A model grass identifies phenotypes associated with mature root and shoot for genetic improvement of well-watered and drought-stressed cereals.
Abstract
Seedling roots enable plant establishment. Their small phenotypes are measured routinely. Adult root systems are relevant to yield and efficiency, but phenotyping is challenging. Root length exceeds the volume of most pots. Field studies measure partial adult root systems through coring or use seedling roots as adult surrogates. Here, we phenotyped 79 diverse lines of the small grass model Brachypodium distachyon to adults in 50-cm-long tubes of soil with irrigation; a subset of 16 lines was droughted. Variation was large (total biomass, ×8; total root length [TRL], ×10; and root mass ratio, ×6), repeatable, and attributable to genetic factors (heritabilities ranged from approximately 50% for root growth to 82% for partitioning phenotypes). Lines were dissected into seed-borne tissues (stem and primary seminal axile roots) and stem-borne tissues (tillers and coleoptile and leaf node axile roots) plus branch roots. All lines developed one seminal root that varied, with branch roots, from 31% to 90% of TRL in the well-watered condition. With drought, 100% of TRL was seminal, regardless of line because nodal roots were almost always inhibited in drying topsoil. Irrigation stimulated nodal roots depending on genotype. Shoot size and tillers correlated positively with roots with irrigation, but partitioning depended on genotype and was plastic with drought. Adult root systems of B. distachyon have genetic variation to exploit to increase cereal yields through genes associated with partitioning among roots and their responsiveness to irrigation. Whole-plant phenotypes could enhance gain for droughted environments because root and shoot traits are coselected.
Adult plant root systems are relevant to the size and efficiency of seed yield. They supply water and nutrients for the plant to acquire biomass, which is positively correlated to the harvest index (allocation to seed grain), and the stages of flowering and grain development. Modeling in wheat (Triticum aestivum) suggested that an extra 10 mm of water absorbed by such adult root systems during grain filling resulted in an increase of approximately 500 kg grain ha−1 (Manschadi et al., 2006). This was 25% above the average annual yield of wheat in rain-fed environments of Australia. This number was remarkably close to experimental data obtained in the field in Australia (Kirkegaard et al., 2007). Together, these modeling and field experiments have shown that adult root systems are critical for water absorption and grain yield in cereals, such as wheat, emphasizing the importance of characterizing adult root systems to identify phenotypes for productivity improvements.
Most root phenotypes, however, have been described for seedling roots. Seedling roots are essential for plant establishment, and hence, the plant’s potential to set seed. For technical reasons, seedlings are more often screened than adult plants because of the ease of handling smaller plants and the high throughput. Seedling-stage phenotyping may also improve overall reproducibility of results because often, growth media are soil free. Seedling soil-free root phenotyping conditions are well suited to dissecting fine and sensitive mechanisms, such as lateral root initiation (Casimiro et al., 2003; Péret et al., 2009a, 2009b). A number of genes underlying root processes have been identified or characterized using seedlings, notably with the dicotyledonous models Arabidopsis (Arabidopsis thaliana; Mouchel et al., 2004; Fitz Gerald et al., 2006; Yokawa et al., 2013) and Medicago truncatula (Laffont et al., 2010) and the cereals maize (Zea mays; Hochholdinger et al., 2001) and rice (Oryza sativa; Inukai et al., 2005; Kitomi et al., 2008).
Extrapolation from seedling to adult root systems presents major questions (Hochholdinger and Zimmermann, 2008; Chochois et al., 2012; Rich and Watt, 2013). Are phenotypes in seedling roots present in adult roots given developmental events associated with aging? Is expression of phenotypes correlated in seedling and adult roots if time compounds effects of growth rates and growth conditions on roots? Watt et al. (2013) showed in wheat seedlings that root traits in the laboratory and field correlated positively but that neither correlated with adult root traits in the field. Factors between seedling and adult roots seemed to be differences in developmental stage and the time that growing roots experience the environment.
Seedling and adult root differences may be larger in grasses than dicotyledons. Grass root systems have two developmental components: seed-borne (seminal) roots, of which a number emerge at germination and continue to grow and branch throughout the plant life, and stem-borne (nodal or adventitious) roots, which emerge from around the three-leaf stage and continue to emerge, grow, and branch throughout the plant life. Phenotypes and traits of adult root systems of grasses, which include the major cereal crops wheat, rice, and maize, are difficult to predict in seedling screens and ideally identified from adult root systems first (Gamuyao et al., 2012).
Phenotyping of adult roots is possible in the field using trenches (Maeght et al., 2013) or coring (Wasson et al., 2014). A portion of the root system is captured with these methods. Alternatively, entire adult root systems can be contained within pots dug into the ground before sowing. These need to be large; field wheat roots, for example, can reach depths greater than 1.5 m depending on genotype and environment. This method prevents root-root interactions that occur under normal field sowing of a plant canopy and is also a compromise.
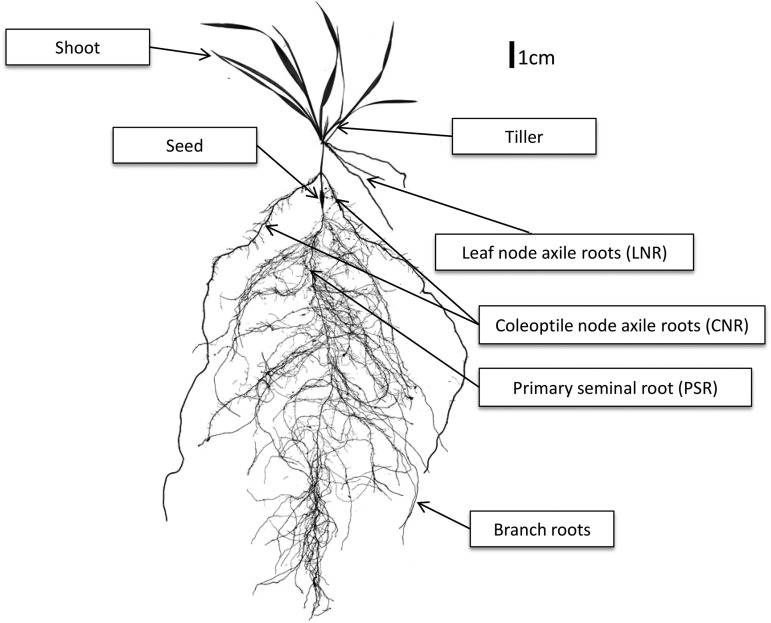
A solution to the problem of phenotyping adult cereal root systems is a model for monocotyledon grasses: Brachypodium distachyon. B. distachyon is a small-stature grass with a small genome that is fully sequenced (Vogel et al., 2010). It has molecular tools equivalent to those available in Arabidopsis (Draper et al., 2001; Brkljacic et al., 2011; Mur et al., 2011). The root system of B. distachyon reference line Bd21 is more similar to wheat than other model and crop grasses (Watt et al., 2009). It has a seed-borne primary seminal root (PSR) that emerges from the embryo at seed germination and multiple stem-borne coleoptile node axile roots (CNRs) and leaf node axile roots (LNRs), also known as crown roots or adventitious roots, that emerge at about three leaves through to grain development. Branch roots emerge from all root types. There are no known anatomical differences between root types of wheat and B. distachyon (Watt et al., 2009). In a recent study, we report postflowering root growth in B. distachyon line Bd21-3, showing that this model can be used to answer questions relevant to the adult root systems of grasses (Chochois et al., 2012).
In this study, we used B. distachyon to identify adult plant phenotypes related to the partitioning among seed-borne and stem-borne shoots and roots for the genetic improvement of well-watered and droughted cereals (Fig. 1; Table I). We had three reasons for phenotyping seed-borne and stem-borne roots separately. First, they are easy to identify from their location and timing of emergence and hence, select rapidly. Second, a number of studies suggest that seed-borne and stem-borne root systems grow and function differently in response to soil conditions, including water (Krassovsky, 1926; Navara et al., 1994), nitrogen, phosphorus (Tennant, 1976; Brady et al., 1995), oxygen (Wiengweera and Greenway, 2004), soil hardness (Acuna et al., 2007), and microorganisms (Sivasithamparam et al., 1978). Of note is the study by Krassovsky (1926), which was the first, to our knowledge, to show differences in function related to water. Krassovsky (1926) showed that seminal roots of wheat absorbed almost 2 times the water as nodal roots per unit dry weight but that nodal roots absorbed a more diluted nutrient solution than seminal roots. Krassovsky (1926) also showed by removing seminal or nodal roots as they emerged that “seminal roots serve the main stem, while nodal roots serve the tillers” (Krassovsky, 1926). Volkmar (1997) showed, more recently, in wheat that nodal and seminal roots may sense and respond to drought differently. In millet (Pennisetum glaucum) and sorghum (Sorghum bicolor), Rostamza et al. (2013) found that millet was able to grow nodal roots in a dryer soil than sorghum, possibly because of shoot and root vigor.
Figure 1.
B. distachyon plant scanned at the fourth leaf stage, with the root and shoot phenotypes studied indicated. Table I shows the full list of the phenotypes measured across lines and in response to well-watered and drought conditions.
Table I. List of phenotypes measured, abbreviations, units, and ranges of variation observed among B. distachyon lines in one single experiment (experiment 6; 36 lines and 3 replicates) or for all of the well-watered experiments presented in this study (79 lines and three to 10 replicates).
