lengthen telomeres. Here, we examine the significance of these short and long telomere length extremes for understanding the molecular basis of age-related disease and cancer.
The origins of telomere human genetics
Telomeres and telomerase were first discovered in Tetrahymena thermophila, a protozoan of no obvious clinical significance[1,2]. Genetic models in yeast, mice, and human cells subsequently established a framework for how chromosome ends are maintained before disease connections crystallized[3]. Now, abnormally short telomeres are appreciated to mediate common age-related disorders such as pulmonary fibrosis and emphysema[4]. While the clear connection between short telomeres and degenerative disease may have suggested a hypothetical benefit for long telomeres, new discoveries have uncovered a potential link between long telomeres and familial cancer syndromes[5–7]. Here, we review how extreme telomere length abnormalities, both short and long, inform understanding the molecular basis of diseases associated with aging as well as cancer.
Telomere length is a “molecular clock” mechanism
Telomere shortening is considered one of the best-characterized mechanisms of cellular aging[4]. This claim builds on the fact that telomere length predicts the onset of replicative senescence[8,9]. Telomeres also shorten in humans with age, and in the past decade, it has become clear that abnormally short telomeres can cause several age-related disease phenotypes[4]. The progressive shortening of the TTAGGG telomeric sequence occurs because DNA polymerases cannot fully copy to chromosome ends[10]. Telomerase offsets this ‘end replication problem’ by synthesizing new telomere sequences[2,11,12]. When telomeres become critically short, they activate a DNA damage response[13], which provokes cellular senescence or apoptosis[14–18]; these responses underlie the progressive natural history of disorders that share the short telomere defect as a driving mechanism.
Several safeguards restrict telomere elongation in favor of net shortening with aging. They include a tight regulation of telomerase levels, as well as intrinsic factors at telomeres that limit excessive elongation by telomerase[19–21]. The expression of the reverse transcriptase component of telomerase, TERT, is also repressed in most adult tissues. In hematopoietic as well as other somatic stem cells, even though telomerase is expressed, its low levels do not offset the telomere shortening that normally occurs with aging[19,22–24]. As we will discuss here, genetic defects that disturb this telomere length homeostasis cause highly penetrant disease phenotypes.
The mammalian short telomere phenotype was first studied in telomerase null mice[14,15,25]. While telomerase loss alone has no clinical consequences in the first generation, late-generation telomerase null mice accumulate short telomeres[14,15,18,25,26]. The short telomeres cause degenerative organ failure indicating that the telomere length, and not telomerase loss, is the primary driver of the phenotype. Late-generation mice with short telomeres develop a stem cell failure phenotype, which is prominent in highly proliferative tissues such as the bone marrow and intestinal tract where the replicative potential of stem cells is critical for homeostasis[14,15,18,25,26]. The human short telomere syndromes recapitulate these phenotypes[21,27].
THE SHORT TELOMERE SYNDROMES
The human short telomere phenotype in high turnover tissues
Studies over the past decade have linked the human short telomere phenotype to a broad spectrum of disease[21]; it spans the entire age spectrum from infancy to adulthood (Figure 1). While at onset their clinical and histopathologic classification may show few shared features, there is growing appreciation for their unified natural history[28]. Their recognition as a single syndromic spectrum is critical for treatment decisions, because even though a single organ presentation may arise initially, the systemic telomere defect complicates treatment. Because some of these complications can be averted, the molecular grouping of disease across organs under the short telomere syndrome umbrella exemplifies a molecular medicine paradigm that directly impacts patient care[27,29,30].
Figure 1. Telomere length extremes and their predominant clinical manifestations.
Telogram showing the telomere length range across the age spectrum with percentile lines defining the normal range at every age. The short telomere syndromes have typical manifestations that are represented by the red circles at the typical age of onset. Familial melanoma and glioma have been linked to mutations that putatively cause long telomeres.
The short telomere phenotype in children and young adults represents more severe disease[4]. Bone marrow failure is its most common first manifestation, and stem cell transplantation alleviates this condition pointing to a stem cell-autonomous defect in this compartment[21,31–34]. Affected individuals are also prone to developing intestinal villous atrophy, immunodeficiency and infertility[21,27,35]. Pediatric presentations may also be recognized in historically defined syndromic entities. Dyskeratosis congenita was the first disorder to be linked to telomerase mutations and short telomeres[36,37]; it is classically defined by abnormalities in the skin, mucosa and nails[38,39]. Hoyeraal-Hreidarsson syndrome manifests in infancy and is characterized by developmental delay, enterocolitis, and immunodeficiency[27,40–42]. The criteria for recognizing Hoyeraal-Hreidarsson syndrome and dyskeratosis congenita may be specific; but they identify only a small subset of all short telomere syndrome presentations[43].
