Abstract
Eicosanoid production by macrophages is an early response to microbial infection that promotes acute inflammation. The intracellular pathogen Listeria monocytogenes stimulates arachidonic acid release and eicosanoid production from resident mouse peritoneal macrophages through activation of group IVA cytosolic phospholipase A2 (cPLA2α). The ability of wild type L. monocytogenes (WTLM) to stimulate arachidonic acid release is partially dependent on the virulence factor listeriolysin O; however, WTLM and L. monocytogenes lacking listeriolysin O (ΔhlyLM) induce similar levels of cyclooxygenase 2. Arachidonic acid release requires activation of MAPKs by WTLM and ΔhlyLM. The attenuated release of arachidonic acid that is observed in TLR2−/− and MyD88−/− macrophages infected with WTLM and ΔhlyLM correlates with diminished MAPK activation. WTLM but not ΔhlyLM increases intracellular calcium, which is implicated in regulation of cPLA2α. Prostaglandin E2, prostaglandin I2, and leukotriene C4 are produced by cPLA2α+/+ but not cPLA2α−/− macrophages in response to WTLM and ΔhlyLM. Tumor necrosis factor (TNF)-α production is significantly lower in cPLA2α+/+ than in cPLA2α−/− macrophages infected with WTLM and ΔhlyLM. Treatment of infected cPLA2α+/+ macrophages with the cyclooxygenase inhibitor indomethacin increases TNFα production to the level produced by cPLA2α−/− macrophages implicating prostaglandins in TNFα down-regulation. Therefore activation of cPLA2α in macrophages may impact immune responses to L. monocytogenes.
The human pathogen Listeria monocytogenes promotes disease through ingestion of contaminated food, and primarily affects individuals with a suppressed immune system (1). L. monocytogenes gains entry through the intestinal tract but can traverse the blood-brain barrier and placenta to infect the brain and fetus resulting in high mortality. L. monocytogenes invades and replicates in a variety of cell types where it shelters itself intracellularly from host antibodies and complement (2). L. monocytogenes has been used extensively as a model organism to study host-pathogen interactions (3).
A number of virulence factors have been identified that contribute to the ability of L. monocytogenes to replicate intracellularly and infect neighboring cells (4). Once L. monocytogenes has invaded cells, the virulence factor listeriolysin O (LLO)2 perforates phagocytic vacuoles, which is essential for escape into the cell cytosol (5–7). Two bacterial phospholipases C (PLC), a phosphatidylinositol-specific PLC (PI-PLC) and broad range PLC (BR-PLC), also contribute to escape of L. monocytogenes from these primary vacuoles (8–11). Once in the cytosol the L. monocytogenes surface protein ActA polymerizes actin, which propels L. monocytogenes through the cytosol and into pseudopods that are engulfed by neighboring cells to form secondary vacuoles. Bacterial escape from these secondary vacuoles spreads infection (3, 12, 13).
In experimental systemic L. monocytogenes infection in mice, the bacteria are rapidly cleared from the bloodstream and taken up by the spleen and liver phagocytes. Resident macrophages in the liver, Kupffer cells, play important roles in bacterial uptake and in controlling L. monocytogenes infection (14). Resident macrophages produce the cytokines interleukin (IL)-6, IL-12, and tumor necrosis factor (TNF)-α, which initiate pro-inflammatory responses that are important for recruiting neutrophils to the liver to kill L. monocytogenes.
Eicosanoid production is an early response to microbial infection that can regulate innate immunity (15, 16). Eicosanoids are oxygenated metabolites of arachidonic acid that exert their potent biological effects by binding to G-protein-coupled receptors (17). Arachidonic acid is metabolized through the cyclooxygenase (COX) and lipoxygenase pathways for the production of prostaglandins and leukotrienes, respectively. Leukotrienes induce acute inflammatory responses such as increased vascular permeability and recruitment of granulocytes. Prostaglandins have pro-inflammatory effects by increasing vascular permeability but also exert immune-suppressive effects (18). Resident peritoneal macrophages have been used extensively as a model to study the regulation of arachidonic acid release and eicosanoid production; however, these responses have not been investigated thoroughly in the context of bacterial infection. In this study we demonstrate that nonopsonized L. monocytogenes activates group IVA cytosolic phospholipase A2 (cPLA2α) in resident peritoneal macrophages resulting in arachidonic acid release and eicosanoid production. Optimal activation of cPLA2α involves the bacterial virulence factor LLO and host toll-like receptor 2 (TLR2). Our results also demonstrate an important role for cPLA2α activation in suppression of TNFα production by macrophages infected with L. monocytogenes, implicating eicosanoids in regulating immune responses to L. monocytogenes.
EXPERIMENTAL PROCEDURES
Materials
Pathogen-free ICR mice were obtained from Harlan Sprague-Dawley and used for all experiments unless otherwise specified. TLR2 and MyD88 knock-out mice (C57BL/6) were generated as described previously (19), and age-matched control C57BL/6 mice were obtained from Harlan Sprague-Dawley. cPLA2α−/− mice were generated using 129 embryonic stem cells in a C57BL/6 strain as described previously (20). The mixed strain was backcrossed onto a Balb/c background and used after 10 generations. All mice were used between 5 and 10 weeks of age for macrophage isolation. [5,6,8,9,11,12,14,15-3H]Arachidonic acid (specific activity 100 Ci/mmol) was from PerkinElmer Life Sciences. Fetal bovine serum (Gemini Bio-Products) was heat-inactivated at 56 °C for 30 min before use. DMEM was from Cambrex BioScience Walkersville, Inc. Hanks’ balanced salts solution was purchased from Invitrogen. Human serum albumin was obtained from Intergen. The cPLA2α inhibitor, pyrrolidine-2, was generously provided by Dr. Michael Gelb (University of Washington, Seattle). Polyclonal antibody to cPLA2α was raised as described (21). Antibodies to phosphorylated extracellular signal-regulated kinases (ERKs) and p38 were obtained from Cell Signaling Technology, Inc. Polyclonal antibody to murine COX2 was obtained from Cayman Chemical Co. Antibody to L. monocytogenes was obtained from Difco (BD Biosciences). Gentamicin and latrunculin A were from Sigma. U0126 and SB202190 were obtained from Calbiochem. Complete protease inhibitor tablets were obtained from Roche Diagnostics. Tryptic soy agar and brain heart infusion broth were purchased from Fluka Bio-Chemika and BD Biosciences, respectively. Cyto-Tox ONE homogeneous membrane integrity assay kit was obtained from Promega. Fura Red-AM was from Invitrogen.
Bacteria
Wild type L. monocytogenes (WTLM) (DP-L10403S), LLO mutant (ΔhlyLM) (DP-L2161), double mutant strain lacking PI-PLC and BR-PLC (ΔplcAΔplcB) (DP-L1936), triple mutant strain (ΔhlyΔplcAΔplcB) (DP-L2319), and WTLM expressing enhanced green fluorescent protein (EGFP) (strain L3-L268) were used in this study. The strains were stored as glycerol stocks at −80 °C. L. monocytogenes were grown overnight with shaking (250 rpm) at 37 °C in brain heart infusion broth and then sub-cultured to an optical density of 0.6. Bacteria were washed once in PBS and resuspended in serum-free DMEM containing 0.1% human serum albumin (stimulation medium) for use in experiments.
Macrophage Isolation and Arachidonic Acid Release Assay
Resident mouse peritoneal macrophages were obtained by peritoneal lavage using 8 ml of DMEM containing 10% heat-inactivated fetal bovine serum, 100 μg/ml streptomycin sulfate, 100 units/ml penicillin G, and 2 mM glutamine (supplemented DMEM) containing 10 units/ml heparin. Cells were plated at 5 × 105 cells/well (48-well plate) and incubated for 2 h at 37 °C in a humidified atmosphere of 7.5% CO2 in air. Cells were washed twice with calcium- and magnesium-free Hanks’ balanced salts solution to remove nonadherent cells followed by one wash with supplemented DMEM. Macrophages were cultured in supplemented DMEM containing [3H]arachidonic acid (0.1 μCi/250 μl/well) for 16–18 h at 37 °C. The cells were washed twice with antibiotic-free stimulation medium to remove unincorporated [3H]arachidonic acid, and then infected with L. monocytogenes in stimulation medium. The culture medium was removed at the indicated times after infection, centrifuged, and the amount of radioactivity in the supernatant measured by scintillation counting. The cell-associated radioactivity was measured following solubilization of the monolayer with 0.1% Triton X-100. The amount of radioactivity released into the culture medium was expressed as percent of the total radioactivity incorporated (cell-associated plus medium).
Cell Cytotoxicity Assay
Resident mouse peritoneal macrophages were plated in 96-well plates (0.16 × 106 cells/well) and cultured as described above. The effect of L. monocytogenes on macrophage viability was assayed using Cyto-Tox ONE homogeneous membrane integrity assay kit according to manufacturer’s protocol using the LS55 spectrofluorometer (PerkinElmer Life Sciences). The macrophage culture medium was collected at various times after infection with L. monocytogenes and 50 μl used to determine the percent lactate dehydrogenase (LDH) release.
L. monocytogenes Uptake Assay
Macrophages plated at 0.5 × 106 cells/well (48-well plate) were cultured overnight in complete medium and then incubated with WTLM and ΔhlyLM (m.o.i. 25) in stimulation medium for 1 h. Macrophages were washed twice and incubated in stimulation medium containing gentamicin (50 μg/ml) for 30 min to kill extracellular bacteria. After washing twice with PBS, macrophages were lysed with 0.1% Triton X-100 in PBS and the intra-cellular L. monocytogenes plated on tryptic soy agar plates. The number of colony-forming units (CFU) was determined after incubation of plates for 22–24 h at 37 °C. For comparing uptake of L. monocytogenes by peritoneal macrophages from cPLA2α+/+ and cPLA2α−/− mice, a similar protocol was used except the macrophages were not incubated with gentamicin. After a 1-h incubation with WTLM, the macrophages were washed six times to remove extracellular L. monocytogenes following a protocol described previously (22).
