Accumulation of an immune coreceptor or its ectodomain leads to detrimental effects on Arabidopsis growth and constitutive activation of immunity.
Abstract
The membrane-bound BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 (BAK1) is a common coreceptor in plants and regulates distinct cellular programs ranging from growth and development to defense against pathogens. BAK1 functions through binding to ligand-stimulated transmembrane receptors and activating their kinase domains via transphosphorylation. In the absence of microbes, BAK1 activity may be suppressed by different mechanisms, like interaction with the regulatory BIR (for BAK1-INTERACTING RECEPTOR-LIKE KINASE) proteins. Here, we demonstrated that BAK1 overexpression in Arabidopsis (Arabidopsis thaliana) could cause detrimental effects on plant development, including growth arrest, leaf necrosis, and reduced seed production. Further analysis using an inducible expression system showed that BAK1 accumulation quickly stimulated immune responses, even under axenic conditions, and led to increased resistance to pathogenic Pseudomonas syringae pv tomato DC3000. Intriguingly, our study also revealed that the plasma membrane-associated BAK1 ectodomain was sufficient to induce autoimmunity, indicating a novel mode of action for BAK1 in immunity control. We postulate that an excess of BAK1 or its ectodomain could trigger immune receptor activation in the absence of microbes through unbalancing regulatory interactions, including those with BIRs. Consistently, mutation of SUPPRESSOR OF BIR1-1, which encodes an emerging positive regulator of transmembrane receptors in plants, suppressed the effects of BAK1 overexpression. In conclusion, our findings unravel a new role for the BAK1 ectodomain in the tight regulation of Arabidopsis immune receptors necessary to avoid inappropriate activation of immunity.
Plants rely on their innate immune system to detect microbes and mount an active defense against pathogens. The plant immune system is traditionally considered to be composed of two layers (Jones and Dangl, 2006). The first one is based on the activity of pattern-recognition receptors (PRRs) that can detect microbe-associated molecular patterns (MAMPs) and trigger what is termed pattern-triggered immunity (PTI; Boller and Felix, 2009). Many plant pathogens can suppress this basal defense response using virulence factors termed effectors. In a second layer of defense, plants can make use of resistance (R) proteins to recognize the presence of pathogen effectors resulting in effector-triggered immunity (ETI), which resembles an accelerated and amplified PTI response (Jones and Dangl, 2006).
Plants utilize plasma membrane-associated receptor-like proteins (RLPs) or receptor-like kinases (RLKs) as PRRs to sense specific signals through their ectodomains (Böhm et al., 2014). RLPs and RLKs require the function of additional RLKs to form active receptor complexes and transfer the external signal to the inside of the cells (Zhang and Thomma, 2013; Cao et al., 2014; Liebrand et al., 2014). The best-known coreceptor is the leucine-rich repeat (LRR)-RLK BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 (BAK1), which was originally identified as a positive regulator and partner for the brassinosteroid (BR) receptor BRASSINOSTEROID INSENSITIVE1 (BRI1; Li et al., 2002; Nam and Li, 2002). BRs refer to phytohormones that promote plant growth and development (Fujioka and Yokota, 2003). Thus, loss-of-function mutations in BAK1 negatively impact Arabidopsis (Arabidopsis thaliana) growth due to improper cell elongation. In short, bak1 mutants display compact rosettes with round-shaped leaves and shorter petioles and phenocopy weak bri1 mutations (Li et al., 2002; Nam and Li, 2002). Conversely, certain mutants affected in the BAK1 ectodomain show increased activity in the BR signaling pathway and share phenotypic similarities with BRI1-overexpressing lines (Wang et al., 2001), including elongated hypocotyls, petioles, and leaf blades and an overall increase in height (Jaillais et al., 2011; Chung et al., 2012).
Furthermore, BAK1 is involved in the containment of cell death, independently of its function in BR signaling. Arabidopsis bak1 knockout mutants exhibit extensive cell death spreading after microbial infection (Kemmerling et al., 2007). In addition, spontaneous cell death develops in Arabidopsis double mutant plants lacking both BAK1 (also named SOMATIC EMBRYOGENESIS RECEPTOR KINASE3 [SERK3]) and its closest homolog BAK1-LIKE1 (BKK1)/SERK4, causing seedling lethality even in the absence of microbes (He et al., 2007). Similar phenotypes are observed in Arabidopsis, rice (Oryza sativa), and Nicotiana benthamiana by lowering the expression of BAK1 and its homologs (Heese et al., 2007; Jeong et al., 2010; Park et al., 2011). Interestingly, typical defense responses, like the production of reactive oxygen species and constitutive callose deposition, are also detected in those plants, although the basis for this phenomenon remains poorly understood (He et al., 2007; Kemmerling et al., 2007; Park et al., 2011; Gao et al., 2013).