Experiment details are in Supplemental Table S1.
| Phenotype | Abbreviation | Unit | Range of Variation |
|
|---|---|---|---|---|
| All Experiments (79 Lines and 582 Plants) | Experiment 6 (36 Lines) | |||
| Whole plant | ||||
| TDW | TDW | Milligrams | 88.6–773.8 (×8.7) | 285.6–438 (×1.5) |
| Shoot | ||||
| SDW | SDW | Milligrams | 56.4–442.5 (×7.8) | 78.2–442.5 (×5.7) |
| No. of tillers | TillerN | Count | 2.8–20.3 (×7.4) | 10–20.3 (×2) |
| Total root system | ||||
| TRL | TRL | Centimeters | 1,050–10,770 (×10.3) | 2,090–5,140 (×2.5) |
| RDW | RDW | Milligrams | 28.9–312.17 (×10.8) | 62.2–179.1 (×2.9) |
| Rootpc | Rootpc | Percentage (of TDW) | 20.5–60.6 (×3) | 20.5–44.3 (×2.2) |
| R/S | R/S | Unitless ratio | 0.26–1.54 (×6) | 0.26–0.80 (×3.1) |
| PSRs | ||||
| Length (including branch roots) | PSRL | Centimeters | 549.1–4,024.6 (×7.3) | 716–2,984 (×4.2) |
| PSRpc | PSRpc | Percentage (of TRL) | 14.9–94.1 (×6.3) | 31.3–72.3 (×2.3) |
| No. of axile roots | PSRcount | Count | 1 | 1 |
| Length of axile root | PSRsum | Centimeters | 17.45–52 (×3) | 17.45–30.3 (×1.7) |
| Branch roots | PSRbranch | Centimeters · (centimeters of axile root)−1 | 19.9–109.3 (×5.5) | 29.3–104.3 (×3.6) |
| CNRs | ||||
| Length (including branch roots) | CNRL | Centimeters | 0–3,856.7 | 0–2,266.5 |
| CNRpc | CNRpc | Percentage (of TRL) | 0–57.1 | 0–49.8 |
| No. of axile roots | CNRcount | Count | 0–2 | 0–2 |
| Cumulated length of axile roots | CNRsum | Centimeters | 0–113.9 | 0–47.87 |
| Branch roots | CNRbranch | Centimeters · (centimeters of axile root)−1 | 0–77.8 | 0–77.8 |
| LNRs | ||||
| Length (including branch roots) | LNRL | Centimeters | 99.5–5,806.5 (×58.5) | 216.1–2,532.4 (×11.7) |
| LNRpc | LNRpc | Percentage (of TRL) | 4.2–72.7 (×17.5) | 6–64.8 (×10.9) |
| LNRcount | LNRcount | Count | 2–22.2 (×11.1) | 3.3–15.3 (×4.6) |
| LNRsum | LNRsum | Centimeters | 25.9–485.5 | 48–232 (×4.8) |
| Branch roots | LNRbranch | Centimeters · (centimeters of axile root)−1 | 2.1–25.4 (×12.1) | 3.2–15.9 (×5) |
The third reason for dissecting the different root types in this study was that they seem to have independent genetic regulation through major genes. Genes affecting specifically nodal root growth have been identified in maize (Hetz et al., 1996; Hochholdinger and Feix, 1998) and rice (Inukai et al., 2001, 2005; Liu et al., 2005, 2009; Zhao et al., 2009; Coudert et al., 2010; Gamuyao et al., 2012). Here, we also dissect branch (lateral) development on the seminal or nodal roots. Genes specific to branch roots have been identified in Arabidopsis (Casimiro et al., 2003; Péret et al., 2009a), rice (Hao and Ichii, 1999; Wang et al., 2006; Zheng et al., 2013), and maize (Hochholdinger and Feix, 1998; Hochholdinger et al., 2001; Woll et al., 2005).
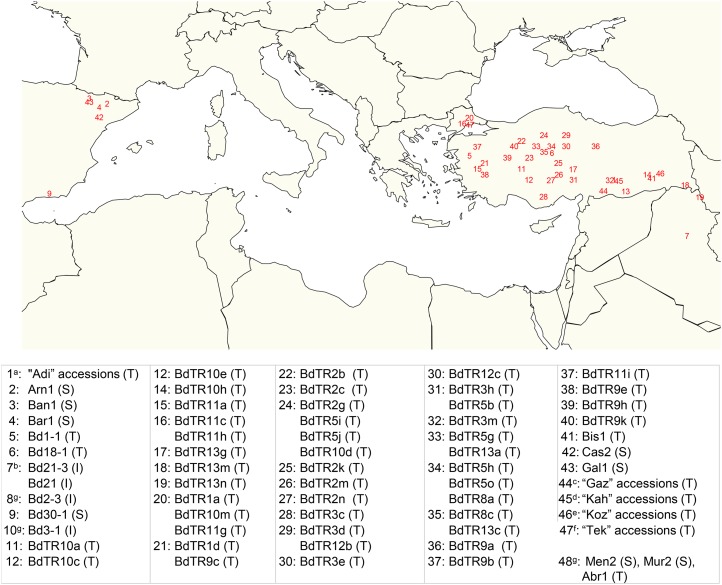
This study explored the hypothesis that adult root systems of B. distachyon contain genotypic variation that can be exploited through phenotyping and genotyping to increase cereal yields. A selection of 79 wild lines of B. distachyon from various parts of the Middle East (Fig. 2 shows the geographic origins of the lines) was phenotyped. They were selected for maximum genotypic diversity from 187 diploid lines analyzed with 43 simple sequence repeat markers (Vogel et al., 2009). We phenotyped shoots and mature root systems concurrently because B. distachyon is small enough to complete its life cycle in relatively small pots of soil with minimal influence of pot size compared with crops, such as wheat. We further phenotyped a subset of this population under irrigation (well watered) and drought to assess genotype response to water supply. By conducting whole-plant studies, we aimed to identify phenotypes that described partitioning among shoot and root components and within seed-borne and stem-borne roots. Phenotypes that have the potential to be beneficial to shoot and root components may speed up genetic gain in future.
Figure 2.
B. distachyon lines phenotyped in this study and their geographical origin. Capital letters in parentheses indicate the country of origin: Turkey (T), Spain (S), and Iraq (I; Vogel et al., 2009). a, Adi3, Adi7, Adi10, Adi12, Adi13, and Adi15; b, Bd21 and Bd21-3 are the reference lines of this study. Bd21 was the first sequenced line (Vogel et al., 2010) and root system (described in detail in Watt et al., 2009), and Bd21-3 is the most easily transformed line (Vogel and Hill, 2008) and parent of a T-DNA mutant population (Bragg et al., 2012); c, Gaz1, Gaz4, and Gaz7; d, Kah1, Kah2, and Kah3. e, Koz1, Koz3, and Koz5; f, Tek1 and Tek6; g, exact GPS coordinates are unknown for lines Men2 (S), Mur2 (S), Bd2.3 (I), Bd3-1 (I), and Abr1 (T).
RESULTS
Heritability and Genetic Correlations
Root and shoot phenotypes were measured on B. distachyon in this study, and these are listed in Table I with acronyms. Heritability ranged from 47% for the total length of nodal axile roots (LNRsum) to 82% for tiller number and total plant dry weight (TDW; Table II shows statistical analyses to assess heritability and robustness of traits of the data set from well-watered conditions). For root traits, heritability ranged from 47% for LNRsum to 80% for the percentage of total root length (TRL) invested in CNR. The most heritable root traits were those related to the partitioning of the percentage of total root length among primary seminal roots (PSRpc), the percentage of total root length among coleoptile node root (CNRpc), and the percentage of total root length among leaf node axile roots (LNRpc) as well as the number of leaf node axile roots (LNRcount). Phenotypes associated with resource allocation between root and shoot, such as percentage of total dry weight in roots (Rootpc) and root to shoot ratio (R/S), were commonly of a lower heritability value than others (around 50%).
Table II. Line-mean and single-plant (in parentheses) heritability and genetic correlations with TRL and TDW for 10 phenotypes across nine experiments presented in this study.
ne, Not estimable; TillerN, number of tillers; *, variance component and genetic correlation are statistically different from 0 at P = 0.05; **, variance component and genetic correlation are statistically different from 0 at P = 0.01; †, variance component and genetic correlation are not statistically different from 0 at P = 0.10; —, not applicable.