Eicosanoid and Cytokine Analysis
Resident mouse peritoneal macrophages were plated as described for arachidonic acid release assays but incubated overnight without [3H]arachidonic acid. Macrophages were incubated with either WTLM or ΔhlyLM for 1 h, washed, and incubated in stimulation medium containing 50 μg/ml gentamicin. The culture medium was harvested 3 h after infection, complete protease inhibitor tablet (1×) added, and stored at −20 °C. Eicosanoids in the culture medium were quantified by enzyme-linked immunosorbent assay (ELISA Tech, Aurora, CO). TNFα in the culture medium was quantified by enzyme-linked immunosorbent assay (ELISA Tech, Aurora, CO) and by Luminex assay, which gave similar results.
Western Blots
Macrophages were washed twice in ice-cold PBS and lysed in buffer containing 50 mM HEPES, pH 7.4, 150 mM sodium chloride, 10% glycerol, 1% Triton X-100, 1 mM EGTA, 1 mM EDTA, 200 μM sodium vanadate, 10 mM tetrasodium pyrophosphate, 100 mM sodium fluoride, 300 nM p-nitro-phenyl phosphate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. After incubation on ice for 30 min, cell lysates were centrifuged at 15,000 rpm for 15 min, and protein concentration in the supernatant was determined by the bicinchoninic acid method. Lysates were boiled for 5 min after addition of Laemmli buffer, and then proteins (10–30 μg) were resolved on 10% SDS-polyacrylamide gels. After transfer, nitrocellulose membranes were incubated in blocking buffer (20 mM Tris-HCl buffer, pH 7.6, containing 137 mM NaCl, 0.05% Tween, and 5% nonfat milk), and then incubated overnight at 4 °C with polyclonal antibodies to cPLA2α, COX2, phospho-ERKs, and monoclonal antibody to phospho-p38. The membranes were incubated with horseradish peroxidase-linked secondary antibodies (1:5000) in blocking buffer for 30 min at room temperature. The immunoreactive proteins were detected using the Amersham Biosciences ECL system.
Production of Recombinant Adenovirus and Microscopy
Macrophages (5 × 105) were plated onto glass-bottomed Mat-Tek dishes in complete medium, incubated for 2 h, and then washed three times. Enhanced cyan fluorescent protein-cPLA2α (ECFP-cPLA2α) was expressed in resident peritoneal macrophages using recombinant adenovirus (23). Human cPLA2α was cloned into the ECFP vector (Clontech), and Ala-206 in ECFP was mutated to Lys to produce the monomeric form. ECFP-cPLA2α was subcloned into pVQ CMV k-NpA vector, and virus (AdECFP-cPLA2α) was produced and purified by ViraQuest Inc. (North Liberty, IA). Macrophages were incubated in stimulation medium (150 μl) containing AdECFP-cPLA2α for 2 h. Supplemented DMEM (1.0 ml) was added, and the macrophages were incubated at 37 °C in 7.5% CO2 for 26 h. Macrophages were washed and incubated in stimulation medium with EGFP-WTLM (m.o.i. 25), and then fixed with 3% paraformaldehyde in PBS containing 3% sucrose for 15 min as described previously (23). In some experiments, paraformaldehyde-fixed macrophages infected with WTLM or ΔhlyLM were probed with rabbit polyclonal antibody to L. monocytogenes followed by Texas Red-conjugated secondary antibody. Golgi was visualized using a rabbit polyclonal antibody to Giantin followed by Texas Red-conjugated secondary antibody. Cells were imaged on an inverted Zeiss 200M microscope with a 175-watt xenon lamp using 63× oil immersion objective. Images were acquired using a CCD camera from Sensicam and data analyzed using Intelligent Imaging Innovations Inc. (3I) software.
Calcium Imaging
Macrophages (5 × 105) plated onto glass-bottomed MatTek dishes were cultured overnight, washed with phenol red-free DMEM containing 2 mM probenecid and 25 mM HEPES, pH 7.4 (imaging medium), and then incubated in imaging medium containing 5 μM Fura Red-AM and 0.02% pluronic at room temperature. After 30 min, the macrophages were washed three times and incubated in imaging medium for an additional 30 min. L. monocytogenes strains were added, and live cell imaging was carried out at room temperature using an inverted Zeiss 200M microscope with a 40× oil immersion objective and a Fura Red long pass dichroic mirror and emission filter set (Chroma) for the calcium indicator. Cells were illuminated at 403 and 490 nm (to detect calcium-bound and calcium-free form of the Fura Red indicator, respectively). Images were acquired using a CCD camera from Sensicam, and data were analyzed using Intelligent Imaging Innovations Inc. (3I) software.
Statistical Analysis
Statistics were calculated in GraphPad using paired t test to obtain two-tailed p values.
RESULTS
L. monocytogenes Stimulates cPLA2α-mediated Arachidonic Acid Release from Resident Peritoneal Macrophages
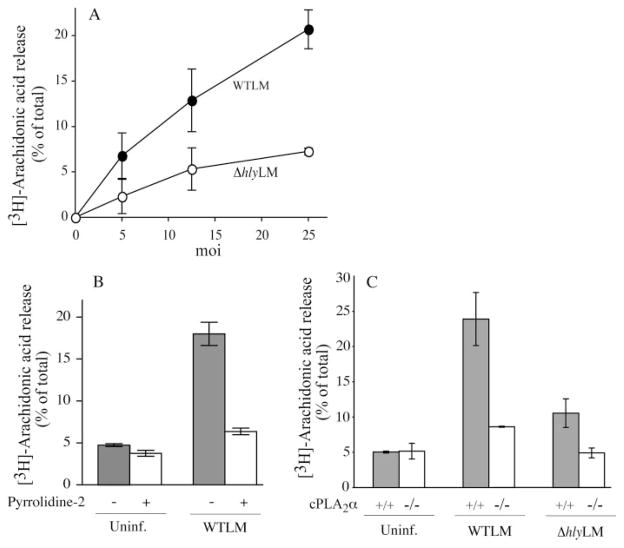
Unopsonized WTLM stimulated arachidonic acid release from resident mouse peritoneal macrophages at multiplicities of infection (m.o.i.) in the range of 5–25 (bacteria:macrophage) (Fig. 1A). An L. monocytogenes mutant strain lacking LLO (ΔhlyLM) induced ~60% less arachidonic acid release than WTLM at 60 min after infection. The role of L. monocytogenes PLCs was also investigated by testing the double mutant lacking PI-PLC and BR-PLC, and the triple mutant strain lacking LLO, PI-PLC, and BR-PLC. Results demonstrated that PLCs do not play a role in stimulating arachidonic acid release because the response to the double PLC mutant was identical to WTLM, and the response to the triple mutant was identical to ΔhlyLM (data not shown). Arachidonic acid release induced by WTLM was inhibited by the cPLA2α inhibitor pyrrolidine-2 (Fig. 1B) and was reduced by 85% in cPLA2α−/− macrophages infected with WTLM and was near background levels in cPLA2α−/− macrophages infected with ΔhlyLM (Fig. 1C). Thus nonopsonized L. monocytogenes stimulates cPLA2α-mediated arachidonic acid release from resident peritoneal macrophages that involves the virulence factor LLO.
FIGURE 1. L. monocytogenes stimulates cPLA2α-mediated arachidonic acid release from mouse peritoneal macrophages.
Macrophages labeled with [3H]arachidonic acid were infected for 60 min with the indicated m.o.i. of WTLM or ΔhlyLM (A) or WTLM and ΔhlyLM (m.o.i. 25) (B and C). B, macrophages were incubated for 30 min with and without 5 μM pyrrolidine-2 prior to adding WTLM. C, macrophages isolated from cPLA2α+/+ and cPLA2α−/− mice (C57BL/6/129 mixed strain) were used. Similar results were obtained with macrophages isolated from cPLA2α+/+ and cPLA2α−/− BALB/c mice. The amount of [3H]arachidonic acid released into the medium from infected and uninfected (Uninf.) macrophages was measured and expressed as a percentage of the total incorporated radioactivity. The results are the averages of two independent experiments in triplicate ± S.D.
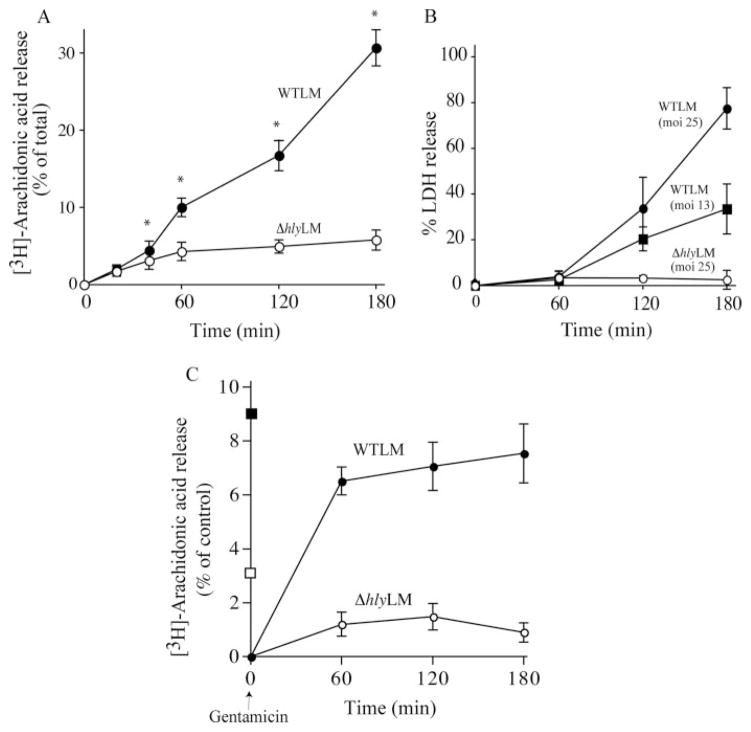
A time course illustrated that the release of arachidonic acid increased continuously from 20 min to 3 h after WTLM infection (Fig. 2A). In contrast, arachidonic acid release increased up to 60 min after ΔhlyLM infection and then leveled off. The amount of arachidonic acid released 20 min after infection with WTLM and ΔhlyLM was not significantly different, but by 40 min WTLM induced significantly higher levels of arachidonic acid release. Because L. monocytogenes proliferates in tissue culture medium, LLO released from growing WTLM over time may promote toxicity in resident peritoneal macrophages. As shown in Fig. 2B, ΔhlyLM was not cytotoxic during the 3 h of infection. In contrast, WTLM at m.o.i. of 13 and 25 induced 38 and 80% release of LDH, respectively, by 3 h. Therefore, the release of arachidonic acid beyond 60 min by WTLM correlated with cytotoxicity. The ability of WTLM and ΔhlyLM to stimulate arachidonic acid release was investigated under conditions in which growth of extracellular L. monocytogenes was inhibited with gentamicin. When macrophage cultures were washed 1 h after addition of WTLM, and then incubated in the presence of gentamicin for 2 h, cytotoxicity was prevented (data not shown). To determine the effect of blocking extracellular growth on arachidonic acid release, macrophages labeled with [3H]arachidonic acid were infected (m.o.i. 25) with L. monocytogenes for 60 min, and the cultures were rinsed and then incubated in media containing gentamicin. The amount of arachidonic acid released into the culture medium was measured at 1, 2, and 3 h after adding gentamicin (Fig. 2C). During the initial 60 min infection (no gentamicin), the level of arachidonic acid released in response to WTLM and ΔhlyLM was 9 and 3.5%, respectively, similar to the results shown in Fig. 2A. After removing the culture medium and adding fresh medium containing gentamicin, the release of arachidonic acid 60 min after adding gentamicin was 5-fold greater from macrophages infected with WTLM than those infected with ΔhlyLM suggesting that cPLA2α remained more active in macrophages infected with WTLM. There was little additional release of arachidonic acid from macrophages infected with WTLM at 120 and 180 min after adding gentamicin, unlike cultures without gentamicin (compare Fig. 2, A and C). The results suggest that extracellular LLO contributes to cPLA2α activation at these later time points as shown in Fig. 2A.