On the other hand, BAK1 is widely studied as a key component of immune signaling pathways due to its known association with different PRRs, including RLKs and RLPs (Kim et al., 2013; Böhm et al., 2014). Upon MAMP perception, PRRs induce signaling and physiological defense responses like mitogen-activated protein kinase (MAPK) activation, reactive oxygen species and ethylene production, and modifications in gene expression, all of which contribute to PTI. Among the best-studied examples of BAK1-regulated PRRs are two LRR-receptor kinases, ELONGATION FACTOR Tu RECEPTOR (EFR), which senses the active epitope elf18 of the bacterial elongation factor Tu, and the flagellin receptor FLAGELLIN SENSING2 (FLS2), which senses the active epitope flg22 of bacterial flagellin (Gómez-Gómez and Boller, 2000; Chinchilla et al., 2006; Zipfel et al., 2006). Immediately after flg22 binding to its LRR ectodomain, FLS2 forms a tight complex with BAK1 (Chinchilla et al., 2007; Sun et al., 2013). This heteromerization step may bring the two kinase domains closer and thereby induce, within seconds, the phosphorylation of BAK1 and FLS2 (Schulze et al., 2010; Schwessinger et al., 2011). These steps are sufficient to initiate the immune signaling pathway, even if the ectodomains and kinase domains are switched between FLS2 and BAK1 (Albert et al., 2013).
While PRRs, such as FLS2 and EFR, are extremely sensitive to even subnanomolar concentrations of their ligands, a tight control of these receptors is expected, since constitutive activation of defense responses in plants dramatically impairs fitness and growth (Tian et al., 2003; Korves and Bergelson, 2004). However, the mechanisms that underlie the attenuation of PRR activation or prevent these receptors from signaling constitutively remain largely unknown (Macho and Zipfel, 2014). Several independent observations indicate that BAK1 and FLS2 are present in close spatial proximity in preformed complexes at the plasma membrane (Chinchilla et al., 2007; Schulze et al., 2010; Roux et al., 2011). Negative regulation of immune signaling prior to ligand perception could happen within the PRR complex and depend on conformational changes following the association of FLS2 with flg22 (Meindl et al., 2000; Schulze et al., 2010; Mueller et al., 2012). Additionally, other partners might prevent the constitutive interaction of BAK1 with FLS2. Such could be the case for the LRR-RLK BAK1-INTERACTING RECEPTOR-LIKE KINASEs (BIRs): BIR2 was recently discovered as a substrate and negative regulator for BAK1, while the absence of BIR1 leads to the activation of defense induction and strong dwarfism (Gao et al., 2009; Halter et al., 2014b). Furthermore, MAMP signaling may be constrained by phosphatases, as suggested in earlier studies (Felix et al., 1994; Gómez-Gómez et al., 2001) and recently shown for the protein phosphatase 2A, which controls PRR activation likely by modulating the BAK1 phosphostatus (Segonzac et al., 2014). These examples illustrate the variety of mechanisms that may tightly control BAK1 activity.
In this work, we show that regulation of BAK1 accumulation is crucial for Arabidopsis fitness, as its overexpression leads to dwarfism and premature death. The phenotype differs from BR mutants and is very reminiscent of or even identical to the autoimmune phenotype of plants showing constitutive activation of R proteins (Oldroyd and Staskawicz, 1998; Bendahmane et al., 2002; Zhang et al., 2003). BAK1 overexpression is associated with constitutive activation of defense pathway(s) involving the general coregulator of RLPs, SUPPRESSOR OF BIR1-1 (SOBIR1; Liebrand et al., 2013, 2014). To our knowledge, this is the first report and comprehensive characterization of such an autoimmunity phenotype for Arabidopsis plants overexpressing BAK1, and it highlights the importance of the regulation of PTI overactivation.