| Phenotype | Line | Line × Experiment | Residual | Heritability (Single-Plant Heritability) | Genetic Correlation with TRL | Genetic Correlation with TDW |
|---|---|---|---|---|---|---|
| TRL | 243,392 ± 159,034* | 560,269 ± 166,005** | 1,586,423 ± 112,735** | 54 (10) | — | 0.93** |
| PSRpc | 58.1 ± 19.3** | 24.2 ± 16.9* | 216.8 ± 16.1** | 72 (19) | −0.05† | 0.28* |
| CNRpc | 57.7 ± 17.9** | 23.2 ± 13.5* | 128.5 ± 11.5** | 80 (28) | 0.79** | 0.06† |
| LNRpc | 70.9 ± 22.7** | 27.7 ± 15.8* | 180.2 ± 13.6** | 79 (25) | −0.96** | −0.65** |
| LNRcount | 2.92 ± 1.12** | 1.00 ± 0.78† | 9.15 ± 0.77** | 75 (22) | 0.09† | 0.03† |
| LNRsum | 33,365 ± 31,970† | 66,459 ± 34,026* | 331,490 ± 27,702** | 47 (8) | 0.35** | 0.91** |
| TDW | 1,675 ± 651** | ne | 4,077 ± 491** | 82 (29) | 0.93** | — |
| Rootpc | 5.22 ± 4.82† | 2.98 ± 5.08† | 49.9 ± 6.4** | 51 (9) | 0.11* | 0.04† |
| R/S | 0.004 ± 0.004† | 0.002 ± 0.004† | 0.041 ± 0.005** | 50 (9) | 0.03† | 0.03† |
| TillerN | 2.51 ± 0.85** | 0.92 ± 0.61* | 5.01 ± 0.44** | 82 (30) | 0.86** | 0.36** |
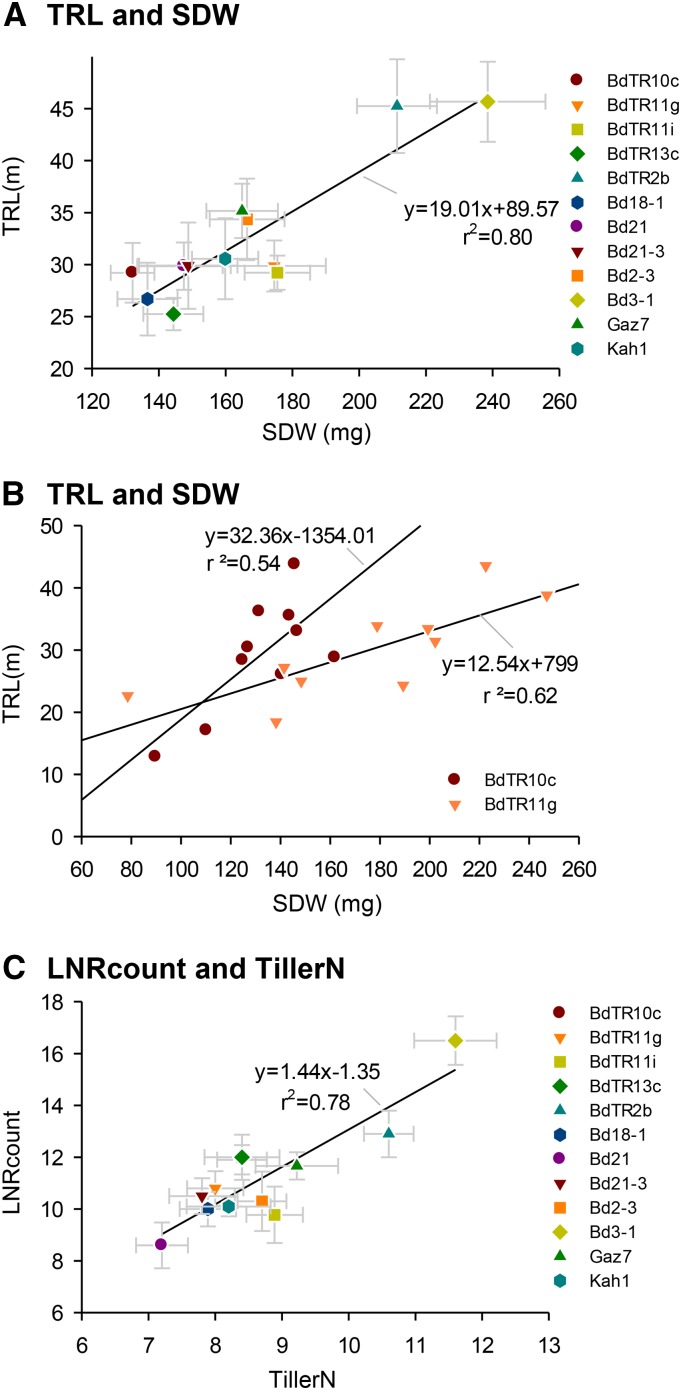
Genetic correlations between different traits were calculated for all genotypes in all of the well-watered experiments (Table II). High and significant correlations were obtained for TRL and TDW (rg = 0.93; P < 0.01), CNRpc and TRL (rg = 0.79; P < 0.01), and LNRpc and TRL (rg = −0.96; P < 0.01). Interestingly, LNRcount did not correlate well with TDW or TRL. This suggests that branch root length per axile root was the main determinant of TDW or TRL because the same TRL could be achieved with different numbers of LNRs.
We conducted nine experiments in this study (Supplemental Table S1). The first four were designed to optimize experimental conditions to maximize genotypic variation between lines and soil water treatments and confidence toward increasing repeatability between similar experiments. For example, during experiments 1 and 2, which were conducted during a 16-h-light/8-h-dark photoperiod, lines Bd21-3, Bd3-1, and BdTR10E flowered during the experiment. Therefore, to avoid the potential confounding effect of differential flowering on shoot and root growth as well as resource partitioning, we switched to a 12-h-light/12-h-dark photoperiod, which inhibited flowering in all lines. Similarly, we adjusted water supply and evaporation during the first four experiments. By comparing experiments 1 and 2, we found that a mulch of polyethylene beads at the surface of the soil reduced evaporation and resulted in more reproducible and consistent CNR and LNR emergence and growth. To make watering more consistent and root growth repeatable, we installed and calibrated an automatic watering system using drippers from experiment 3 onward. The experiments resulted in high repeatability and heritability among traits, notably phenotypes associated with partitioning among adult root system components (Table II).
Variation in Adult Root Systems between Reference Lines Bd21 and Bd21-3 and across 79 Lines in Well-Watered Conditions
Lines Bd21 and Bd21-3 were included in all experiments as reference lines. Bd21 was the first line to be sequenced, and its genome is still used as a reference to others (Vogel et al., 2010); it was also the line used to characterize B. distachyon root systems (Watt et al., 2009). Bd21-3 is the parent of a publically available transfer DNA (T-DNA) mutant population (Bragg et al., 2012).
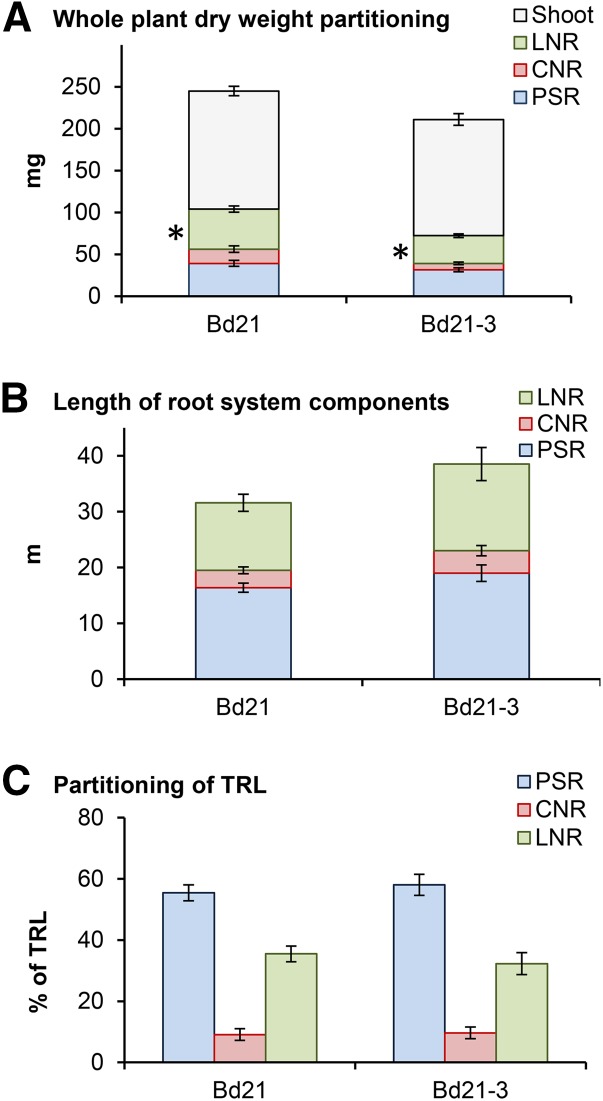
Because Bd21 and Bd21-3 were included in all experiments, a high replication level was available for each for comparison (Fig. 3). Bd21 and Bd21-3 had a similar TRL and root dry weight (RDW) as well as shoot dry weight (SDW; Fig. 3). No statistically significant difference (P = 0.085) was found in TRL (Fig. 3B) or its distribution into nodal root types (P = 0.73, P = 0.65, and P = 0.54 for percentage of TRL into PSR, CNR, and LNR, respectively; Fig. 3). Root systems of both lines were dominated by PSR length, which represented around 55% to 60% of TRL in these well-watered conditions. A small percentage (approximately 10% of TRL) was present in CNR; the rest (approximately 30% of TRL) was partitioned into LNR. The only significant difference observed between the two lines in a two-way ANOVA was LNR dry weight (P = 0.01; Fig. 3A). Above ground, both lines were again similar in terms of the timing of the appearance of leaves, the timing of the appearance of tillers, and total plant height (Supplemental Fig. S1).
Figure 3.
Partitioning of root and shoot tissues in Bd21 and Bd21-3 in well-watered soil. Bars are means from 38 plants for Bd21 and 42 plants for Bd21-3 over seven independent experiments; error bars represent sem. A, Whole-plant dry weight partitioning (milligrams). *, ANOVA shows that only LNR dry weights are statistically significantly different at P < 0.05 (P = 0.917 for PSR dry weight, P = 0.067 for CNR dry weight, P = 0.01 for LNR dry weight, and P = 0.854 for SDW). B, Length of root system components (in meters per plant; no significant difference at P < 0.05). C, Partitioning of TRL (percentage of TRL into each root component; no significant difference at P < 0.05).
Across 79 lines, most root phenotypes varied at least 2-fold beyond that of reference lines Bd21 and Bd21-3 (Table I; Supplemental Figs. S2 and S8). The only phenotype with no significant variation was the number of PSRs; all lines consistently grew a single PSR axile root. The CNR number varied between 0 and 2, whereas the LNR number varied much more (from 2–22.2; Table I). The most diverse phenotype that we measured across the set of lines was the partitioning of TRL among the three root types (PSR, CNR, and LNR with heritability values of 72%, 80%, and 79%, respectively). Notably, the percentage of total length (including branch root length) allocated to the LNR varied by a factor of 10.9 between the two most extreme lines (4.2% for Adi12 in experiment 4 and 72.7% for B21-3 in experiment 3; Table I).