FIGURE 2. Time course of arachidonic acid release and cytotoxicity in response to WTLM and ΔhlyLM.
Macrophages were infected with WTLM and ΔhlyLM (m.o.i. 25), and release of [3H]arachidonic acid (A) and LDH (B) was monitored at the indicated times after infection in the absence of gentamicin. C, [3H]Arachidonic acid-labeled macrophages were incubated with WTLM or ΔhlyLM for 60 min, washed, and then incubated in fresh medium containing 50 μg/ml gentamicin. The amount of [3H]arachidonic acid released was determined after the initial 60 min of infection in the absence of gentamicin in response to WTLM (■) or ΔhlyLM (□), and then at the indicated times after washing and addition of fresh medium containing gentamicin (50 μg/ml). The amount of [3H]arachidonic acid released into the medium from infected and uninfected cells was measured and expressed as a percentage of the total incorporated radioactivity after subtracting background arachidonic acid release from uninfected macrophages. The results are the averages of three experiments ±S.E. (A and C) or the averages of two experiments ±S.D. (B). [3H]arachidonic acid release because of WTLM and ΔhlyLM was insignificant at 20 min but was significantly (*, p < 0.05) higher in response to WTLM than ΔhlyLM at 40 min and thereafter (A).
Role of L. monocytogenes Internalization in Regulating cPLA2α Activation
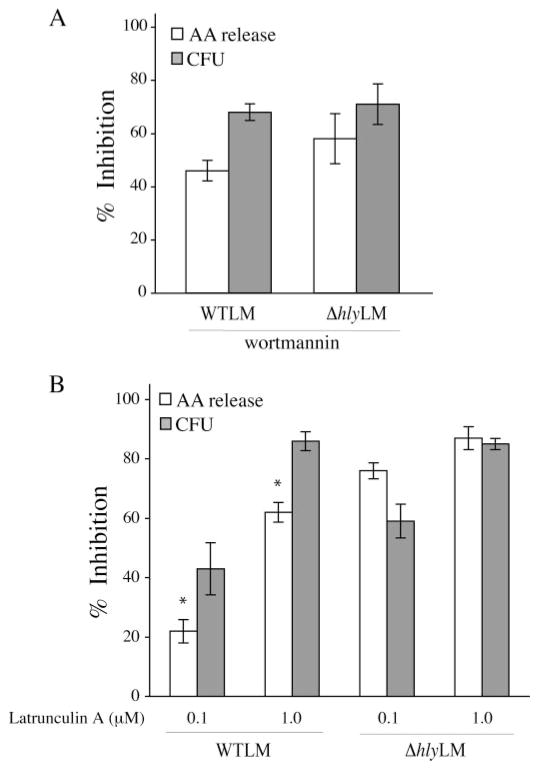
The increased cPLA2α activation by WTLM could in part be due to its ability to escape into the cytosol and proliferate. Alternatively, LLO could play an extracellular role by inducing signals that contribute to cPLA2α activation. This latter process would presumably not be dependent on WTLM internalization. Thus, experiments were carried out to determine whether internalization of WTLM and ΔhlyLM is required for stimulation of arachidonic acid release. It has been reported that inhibiting phosphoinositide 3-kinase (PI 3-kinase) with wortmannin blocks internalization of L. monocytogenes into epithelial cells (24). As shown in Fig. 3A, wortmannin also inhibited internalization of WTLM and ΔhlyLM by resident peritoneal macrophages. This inhibition was similar for WTLM and ΔhlyLM (68 and 71%, respectively) by 60 min after infection. In control macrophages, not treated with wortmannin, there was greater uptake of ΔhlyLM (2.7 × 105 CFU/well) than WTLM (1.1 × 105 CFU/well). J774 macrophages have been reported to internalize ΔhlyLM to a greater extent than WTLM (25). Wortmannin inhibited internalization to a greater extent than arachidonic acid release in response to WTLM suggesting that arachidonic acid release is in part stimulated extracellularly.
FIGURE 3. Role of L. monocytogenes internalization in regulating arachidonic acid release.
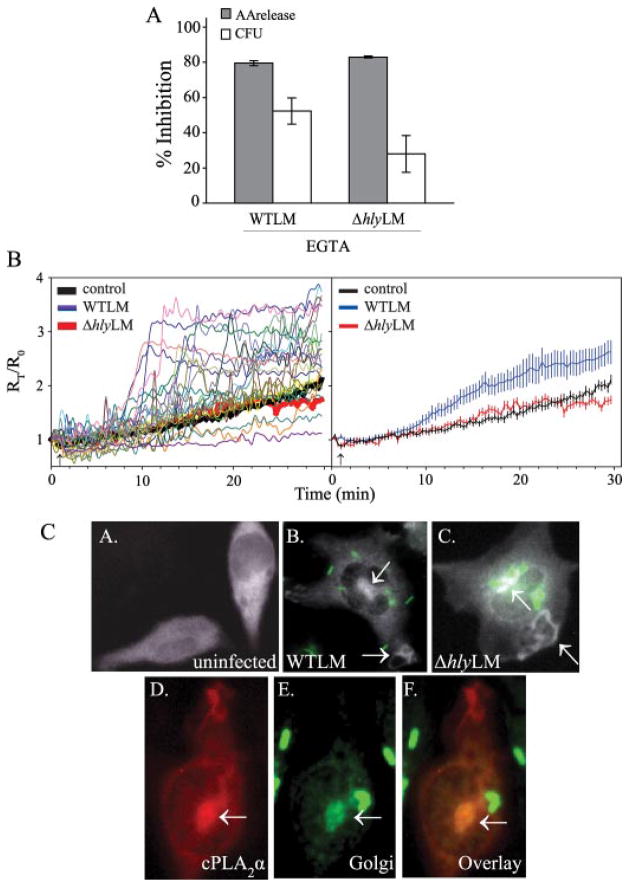
[3H]Arachidonic acid-labeled (open bars) or unlabeled macrophages (gray bars) were incubated in the absence or presence of 0.1 μM wortmannin (A), or 0.1 or 1.0 μM latrunculin A (B) for 30 min. After 60 min of incubation with WTLM and ΔhlyLM (m.o.i. 25), [3H]arachidonic acid (AA) release (open bars) or bacterial uptake (CFU) (gray bars) were measured. The results are averages ±S.E. of a minimum of three independent experiments. The data are expressed as the % inhibition relative to WTLM or ΔhlyLM controls not treated with inhibitors. WTLM- and ΔhlyLM-infected control macrophages released 18.5 and 10.5% AA (A) and 19.3 and 10.9% AA (B), respectively. There was significantly (*, p < 0.05) less inhibition of [3H]arachidonic acid release by latrunculin A (0.1 and 1.0 μM) from macrophages stimulated with WTLM than ΔhlyLM.
Because inhibition of PI 3-kinase could affect signaling pathways that regulate cPLA2α activation, the effect of latrunculin A, which depolymerizes actin, on internalization of L. monocytogenes and arachidonic acid release was also determined (Fig. 3B). Latrunculin A at 0.1 and 1 μM inhibited internalization of WTLM (47 and 87%, respectively) and ΔhlyLM (59 and 85%, respectively) to a similar extent. As observed for experiments in Fig. 3A, there was greater uptake of ΔhlyLM (2.9 × 105 CFU/well) than WTLM (1.3 × 105 CFU/well) by control macrophages not treated with latrunculin A. There was a direct correlation between blocking internalization of ΞhlyLM with latrunculin A and inhibition of arachidonic acid release, indicating that internalization of ΔhlyLM is required for cPLA2α activation. However, there was significantly less inhibition of arachidonic acid release by latrunculin A (0.1 and 1.0 μM) from macrophages infected with WTLM (22 and 62%, respectively) than ΔhlyLM (76 and 87%, respectively). Therefore, cPLA2α activation by ΔhlyLM is dependent on internalization, whereas internalization-dependent and -independent pathways are involved in cPLA2α activation by WTLM. The results suggest that LLO exerts an extracellular effect on macrophages leading to greater stimulation of cPLA2α.