RESULTS
Arabidopsis Plants Overexpressing BAK1 Show Stunted Growth and Extensive Cell Death
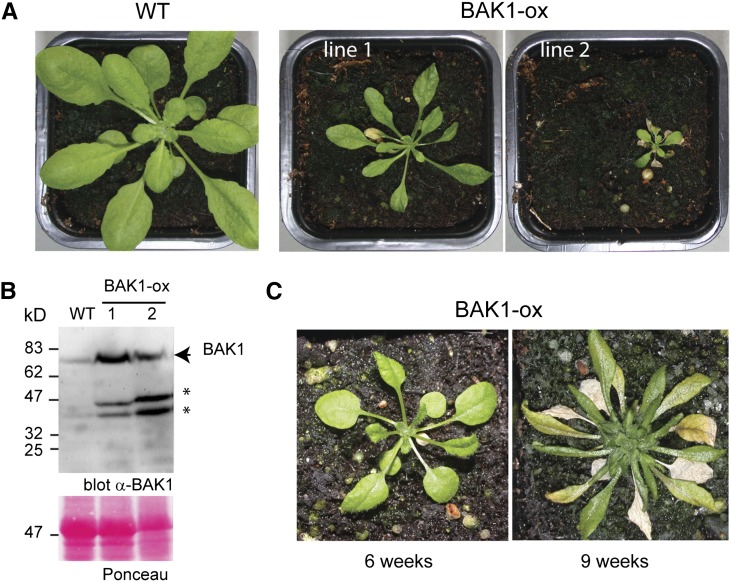
To study the effect of an increased dose of BAK1, transgenic Arabidopsis lines overexpressing BAK1 (called BAK1-ox) were generated. All BAK1-ox plants showed a dramatically stunted phenotype, albeit with variable severity, and stopped growing soon after germination (Fig. 1). Moreover, leaf necrosis could be observed on all BAK1-ox plants, and this phenomenon increased as leaves aged (Fig. 1C). While 75% of the overexpressing lines died prematurely, some of the surviving plants did flower but showed reduced seed production. The phenotype caused by BAK1 overexpression was highly reproducible, observed for 60 independent T1 lines in more than 10 different selection assays. It also occurred when seedlings were grown under axenic conditions (Supplemental Fig. S1).
Figure 1.
BAK1 overexpression causes necrosis and growth defects in Arabidopsis. A, Photographs of two transgenic T1 plants overexpressing BAK1 under the control of the 2× 35S promoter from Cauliflower mosaic virus (BAK1-ox) exhibiting a strongly stunted phenotype in contrast to wild-type (WT) Columbia-0 (Col-0) plants after 6 weeks of growth. B, Western-blot analysis of whole extract from the two presented lines with anti-BAK1 antibodies. The arrowhead indicates full-length BAK1, and the asterisks signal the presence of two additional bands. Protein loading control by Ponceau S is shown. C, Of the 60 independent T1 lines isolated from 10 independent selections, about 50% had a milder phenotype and developed symptoms at a later time point.
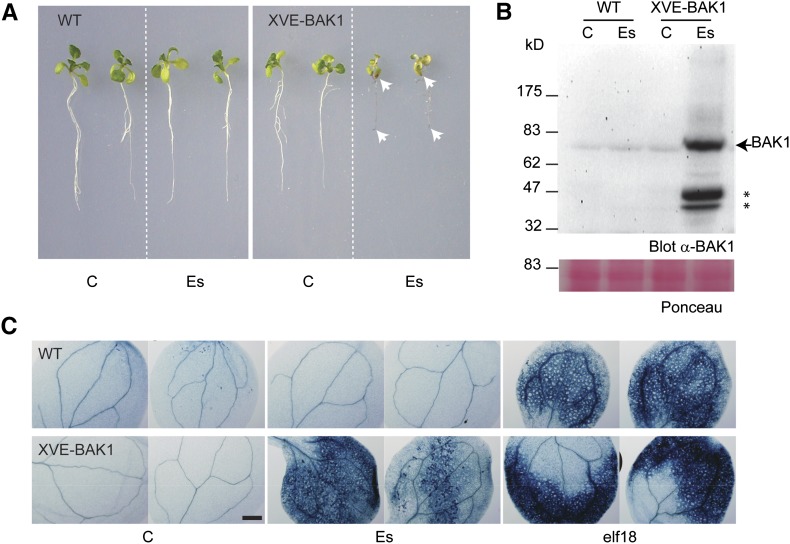
Growth arrest and massive cell death independent of pathogen or MAMP exposure are reminiscent of plants with constitutively activated defense responses (Bowling et al., 1994; Rate et al., 1999; Lorrain et al., 2003). To test the induction of immune responses, transgenic plants expressing BAK1 under the control of the chimeric transactivator XVE (for composition, see “Materials and Methods”; Zuo et al., 2000) were generated to allow conditional BAK1 overexpression upon estradiol treatment (XVE-BAK1; Fig. 2; Supplemental Fig. S2). While estradiol treatment had no obvious effect on wild-type seedlings, it caused a severe growth arrest on the XVE-BAK1 seedlings (Fig. 2, A and B; Supplemental Fig. S2). Intriguingly, estradiol-induced expression of BAK1 had a stronger effect on seedling growth than treatment with the active MAMP flg22 (Supplemental Fig. S2). Whereas flg22-treated seedlings remained green, estradiol-treated XVE-BAK1 seedlings exhibited roots and cotyledons with a brownish coloration and necrotic appearance. Trypan blue staining and microscopic examination of seedling tissue confirmed massive cell death in the mesophyll of cotyledons overexpressing BAK1 (Fig. 2C), a result that could also be obtained after treatment with some MAMPs, like elf18 (Fig. 2C).
Figure 2.