The slow turnover phenotype in short telomere syndromes
Lung disease is the most common presentation of short telomere syndromes and it represents an attenuated, adult-onset phenotype (Figure 1)[43]. Two types of lung disease have been linked to mutant telomerase and telomere genes. Idiopathic pulmonary fibrosis and the related interstitial diseases are marked by progressive lung scarring. Familial pulmonary fibrosis is a common manifestation of short telomere syndromes[43], and six mutant telomerase and telomere genes explain one-third of cases[44–49]. Telomerase mutations have also been recently linked to the risk of emphysema[50]. The frequency of telomerase mutations in severe emphysema rivals alpha-1 antitrypsin deficiency, which until recently was its only known Mendelian cause[50]. In families with telomerase mutations, emphysema appears in smokers, while pulmonary fibrosis is the predominant pathology in never smokers[50]. This apparent phenotypic heterogeneity points to a profound gene-environment interaction in a Mendelian disorder in which a single mutation causing telomere shortening provokes fibrotic scarring in never smokers and emphysematous airspace destruction in smokers[50]. In animal models, telomere dysfunction in alveolar stem cells triggers cellular senescence and recapitulates many features of the human lung pathology including recruitment of an inflammatory response[51]. The fibrosis-emphysema caused by short telomeres may therefore be driven by alveolar stem cell failure, and this biology points to potential new approaches to their treatment[4,51]. Pulmonary fibrosis and emphysema are estimated to affect 100,000 and 5 million individuals, respectively, in the United States alone[52,53]. Their disease burden, along with their close association with telomere genetics, makes the short telomere syndromes the most prevalent among the premature aging disorders.
Cancer in the short telomere syndromes
Although the majority of the premature mortality in the short telomere syndromes is caused by degenerative organ failure, there is also an increased risk for cancer. Cancer is estimated to affect 10% of dyskeratosis congenita cases, and when it arises, it is diagnosed at a younger age than the general population[54]. The basis of the cancer prone state in the short telomere syndromes is not understood, but its predilection appears to be for high turnover tissues where stem cell failure also occurs. Short telomere syndromes are associated with an increased incidence of non-melanoma skin cancers, as well as squamous cell carcinomas of the head and neck[54], but the highest risk is for myelodysplasia and acute myeloid leukemia. These latter bone marrow-derived malignancies are sometimes a first manifestation of telomere-mediated disease[54,55]. In the bone marrow, the stem cell failure state and progressive stem cell dropout may cause replication errors in surviving stem cells that could lead to a clonal advantage. Short telomere patients may also have impaired cancer surveillance because of immunosenescence[21]. All in all, even though the rate of some types of cancers in short telomere syndromes is higher than in the general population, its overall incidence is dwarfed by organ failure which accounts for 90% of the mortality[38].