Activation of MAPKs by L. monocytogenes Regulates Arachidonic Acid Release
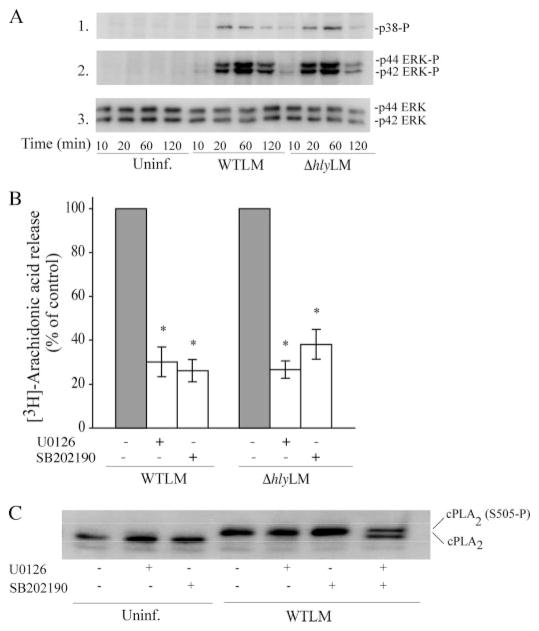
Activation of MAPKs regulates cPLA2α-mediated arachidonic acid release from resident peritoneal macrophages in response to a variety of agonists (26). The ability of L. monocytogenes to activate MAPKs in resident peritoneal macrophages was investigated by Western blotting using phospho-specific antibodies to the activated forms of p38 and p42/p44 ERKs (Fig. 4A). WTLM and ΔhlyLM activated p38 and ERKs to a similar extent, which occurs between 20 and 60 min and then diminishes by 120 min. The MEK1 inhibitor U0126 and the p38 inhibitor SB202190 significantly blocked arachidonic acid release implicating a role for these MAPKs in regulating cPLA2α activation in response to L. monocytogenes (Fig. 4B). In control experiments, the MAPK inhibitors did not block internalization of L. monocytogenes (data not shown). cPLA2α is phosphorylated by p38 and ERKs on Ser-505, which causes a decrease in electrophoretic mobility on SDS-polyacrylamide gels (27, 28). As shown in Fig. 4, WTLM induced a cPLA2α gel shift indicating phosphorylation on Ser-505. Preincubation of macrophages with either the MEK1 or p38 inhibitors prior to infection with L. monocytogenes had no effect on Ser-505 phosphorylation. Treatment of macrophages with both U0126 and SB202190 partially blocked the gel shift, suggesting that ERKs and p38 contribute to the phosphorylation of cPLA2α in response to L. monocytogenes. However, the MAPK inhibitors significantly blocked arachidonic acid release under conditions that did not detectably inhibit cPLA2α Ser-505 phosphorylation, suggesting that MAPKs have additional roles in regulating arachidonic acid release induced by L. monocytogenes.
FIGURE 4. Activation of ERKs and p38 by WTLM and ΔhlyLM contributes to cPLA2α-mediated AA release.
A, cell lysates were prepared from uninfected (Uninf.) or L. monocytogenes-infected macrophages (m.o.i. 25) at the indicated times. Activation of MAPKs was determined by probing for phosphorylated p38 (p38-P) or ERKs (ERK-P) on Western blots using phosphospecific antibodies. The samples were probed for total ERK protein (panel 3) as a control for sample loading. B, [3H]arachidonic acid-labeled macrophages were preincubated with vehicle (Me2SO), 10 μM U0126 for 15 min, or 10 μM SB202190 for 60 min followed by infection (m.o.i. 25) with WTLM or ΔhlyLM for 60 min. The amount of [3H]arachidonic acid released into the media is expressed as a percentage of release from control macrophages (gray bars) not treated with inhibitors (100%). WTLM- and ΔhlyLM-infected control macrophages not treated with inhibitors released 18.6 and 8.4% AA, respectively. C, cPLA2α gel shift was analyzed by immunoblotting lysates from macrophages treated as described above. Results are representative of two independent experiments (A and C) or the averages ± S.E. of three independent experiments (B). Arachidonic acid release was significantly lower (*, p < 0.05) from macrophages treated with MAPK inhibitors compared with controls (B).
Role of Calcium in Regulating cPLA2α Activation
cPLA2α is regulated by increases in concentrations of intracellular calcium ([Ca2+]i) that binds to the C2 domain and promotes its translocation from cytosol to membrane where it hydrolyzes arachidonic acid from phospholipid (29). To determine the role of extracellular calcium in regulating arachidonic acid release and internalization of L. monocytogenes, macrophages were incubated in medium containing EGTA. Chelating extracellular calcium significantly blocked both arachidonic acid release stimulated by WTLM and ΔhlyLM (80 and 83%, respectively) and internalization (53 and 30%, respectively) (Fig. 5A). In control macrophages not treated with EGTA, the numbers of intracellular ΔhlyLM (3.9 × 105 CFU/well) were greater than WTLM (1.4 × 105 CFU/well). Although chelating extracellular calcium with EGTA tended to block internalization of WTLM to a greater extent than ΔhlyLM, this did not reach statistical significance. The results suggest that calcium has a dual role and regulates cPLA2α activation and internalization of L. monocytogenes. The ability of WTLM and ΔhlyLM to induce increases in [Ca2+]i was compared (Fig. 5B). Live cell imaging of calcium mobilization over time in macrophages loaded with Fura Red-AM demonstrated a heterogeneous response of individual cells to WTLM, but increases in [Ca2+]i were evident in many cells ~10 min after adding WTLM. The heterogeneous response may be due to the timing and number of WTLM that interact with macrophages. In contrast, increases in [Ca2+]i could not be detected in most macrophages incubated with ΔhlyLM.
FIGURE 5. Role of calcium in regulating cPLA2α activation by L. monocytogenes.
A, [3H]arachidonic acid-labeled (gray bars) or unlabeled macrophages (open bars) were incubated in the absence or presence of 10 mM EGTA for 15 min. After a 60-min incubation with WTLM and ΔhlyLM (m.o.i. 25), [3H]AA release or bacterial uptake (CFU) was measured. The amount of [3H]AA released into the media is expressed as a percentage of release from control macrophages (gray bars) not treated with EGTA (100%). WTLM- and ΔhlyLM-infected macrophages not treated with EGTA released 21.4 and 8.8% AA, respectively. The results are averages ±S.E. of three independent experiments. There was significantly (p < 0.05) less [3H]AA release and CFU from macrophages in the presence of EGTA. B, macrophages loaded with Fura Red-AM were incubated without (control) or with (m.o.i. 25) WTLM or ΔhlyLM. [Ca2+]i changes were determined by live cell imaging and calculated as a ratio of the fluorescence intensities of bound to unbound calcium (F403/F490). Each point is expressed relative to time 0 (Rt/R0). In the graph on the left, data from 16 to 25 control (solid black line) or ΔhlyLM infected (solid red line) macrophages were averaged. For macrophages infected with WTLM, data from individual macrophages are shown as different colored lines. In the graph on the right, averages ± S.E. are shown. C, macrophages expressing ECFP-cPLA2α were incubated without (uninfected, panel A) or with (m.o.i. 25) EGFP-WTLM (panel B) or ΔhlyLM (panel C) for 60 min and then fixed for fluorescence microscopy. For visualizing ΔhlyLM (panel C) or WTLM shown in panels E and F, macrophages were probed with antibody to L. monocytogenes. Panel D shows macrophages expressing ECFP-cPLA2α (shown as pseudo-red) after incubation with WTLM for 30 min. The same cell probed with rabbit polyclonal antibodies to the Golgi marker Giantin (pseudo-green) and to L. monocytogenes followed by Texas Red secondary antibody (shown as pseudo-green) is shown in panel E. Although the secondary antibody depicts both Golgi and L. monocytogenes, bacteria are readily distinguished from Golgi (arrow), where localization of cPLA2α is evident (overlay, panel F).
We previously reported that cPLA2α translocates to the phagosome during internalization of zymosan by resident peritoneal macrophages (23). To determine whether this occurs during phagocytosis of L. monocytogenes, the subcellular localization of ECFP-cPLA2α in macrophages infected with WTLM or ΔhlyLM was monitored (Fig. 5C). In uninfected macrophages, ECFP-cPLA2α exhibited diffuse cytoplasmic localization (Fig. 5C, panel A). Infection of macrophages with WTLM and ΔhlyLM caused considerable cell spreading (Fig. 5C, panels B and C). In macrophages incubated with EGFP-tagged WTLM, ECFP-cPLA2α localized to a juxtanuclear region (Fig. 5C, panel B) indicative of Golgi, which was confirmed by showing co-localization with the Golgi marker Giantin (Fig. 5C, panels D–F). Localization of ECFP-cPLA2α to membrane ruffles also occurred in infected macrophages. A similar pattern of ECFP-cPLA2α localization was observed in macrophages infected with ΔhlyLM (Fig. 5C, panel C). There was no evidence of ECFP-cPLA2α fluorescence surrounding either WTLM or ΔhlyLM, indicating that cPLA2α does not translocate to the phagosome.