Inducible BAK1 overexpression causes growth inhibition and mesophyll cell death in Arabidopsis seedlings. Transgenic plants expressing the BAK1 genomic sequence under the control of the estradiol-inducible system (XVE-BAK1) were analyzed versus wild-type (WT) Col-0 plants. A, Seedling phenotype after control (C) or estradiol (Es; 1 μm) treatment. White arrowheads indicate a brownish coloration of plant tissue. B, Western-blot analysis of the same samples with anti-BAK1 antibodies. The arrowhead indicates full-length BAK1, and the asterisks signal truncated forms of BAK1. Ponceau S staining shows equal loading of proteins. C, Micrograph images of Trypan blue-stained cotyledons of wild-type or XVE-BAK1 seedlings grown under axenic conditions after control (C), 1 μm estradiol (Es), or 1 μm elf18 (elf18) treatment. As flg22 did not trigger cell death reproducibly, elf18 was used as positive control. Two representative images are presented after examination of more than 20 individuals in four independent experiments. Bar = 0.5 mm.
BAK1 Overexpression Induces the Activation of Immune Responses
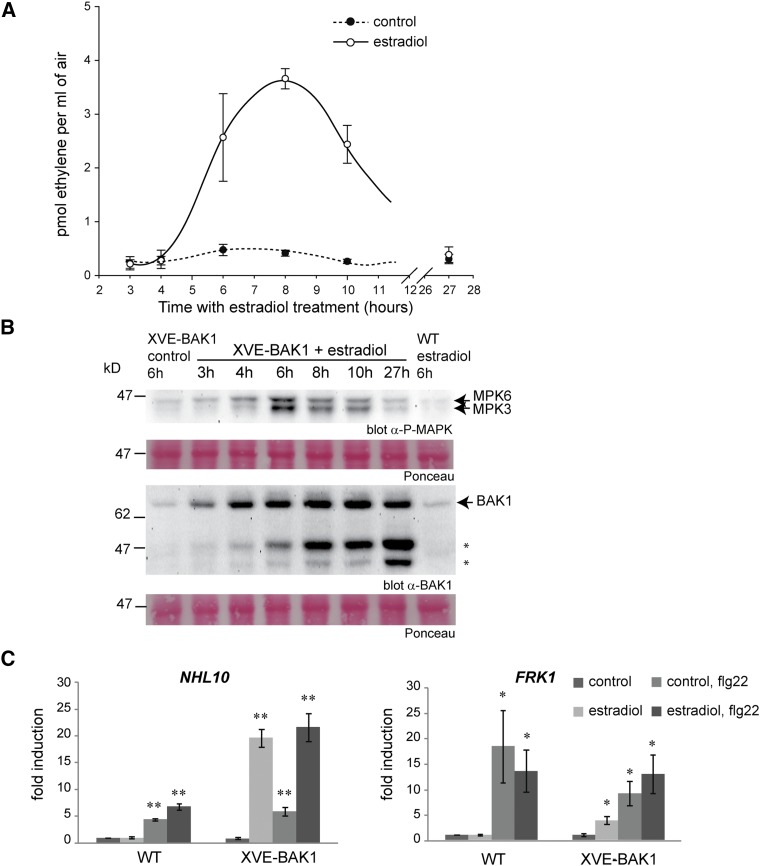
A time-course experiment was performed to evaluate the kinetics of BAK1 expression after estradiol treatment (Fig. 3). A tight correlation was observed between BAK1 accumulation and MAPK activation deduced from their phosphorylation status, as well as induction of the stress hormone ethylene biosynthesis (Fig. 3). MAPK phosphorylation and ethylene production were clearly induced 4 and 6 h after estradiol treatment and reached maxima at 6 and 8 h, respectively. In contrast, no activation of MAPKs, or of ethylene accumulation, was detected in nonoverexpressing tissue of either wild-type or transgenic plants (Fig. 3; Supplemental Fig. S3). Interestingly, the ethylene accumulation triggered by BAK1 overexpression often reached higher levels than those achieved after flg22 treatment in wild-type or noninduced plants. Moreover, the ethylene response seemed saturated, as subsequent flg22 treatment did not lead to further stimulation of ethylene biosynthesis (Supplemental Fig. S3B). Activation of the MAPKs, MPK3 and MPK6, in BAK1-overexpressing seedlings was sustained for several hours after estradiol treatment (Fig. 3B), in contrast to the more transient kinetics of flg22-induced activation, which is maximal after 15 to 30 min and strongly declines at 60 min (Suarez-Rodriguez et al., 2007; Cheng et al., 2013). In estradiol-treated XVE-BAK1 seedlings, the increase in BAK1 levels was accompanied by the accumulation of two BAK1 truncated forms (Fig. 3B), which opened the possibility that these polypeptides might cause some of the observed effects.
Figure 3.