The genetics of short telomere syndromes
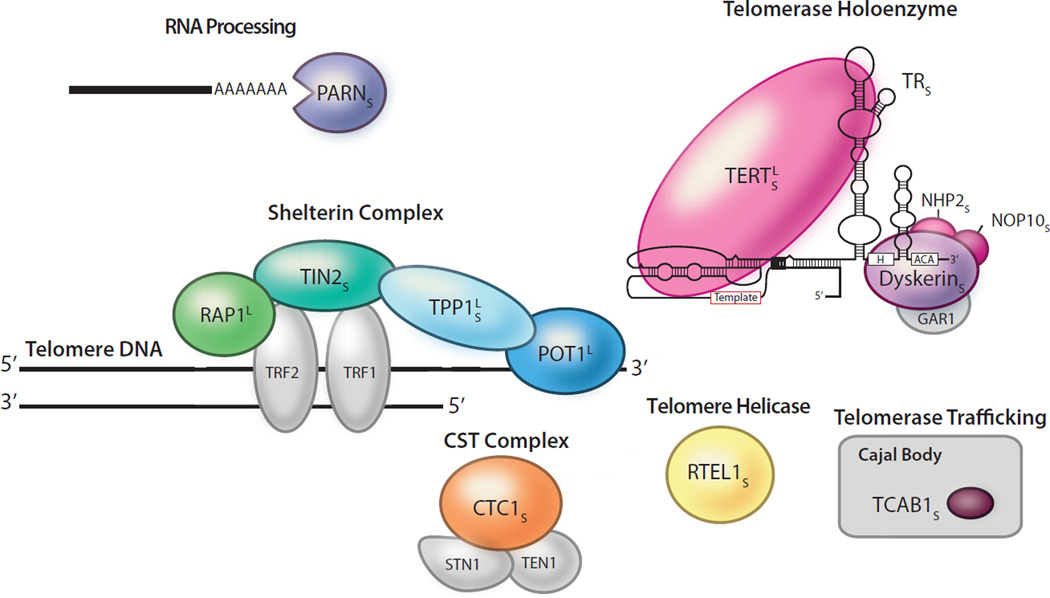
Short telomere syndromes show Mendelian inheritance, and as of this writing, 11 genes have been identified[34,36,48,56–67] (Figure 2). Together, they explain 50–70% of the short telomere Mendelian phenotype. Mutations in these genes disturb telomere homeostasis by impairing telomerase biogenesis, affecting its catalytic functions, its recruitment by shelterin, or the stability of telomere replication machinery components (Figure 2). The predominant phenotype and age of onset do not depend on the gene or the mutation type, but is determined by the extent by which a given mutation causes telomere shortening[68]. The most prevalent cause of short telomere syndromes is heterozygous loss-of-function mutations in TERT, which manifest in adults as autosomal dominant familial pulmonary fibrosis[4] (Figures 3A). In rare cases, biallelic TERT mutations have also been described and they are associated with more severe short telomere defects and early-onset disease[69]. Mutations in RTEL1 and PARN also show a similar pattern of adult- and pediatric-onset disease depending on whether one or two alleles are affected, respectively[48,63,70].
Figure 2. Telomerase and telomere components mutated in both short and long telomere syndromes.
The ‘S’ subscript indicates a link to short telomere syndromes (n = 11 genes), while the ‘L’ superscript indicates a link to long telomere syndromes (n = 4 genes). Mutant components are shown in color and gray denotes telomere components not known to be linked to disease. These mutations affect telomerase catalytic activity or processivity (TERT and TR), telomerase biogenesis (dyskerin encoded by DKC1), NOP10 and NHP2, or telomerase trafficking (TCAB1 also known as WRAP53). Mutations in telomere syndromes may also fall in the shelterin components: TIN2 (encoded by TINF2), TPP1 (encoded by ACD), POT1, or RAP1 (encoded by TERF2IP). CTC1 and RTEL1 affect lagging strand synthesis and telomere replication, respectively. PARN is involved in RNA processing and deadenylation.
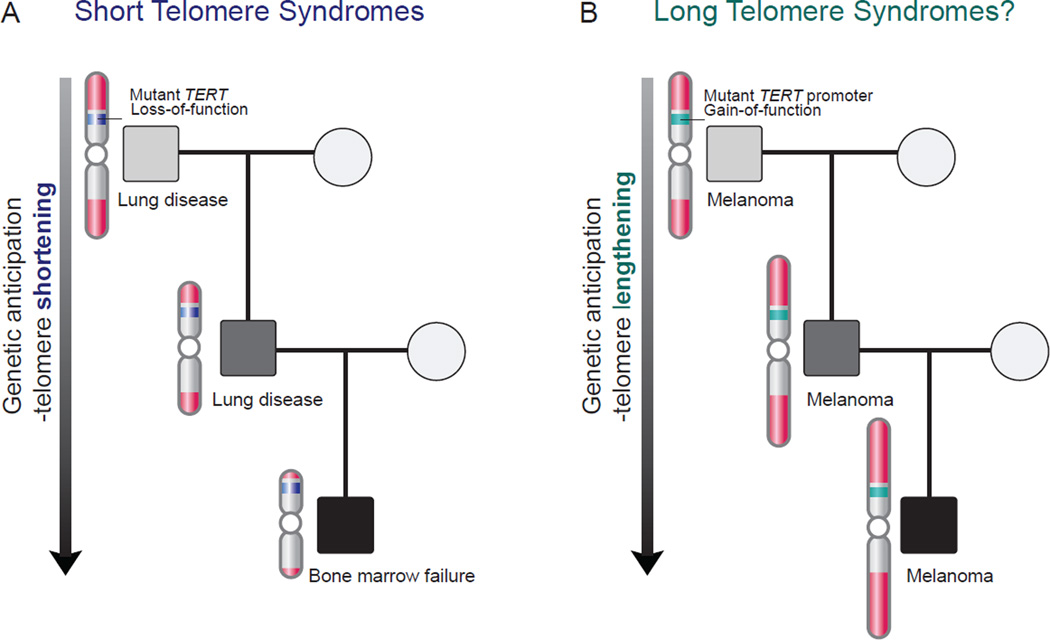
Figure 3. Genetic anticipation in the autosomal dominant short and long telomere syndromes.