Variation in Partitioning of Adult Root System Components
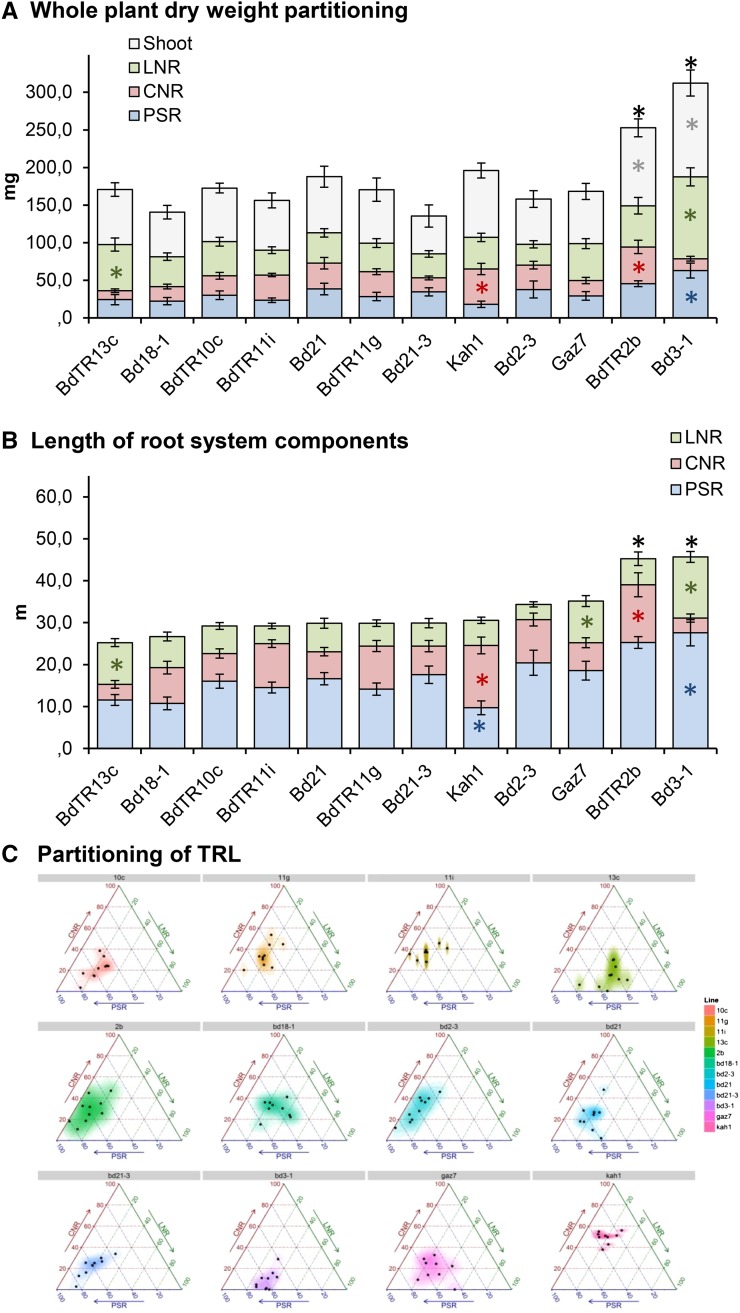
We further explored the wide variation in partitioning within and between roots and shoots reported among 79 lines above, with greater replication of a subsample of 12 lines in experiment 9 (Table I). These 12 lines were selected because they had similar TRLs (H2 = 54%; Table II) but different partitioning to root system components (Fig. 4). Root system length ranged from 27 to 47 m per plant among 12 lines, with most around 30 m plant−1 (Fig. 4B).
Figure 4.
Comparison among 12 lines of B. distachyon for partitioning between root and shoot tissues. Error bars represent sem (n = 10). For all three sections, black asterisks above the bar show significant difference with Bd21-3 (P < 0.05) in TDW (A) or TRL (B). Colored asterisks within the bars show significant difference with Bd21-3 for the selected trait (P < 0.05). C, Ternary plots illustrating TRL distribution among the three root types. Each dot represents one plant plotted against three axes, with each representing one of three root types: PSR (blue), CNR (red), and LNR (green).
Partitioning of total root system length into its different components varied among 12 lines, with the widest variation in the extent to which TRL was allocated to the two nodal axile root types: the CNRs and the LNRs (Fig. 4C). For example, lines such as Bd21-3, Gaz-7, and Bd2-3, allocated up to 60% of the TRL into the PSR (H2 = 72%; Table II), whereas Kah1 allocated one-half of its TRL to the PSR (around 30%), despite having a similar TRL to the other three lines (approximately 35 m per plant; no significant difference at P < 0.05; Fig. 4, B and C). Partitioning to the CNR system diverged 4-fold among genotypes (H2 = 80%; Table II) from less than 10% of the TRL for lines number Bd3-1 to up to 50% of the TRL in Kah1. LNR partitioning (H2 = 79%; Table II) varied as widely as that of CNRs: from 10% in Bd2-3 to 40% in BdTR13c. It is also interesting to point out that Bd3-1 and BdTR2b, with similar TRLs and PSR lengths, divided the remainder of their root length into different nodal root types (Fig. 4, B and C).
Allometry and Shoot and Root Partitioning
TRL and SDW were positively correlated across the subset of 12 lines (rg = 0.80; Fig. 5). This may have been driven, in part, by the strong positive correlation (r2 = 0.78) observed between the number of LNRs and the number of shoot tillers in these well-watered conditions (Fig. 5; Supplemental Fig. S3). There were interesting differences, however, among lines in the relationships between shoot and root partitioning (Fig. 5A). For example, the mean SDW of BdTR11g was 38% greater than that of BdTR10c, but both lines had a similar TRL. The pattern of partitioning is shown in Figure 5B. The trend line through 10 individual plants of BdTR10c is 3 times steeper than that through the BdTR11g individuals, highlighting lower partitioning to SDW for a given root length in BdTR10c than BdTR11g. BdTR10c also allocated almost 3 times more root length for a given SDW than BdTR11g. Allometric relationships are further analyzed for lines BdTR10c and BdTR11g in Supplemental Figure S3. Both have strong correlation between TRL and SDW. However, the different slopes and intercepts suggest that these traits respond to not only plant size but also, factors that regulate partitioning and vary between genotypes.
Figure 5.
A, Root and shoot partitioning among 12 B. distachyon lines in experiment 9 (for details about the experiment, see Supplemental Table S1). B, Relationship between TRL and SDW in lines BdTR10c and BdTR11g only. C, Relationship between LNRcount and the number of tillers (TillerN) in 12 genotypes. Error bars represent sem (n = 10).
Root System Partitioning to Branch Root Length
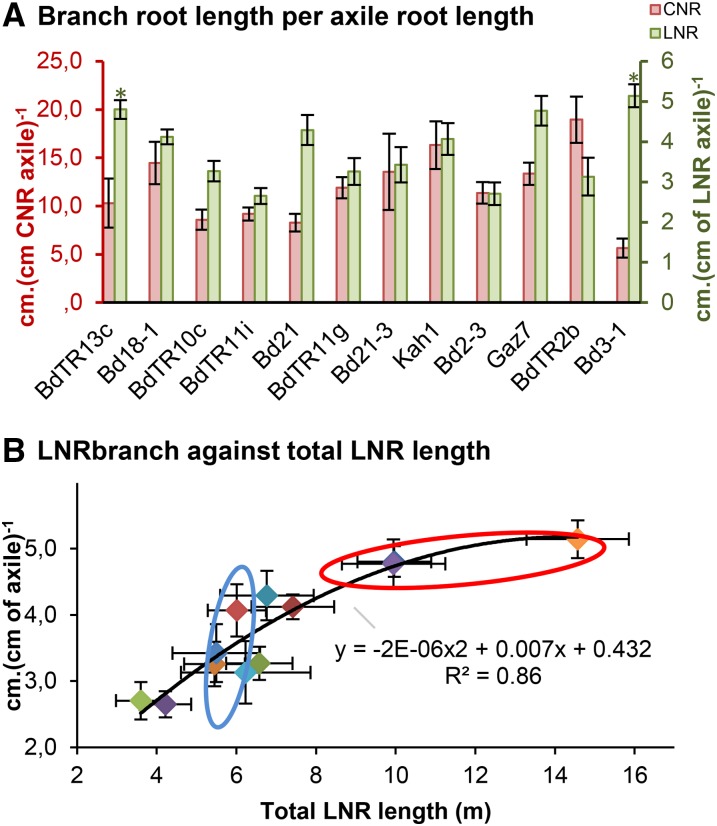
Partitioning to branch roots was expressed as centimeters of length of branch root per centimeters of length of parent axile root. We observed 7-fold variation for this phenotype (Table I) from 33.5 to 245 cm per 1 cm of axile root. This variation was further examined in the intensely sampled 12 lines of experiment 9, and wide variation was observed again, particularly for nodal root types (CNR and LNR), which expressed a 4-fold difference in partitioning to branch roots between extreme lines (Fig. 6). Values ranged from 5 to 20 cm of branch root per 1 cm of axile root for CNR to 2 to 5 cm of branch root per 1 cm of axile root for LNR. This branch root length would have arisen from initiation of branch roots from the parent axile root or elongation of branch roots.
Figure 6.
Branch root length of 12 lines of B. distachyon from experiment 9 (for detailed conditions, see Supplemental Table S1). A, Branch root length of nodal roots expressed in centimeters (centimeters of axile root)−1 for CNR (red; left axis) and LNR (green; right axis). Error bars represent sem (n = 10). *, Significant difference with Bd21-3 (P < 0.05). B, Branch root length in centimeters (centimeters of LNR)−1 for LNR against total LNR length in meters per plant. Blue circle encompasses lines with similar LNR length but different branching values. Red circle encompasses lines with similar branching but different total LNR length. Error bars represent sem (n = 10).
A strong correlation between total LNR length and LNR branching was observed (Fig. 6B). However, deeper analysis showed that no general rule could be drawn from across-line data and that each line required individual consideration, similar to the root and shoot partitioning described above. Indeed, lines included in the red circle in Figure 6 showed different total LNR length, despite a similar branching ratio. This means that Bd3-1 (with the longest LNR in this category) achieved a longer LNR by growing more LNRs and not by increasing branching rate. However, lines circled in blue in Figure 6 showed similar LNR length, despite a different branching ratio, indicating that they managed to produce the same LNR length by different strategies: in one case, by increasing branching and minimizing new axile roots and in the other case, by growing more axile roots and restricting branching of existing axile roots.
Root and Shoot System Variation among Lines in Their Response to Drought
In experiment 4, 16 lines were exposed to two watering conditions: well-watered and drought soil profiles (conditions are in Supplemental Table S1). Water content was measured at three different soil depths in both conditions (Supplemental Fig. S4), showing that, in the drought treatment, wilting point was achieved after 5 weeks only in the top few centimeters of soil. Water was still available for the plant in most of the pot volume.