Arachidonic Acid Release Stimulated by L. monocytogenes Involves TLR2 and MyD88
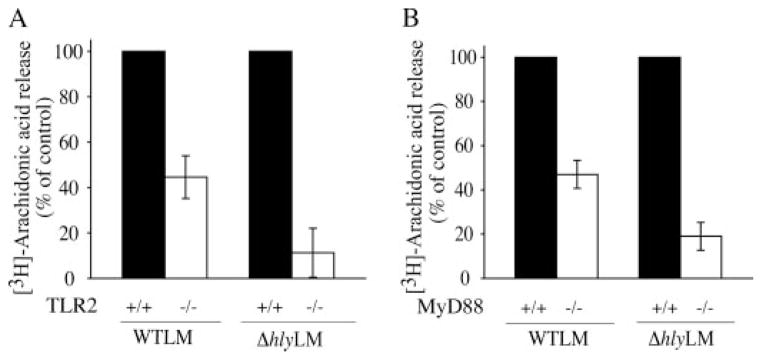
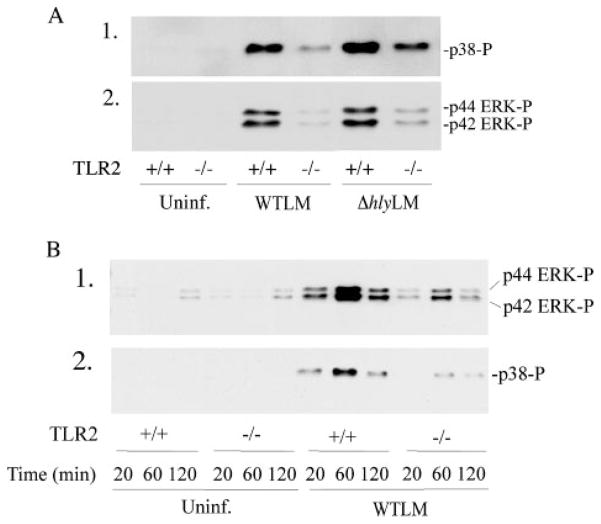
MyD88-deficient mice are highly susceptible to L. monocytogenes infection; however, contradictory results have been reported for the involvement of TLR2 in controlling infection (30–32). The role of TLR2 and MyD88 in regulating cPLA2α activation was investigated by comparing the ability of L. monocytogenes to induce arachidonic acid release from resident peritoneal macrophages isolated from wild type, TLR2, or MyD88 knock-out mice. Arachidonic acid release in response to WTLM and ΔhlyLM was attenuated in TLR2−/− macrophages by 55 and 90%, respectively (Fig. 6A). The extent of L. monocytogenes internalization did not differ in TLR2+/+ and TLR2−/− macrophages (data not shown). As shown in Fig. 6B, arachidonic acid release was attenuated to a similar extent in MyD88−/− macrophages as TLR2−/− macrophages indicating that TLR2 is the principal MyD88-dependent receptor involved in regulating cPLA2α activation in response to L. monocytogenes. The attenuated release of arachidonic acid from TLR2−/− macrophages correlated with less activation of p38 and p42/p44 ERKs in response to WTLM and ΔhlyLM (Fig. 7A). The defect in the activation of these MAPKs in TLR2−/− macrophages was evident from 20 to 120 min after addition of L. monocytogenes (Fig. 7B).
FIGURE 6. Role of TLR2 and MyD88 in regulating arachidonic acid release in response to WTLM and ΔhlyLM.
[3H]Arachidonic acid-labeled resident mouse peritoneal macrophages isolated from wild type (solid bars), TLR2−/− (open bars) (A) or MyD88−/−(open bars) (B) mice were infected (m.o.i. 25) with WTLM or ΔhlyLM for 60 min. The amount of [3H]arachidonic acid released into the media is expressed as a percentage of release from wild type macrophages (100%). WTLM- and ΔhlyLM-infected wild type macrophages released 13.9 and 6.1% AA (A) and 23.2 and 12.5% AA (B), respectively. Results are the averages ± S.D. of two independent experiments.
FIGURE 7. TLR2 regulates p38 and ERK activation in macrophages infected with WTLM and ΔhlyLM.
Resident mouse peritoneal macrophages isolated from TLR2−/−and TLR2−/−mice were infected (m.o.i. 25) with WTLM or ΔhlyLM for 60 min (A) or for the times indicated (B). Activation of MAPKs was determined by probing for phosphorylated p38 (p38-P) or ERKs (ERK-P) on Western blots using phospho-specific antibodies. Uninf, uninfected.
COX2 Expression and Eicosanoid Production Induced by L. monocytogenes
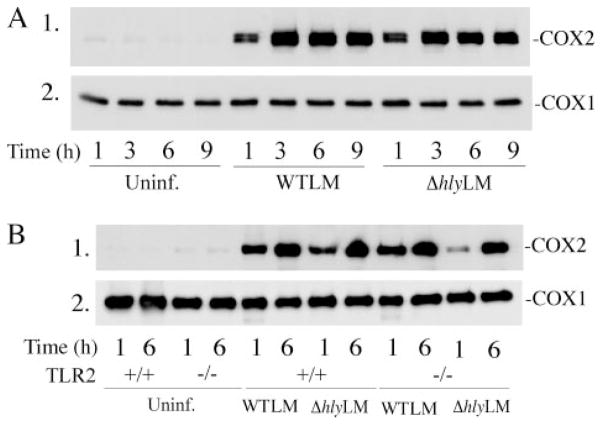
WTLM and ΔhlyLM induced the expression of COX2 to a similar extent with maximal up-regulation occurring 3 h after infection (m.o.i. 25) (Fig. 8A). Expression of COX2 induced by WTLM and ΔhlyLM did not involve TLR2 because similar levels of expression occurred in TLR2+/+ and TLR2−/− macrophages (Fig. 8B). Levels of COX1 were unaffected in macrophages infected with L. monocytogenes and were similar in TLR2+/+ and TLR2−/− macrophages.
FIGURE 8. COX2 expression is induced by WTLM and ΔhlyLM and is independentofTLR2.
Resident mouse peritoneal macrophages isolated from ICR (A) and TLR2−/−and TLR2+/+mice (B) were infected (m.o.i. 25) with WTLM or ΔhlyLM. After a 1-h incubation with L. monocytogenes, the macrophages were washed and then incubated in medium containing gentamicin. Expression of COX2 and COX1 was determined by Westernblotting of macrophage lysates prepared at the times indicated after addition of L. monocytogenes. Uninf, uninfected.
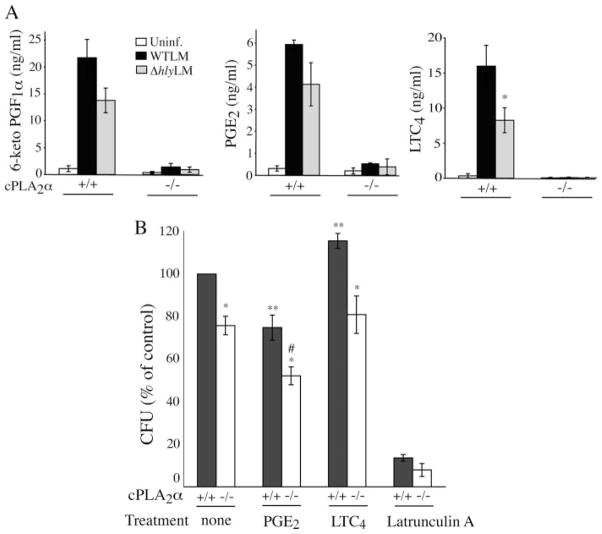
L. monocytogenes stimulated the production of PGE2, PGI2, and LTC4 by cPLA2α+/+ macrophages, but eicosanoid production was almost completely ablated in cPLA2α−/− macrophages (Fig. 9A). The level of prostanoids produced by cPLA2α+/+ macrophages 3 h after infection was lower in macrophages infected with ΔhlyLM than WTLM, although this did not reach statistical significance. However, WTLM stimulated significantly more LTC4 production than ΔhlyLM consistent with the ability of WTLM to increase [Ca2+]i, which regulates 5-lipoxygenase activation. Thus some of the increased arachidonic acid released by WTLM is diverted through the 5-lipoxy-genase pathway.
FIGURE 9. cPLA2α is required for eicosanoid production by macrophages infected with L. monocytogenes.
A, resident peritoneal macrophages isolated from cPLA2α−/− and cPLA2α−/− mice (BALB/c) were incubated with WTLM or ΔhlyLM (m.o.i. 25) for 60 min, washed, and then incubated in fresh medium containing gentamicin. Levels of PGE2, 6-keto prostaglandin F1α (6-keto PGF1α, the stable metabolite of PGI2), and leukotriene C4 (LTC4) were measured in the culture medium collected 3 h after adding L. monocytogenes. The results are the averages ± S.E. of five independent experiments for cPLA2α+/+ macrophages and averages ± S.D. of two experiments for cPLA2α−/− macrophages. There was significantly (*, p < 0.05) less LTC4 production by cPLA2α+/+ macrophages infected with ΔhlyLM than WTLM. B, resident peritoneal macrophages isolated from cPLA2α+/+ and cPLA2α−/− mice were incubated with or without (none) PGE2 (4 ng/ml), leukotriene C4 (10 ng/ml), or latrunculin A (1 μM) for 15 before addition of WTLM (m.o.i. 25). After incubation for 1 h, the macrophages were washed six times and lysed with 0.1% Triton X-100 for determination of CFU. The results are the average ± S.E. of at least three independent experiments. There was significantly (*, p < 0.05) less CFU in untreated cPLA2α−/− macrophages than cPLA2α+/+ macrophages and in PGE2- or LTC4-treated cPLA2α−/− macrophages than similarly treated cPLA2α+/+ macrophages. There was significantly (**, p < 0.05) less CFU in cPLA2α+/+ macrophages treated with PGE2 and more CFU in cPLA2α+/+ macrophages treated with LTC4 compared with untreated cPLA2α+/+ macrophages (none). There was significantly (#, p < 0.05) less CFU in cPLA2α−/− macrophages treated with PGE2 than in untreated cPLA2α−/− macrophages. Uninf, uninfected.