(a) In the short telomere syndromes, successive telomere shortening across generations manifests in disease that shows an earlier age of onset. There is also an evolving pattern with ancestors developing lung disease and their progeny having a higher incidence of high turnover phenotypes such as bone marrow failure. (b) Long telomere syndromes appear to also show genetic anticipation of the melanoma phenotype; this is presumably caused by inheritance of long telomeres (right panel). Mutations in TERT have been linked to both syndromes with short telomere syndromes being caused by loss-of-function mutations (a), while familial melanoma is caused by promoter gain-of-function mutations that turn on TERT transcription (b).
Genetic anticipation and the evolving disease pattern in the telomere syndromes
Two features distinguish the genetics of autosomal dominant short telomere syndromes from other Mendelian disorders. They also explain the unique natural history of these disorders. The first is that autosomal dominant families with short telomere syndromes show genetic anticipation, an earlier and more severe onset of disease in successive generations[57,71] (Figure 3A). Telomere shortening is a second established molecular mechanism for genetic anticipation in addition to trinucleotide repeat expansion. The successive telomere shortening eventually precipitates severe pediatric disease and/or infertility, leading to loss of the mutation in that lineage[46,68]. Telomere gene mutations thus tend to be private to each autosomal dominant kindred, leading to significant allele heterogeneity. In addition to the earlier onset in later generations, the disease evolves from slow-turnover tissues in older generations (e.g. pulmonary fibrosis-emphysema) to a high-turnover phenotype in younger generations (e.g. bone marrow failure)[68]. The rate of genetic anticipation and disease evolution within a family depends on the extent of telomere shortening caused by a given mutant allele across a generation[72]. As we will highlight below, cancer prone families with mutations that conversely promote telomere lengthening also show evidence of genetic anticipation.
THE LONG TELOMERE SYNDROMES
The price of long telomeres in an increased risk of melanoma
The premature aging phenotypes caused by abnormally short telomeres may intuitively suggest that long telomeres confer an advantage for health and lifespan. However, there is increasing evidence that such a view may be overly simplistic. In the past two years, mutations that appear to lengthen telomeres have been linked to an increased risk of cancer. It is these familial cancer syndromes that we posit here are long telomere syndromes (Figures 1). They are also caused by mutations in telomerase and shelterin genes and two of these genes have been implicated also in the short telomere syndromes (Figures 2 and 3). The cancer spectrum in these putative long telomere syndromes is not yet fully defined, but so far it appears to be particularly enriched for melanoma and glioma[5–7,73,74]. Notably, these cancers are different from the squamous cell and hematologic malignancies that arise in the short telomere syndromes.
Mutations in telomere genes that cause familial cancers
Evidence that mutant genes that may promote telomere lengthening cause familial cancer came first from a large melanoma kindred that carried an activating mutation in the TERT promoter[5]. This mutation creates an E-twenty-six (ETS) binding site that turns on TERT transcription[5,75,76]. Such a mechanism contrasts with the TERT loss-of-function mutations seen in the short telomere syndromes that impair the enzyme’s catalytic functions[57] (Figure 3A). Somatic TERT promoter mutations are also found in 70% of melanomas[75] as well as a number of other solid tumors[77]. Their high prevalence makes TERT promoter mutations some of the most common somatic mutations seen in human cancer and points to telomerase abundance as critical in cancer initiation and progression.