We found wide and significant variation in the responses of the lines to irrigation or drought in terms of SDWs, RDWs, TRL, and partitioning to components of the root system length (Fig. 7 presents a subset; Supplemental Fig. S5 shows 16 lines). Lines that had a significant reduction in SDW in response to drought also had had significant reduction in TRL (4 of 16 in Supplemental Fig. S5).
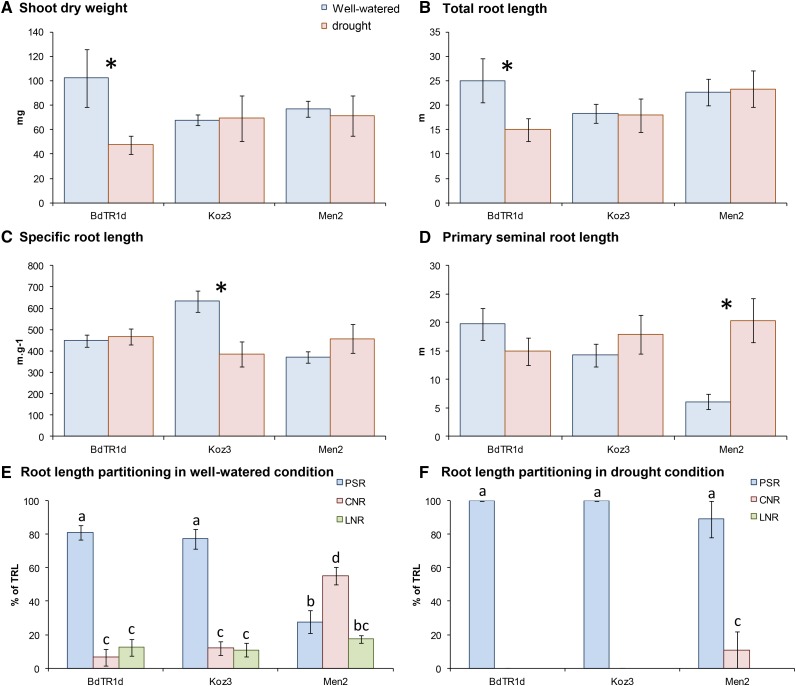
Figure 7.
Response of a subset of three lines of B. distachyon in root and shoot partitioning to well-watered and drought conditions (experiment 4; for all of the lines in this experiment, see Supplemental Fig. S5; for detailed conditions, see Supplemental Table S1). A, Shoot dry weight. B, Total root length. C, Specific root length. D, Primary seminal root length. E, Root length partitioning in well-watered condition. F, Root length partitioning in drought condition. Error bars represent sems (n = 4). *, Significant differences between the treatments in A to D. Similar letters (a–c) in E and F show groups with no significant difference (P < 0.05): PSR (blue), CNR (red), and LNR (green).
BdTR1d, Koz3, and Men2 are highlighted in Figure 7 to show the wide diversity in growth among shoots and root types in response to water supply. BdTR1d had a reduction in SDW in droughted compared with well-watered soil (P < 0.05) but were the only lines (of the three presented in Fig. 7) to have greater (67%; P < 0.05) TRL in the well-watered condition compared with the drought condition (Fig. 7B; e.g. showed water responsiveness). BdTR1d had the same PSR in both conditions; the greater TRL with irrigation was caused entirely by extra nodal root length (Fig. 7E). In contrast to BdTR1d, Koz3 and Men2 had similar SDWs in droughted and well-watered soils (Fig. 7A). Koz3 had similar TRLs between the two conditions (Fig. 7B), but in the droughted condition, it had a significantly lower specific root length (root length per unit of dry weight; P < 0.05; Fig. 7C), suggesting either thicker roots or dense/less porous roots in the drought condition. Men2 also grew the same TRL in both conditions, but in contrast, it grew significantly more (4-fold) PSR length in the drought condition compared with the well-watered pots. Men2 grew extensive CNR (45% of TRL) and LNR (20% of TRL) systems, leaving only about 30% of TRL to the PSR (Fig. 7E) in the well-watered condition. Men2 maintained the same TRL across water regimes, but development of nodal root system in the well-watered pots was achieved at the cost of PSR.
The phenotype observed consistently in response to water supply was changes in partitioning of TRL among seminal and nodal root types. Most obvious was the strong inhibition of nodal root growth in drying soil (Fig. 7, E and F). Indeed, in the well-watered conditions, most lines had a root system that constituted a mix of about 60% to 80% of TRL in PSRs, and the rest was divided between CNRs and LNRs (Fig. 7, E and F; Supplemental Fig. S5, E and F). However, in the drying soil profile pots, only 4 of 64 plants (16 lines × 4 replicates) were able to grow CNRs or LNRs. These plants were from different lines, such that we did not find a line that consistently expressed an ability to grow nodal roots when the soil was left to dry down.
DISCUSSION
Phenotypic Variation among B. distachyon Lines
The adult root systems of natural lines of B. distachyon had wide, repeatable, and heritable phenotypic variation under the conditions in which we measured them. Phenotypic variation was observed for all of the characteristics measured (Tables I and II), notably those related to partitioning with and between seed-borne and stem-borne tissues in response to well-watered and drought conditions. For some traits, up to 80% of the variability observed was explained by genotype. High heritability values across experiments were obtained, despite widely expected inherent variability of root traits because of high replication within and across experiments and reduced line × experiment interaction resulting from careful control of growth conditions. These results suggest that the B. distachyon root traits presented in this article can ultimately be applied toward the genetic improvement of wheat and other grasses. Robust phenotyping and the close-to-field soil conditions give confidence for additional analysis and interpretation from the data.
It is important, however, to recognize that diversity was found in controlled conditions before plants had reached flowering and may not translate to field conditions and agronomic scenarios at yield. Although soil characteristics and water regimes fall within the soil types and water availability levels encountered in fields with sandy-loam soils that are subject to rainfall and periods of drought, the pots would have restricted root spread, root-root and shoot-shoot interactions were likely limited compared with those of a crop canopy, and shoot light and temperature conditions were different from those in a field. The advantage of using a plant model, such as B. distachyon, was the ability to phenotype adult roots in field-like conditions, but validation is required in the future with B. distachyon in the field directly or cereals with similar genotypic and phenotypic variations.
Exploring Reasons for Diversity among B. distachyon Lines
The environmental characteristics (climate, elevation, and soil properties) of the geographical origins of the lines may have explained the phenotypic diversity. However, no correlation could be found between root phenotypes and the location of collection sites (Supplemental Fig. S6). Similarly, no correlation was found with climatic data, such as average temperature or total rainfall (Supplemental Fig. S7). It is possible that variation in soil composition may correlate with differences among root phenotypes, but detailed data on soil composition were not collected with the seeds. It may be possible to sample soil from the original collection sites using the Global Positioning System coordinates available for most lines in the future to find an association with the phenotypes, soil types, and their expression in well-watered or drought conditions. It is also possible that the reverse is true—that phenotypes measured in this study do not confer climatic or soil-type adaptations. We tested if the diversity was simply because of allometry because we found wide variation in plant size across experiments, and differences between genotypes in root traits may have been driven by different plant stages and sizes at the time of sampling. There was, indeed, a general correlation among a number of root traits (Supplemental Fig. S3) and SDWs across lines. However, for root traits analyzed more deeply, even when the correlation with SDW was strong, genotypes had different slopes and intercepts, indicating that partitioning to roots and shoots was regulated by mechanisms other than plant size. Allometry alone does not explain diversity in root and shoot partitioning in B. distachyon. This was also found to be the case in millet and sorghum in response to irrigation and drought (Rostamza et al., 2013).
Diversity in the ways that root traits are linked to shoot size (referred to here as partitioning phenotypes) may be valuable for speeding up whole-plant genotypic gain in breeding programs for cereals. This study suggests that B. distachyon is a useful cereal model to select whole-plant phenotypes at the adult stage because of its small size and diversity.
Phylogenetic Associations among Phenotypes
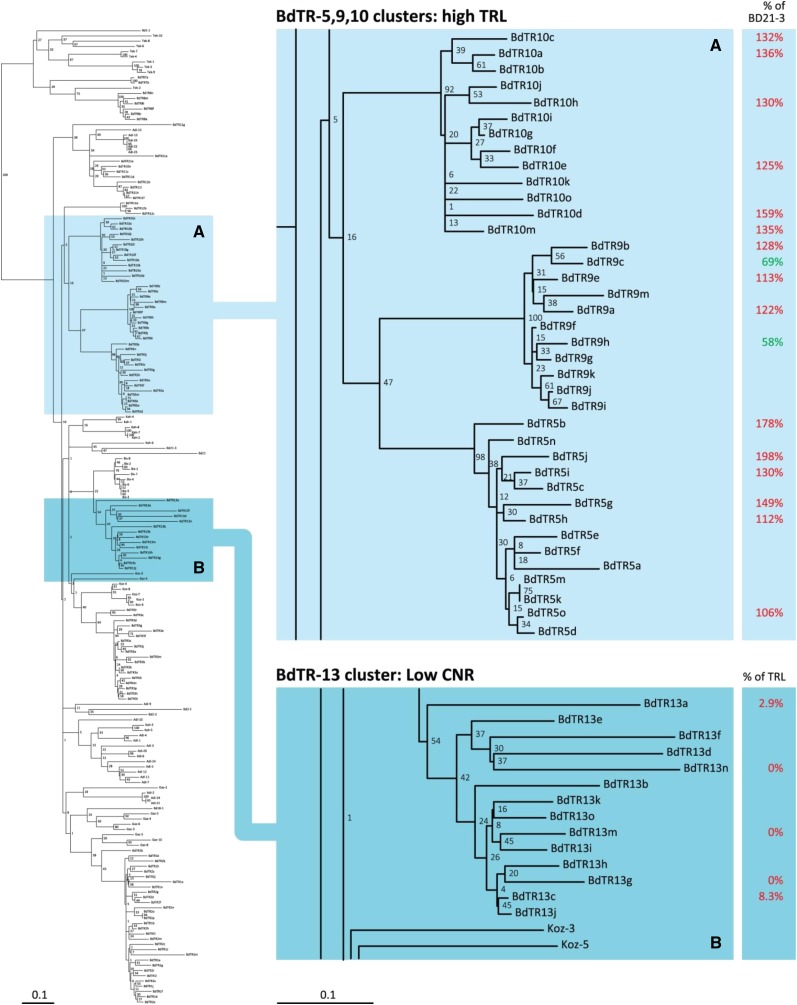
We looked for evidence that root phenotypes were more or less associated with genetically similar lines (Fig. 8). Data from the independent experiments were normalized to Bd21-3 and aligned with the phylogenetic tree designed by Vogel et al., (2009) (Fig. 8). Interestingly, all lines tested in the groups BdTR5, BdTR9, and BdTR10 had longer TRLs than Bd21-3 (up to 200% of Bd21-3’s TRL for BdTR5j), with only two exceptions in 17 lines: BdTR9h and BdTR9c (Fig. 8A). Similarly, Bd21, Bd21-3, and the BdTR3 and BdTR13 lines, closer together than the BdTR5-9-10 group, had similar TRLs (between 80% and 120% of Bd21-3 root length). As for partitioning of TRL among root types, CNRs were smaller in the BdTR13 cluster than for lines in the rest of the tree (Fig. 8B).