Defense responses are constitutively activated in the presence of BAK1 accumulation. A, Ethylene accumulation was assessed using wild-type (WT) Col-0 or estradiol-inducible BAK1-overexpressing (XVE-BAK1) seedlings at the indicated times after treatment with estradiol (1 μm) or ethanol (control). Graphs show average and sd of four technical replicates. B, MAPK activation was examined using antibodies recognizing the phosphorylated forms of MPK3 and MPK6. Accumulation of BAK1 was analyzed using anti-BAK1 antibodies. Ponceau S staining shows equal loading of proteins. C, Accumulation of NHL10 and FRK1 transcripts was analyzed by quantitative PCR in wild-type and XVE-BAK1 seedlings in response to a 6-h treatment with 1 μm estradiol or control. Where stated, a 1-h treatment with 1 μm flg22 was included as a positive control. The bars show averages of three biological replicates with error bars indicating se. Significant differences according to an ANOVA test are marked by asterisks: *, P < 0.05; and **, P < 0.01. All experiments were performed three times with similar results.
Upon MAMP perception, the activation of cytoplasmic kinases, such as MAPKs or calcium-dependent protein kinases, controls the expression of various defense genes, including NDR1/HIN1 LIKE1 (NHL10) and FLG22-INDUCED RECEPTOR KINASE1 (FRK1; Asai et al., 2002; Boudsocq et al., 2010). Our results showed that NHL10 and FRK1 transcripts clearly accumulated in seedlings overexpressing BAK1 in the absence of elicitor (Fig. 3C; Supplemental Fig. S3D). In fact, NHL10 induction was higher in response to BAK1 overexpression than to flg22 perception.
Taken together, these results showed that BAK1 overexpression caused the induction of multiple immune responses in Arabidopsis in the absence of MAMPs or microbes. BAK1, also termed SERK3, belongs to a five-membered gene family (Li et al., 2002; Nam and Li, 2002). We thus tested the effect of overexpression of the BAK1 closest homolog, SERK4, and the more distantly related SERK1 using the inducible XVE system in transformed Arabidopsis. Similar to BAK1, overexpression of both SERKs led to the activation of ethylene production and growth inhibition (Supplemental Fig. S4).
BAK1 Overexpression Induces Resistance to Pseudomonas syringae pv tomato DC3000 in Arabidopsis
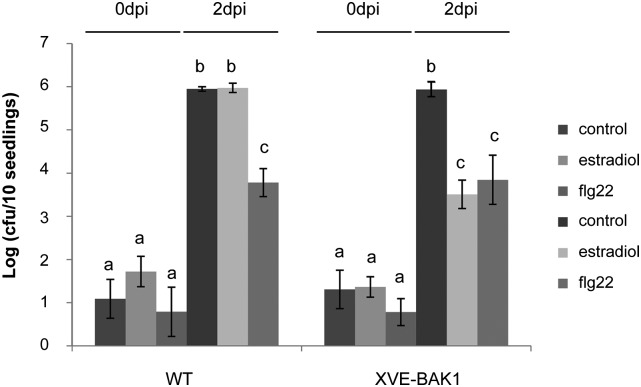
Since BAK1 overexpression induces defense responses, we investigated the resistance of BAK1-overexpressing seedlings to P. syringae pv tomato strain DC3000 when compared with wild-type seedlings. No obvious symptoms could be observed on young seedlings in our experimental conditions. Quantification of in planta bacterial growth was used to evaluate disease resistance. There was no significant difference in bacteria number between the genotypes just after dip inoculation (0 d post inoculation [dpi]; Fig. 4). In contrast, seedlings overexpressing BAK1 displayed a 100-fold decrease in bacteria titer when compared with wild-type seedlings at 2 dpi (Fig. 4). This effect was as strong as the immune response induced by flg22 pretreatment. Thus, activation of defense responses by BAK1 overexpression resulted in effective resistance against P. syringae pv tomato DC3000.
Figure 4.
BAK1 overexpression restricts bacterial growth. After a pretreatment with estradiol, flg22, or both solvents as a control, seedlings were dip inoculated with P. syringae pv tomato DC3000. Bacteria were extracted and counted 4 h (0 dpi) and 48 h post inoculation (2 dpi). The bars show averages of three samples with error bars indicating se. Significant differences (ANOVA test, P < 0.01) are indicated by different letters. cfu, Colony-forming units. Similar results were obtained in three independent experiments.