Eicosanoids have been reported to differentially affect bacterial internalization. PGE2 suppresses phagocytosis of L. monocytogenes but leukotrienes enhance phagocytosis of Klebsiella pneumoniae by macrophages (22, 33). cPLA2α+/+ and cPLA2α−/− peritoneal macrophages were compared to determine whether the decreased production of diverse lipid mediators by cPLA2α−/− macrophages affects internalization of WTLM. For these experiments, CFUs were determined after incubation of macrophages with WTLM for 60 min followed by extensive washing to remove extracellular bacteria, rather than using gentamicin, to compare our results with the study of Hutchison and Myers (22). To ensure that the washing procedure sufficiently removed extracellular WTLM, macrophages were treated with latrunculin A, which blocks WTLM internalization (Fig. 9B). Latrunculin A blocked WTLM uptake by 90% indicating that the CFU represent internalized bacteria and not extracellular bacteria bound to the cell surface. Using the washing technique, the number of WTLM internalized by cPLA2α+/+ macrophages (1.3 × 106 CFU/well) was higher than obtained following gentamicin treatment. Treating cell cultures with gentamicin is a commonly used protocol to kill extracellular L. monocytogenes and is essential for preventing growth of extracellular bacteria during long term incubations. However, it has been reported that gentamicin can gain access to the phagosomal compartment and contribute to killing of intracellular L. monocytogenes, which may explain the higher CFU obtained with the washing protocol (34). A comparison of the number of internalized WTLM in cPLA2α+/+ and cPLA2α−/− macrophages after incubation with bacteria for 60 min revealed that cPLA2α−/− macrophages internalized significantly fewer WTLM (25% less CFU) than cPLA2α+/+ macrophages (Fig. 9B). The direct addition of PGE2, at a concentration in the range produced by cPLA2α+/+ macrophages, significantly blocked internalization of WTLM by cPLA2α+/+ and cPLA2α−/− macrophages. In contrast, LTC4 addition induced a small (15%) but significant increase in WTLM uptake by cPLA2α−/− macrophages but not by cPLA2α−/− macrophages. Thus the decreased uptake of WTLM by cPLA2α+/+ macrophages is not solely explained by the lack of LTC4 production.
cPLA2α-mediated Prostaglandin Production Down-regulates WTLM-stimulated TNFα Production
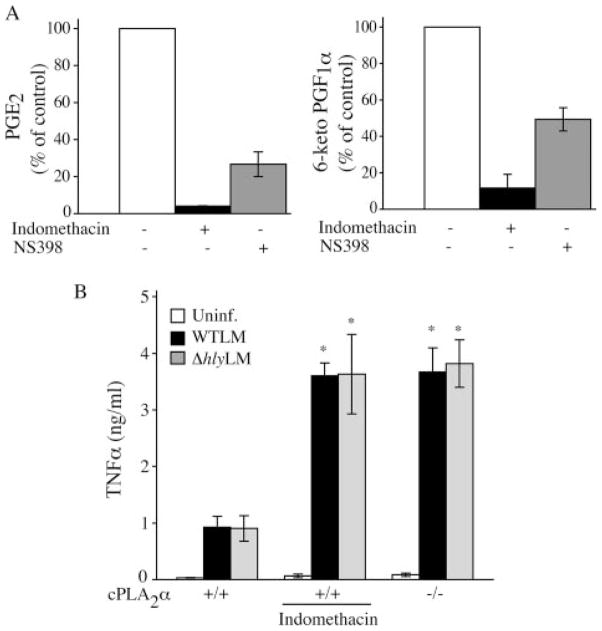
We investigated the contribution of COX1 and COX2 in mediating prostaglandin production in macrophages infected with WTLM. As shown in Fig. 10A, the COX2 inhibitor NS398 blocked PGE2 and PGI2 production by 72 and 55%, respectively, whereas the nonselective COX inhibitor indomethacin blocked prostaglandin production by over 90%. The results suggest a role for both COX2 and COX1 in prostaglandin production in response to L. monocytogenes.
FIGURE 10. Regulation of TNFα production by prostaglandins.
A, resident peritoneal macrophages isolated from cPLA2α+/+ mice were incubated for 30 min with or without 20 μM indomethacin or 10 μM NS398 and then incubated with WTLM for 3 h as described above for Fig. 9A. Control macrophages not treated with inhibitors produced 9.9 ng/ml PGE2 and 9.1 ng/ml 6-keto PGF1α.B, cPLA2α+/+ macrophages, incubated with or without 20 μMindo-methacin, and cPLA2α−/− macrophages incubated without indomethacin, were treated as described in Fig. 9A. The culture media were collected 3 h after adding WTLM or ΔhlyLM for TNFα analysis. Results are the averages of two independent experiments ± S.D. (A) or the averages of three independent experiments ± S.E. (B). cPLA2α−/− macrophages and indomethacin-treated cPLA2α+/+ macrophages produced significantly more (p < 0.05) TNFα production than untreated cPLA2α+/+ macrophages.
Several reports have demonstrated a role for prostanoids in regulating cytokine production by macrophages (35–43). Considering the importance of TNFα in regulating immune responses to L. monocytogenes infection, we compared TNFα production by cPLA2α+/+ and cPLA2α−/− macrophages in response to L. monocytogenes. cPLA2α−/− macrophages produced significantly higher levels of TNFα than cPLA2α+/+ macrophages (Fig. 10B). Treating cPLA2α+/+ macrophages with the COX inhibitor indomethacin increased TNFα production to the levels produced by cPLA2α−/− macrophages implicating a role for prostaglandins in suppressing TNFα production.
DISCUSSION
The ability of L. monocytogenes to disseminate and evade host responses is in part because of its ability to survive and multiply within a variety of cell types, including tissue macrophages (2, 44, 45). The results of this study demonstrate that L. monocytogenes stimulates cPLA2α activation and eicosanoid production in resident peritoneal macrophages and occurs in the absence of serum with un-opsonized bacteria. WTLM and ΔhlyLM stimulate similar levels of arachidonic acid release at early times (20 min), but at later times (40–60 min) WTLM stimulates to a greater extent than ΔhlyLM. The results demonstrate that LLO augments cPLA2α activation but is not essential. In contrast, the activation of host phospholipases C and D in J774 macrophages by WTLM is absolutely dependent on LLO (46). The increased arachidonic acid release by WTLM could in part be due to LLO-dependent escape from the primary phagosome and proliferation in the cytosol, which occurs within the time frame observed for enhancement of arachidonic acid release by WTLM. However, if LLO-dependent escape of WTLM from the primary vacuole or cell-to-cell spread contributed to cPLA2α activation, we would have expected the double PLC mutant to be less effective at inducing arachidonic acid release because PLCs contribute to escape and spread (8–11). However, the double L. monocytogenes mutant lacking PI-PLC and BR-PLC stimulated arachidonic acid release as effectively as WTLM. Experiments also demonstrated that L. monocytogenes mutants lacking the virulence factors p60, an autolysin that hydrolyzes peptidoglycan, and ActA, a protein important for actin-based L. monocytogenes motility, stimulated arachidonic acid release as effectively as WTLM (data not shown) (47–49).
Results using latrunculin A, which blocks internalization of WTLM to a greater extent than arachidonic acid release, suggest that LLO may act extracellularly to promote cPLA2α activation. In contrast, arachidonic acid release induced by ΔhlyLM is more dependent on internalization than for WTLM. The delay in the enhancement of arachidonic acid release by WTLM may be due to the time-dependent production and secretion of extracellular LLO to levels needed for augmenting arachidonic acid release. LLO may play a signaling role at the plasma membrane in addition to its role in promoting escape of L. monocytogenes from the primary phagosome (3). Recent studies have demonstrated that LLO exhibits cholesterol-dependent pore-forming ability at physiological pH and may induce cell signaling by aggregating lipid rafts (50, 51). It has been shown that L. monocytogenes-stimulated leukotriene production by human neutrophils is completely dependent on LLO. It occurs independently of phagocytosis and by purified LLO (52). In macrophages, we find that both LLO-dependent and -independent mechanisms contribute to cPLA2α activation. Heat-killed L. monocytogenes also stimulated arachidonic acid release (data not shown), although more weakly than ΔhlyLM, suggesting that a cell wall component of L. monocytogenes contributes to cPLA2α activation.
To mediate arachidonic acid release, cPLA2α must translocate from the cytosol to the membrane to access substrate. This is an important regulatory step induced by elevations in [Ca2+]i that binds to the cPLA2α C2 domain increasing its affinity for membrane (29, 53, 54). However, in response to certain stimuli cPLA2α-mediated arachidonic acid release from macrophages occurs with no detectable increase in [Ca2+]i, although resting levels of calcium are required (55–58). Previous studies have shown that WTLM stimulates [Ca2+]i increases in endothelial cells, epithelial cells, and J774 macrophages to a greater extent than ΔhlyLM, and that LLO forms calcium-permeable pores in cells (25, 59–62). Similarly, we found in resident peritoneal macrophages that WTLM but not ΔhlyLM increases [Ca2+]i, which may contribute to the ability of WTLM to stimulate greater arachidonic acid release. However, arachidonic acid release stimulated by ΔhlyLM is blocked by chelating extracellular calcium. This suggests a role for resting [Ca2+]i, which may be lowered by extracellular EGTA. It is also possible that ΔhlyLM stimulates local, transient increases in [Ca2+]i that we were not able to detect. During zymosan phagocytosis, cPLA2α translocates to the forming phagosome, membrane ruffles, and Golgi (23). Similarly, cPLA2α translocates to Golgi and membrane ruffles in macrophages infected with L. monocytogenes. However, we found no evidence that cPLA2α translocates to the L. monocytogenes phagosome suggesting differences in the properties of the phagosome membrane.
Activation of cPLA2α in resident peritoneal macrophages is partially dependent on engagement of TLR2 by L. monocytogenes. Arachidonic acid release is more dependent on TLR2 in response to ΔhlyLM than to WTLM, consistent with a TLR2-independent role for LLO in cPLA2α activation in response to WTLM. Resident peritoneal macrophages lacking MyD88 exhibited a similar decrease in arachidonic acid release as TLR2−/− macrophages, in response to WTLM and ΔhlyLM. This suggests that TLR2 engagement is the principal MyD88-dependent pathway involved in regulating cPLA2α activation in response to L. monocytogenes. Our results demonstrate that TLR2 is required for optimal activation of ERKs and p38, which regulate activation of cPLA2α-mediated arachidonic acid release in response to L. monocytogenes. In contrast to reports using nonphagocytic cells, MAPKs are activated to a similar extent by WTLM and ΔhlyLM in peritoneal macrophages, and are not required for L. monocytogenes internalization (63–65). MAPKs (ERKs and p38) phosphorylate cPLA2α on Ser-505, which enhances activity of cPLA2α and promotes arachidonic acid release (27, 28, 66). L. monocytogenes induces the stoichiometric phosphorylation of cPLA2α on Ser-505 as evidenced by the complete shift in electrophoretic mobility on SDS-polyacrylamide gels. However, inhibition of either ERKs or p38 blocked L. monocytogenes-induced arachidonic acid release under conditions that did not prevent phosphorylation of cPLA2α on Ser-505. These results suggest an additional role independent of Ser-505 phosphorylation for MAPKs in regulating cPLA2α in response to L. monocytogenes, as observed in other cell models (26, 67). Targeted disruption of MAPK-activated protein kinase, a substrate for p38 and ERKs, increases susceptibility of mice to L. monocytogenes infection (68). Our data suggest another role for MAPKs, promoting cPLA2α activation, in regulating immune responses to L. monocytogenes.