Figure 8.
Alignment on parts of the phylogenetic tree from the work by Vogel et al. (2009) of two traits measured in the natural lines of B. distachyon. A, TRLs as a percentage of the TRL of Bd21-3. Red numbers show root systems bigger than Bd21-3, and green numbers show smaller root systems compared with Bd21-3. B, CNRpc.
We were, thus, able to find groups of genetically related lines with similar root phenotypes. This suggests a common genetic basis for some traits. Although these data are insufficient to identify candidate genes through an ecotilling approach (Comai et al., 2004), these results suggest that analyzing more natural lines more deeply could be a powerful strategy to quickly identify genes associated with adult root systems of grasses.
Partitioning among Root System Components
The lines showed contrasting phenotypes of partitioning among PSR, CNR, and LNR types that had a high heritability (Table II), suggesting that particular partitioning patterns can be selected through breeding. Interestingly, we did not find any line completely devoid of one root type or with more than one primary seminal axile root. The first hypothesis is that we did not test enough lines to find these phenotypes, which may be uncommon. The second hypothesis is that the existence of the three root types offers a selective advantage to the species, providing more plasticity and flexibility to cope more readily and efficiently with environments as the plant ages.
The diversity in partitioning among root types found here presents important questions around the function of the cereal root system components. Studies have shown that root types of cereals differ in their responses to soil conditions and their contributions to growth and yield (introduction and references therein). This is partly because of their timing of emergence because seminal axile roots are restricted to emerging at seedling establishment, whereas nodal axile roots emerge through the life of the plant. Seminal roots can have higher specific activities of uptake, but nodal roots can become important as the plant ages (Navara et al., 1994). This may be explained by the higher specific root length of seminal roots and the younger ages of the nodal roots. For example, nodal roots took up more nitrate than seminal roots in wheat after rewetting of dry surface soil, possibly because they were younger and had less degraded cortices than the seminal roots (Brady et al., 1995) or possibly because they more readily developed branch roots from younger pericycles. Interestingly, vascular transport and signals to the shoots may differ from the root types. Vegetative growth of wheat was inhibited more by nodal root infection than seminal root infection (Sivasithamparam and Parker, 1978), and wheat nodal roots in droughted soil limited shoot growth and relative water content less than seminal roots in droughted soil (Volkmar, 1997). Nodal roots may be particularly important in conditions where additional root growth is needed because seminal root number is set after germination.
The CNR particularly attracted our attention. It was the only root type that we found to vary between and within genotypes. A few lines presented very little CNR systems, whereas it was the most developed system in other lines. They seem to be intermediate between PSRs and LNRs. They typically emerge after the seminal root but before LNR. The basal internal anatomy of CNRs is typical of nodal roots, but they are connected to the mesocotyl (between the seed and the leaf nodes), unlike the LNRs, which are directly connected to the leaf nodes and the tillers (Watt et al., 2009). Krassovsky (1926) suggested that the seminal roots in wheat and barley (Hordeum vulgare) mainly feed the main stem (and head with developing grain), whereas LNRs feed their associated tiller primarily, providing the main tiller small fractions of the water and nutrients that they collect. Water and nutrients collected by the CNRs may follow the same path as water and nutrients collected by the PSRs, which suggests that CNRs would be functionally closer to seminal roots and possibly, main stem and heads than LNRs. Our data also show that CNRs are often as long and deep as the seminal root axes, unlike LNRs, which are more numerous but often grow shallower. Hence, CNRs may be important for accessing water and nutrients at depth.
Responsiveness to Soil Moisture
B. distachyon was shown to be a useful model for grass responses to severe drought by Luo et al. (2011). Luo et al. (2011) measured the severity of leaf wilting on plants grown in pots much shallower than those used here. Wilting point was reached as early as 6 d after the drought treatment, whereas our plants were still growing (some at a slower rate) as late as 5 weeks after irrigation was stopped. Our plants did not reach the wilting point because they were supported by the available moisture measured in our long tubes at the end of the experiment (Supplemental Fig. S4). Luo et al. (2011) identified variation among some of the germplasm examined in time to wilting upon soil drying. We show that B. distachyon is also a source of variation in phenotypes expressed in less severe droughts, likely to be found in agronomically productive field conditions, rather than under severe drought.
In droughted pots, we observed consistent and strong inhibition of CNR and LNR nodal root growth. Root primordia were visible in the dry upper layer of soil, but roots did not elongate. Our results in B. distachyon are consistent with observations in other species, such as Phalaris spp., ryegrass (Lolium spp.; Cornish, 1982; Cornish et al., 1984), sorghum (Soman and Seetharama, 1992), barley (Crossett et al., 1975), and wheat (Passioura, 1972). Volkmar (1997), however, observed that soil drying around an already emerged nodal root system of wheat induced a 4-fold increase in its growth compared with the control, where no drought treatment was applied. This may have been because branch root growth was stimulated in drying soil. Millet branch roots on nodal axile roots were also stimulated in drying soil (Rostamza et al., 2013). The regulation of nodal axile and branch roots seems to be caused by local soil moisture rather than water available to the entire root system. In the experiments conducted here, water was available in the middle and lower layers of the pot and could have been supplied to the nodal axile roots in the drying upper soil through the phloem (Boyer et al., 2010).
The B. distachyon lines expressed variation in partitioning between and within shoots and roots in response to soil moisture. Root to shoot signaling is widely documented in the literature (Munns and Sharp, 1993; Wilkinson and Davies, 2002; Clark et al., 2005; Dodd, 2005). Shoot responses to drying soil are not only the result of a reduced or insufficient water uptake, but also, they activate signals likely sent from the roots in the form of hormones.
Diversity in responsiveness to drying soil in B. distachyon at the whole-plant level may eventually allow one to design environment-specific varieties of wheat or other temperate cereals. A new variety that lacks nodal root growth inhibition by dry top soils would gain the ability to take up more of the deep water, thus having the opportunity to yield more in drought-prone environments. We suggest that the trait able to grow nodal roots in dry top soil be added to the ideotype proposed by Lynch (2013). This is notably the case where crops rely on deep water fallen between two cropping seasons and stored in deep soil. Equally interesting was that there was wide variation in the response to irrigation among the lines in shoot and nodal root growth. Regulation of the stimulation of nodal axile roots by water after drought stress is poorly understood. Insights may come from the hydropatterning mapped by Bao et al. (2014) in the branch root cells of Arabidopsis and maize seedling primary roots. Water responsiveness in roots may be equally important to understand for response to drought for crop productivity in the future.
The diversity in phenotypes observed in our study poses the question of underlying genetic regulation. Transcriptomics approaches and differential gene expression analyses between the different components of the root system and in contrasting watering conditions (RNA sequencing and microarrays) are potential ways to obtain a list of candidate genes specific to each root type and condition. Another approach is to use these data to select parents for crossing according to a phenotype of interest. Analyzing the progeny of such crosses is useful to identify single nucleotide polymorphisms, genomic regions, and ultimately, genes specific to the phenotype for which the parents have been chosen.
CONCLUSION
We analyzed the adult root systems of 79 lines of B. distachyon under controlled conditions with or without irrigation and observed considerable natural diversity in the phenotypes related to partitioning among the major seed-borne and stem-borne shoot and root tissues of grasses depending on drought. This model and its genetic resources can now be used to test the functionality and genetic basis for these phenotypes because they are readily achieved and measured in controlled conditions.
Most root phenotypes described on seedlings or larger root systems ignore the nature or origin of the root considered. Do seminal and nodal roots have the same ability to absorb and conduct water? Do they have similar ability to absorb nutrients? Do they present the same transporters at their surfaces? Does each root system component have specific interactions with bacteria and symbiotic fungi? A better understanding of the roles of each root type is required to determine the best combination of seminal and nodal root traits for a given environment. Thus, we are encouraged that our results can be used as a starting point for future studies to design and understand the contribution of phenotypes with variation in partitioning to drought tolerance, irrigation responsiveness, pathogen resistance, and rhizosphere interactions. Such knowledge is likely to be useful for creating improved varieties (Chochois et al., 2012).
MATERIALS AND METHODS
Plant Material
Seventy-nine lines of Brachypodium distachyon from two main geographic regions (Fig. 2) were phenotyped with a focus on characterizing adult root systems (Fig. 1; Table I). The genotypic diversity of these lines (except those from Spain) was previously assessed using SSR markers (Vogel et al., 2009), and the phylogenetic tree generated therein was used to select a subset of 79 lines representing maximum diversity for phenotyping. Lines were referenced against Bd21 and Bd21-3; Bd21 is the line from which the reference genome sequence was produced (Vogel et al., 2010) and the first to be phenotyped in detail for shoot and root development (Watt et al., 2009). Bd21-3 is the most easily transformed (Vogel and Hill, 2008) and parent of over 20,000 T-DNA lines (Bragg et al., 2012; http://jgi.doe.gov/our-science/science-programs/plant-genomics/brachypodium/brachypodium-t-dna-collection/; Fig. 2).