Since the initial description of a TERT promoter mutation in a family with melanoma, germline mutations in three other telomere gene, all encoding shelterin components, have been linked to familial melanoma and glioma[6,7,73,74] (Figure 2). They include POT1, TPP1 and RAP1[6,7] (Figure 2). Like TERT, mutations in TPP1 can cause both short and long telomere syndromes. TPP1 is a shelterin component that has been implicated in telomerase recruitment, but it also functions to prevent telomerase access to the telomere[78–80]. Its dual functions explain the contrasting net effect of TPP1 mutations on telomere length. The TPP1 single amino acid deletion falls in the TEL patch domain; this impairs telomerase recruitment resulting in net shortening[61,62]. The resulting disease phenotype is marked by stem cell failure and a small increased cancer risk. In contrast, TPP1 nonsense mutations in cancer prone families disrupt protein stability, and are predicted to allow improved telomerase access and promote telomere elongation[62,73,80]. Based on the genetic anticipation documented for short telomere syndromes, it would be expected that long telomere length may similarly be expected to be inherited across generations[18,26,57,81]. A close examination of the published pedigrees interestingly shows a pattern of genetic anticipation for the age of melanoma-related diagnosis and mortality[6,7,73]. While it is theoretically possible that lead time bias (i.e. the earlier detection of cancers because of earlier screening) could explain this pattern, the fact that this genetic anticipation occurs for the age at death as well as diagnosis favors a model where progressively longer telomeres promote an earlier, more aggressive cancer course in later generations (Figure 3B).
How would abnormally long telomeres predispose to melanoma?
Studies over the past two decades in animal models shed light on how long telomeres may promote cancer-related mortality. Although mice with short telomeres show limited survival because of their stem cell failure phenotypes, in cancer prone contexts they show a paradoxical advantage in overall survival[82–85]. This benefit has been reproduced across several cancer prone models, including oncogene-driven cancers, such as Myc and K-ras, as well as those driven by loss of tumor suppressors such as Apc and Ink4a[82–85]. The survival advantage is seen despite the fact that short telomere mice accumulate more micro-tumors, because short telomeres provoke apoptosis and senecence checkpoints in pre-cancers[83,86]. Thus, short telomeres confer an overall benefit at the organismal level in animal models where cancer is induced by a single driving mutation.
Evidence from population studies also supports an association between long telomeres and cancer risk. This association is best docuemented in cutaneous malignant melanoma[87–90], an intriguing observation in light of the cutaneous melanoma-rich phenotype documented in the long telomere syndromes. Cutaneous malignant melanoma is marked by some of the highest mutation burdens among human cancers because of the mutagenic effects of ultraviolet light[91]. Its clustering in families and individuals who have long telomeres underscores an important role for the telomere-mediated replicative senescence checkpoint in suppressing tumors where mutagenesis is environmentally induced. Unrestricted proliferation when telomeres are long would increase the likelihood of sustaining driver mutations that eventually promote a clonal advantage and metastasis. The role of long telomeres in promoting cancer is however nuanced, and the association with cancer risk may be tissue and cell type specific. For example, the risk of non-melanoma skin cancers (e.g. squamous cell carcinoma) is associated with short telomere length[92]. The available evidence thus suggests that distinct cancer phenotypes are associated with both short and long telomere syndrome extremes, and that a melanoma-rich phenotype will be a hallmark of long telomere syndromes.
SUMMARY AND LOOKING AHEAD
Emerging discoveries have painted a rich mosaic of how telomere length abnormalities at the extremes play a role in disease. The short telomere syndromes unite a group of stem cell failure disorders that share a single molecular pathology. Their grouping informs treatment and pathogenesis paradigms for common, poorly understood conditions especially lung disease. The short telomere phenotype overlaps with diseases that are normally acquired with aging, and it is associated with a modest cancer risk. At the other extreme, the long telomere syndromes manifest as a highly penetrant cancer-prone state. They are enriched for cutaneous melanoma presumably because in the setting of environmentally induced mutagenesis, the loss of the replicative senescence checkpoint promotes carcinogenesis. Both disease extremes point to the importance of a telomere length equilibrium in maintaining stem cell homeostasis with aging while simultaneously minimizing cancer risk.