We previously reported that engagement of TLR2 is not sufficient for cPLA2α activation. The TLR2 ligand MALP2 poorly induces arachidonic acid release but acts synergistically with the dectin-1 agonist particulate β-glucan (69). Therefore it is likely that engagement of an unknown receptor by L. monocytogenes, on resident peritoneal macrophages, mediates internalization and induces signals for cPLA2α activation cooperatively with TLR2. We found that internalization of WTLM and ΔhlyLM is blocked by inhibition of PI 3-kinase and actin polymerization, and to a lesser extent by chelating extracellular calcium. Uptake of L. monocytogenes into nonphagocytic cells is mediated in part by internalin A and internalin B, which interact with E-cadherin and Met receptors on host cells, respectively (70, 71). Internalin B binding to the tyrosine kinase receptor Met mediates internalization of L. monocytogenes through activation of PI 3-kinase, MAPK, and actin polymerization (3, 24, 65, 70). Internalin B also exhibits divalent-cation dependent binding to C1q-R, the receptor for the complement component C1q that is expressed on many cells, including peritoneal macrophages (72, 73). In J774 macrophages, internalin B mediates activation of Ras and PI 3-kinase, and internalin A mediates phagocytosis of L. monocytogenes (74, 75). However, we found that mutant L. monocytogenes lacking both internalin A and internalin B stimulates arachidonic acid release in resident peritoneal macrophages as effectively as WTLM (data not shown) suggesting that the internalins are not involved in mediating cPLA2α activation.
L. monocytogenes induces transcriptional responses in macrophages by interaction of cell wall components with pattern recognition receptors such as TLRs, and by MyD88-independent mechanisms that are triggered by escape into the cytosol (76). The cytosolic specific transcriptional response induced by WTLM but not ΔhlyLM, in bone marrow-derived macrophages, involves activation of p38 (77). In contrast, our results demonstrate that WTLM and ΔhlyLM both stimulate the TLR2-dependent early activation of ERKs and p38 in resident peritoneal macrophages. Activation of MAPKs is not completely ablated in TLR2- or MyD88-deficient resident peritoneal macrophages implicating a role for another signaling pathway independent of cytosolic sensing. In addition, ΔhlyLM induces similar levels of COX2 expression as WTLM by a TLR2-independent mechanism. The results demonstrate that the signals induced by L. monocytogenes for eicosanoid production, including acute activation of cPLA2α and up-regulation of COX2, are not dependent on cytosolic sensing and involve TLR2-dependent and -independent pathways. The data implicate the presence of another signaling pathway triggered by interaction of L. monocytogenes with a receptor on macrophages that remains to be identified.
The production of eicosanoids by resident peritoneal macrophages infected with L. monocytogenes was dependent on cPLA2α. Therefore a comparison of macrophages from cPLA2α knock-out and wild type mice provided a model system to determine whether production of eicosanoids influences macrophage responses to L. monocytogenes infection. WTLM induced a similar degree of cytotoxicity in cPLA2α+/+ and cPLA2α−/− macrophages 24 h after infection despite incubation in medium containing gentamicin (data not shown). This suggests that cPLA2α activation does not influence escape, intracellular growth, and killing of resident peritoneal macrophages by WTLM.
A comparison of the ability of cPLA2α+/+ and cPLA2α−/− macrophages to internalize WTLM revealed a small (25%) but significant decrease in uptake of WTLM by cPLA2α−/−. We found opposing effects of adding PGE2 and LTC4 on internalization of WTLM. PGE2 blocked uptake of WTLM consistent with a previous report showing a decrease in phagocytosis of WTLM by peritoneal macrophages as determined by microscopic evaluation (22). The lack of PGE2 production by cPLA2α−/− would be expected to result in increased WTLM uptake, which was not observed. Addition of LTC4 to cPLA2α+/+ macrophages slightly increased phagocytosis of WTLM, a trend similar to that observed for phagocytosis of K. pneumoniae by rat alveolar macrophages (33). However, LTC4 addition did not reverse the defective ability of cPLA2α−/− peritoneal macrophages to internalize WTLM. cPLA2α initiates the production of numerous COX and lipoxygenase-derived lipid mediators that can differentially effect cellular responses depending on the profile of eicosanoid receptor expression. Therefore, it is unlikely that the decrease in phagocytosis of WTLM by cPLA2α−/− macrophages can be ascribed to the loss of one particular lipid mediator.
We found that cPLA2α activation influences TNFα production, a cytokine essential for combating L. monocytogenes infection (78–80). cPLA2α+/+ macrophages produced significantly less TNFα than cPLA2α−/− macrophages. The ability of indo-methacin to enhance production of TNFα in cPLA2α+/+ macrophages to the level produced by cPLA2α−/− macrophages in response to L. monocytogenes suggests that prostaglandins act in an autocrine fashion to suppress TNFα production. It has been reported that TNFα production by human and mouse macrophages, including Kupffer cells, is suppressed by exogenous addition of prostaglandins and enhanced by blocking endogenous prostaglandin production with cyclooxygenase inhibitors (35–43). We show that this regulatory program is initiated by the activation of cPLA2α in response to L. monocytogenes infection. The receptors for PGE2 (EP2 and EP4) and PGI2 (IP) mediate increases in cAMP, which down-regulates TNFα production (35, 42, 81). It has been shown that treatment with indomethacin increases susceptibility of mice infected intraperitoneally with L. monocytogenes suggesting that prostaglandins or thromboxane A2 has a protective role (82). In contrast, administering PGE1 suppresses cellular immunity in mice infected intravenously with L. monocytogenes (83). It is difficult to reconcile the role of COX-derived metabolites from these studies because of the differences in route of infection and use of indomethacin, which blocks production of all prostanoids and thromboxane A2, versus using a single prostaglandin. However, they do point to a role for lipid mediators in regulating immune response to L. monocytogenes. Prostaglandins may have multiple effects in regulating immunity. In addition to affecting TNFα levels, prostaglandins suppress production of a variety of chemokines. They also up-regulate IL-10 and IL-6 production (36, 84, 85) and will have differential effects that depend on the types of prostaglandin receptors expressed on target cells (86). In addition to regulating cytokine production, prostaglandins promote increases in vascular permeability and blood flow, which regulate the migration of effector cells from the blood to the site of infection (86). Our results demonstrate that regulation of cytokine production by prostaglandins occurs as a result of cPLA2α activation by L. monocytogenes, suggesting that its early activation in tissue macrophages may have an impact on immune responses to microbial infection.
Footnotes
The abbreviations used are: LLO, listeriolysin O; PLA2, phospholipase A2; cPLA2, cytosolic PLA2; WTLM, wild type L. monocytogenes; ΔhlyLM, LLO-deficient L. monocytogenes; COX, cyclooxygenase; PLC, phospholipase C; IL, interleukin; TNF, tumor necrosis factor; TLR2, toll-like receptor 2; DMEM, Dulbecco’s modified Eagle’s medium; EGFP, enhanced green fluorescent protein; ECFP, enhanced cyan fluorescent protein; ERK, extracellular signal regulated kinase; MAPK, mitogen-activated protein kinase; PI 3-kinase, phosphoinositide 3-kinase; m.o.i., multiplicity of infection; LDH, lactate dehydrogenase; BR-PLC, broad range PLC; PBS, phosphate-buffered saline; CFU, colony-forming unit; AA, arachidonic acid.
This work was supported by National Institutes of Health Grants HL34303 (to C. C. L.) and AI065638 (to L. L. L.).