Soil
The soil was a 1:1 blend of an organic matter, loam, and sand mix called Wheat Special (used routinely at the Commonwealth Scientific and Industrial Research Organisation Black Mountain Laboratories to grow wheat [Triticum aestivum] plants up to maturity) andcoarse river sand. We used this soil because previous studies showed that wheat roots grow well in it (Boyer et al., 2010) and have similar phenotypes to roots grown in the field (Watt et al., 2013); also, B. distachyon and wheat roots show similar development in this soil (Watt et al., 2009).
Before potting the soil, it was sieved to 2 mm to prevent root growth through larger organic matter particles and to increase ease of washing, and then it was mixed with Aboska fertilizer (1 g L−1). Aboska fertilizer was also in Wheat Special before the river sand addition at the following concentration: N:P:K at 14:6:5, 4.5% (w/w) calcium, 5.5% (w/w) sulfur, 2% (w/w) magnesium, and 0.13% (w/w) iron. The final nutrient composition of the soil used to grow B. distachyon is presented in Supplemental Table S2.
Soil was packed loosely into 50-cm-tall and 9-cm-diameter polyvinyl chloride tubes to a bulk density of 1.11 g cm−3 (±0.012; n = 3). This bulk density is similar to that of a plowed field soil and would not be expected to impede root growth (Passioura, 1991). Tubes were sealed at the bottom with a cap pierced with seven holes (2-mm diameters) to allow drainage, saturated with water, and left to reach field capacity when drainage stopped. A water retention curve was generated for the soil in a pressurized chamber and fitting the van Genuchten model (van Genuchten et al., 1991; Supplemental Fig. S4A). It showed that the soil behaves between typical sandy loam and loamy sand soils, agreeing with its 50% river sand composition. The gravimetric water contents of soil at different levels in the tubes with irrigation and drought conditions ranged from 3.34 to 21.33 g of water per g of dry soil for droughted surface soil and deep well-watered soil, respectively (Supplemental Fig. S4B). These corresponded to a soil water tension below 5 kPa (well-watered deep soil) and above 1,500 kPa (droughted surface soil). These conditions would provide sufficient plant-available water in all conditions, except in the top soil of the droughted experiment (described below).
Growth Conditions
Nine independent experiments were conducted in the same growth cabinet (Conviron, Canada) at the Commonwealth Scientific and Industrial Research Organization Black Mountain Laboratories (Supplemental Table S1). Seeds, including husks, were sown directly into the soil. Because sowing depth can have a significant impact on nodal root emergence, each seed was carefully planted at a depth of 2 cm with embryo facing downward. After sowing, pots were watered and placed in a cold room (6°C) for stratification for 7 d, and then they were moved to the growth cabinet for the duration of the experiment. Conditions for the experiments changed over the first four experiments to optimize for screening mature root systems and testing effects of irrigation (Supplemental Table S1; experiment 4 is described below). Experiments 5 to 8 focused on covering the widest genotypic diversity, and experiment 9 repeated and confirmed a selected set of lines with high replication.
In experiment 4, the root systems of a subset of 16 lines were analyzed in two different moisture conditions. Eight pots of each line were sown with seed, watered, and stratified at 6°C for 1 week, and then they were placed in the growth chamber. Upon transfer from the cold room to the growth chamber, all pots were well watered several times over the course of the day and left to drain overnight. Polyethylene beads were added to the surface to prevent evaporation. Four pots for each line were equipped with drippers that delivered 80 mL of water every 3 d (the well-watered treatment). The other four pots for each line were not watered throughout the experiment, letting plants grow on water stored in the pot from the initial watering event (the drought treatment). Plants were harvested 38 d after sowing, and root systems were washed as described below. Water contents and their suctions at different levels in the pots of well-watered and drought treatments are presented in Supplemental Figure S4B.
Root and Shoot Phenotyping
Roots were quantified among different root types: PSRs, CNRs, and LNRs (Fig. 1). CNRs and LNRs are commonly referred to as crown roots or adventitious roots; however, we retain the nomenclature proposed by Weaver (1926), Wenzel et al. (1989), Pellerin and Pages (1994), and Watt et al. (2009), which classifies root types based on the tissues from which they originate.
To harvest the shoots and roots, pots containing intact plants were saturated with water and then gently tipped to slide soil and plant onto a mesh tray. Soil was washed from roots with a gentle spray, and whole plants were transferred to 50% ethanol and stored until processed. Processing involved untangling the root systems of each plant in a large tray of shallow water and excising the PSRs, CNRs, and LNRs at the seed and root-shoot junction; finally, each type was further untangled, spread out, and scored for branch root length (Fig. 1).
The length of each axile root was measured manually with a ruler and then scanned at a resolution of 400 dots per inch on a flatbed scanner (EPSON) equipped with a transparency unit (Regent). We obtained at least one image for each root type (sometimes more for bigger root systems), and all images were batch processed using WinRhizo (Regent), which gave the TRL. After length processing, each root type from each plant was oven dried (65°C) for 7 d and weighed to obtain the RDW. Shoot phenotypes measured at harvest included the number of leaves along the main stem, the number of tillers, and the length of the longest leaf. Total SDW was determined as for roots.
Statistics
Data were analyzed statistically for heritability and genetic correlations after first checking for normality and error variance heterogeneity across environments. The distribution of residuals for percentage traits (i.e. PSRpc, CNRpc, LNRpc, and Rootpc) was binomial, and therefore, data were arcsine transformed to normalize before analysis. A combined ANOVA and covariance over all experiments was then performed for all root and shoot characters using the SAS mixed linear models procedure MIXED (Littell et al., 1996). Variance and covariance components for line and line × experiment interaction effects were estimated assuming that lines and experiments were random effects. Broad-sense heritabilities were estimated on single-plant and line-mean bases. Genetic correlations and ses were estimated between TRL and TDW using the SAS procedure MIXED (Holland, 2006). Graphics were generated using Microsoft Excel (2007), Sigmaplot version 12.3 (Systat Software Inc.), or R (version 3.1.1) with the ggplot package. Maps were generated in R using either the package ggmap and google maps (Supplemental Fig. S6; http://maps.google.com) or the packages map and maproj (Fig. 2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Shoot development of lines Bd21 and Bd21-3.
Supplemental Figure S2. Summary of the complete data set of all the experiments presented in this study.
Supplemental Figure S3. Allometric relationships between components of the root system and SDW.
Supplemental Figure S4. Water retention characteristics of soil used in this study.
Supplemental Figure S5. Response of 16 lines of B. distachyon in root and shoot partitioning to irrigation and drought.
Supplemental Figure S6. Overlay of six traits on a map of the Middle East region.
Supplemental Figure S7. Relationship between key climate parameters and root phenotypes.
Supplemental Figure S8. Typical scans of the root system of two genotypes.
Supplemental Table S1. Experiments and growth conditions to assess variation in adult plant phenotypes among 79 B. distachyon lines.
Supplemental Table S2. Nutrient composition of the soil used in this study.
Supplementary Material
Acknowledgments
We thank Iain Wilson and Ludmila Tyler for providing seed stocks, Mick Weiss and Sam Walker for excellent technical support, Alan Severini for help in soil characterization, and Carl Davies for figure graphics.
Glossary
- CNR
coleoptile node axile root
- CNRpc
percentage of total root length among coleoptile node axile roots
- LNR
leaf node axile root
- LNRcount
number of leaf node axile roots
- LNRpc
percentage of total root length among leaf node axile roots
- LNRsum
total length of nodal axile roots
- PSR
primary seminal root
- PSRpc
percentage of total root length among primary seminal roots
- RDW
root dry weight
- Rootpc
percentage of total dry weight in roots
- R/S
root to shoot ratio
- SDW
shoot dry weight
- TDW
total plant dry weight
- TRL
total root length
Footnotes
This work was supported by the Australian Grain Research and Development Corporation (grant no. CSP00129) and the Commonwealth Scientific and Industrial Research Organization.
Articles can be viewed without a subscription.