BAK1 Truncated Proteins Are Detected in BAK1-Overexpressing and Wild-Type Plants
Apart from intact BAK1, anti-BAK1 antibodies, directed against the C-terminal domain of BAK1, detected two further polypeptides migrating with molecular masses of approximately 47 and 39 kD (marked by asterisks in Figs. 1–3; Supplemental Fig. S2). These polypeptides were also detected in immunoprecipitates. Analysis by tandem mass spectrometry (MS/MS) confirmed their identity as N-terminally truncated forms of BAK1 (Supplemental Tables S1 and S2). The 47-kD form includes, at least, the whole transmembrane, juxtamembrane, and kinase domains, while the 39-kD polypeptide might correspond to the kinase and C-terminal domains of BAK1 (Supplemental Fig. S5). As the coverage for the full-length BAK1 was only partial (Supplemental Tables S1 and S2), we could not conclude on the precise sequence of these two truncated forms. A qualitatively similar pattern of truncated forms was obtained with immunoprecipitates from wild-type plants, indicating that truncation was not a specific trait of the BAK1-overexpressing plants. Truncated forms seem not to originate from alternative splicing, since they also accumulated when the complementary DNA (cDNA) sequence of BAK1 was used for overexpression (Fig. 5).
Figure 5.
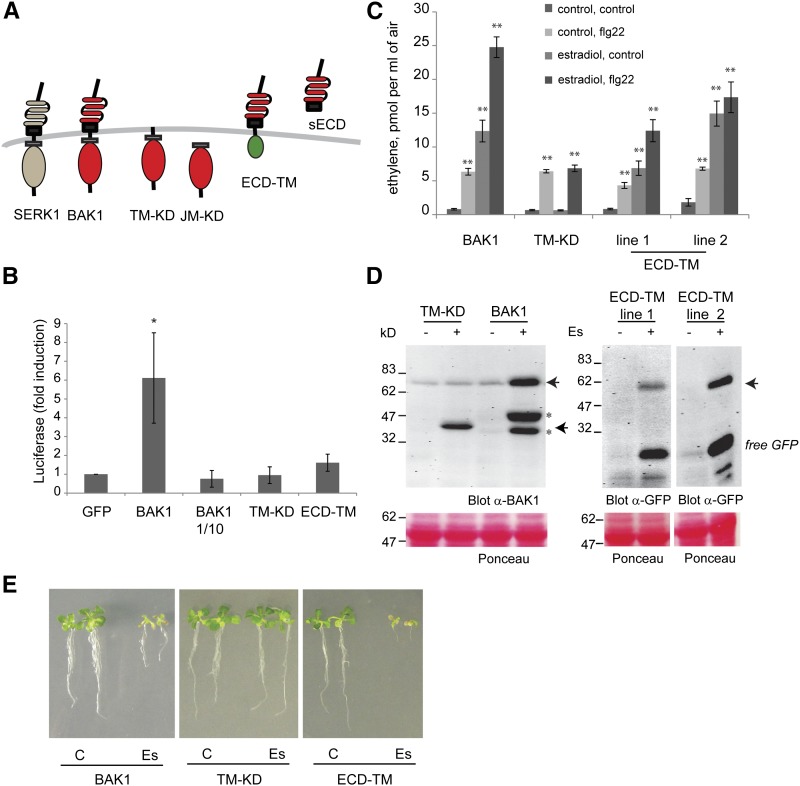
Expression of the BAK1 ectodomain mimics the overexpression of full-length BAK1. A, The following variants of the BAK1 sequence were cloned downstream of the constitutive 2× 35S promoter and/or the estradiol-inducible promoter to express full-length BAK1 (BAK1), a fusion of the signal peptide of BAK1 to its transmembrane and cytoplasmic domains (TM-KD), the cytoplasmic domain of BAK1 (JM-KD), a BAK1 devoid of its cytoplasmic domain and fused to a GFP tag (ECD-TM), and a similar construct devoid of the TM domain (sECD). B, Quantification of the luciferase basal activity in bak1-4 protoplasts cotransformed with the reporter construct pFRK1-Luc and the construct of interest. The BAK1 plasmid was also tested at a one-tenth concentration (1/10). Bars and error bars represent means and sd of three independent experiments. Significant differences from the GFP control are marked by asterisks (*, P < 0.05, ANOVA test). C, Ethylene production in one XVE-TM-KD and two XVE-ECD-TM lines by comparison with an XVE-BAK1 line (generated with the BAK1 cDNA sequence) in response to estradiol and/or flg22 treatment. Graphs show means of six technical replicates, and error bars represent se. Similar results were obtained in three independent experiments. D, Accumulation of the BAK1 variants (arrowheads) in extracts of the samples from C, detected by western blot using anti-BAK1 and anti-GFP antibodies. E, Growth phenotype of transgenic seedlings after 10 d of control (C) or estradiol (Es) treatment. Results presented here are representative of three independent experiments.