The profound disease phenotypes caused by extreme telomere length disturbances raise the possibility that targeting telomere length may be a plausible therapy strategy. In the short telomere syndromes, replenishing defective stem cells, such as is currently done with bone marrow transplantation, or aiming to elongate telomeres, could be clinically beneficial. In contrast, for long telomere-associated cancers, inhibiting telomerase could be effective in preventing or treating cancer. How much leeway is available to exploit this delicate system therapeutically remains to be determined, and any treatment approach will have to consider these multiple safeguards at telomeres that favor moderation over excess.
Acknowledgements
We are grateful to a number of colleagues for helpful comments on this manuscript. Work in our lab is supported by the National Institutes of Health (RO1 HL119476 and CA160433), the Commonwealth Foundation and the Flight Attendants Medical Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 4.Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest. 2013;123:996–1002. doi: 10.1172/JCI66370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. This and the study in reference 75 identified TERT promoter mutations in melanoma.**
- 6.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, Pooley KA, Pritchard AL, Tiffen JC, Petljak M, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet. 2014;46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, Yang XR, Ballew B, Rotunno M, Calista D, Fargnoli MC, Ghiorzo P, Bressac-de Paillerets B, Nagore E, Avril MF, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet. 2014;46:482–486. doi: 10.1038/ng.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 9.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greider CW. Cellular responses to telomere shortening: cellular senescence as a tumor suppressor mechanism. Harvey Lect. 2000;96:33–50. [PubMed] [Google Scholar]
- 11.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 12.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 13.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 14.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 15.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 16.Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo N, Parry EM, Li LS, Kembou F, Lauder N, Hussain MA, Berggren PO, Armanios M. Short telomeres compromise beta-cell signaling and survival. PloS one. 2011;6:e17858. doi: 10.1371/journal.pone.0017858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greider CW. Telomerase RNA levels limit the telomere length equilibrium. Cold Spring Harb Symp Quant Biol. 2006;71:225–229. doi: 10.1101/sqb.2006.71.063. [DOI] [PubMed] [Google Scholar]
- 20.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 21.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 23.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 24.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 26.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Jonassaint NL, Guo N, Califano JA, Montgomery EA, Armanios M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell. 2013;12:319–323. doi: 10.1111/acel.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dietz AC, Orchard PJ, Baker KS, Giller RH, Savage SA, Alter BP, Tolar J. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.65. These references highlight the importance of the short telomere syndrome diagnosis in evaluation and treatment of bone marrow failure and pulmonary fibrosis, respectively. **
- 30. Silhan LL, Shah PD, Chambers DC, Snyder LD, Riise GC, Wagner CL, Hellstrom-Lindberg E, Orens JB, Mewton JF, Danoff SK, et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J. 2014;44:178–187. doi: 10.1183/09031936.00060014. These references highlight the importance of the short telomere syndrome diagnosis in evaluation and treatment of bone marrow failure and pulmonary fibrosis, respectively. **
- 31.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359:2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 32.Dokal I. Inherited aplastic anaemia. Hematol J. 2003;4:3–9. doi: 10.1038/sj.thj.6200215. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 35.Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. 2014 doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 38.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 39.de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatr Transplant. 2007;11:584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 40.Glousker G, Touzot F, Revy P, Tzfati Y, Savage SA. Unraveling the pathogenesis of Hoyeraal-Hreidarsson syndrome, a complex telomere biology disorder. Br J Haematol. 2015 doi: 10.1111/bjh.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyeraal HM, Lamvik J, Moe PJ. Congenital hypoplastic thrombocytopenia and cerebral malformations in two brothers. Acta Paediatr Scand. 1970;59:185–191. doi: 10.1111/j.1651-2227.1970.tb08986.x. [DOI] [PubMed] [Google Scholar]