References
- Acuna TL, Pasuquin E, Wade LJ (2007) Genotypic differences in root penetration ability of wheat through thin wax layers in contrasting water regimes and in the field. Plant Soil 301: 135–149 [Google Scholar]
- Bao Y, Aggarwal P, Robbins NE II, Sturrock CJ, Thompson MC, Tan HQ, Tham C, Duan L, Rodriguez PL, Vernoux T, et al. (2014) Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc Natl Acad Sci USA 111: 9319–9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS, Silk WK, Watt M (2010) Path of water for root growth. Funct Plant Biol 37: 1105–1116 [Google Scholar]
- Brady DJ, Wenzel CL, Fillery IR, Gregory PJ (1995) Root-growth and nitrate uptake by wheat (Triticum aestivum L) following wetting of dry surface soil. J Exp Bot 46: 557–564 [Google Scholar]
- Bragg JN, Wu J, Gordon SP, Guttman ME, Thilmony R, Lazo GR, Gu YQ, Vogel JP (2012) Generation and characterization of the Western Regional Research Center Brachypodium T-DNA insertional mutant collection. PLoS ONE 7: e41916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, et al. (2011) Brachypodium as a model for the grasses: today and the future. Plant Physiol 157: 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Chochois V, Vogel JP, Watt M (2012) Application of Brachypodium to the genetic improvement of wheat roots. J Exp Bot 63: 3467–3474 [DOI] [PubMed] [Google Scholar]
- Clark LJ, Gowing DJ, Lark RM, Leeds-Harrison PB, Miller AJ, Wells DM, Whalley WR, Whitmore AP (2005) Sensing the physical and nutritional status of the root environment in the field: a review of progress and opportunities. J Agric Sci 143: 347–358 [Google Scholar]
- Comai L, Young K, Till BJ, Reynolds SH, Greene EA, Codomo CA, Enns LC, Johnson JE, Burtner C, Odden AR, et al. (2004) Efficient discovery of DNA polymorphisms in natural populations by Ecotilling. Plant J 37: 778–786 [DOI] [PubMed] [Google Scholar]
- Cornish PS. (1982) Root development in seedlings of ryegrass (Lolium-Perenne L) and phalaris (Phalaris-Aquatica L) sown onto the soil surface. Aust J Agric Res 33: 665–677 [Google Scholar]
- Cornish PS, Mcwilliam JR, So HB (1984) Root morphology, water-uptake, growth and survival of seedlings of ryegrass and phalaris. Aust J Agric Res 35: 479–492 [Google Scholar]
- Coudert Y, Périn C, Courtois B, Khong NG, Gantet P (2010) Genetic control of root development in rice, the model cereal. Trends Plant Sci 15: 219–226 [DOI] [PubMed] [Google Scholar]
- Crossett RN, Campbell DJ, Stewart HE (1975) Compensatory growth in cereal root systems. Plant Soil 42: 673–683 [Google Scholar]
- Dodd IC. (2005) Root-to-shoot signalling: assessing the roles of 'up' in the up and down world of long-distance signalling in planta. Plant Soil 274: 251–270 [Google Scholar]
- Draper J, Mur LA, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge AP (2001) Brachypodium distachyon: a new model system for functional genomics in grasses. Plant Physiol 127: 1539–1555 [PMC free article] [PubMed] [Google Scholar]
- Fitz Gerald JN, Lehti-Shiu MD, Ingram PA, Deak KI, Biesiada T, Malamy JE (2006) Identification of quantitative trait loci that regulate Arabidopsis root system size and plasticity. Genetics 172: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamuyao R, Chin JH, Pariasca-Tanaka J, Pesaresi P, Catausan S, Dalid C, Slamet-Loedin I, Tecson-Mendoza EM, Wissuwa M, Heuer S (2012) The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488: 535–539 [DOI] [PubMed] [Google Scholar]
- Hao ZB, Ichii M (1999) A mutant RM109 of rice (Oryza sativa L.) exhibiting altered lateral root initiation and gravitropism. Jpn J Crop Sci 68: 245–252 [Google Scholar]
- Hetz W, Hochholdinger F, Schwall M, Feix G (1996) Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J 10: 845–857 [Google Scholar]
- Hochholdinger F, Feix G (1998) Early post-embryonic root formation is specifically affected in the maize mutant lrt1. Plant J 16: 247–255 [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Feix GH (2001) Cooperative action of SLR1 and SLR2 is required for lateral root-specific cell elongation in maize. Plant Physiol 125: 1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Zimmermann R (2008) Conserved and diverse mechanisms in root development. Curr Opin Plant Biol 11: 70–74 [DOI] [PubMed] [Google Scholar]
- Holland JB. (2006) Estimating genotypic correlations and their standard errors using multivariate restricted maximum likelihood estimation with SAS Proc MIXED. Crop Sci 46: 642–654 [Google Scholar]
- Inukai Y, Miwa M, Nagato Y, Kitano H, Yamauchi A (2001) Characterization of rice mutants deficient in the formation of crown roots. Breed Sci 51: 123–129 [Google Scholar]
- Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17: 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard JA, Lilley JM, Howe GN, Graham JM (2007) Impact of subsoil water use on wheat yield. Aust J Agric Res 58: 303–315 [Google Scholar]
- Kitomi Y, Ogawa A, Kitano H, Inukai Y (2008) CRL4 regulates crown root formation through auxin transport in rice. Plant Root 2: 19–28 [Google Scholar]
- Krassovsky I. (1926) Physiological activity of the seminal and nodal roots of crop plants. Soil Sci 21: 307–325 [Google Scholar]
- Laffont C, Blanchet S, Lapierre C, Brocard L, Ratet P, Crespi M, Mathesius U, Frugier F (2010) The Compact Root Architecture1 gene regulates lignification, flavonoid production, and polar auxin transport in Medicago truncatula. Plant Physiol 153: 1597–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS System for Mixed models. SAS Institute Inc., Cary, NC [Google Scholar]
- Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43: 47–56 [DOI] [PubMed] [Google Scholar]
- Liu S, Wang J, Wang L, Wang X, Xue Y, Wu P, Shou H (2009) Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res 19: 1110–1119 [DOI] [PubMed] [Google Scholar]
- Luo N, Liu J, Yu X, Jiang Y (2011) Natural variation of drought response in Brachypodium distachyon. Physiol Plant 141: 19–29 [DOI] [PubMed] [Google Scholar]
- Lynch JP. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot (Lond) 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeght JL, Rewald B, Pierret A (2013) How to study deep roots-and why it matters. Front Plant Sci 4: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschadi AM, Christopher J, Devoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33: 823–837 [DOI] [PubMed] [Google Scholar]
- Mouchel CF, Briggs GC, Hardtke CS (2004) Natural genetic variation in Arabidopsis identifies BREVIS RADIX, a novel regulator of cell proliferation and elongation in the root. Genes Dev 18: 700–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Sharp RE (1993) Involvement of abscisic-acid in controlling plant-growth in soils of low water potential. Aust J Plant Physiol 20: 425–437 [Google Scholar]
- Mur LA, Allainguillaume J, Catalán P, Hasterok R, Jenkins G, Lesniewska K, Thomas I, Vogel J (2011) Exploiting the Brachypodium Tool Box in cereal and grass research. New Phytol 191: 334–347 [DOI] [PubMed] [Google Scholar]
- Navara J, Jesko T, Duchoslav S (1994) Participation of seminal roots in water-uptake by maize root-system. Biologia 49: 91–95 [Google Scholar]
- Passioura JB. (1972) Effect of root geometry on yield of wheat growing on stored water. Aust J Agric Res 23: 745–752 [Google Scholar]
- Passioura JB. (1991) Soil structure and plant growth. Aust J Soil Res 29: 717–728 [Google Scholar]
- Pellerin S, Pages L (1994) Evaluation of parameters describing the root-system architecture of field-grown maize plants (Zea Mays L): 1. Elongation of seminal and nodal roots and extension of their branched zone. Plant Soil 164: 155–167 [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ (2009a) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Péret B, Larrieu A, Bennett MJ (2009b) Lateral root emergence: a difficult birth. J Exp Bot 60: 3637–3643 [DOI] [PubMed] [Google Scholar]
- Rich SM, Watt M (2013) Soil conditions and cereal root system architecture: review and considerations for linking Darwin and Weaver. J Exp Bot 64: 1193–1208 [DOI] [PubMed] [Google Scholar]
- Rostamza M, Richards RA, Watt M (2013) Response of millet and sorghum to a varying water supply around the primary and nodal roots. Ann Bot (Lond) 112: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasithamparam K, Parker CA (1978) Effect of infection of seminal and nodal roots by Take-All fungus on tiller numbers and shoot weight of wheat. Soil Biol Biochem 10: 365–368 [Google Scholar]
- Soman P, Seetharama N (1992) Genotypic and environmental variation in nodal root-growth of post-rainy season (rabi) sorghum. Exp Agric 28: 331–341 [Google Scholar]
- Tennant D. (1976) Root growth of wheat: I. Early patterns of multiplication and extension of wheat roots including effects of levels of nitrogen, phosphorus and potassium. Aust J Agric Res 27: 183–196 [Google Scholar]
- van Genuchten MT, Leij FJ, Yates SR (1991) The RETC code for quantifying the hydraulic functions of unsaturated soils. EPA 600: 2–91 [Google Scholar]
- Vogel J, Hill T (2008) High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep 27: 471–478 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Garvin DF, Mockler TC, Schmutz J, Rokhsar D, Bevan MW, Barry K, Lucas S, Harmon-Smith M, Lail K, et al. ; International Brachypodium Initiative (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463: 763–768 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Tuna M, Budak H, Huo N, Gu YQ, Steinwand MA (2009) Development of SSR markers and analysis of diversity in Turkish populations of Brachypodium distachyon. BMC Plant Biol 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar KM. (1997) Water stressed nodal roots of wheat: effects on leaf growth. Aust J Plant Physiol 24: 49–56 [Google Scholar]
- Wang H, Taketa S, Miyao A, Hirochika H, Ichii M (2006) Isolation of a novel lateral-rootless mutant in rice (Oryza sativa L.) with reduced sensitivity to auxin. Plant Sci 170: 70–77 [Google Scholar]
- Wasson AP, Rebetzke GJ, Kirkegaard JA, Christopher J, Richards RA, Watt M (2014) Soil coring at multiple field environments can directly quantify variation in deep root 7 traits to select wheat genotypes for breeding. J Exp Bot 65: 6231–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M, Moosavi S, Cunningham SC, Kirkegaard JA, Rebetzke GJ, Richards RA (2013) A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann Bot (Lond) 112: 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M, Schneebeli K, Dong P, Wilson IW (2009) The shoot and root growth of Brachypodium and its potential as a model for wheat and other cereal crops. Funct Plant Biol 36: 960–969 [DOI] [PubMed] [Google Scholar]
- Weaver B. (1926) Root Development of Field Crops. McGraw-Hill Book Company, Inc., New York [Google Scholar]
- Wenzel CL, McCully ME, Canny MJ (1989) Development of water conducting capacity in the root systems of young plants of corn and some other c4 grasses. Plant Physiol 89: 1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H (2004) Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. J Exp Bot 55: 2121–2129 [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25: 195–210 [DOI] [PubMed] [Google Scholar]
- Woll K, Borsuk LA, Stransky H, Nettleton D, Schnable PS, Hochholdinger F (2005) Isolation, characterization, and pericycle-specific transcriptome analyses of the novel maize lateral and seminal root initiation mutant rum1. Plant Physiol 139: 1255–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokawa K, Kagenishi T, Baluška F (2013) Root photomorphogenesis in laboratory-maintained Arabidopsis seedlings. Trends Plant Sci 18: 117–119 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hu Y, Dai M, Huang L, Zhou DX (2009) The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 21: 736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li S, Ren B, Zhang J, Ichii M, Taketa S, Tao Y, Zuo J, Wang H (2013) LATERAL ROOTLESS2, a cyclophilin protein, regulates lateral root initiation and auxin signaling pathway in rice. Mol Plant 6: 1719–1721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.