Overexpression of the BAK1 Ectodomain Partially Mimics BAK1 Overexpression
In order to determine whether intact BAK1 or a subpart of BAK1 was required or sufficient for the induction of defense responses, several BAK1 derivatives with truncations of the N or C terminus (Fig. 5A) were generated and assayed in mesophyll protoplasts from bak1-4 Arabidopsis. This analysis was done using cotransformation with the BAK1 derivatives and the Luciferase (Luc) reporter gene under the control of the flg22-responsive FRK1 promoter, a well-established and highly sensitive monitoring system for plant immunity (Yoo et al., 2007; Mueller et al., 2012; Albert et al., 2013). Protoplasts cotransformed with GFP (used as a negative control), sECD (for soluble ectodomain of BAK1), and TM-KD or JM-KD (cytoplasmic domains of BAK1) exhibited a low background activity of luciferase. By contrast, protoplasts expressing BAK1 or SERK1 exhibited high luciferase activity without MAMP treatment (Fig. 5B; Supplemental Figs. S6 and S7). Interestingly, lowering the amounts of BAK1 plasmid used for cell transformation also lowered the induction of luciferase (Fig. 5B; Supplemental Fig. S7), indicating a dose-dependent effect of BAK1 in these cells. Unfortunately, the ECD-TM protein (for ectodomain of BAK1 anchored to the membrane) did not accumulate in large amounts in protoplasts (Supplemental Fig. S7B) and only caused a slight (approximately 2-fold) activation of the FRK1 promoter (Fig. 5B; Supplemental Fig. S7). However, further tests with stably transformed Arabidopsis plants (named XVE-ECD-TM) showed that estradiol treatment induced a strong expression of ECD-TM (Fig. 5D) and also caused a strong induction of ethylene production (Fig. 5C). Consistently, the expression of ECD-TM, like the overexpression of BAK1, inhibited root and shoot development and caused yellowing of cotyledons (Fig. 5E). Thus, ECD-TM seemed to mimic the overexpression of intact BAK1 with respect to the induction of defense responses and seedling growth inhibition. In contrast, the expression of TM-KD in protoplasts or in stable transformed plants (XVE-TM-KD) did not induce defense responses or growth inhibition (Fig. 5).
The Ectodomain of BAK1 Suppresses MAMP-Induced Responses and Interacts with FLS2 in an flg22-Dependent Manner
We also investigated the effects of BAK1 derivatives on MAMP responses. In bak1-4 cells transformed with GFP, the basal luciferase activity did not increase in response to flg22 (Supplemental Figs. S6 and S7). However, a clear response to elf18 was observed, confirming that the presence of BAK1 is less important for the activation of EFR by elf18 than of FLS2 by flg22 (Chinchilla et al., 2007; Roux et al., 2011). Cotransformation with intact BAK1, but not with any of the truncated BAK1 versions, led to the restoration of flg22 responsiveness in bak1-4 protoplasts (Fig. 6; Supplemental Figs. S6 and S7). Expression of sECD, TM-KD, and JM-KD did not alter flg22 or elf18 responses in these cells. However, transformation with ECD-TM not only failed to restore flg22 responsiveness but also caused a clear reduction in the response to elf18 (Fig. 6A; Supplemental Fig. S7), indicating that the ectodomain of BAK1 might act as an antagonist of SERKs required for the activation of EFR by elf18.
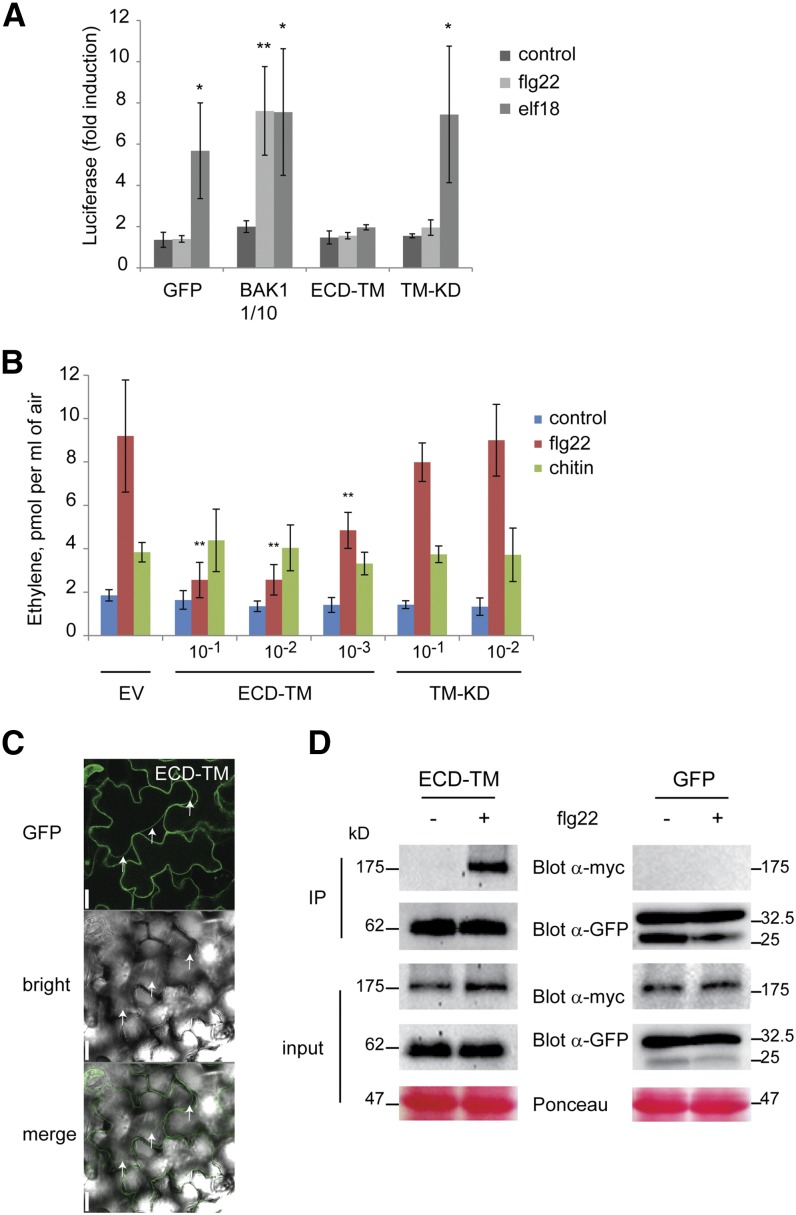
Figure 6.