- 42.Hreidarsson S, Kristjansson K, Johannesson G, Johannsson JH. A syndrome of progressive pancytopenia with microcephaly, cerebellar hypoplasia and growth failure. Acta Paediatr Scand. 1988;77:773–775. doi: 10.1111/j.1651-2227.1988.tb10751.x. [DOI] [PubMed] [Google Scholar]
- 43.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 45.Alder JK, Parry EM, Yegnasubramanian S, Wagner CL, Lieblich LM, Auerbach R, Auerbach AD, Wheelan SJ, Armanios M. Telomere Phenotypes in Females with Heterozygous Mutations in the Dyskeratosis Congenita 1 (DKC1) Gene. Hum Mutat. 2013 doi: 10.1002/humu.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M. Exome Sequencing Identifies Mutant TINF2 in a Family With Pulmonary Fibrosis. Chest. 2015;147:1361–1368. doi: 10.1378/chest.14-1947. These three recent studies identified three new telomere genes in autosomal dominant families with pulmonary fibrosis, underscoring the importance of short telomeres in the pathogenesis of this lung disease. **
- 47. Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, Garnett ET, Montgomery KH, Mason WR, McKean DF, et al. Rare Variants in RTEL1 are Associated with Familial Interstitial Pneumonia. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201408-1510OC. These three recent studies identified three new telomere genes in autosomal dominant families with pulmonary fibrosis, underscoring the importance of short telomeres in the pathogenesis of this lung disease. **
- 48. Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, Choi M, Dharwadkar P, Torres F, Girod CE, et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet. 2015;47:512–517. doi: 10.1038/ng.3278. These three recent studies identified three new telomere genes in autosomal dominant families with pulmonary fibrosis, underscoring the importance of short telomeres in the pathogenesis of this lung disease. **
- 49.Kropski JA, Mitchell DB, Markin C, Polosukhin VV, Choi LA, Johnson JE, Lawson WE, Phillips JA, 3rd, Cogan JD, Blackwell TS, et al. A novel dyskerin (DKC1) mutation is associated with Familial Interstitial Pneumonia. Chest. 2014 doi: 10.1378/chest.13-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stanley SE, Chen JJ, Podlevsky JD, Alder JK, Hansel NN, Mathias RA, Qi X, Rafaels NM, Wise RA, Silverman EK, et al. Telomerase mutations in smokers with severe emphysema. J Clin Invest. 2015;125:563–570. doi: 10.1172/JCI78554. This study identified telomerase mutations as a risk factor for emphysema, implicating this phenotype in one of the most common causes of mortality worldwide. **
- 51. Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BL, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci U S A. 2015;112:5099–5104. doi: 10.1073/pnas.1504780112. This study showed alveolar stem cell senescence caused by telomere dysfunction is sufficient to induce inflammation pointing to stem cell regenerative approaches rather than anti-inflammatories as a rational therapy in telomere-mediated lung disease. **
- 52.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 53.Castaldi PJ, Dy J, Ross J, Chang Y, Washko GR, Curran-Everett D, Williams A, Lynch DA, Make BJ, Crapo JD, et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax. 2014;69:415–422. doi: 10.1136/thoraxjnl-2013-203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirwan M, Vulliamy T, Marrone A, Walne AJ, Beswick R, Hillmen P, Kelly R, Stewart A, Bowen D, Schonland SO, et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum Mutat. 2009;30:1567–1573. doi: 10.1002/humu.21115. [DOI] [PubMed] [Google Scholar]
- 56.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 57.Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kocak H, Ballew BJ, Bisht K, Eggebeen R, Hicks BD, Suman S, O'Neil A, Giri N, Maillard I, Alter BP, et al. Hoyeraal-Hreidarsson syndrome caused by a germline mutation in the TEL patch of the telomere protein TPP1. Genes Dev. 2014;28:2090–2102. doi: 10.1101/gad.248567.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y, Kartawinata M, Li J, Pickett HA, Teo J, Kilo T, Barbaro PM, Keating B, Chen Y, Tian L, et al. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood. 2014;124:2767–2774. doi: 10.1182/blood-2014-08-596445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tummala H, Walne A, Collopy L, Cardoso S, de la Fuente J, Lawson S, Powell J, Cooper N, Foster A, Mohammed S, et al. Poly(A)-specific ribonuclease deficiency impacts telomere biology and causes dyskeratosis congenita. J Clin Invest. 2015;125:2151–2160. doi: 10.1172/JCI78963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, Alter BP, Savage SA. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum Genet. 2013;132:473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional Mutations in RTEL1 Cause Severe Dyskeratosis Congenita. Am J Hum Genet. 2013;92:448–453. doi: 10.1016/j.ajhg.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Guen T, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, Carpentier W, Nitschke P, Picard C, Couillault G, et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- 67.Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marrone A, Walne A, Tamary H, Masunari Y, Kirwan M, Beswick R, Vulliamy T, Dokal I. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110:4198–4205. doi: 10.1182/blood-2006-12-062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanley SE, Noth I, Armanios M. What the genetics "RTEL"ing us about telomeres and pulmonary fibrosis. Am J Respir Crit Care Med. 2015;191:608–610. doi: 10.1164/rccm.201501-0119ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004 doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 72.Alder JK, Cogan JD, Brown AF, Anderson CJ, Lawson WE, Lansdorp PM, Phillips JA, 3rd, Loyd JE, Chen JJ, Armanios M. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS genetics. 2011;7:e1001352. doi: 10.1371/journal.pgen.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aoude LG, Pritchard AL, Robles-Espinoza CD, Wadt K, Harland M, Choi J, Gartside M, Quesada V, Johansson P, Palmer JM, et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju408. These studies extended the role of telomere gene mutations from TERT to shelterin components in families with melanoma and the latter expanded this spectrum to familial glioma, implicating long telomeres as potentially relevant to the risk of these cancers. *
- 74. Bainbridge MN, Armstrong GN, Gramatges MM, Bertuch AA, Jhangiani SN, Doddapaneni H, Lewis L, Tombrello J, Tsavachidis S, Liu Y, et al. Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst. 2015;107:384. doi: 10.1093/jnci/dju384. These studies extended the role of telomere gene mutations from TERT to shelterin components in families with melanoma and the latter expanded this spectrum to familial glioma, implicating long telomeres as potentially relevant to the risk of these cancers. *
- 75. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. This and the study in reference 5 identified TERT promoter mutations in melanoma. **
- 76.Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SF, Nagarajan RP, Choi S, Hong C, He D, Pekmezci M, et al. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015 doi: 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heidenreich B, Rachakonda PS, Hemminki K, Kumar R. TERT promoter mutations in cancer development. Curr Opin Genet Dev. 2014;24:30–37. doi: 10.1016/j.gde.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492:285–289. doi: 10.1038/nature11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 80.Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 2004;18:1649–1654. doi: 10.1101/gad.1215404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012;730:68–74. doi: 10.1016/j.mrfmmm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11:461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28:155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- 84.Perera SA, Maser RS, Xia H, McNamara K, Protopopov A, Chen L, Hezel AF, Kim CF, Bronson RT, Castrillon DH, et al. Telomere dysfunction promotes genome instability and metastatic potential in a K-ras p53 mouse model of lung cancer. Carcinogenesis. 2008;29:747–753. doi: 10.1093/carcin/bgn050. [DOI] [PubMed] [Google Scholar]
- 85.Greenberg RA, Chin L, Femino A, Lee KH, Gottlieb GJ, Singer RH, Greider CW, DePinho RA. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell. 1999;97:515–525. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 86.Feldser DM, Hackett JA, Greider CW. Telomere dysfunction and the initiation of genome instability. Nat Rev Cancer. 2003;3:623–627. doi: 10.1038/nrc1142. [DOI] [PubMed] [Google Scholar]
- 87.Iles MM, Bishop DT, Taylor JC, Hayward NK, Brossard M, Cust AE, Dunning AM, Lee JE, Moses EK, Akslen LA, et al. The effect on melanoma risk of genes previously associated with telomere length. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burke LS, Hyland PL, Pfeiffer RM, Prescott J, Wheeler W, Mirabello L, Savage SA, Burdette L, Yeager M, Chanock S, et al. Telomere length and the risk of cutaneous malignant melanoma in melanoma-prone families with and without CDKN2A mutations. PLoS One. 2013;8:e71121. doi: 10.1371/journal.pone.0071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Llorca-Cardenosa MJ, Pena-Chilet M, Mayor M, Gomez-Fernandez C, Casado B, Martin-Gonzalez M, Carretero G, Lluch A, Martinez-Cadenas C, Ibarrola-Villava M, et al. Long telomere length and a TERT-CLPTM1 locus polymorphism association with melanoma risk. Eur J Cancer. 2014;50:3168–3177. doi: 10.1016/j.ejca.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 90.Nan H, Du M, De Vivo I, Manson JE, Liu S, McTiernan A, Curb JD, Lessin LS, Bonner MR, Guo Q, et al. Shorter telomeres associate with a reduced risk of melanoma development. Cancer Res. 2011;71:6758–6763. doi: 10.1158/0008-5472.CAN-11-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anic GM, Sondak VK, Messina JL, Fenske NA, Zager JS, Cherpelis BS, Lee JH, Fulp WJ, Epling-Burnette PK, Park JY, et al. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol. 2013;37:434–439. doi: 10.1016/j.canep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]