References
- 1.Farber JM, Peterkin PI. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamon M, Bierne H, Cossart P. Nat Rev Microbiol. 2006;4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 4.Dussurget O, Pizarro-Cerda J, Cossart P. Annu Rev Microbiol. 2004;58:611–647. doi: 10.1146/annurev.micro.57.030502.090934. [DOI] [PubMed] [Google Scholar]
- 5.Portnoy DA, Jacks PS, Hinrichs DJ. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayal S, Charbit A. FEMS Microbiol Rev. 2006;30:514–529. doi: 10.1111/j.1574-6976.2006.00021.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. Infect Immun. 1995;63:3241–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilli A, Tilney LG, Portnoy DA. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazquez-Boland JA, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti-Segui C, Goeden KR, Higgins DE. Cell Microbiol. 2007;9:179–195. doi: 10.1111/j.1462-5822.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith GA, Portnoy DA, Theriot JA. Mol Microbiol. 1995;17:945–951. doi: 10.1111/j.1365-2958.1995.mmi_17050945.x. [DOI] [PubMed] [Google Scholar]
- 13.Portnoy DA, Auerbuch V, Glomski IJ. J Cell Biol. 2002;158:409–414. doi: 10.1083/jcb.200205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousens LP, Wing EJ. Immunol Rev. 2000;174:150–159. doi: 10.1034/j.1600-0528.2002.017407.x. [DOI] [PubMed] [Google Scholar]
- 15.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. J Immunol. 2004;173:589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- 16.Harizi H, Gualde N. Tissue Antigens. 2005;65:507–514. doi: 10.1111/j.1399-0039.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 17.Funk CD. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 18.Rocca B, Fitzgerald GA. Int Immunopharmacol. 2002;2:603–630. doi: 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 20.Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, Sapirstein A. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 21.de Carvalho MS, McCormack FX, Leslie CC. Arch Biochem Biophys. 1993;306:534–540. doi: 10.1006/abbi.1993.1549. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison DL, Myers RL. Cell Immunol. 1987;110:68–76. doi: 10.1016/0008-8749(87)90102-x. [DOI] [PubMed] [Google Scholar]
- 23.Girotti M, Evans JH, Burke D, Leslie CC. J Biol Chem. 2004;279:19113–19121. doi: 10.1074/jbc.M313867200. [DOI] [PubMed] [Google Scholar]
- 24.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 25.Wadsworth SJ, Goldfine H. Infect Immun. 1999;67:1770–1778. doi: 10.1128/iai.67.4.1770-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. J Biol Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- 27.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 28.Kramer RM, Roberts EF, Um SL, Börsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. J Biol Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- 29.Evans JH, Spencer DM, Zweifach A, Leslie CC. J Biol Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 30.Torres D, Barrier M, Bihl F, Quesniaux JF, Maillet I, Akira S, Ryffel B, Erard F. Infect Immun. 2004;72:2131–2139. doi: 10.1128/IAI.72.4.2131-2139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelson BT, Unanue ER. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 32.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 33.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. Infect Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drevets DA, Canono BP, Leenen PJM, Campbell PA. Infect Immun. 1994;62:2222–2228. doi: 10.1128/iai.62.6.2222-2228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong WW, Burke PA, Drotar ME, Chavali SR, Forse RA. Immunology. 1995;84:446–452. [PMC free article] [PubMed] [Google Scholar]
- 36.Shinomiya S, Naraba H, Ueno A, Utsunomiya I, Maruyama T, Ohuchida S, Ushikubi F, Yuki K, Narumiya S, Sugimoto Y, Ichikawa A, Oh-ishi S. Biochem Pharmacol. 2001;61:1153–1160. doi: 10.1016/s0006-2952(01)00586-x. [DOI] [PubMed] [Google Scholar]
- 37.Rouzer CA, Kingsley PJ, Wang H, Zhang H, Maorrow JD, Dey SK, Marnett LJ. J Biol Chem. 2004;279:34256–34268. doi: 10.1074/jbc.M402594200. [DOI] [PubMed] [Google Scholar]
- 38.Kunkel SL, Spengler M, May MA, Spengler R, Larrick J, Remick D. J Biol Chem. 1988;263:5380–5384. [PubMed] [Google Scholar]
- 39.Marcinkiewica J. Cytokine. 1991;3:327–332. doi: 10.1016/1043-4666(91)90501-4. [DOI] [PubMed] [Google Scholar]
- 40.Roland CR, Goss JA, Mangino MJ, Hafenrichter D, Flye MW. Ann Surg. 1994;219:389–399. doi: 10.1097/00000658-199404000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fieren MW, van den Bemd GJ, Ben-Efraim S, Bonta IL. Immunol Lett. 1992;31:85–90. doi: 10.1016/0165-2478(92)90015-g. [DOI] [PubMed] [Google Scholar]
- 42.Natarajan M, Lin KM, Hsueh RC, Sternweis PC, Ranganathan R. Nat Cell Biol. 2006;8:571–580. doi: 10.1038/ncb1418. [DOI] [PubMed] [Google Scholar]
- 43.Fennekohl A, Sugimoto Y, Segi E, Maruyama T, Ichikawa A, Puschel GP. J Hepatol. 2002;36:328–334. doi: 10.1016/s0168-8278(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 44.Goldfine H, Wadsworth SJ. Microbes Infect. 2002;4:1335–1343. doi: 10.1016/s1286-4579(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 45.Shaughnessy LM, Swanson JA. Front Biosci. 2007;12:2683–2692. doi: 10.2741/2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldfine H, Wadsworth SJ, Johnston NC. Infect Immun. 2000;68:5735–5741. doi: 10.1128/iai.68.10.5735-5741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humann J, Bjordahl R, Andreasen K, Lenz LL. J Immunol. 2007;178:2407–2414. doi: 10.4049/jimmunol.178.4.2407. [DOI] [PubMed] [Google Scholar]
- 48.Lenz LL, Hohammadi S, Geissler A, Portnoy DA. Proc Natl Acad Sci, U S A. 2003;100:12432–12437. doi: 10.1073/pnas.2133653100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 50.Bavdek A, Gekara NO, Priselac D, Aguirre IG, Darji A, Chakraborty T, Macek P, Lakey JH, Weiss S, Anderluh G. Biochemistry. 2007;46:4425–4437. doi: 10.1021/bi602497g. [DOI] [PubMed] [Google Scholar]
- 51.Gekara NO, Jacobs T, Chakraborty T, Weiss S. Cell Microbiol. 2005;7:1345–1356. doi: 10.1111/j.1462-5822.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 52.Sibelius U, Schulz EC, Rose F, Hattar K, Jacobs T, Weiss S, Chakraborty T, Seeger W, Grimminger F. Infect Immun. 1999;67:1125–1130. doi: 10.1128/iai.67.3.1125-1130.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Towler PS, Knopf JL, Clark JD. J Biol Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- 54.Perisic O, Paterson HF, Mosedale G, Lara-González S, Williams RL. J Biol Chem. 1999;274:14979–14987. doi: 10.1074/jbc.274.21.14979. [DOI] [PubMed] [Google Scholar]
- 55.Qiu ZH, Gijón MA, de Carvalho MS, Spencer DM, Leslie CC. J Biol Chem. 1998;273:8203–8211. doi: 10.1074/jbc.273.14.8203. [DOI] [PubMed] [Google Scholar]
- 56.Gijón MA, Spencer DM, Kaiser AL, Leslie CC. J Cell Biol. 1999;145:1219–1232. doi: 10.1083/jcb.145.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Gijón MA, Leslie CC. J Leukocyte Biol. 1999;65:330–336. doi: 10.1002/jlb.65.3.330. [DOI] [PubMed] [Google Scholar]
- 59.Repp H, Pamulkci Z, Koschinski A, Domann E, Darji A, Birringer J, Brockmeier D, Chakraborty T, Dreyer F. Cell Microbiol. 2002;4:483–491. doi: 10.1046/j.1462-5822.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 60.Dramsi S, Cossart P. Infect Immun. 2003;71:3614–3618. doi: 10.1128/IAI.71.6.3614-3618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rose F, Zeller SA, Chakraborty T, Domann E, Machleidt T, Kronke M, Seeger W, Grimminger F, Sibelius U. Infect Immun. 2001;69:897–905. doi: 10.1128/IAI.69.2.897-905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuchiya K, Kawamura I, Takahashi A, Nomura T, Kohda C, Mitsuyama M. Infect Immun. 2005;73:3869–3877. doi: 10.1128/IAI.73.7.3869-3877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang P, Rosenshine I, Cossart P, Finlay BB. Infect Immun. 1996;64:2359–2361. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiglein I, Goebel W, Troppmair J, Rapp UR, Demuth A, Kuhn M. FEMS Microbiol Lett. 1997;148:189–195. doi: 10.1111/j.1574-6968.1997.tb10287.x. [DOI] [PubMed] [Google Scholar]
- 65.Tang P, Sutherland CL, Gold MR, Finlay BB. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hefner Y, Borsch-Haubold AG, Murakami M, Wilde JI, Pasquet S, Schieltz D, Ghomashchi F, Yates JR, III, Armstrong CG, Paterson A, Cohen P, Fukunaga R, Hunter T, Kudo I, Watson SP, Gelb MH. J Biol Chem. 2000;275:37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- 67.Evans JH, Fergus DJ, Leslie CC. BMC Biochem. 2002;3:30. doi: 10.1186/1471-2091-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lehner MD, Schwoebel F, Kotlyarov A, Leist M, Gaestel M, Hartung T. J Immunol. 2002;168:4667–4673. doi: 10.4049/jimmunol.168.9.4667. [DOI] [PubMed] [Google Scholar]
- 69.Suram S, Brown GD, Ghosh M, Gordon S, Loper R, Taylor PR, Akira S, Uematsu S, Williams DL, Leslie CC. J Biol Chem. 2006;9:5506–5514. doi: 10.1074/jbc.M509824200. [DOI] [PubMed] [Google Scholar]
- 70.Shen Y, Naujokas M, Park M, Ireton K. Cell. 2000;103:501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 71.Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 72.Braun L, Ghebrehiwet B, Cossart P. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wing MG, Seilly DJ, Nicholas RS, Rahman S, Zajicek J, Lachmannn PJ, Compston DA. J Neuroimmunol. 1999;94:74–81. doi: 10.1016/s0165-5728(98)00227-6. [DOI] [PubMed] [Google Scholar]
- 74.Mansell A, Khelef N, Cossart P, O’Neill LA. J Biol Chem. 2001;276:43597–43603. doi: 10.1074/jbc.M105202200. [DOI] [PubMed] [Google Scholar]
- 75.Sawyer RT, Drevets DA, Campbell PA, Potter TA. J Leukocyte Biol. 1996;60:603–610. doi: 10.1002/jlb.60.5.603. [DOI] [PubMed] [Google Scholar]
- 76.McCaffrey RL, Fawcett P, O’Riordan M, Lee KD, Havell EA, Brown PO, Portnoy DA. Proc Natl Acad Sci U S A. 2004;101:11386–11391. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Proc Natl Acad Sci U S A. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluthmann H. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 79.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 80.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. J Immunol. 2005;174:595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 82.Tripp CS, Needleman P, Unanue ER. J Clin Investig. 1987;79:399–403. doi: 10.1172/JCI112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petit JC, Richard G, Burghoffer B, Daguet GL. Infect Immun. 1985;49:383–388. doi: 10.1128/iai.49.2.383-388.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akaogi J, Yamada H, Kuroda Y, Nacionales DC, Reeves WH, Satoh M. J Leukocyte Biol. 2004;76:227–236. doi: 10.1189/jlb.1203627. [DOI] [PubMed] [Google Scholar]
- 85.Chen BC, Liao CC, Hsu MJ, Liao YT, Lin CC, Sheu JR, Lin CH. J Immunol. 2006;177:681–693. doi: 10.4049/jimmunol.177.1.681. [DOI] [PubMed] [Google Scholar]
- 86.Tilley SL, Coffman TM, Koller BH. J Clin Investig. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]