The ectodomain of BAK1 has a dominant negative effect on MAMP responses and is sufficient to interact with FLS2 in an flg22-dependent manner. A, Quantification of luciferase activity in bak1-4 protoplasts cotransformed with the pFRK1-Luc and indicated constructs treated with buffer as a control, 100 nm flg22, or 100 nm elf18. The BAK1 plasmid was used at a low concentration to avoid constitutive activity. Results shown represent three independent experiments. B, Ethylene production by N. benthamiana leaves transformed with ECD-TM, TM-KD, or empty vector (EV) at various optical densities (as indicated on the x axis) after treatment with flg22 (1 μm), chitin (1 mg mL−1), or buffer (control). The graph shows one representative experiment out of two (n = 6 replicates). Bars and error bars represent means and sd. Significant differences from the control (A) and from the results with EV (B) are marked by asterisks: *, P < 0.05; **, and P < 0.01 (ANOVA test). C, Subcellular localization of ECD-TM in Arabidopsis epidermal cells. Standard confocal micrographs show optical sections of cells after plasmolysis. Bars = 20 mm. D, Coimmunoprecipitation (IP) of FLS2-myc and ECD-TM, or GFP as a control, isolated from N. benthamiana leaves was tested after 15 min of mock or flg22 treatment. Top, Western blot with anti-Myc antibodies; bottom, reanalysis of the blot with anti-GFP antibodies. One-tenth of the total amount of proteins used for coimmunoprecipitation was analyzed and shown as input. Results are representative of three independent experiments.
In N. benthamiana, BAK1 is also known to play a crucial function for MAMP signaling (Heese et al., 2007). Overexpression of Arabidopsis BAK1 or its truncated version ECD-TM in N. benthamiana leaves also caused an increased production of ethylene even in the absence of MAMPs, an effect that correlated with the bacterial titer used in the transformation step (Supplemental Fig. S8). On the other hand, the expression of ECD-TM, but not of TM-KD, inhibited flg22-induced ethylene production in N. benthamiana leaves (Fig. 6B). This effect could only be studied at low ECD-TM accumulation levels, obtained with reduced inocula of Agrobacterium tumefaciens that did not stimulate a response in the absence of MAMP treatment. The inhibitory activity of ECD-TM proved to be dose dependent and, notably, had no effect on the induction of ethylene production by chitin, a fungal MAMP, which does not involve BAK1 for activation of its PRR (Heese et al., 2007). These data indicated that ECD-TM inhibited the endogenous FLS2 receptor activity, but not the chitin receptor, and they confirmed the antagonistic effect found with Arabidopsis protoplasts above.
The most straightforward explanation of this antagonistic effect is that ECD-TM could bind to ligand-associated PRRs like intact SERKs do and, due to the absence of the kinase domain, keep these complexes in a nonactivated state. Note that the soluble ectodomain of BAK1, sECD, did not show any obvious inhibitory activity on MAMP signaling (Supplemental Fig. S6), suggesting that membrane localization of the truncated form is important for its activity. The subcellular localization of ECD-TM was examined in Arabidopsis protoplasts and epidermal cells of XVE-ECD-TM transgenic plants using confocal microscopy (Fig. 6C; Supplemental Fig. S7C). In comparison with the GFP control distributed in the cytoplasm, fluorescence emitted by ECD-TM was located homogenously at the periphery of the transformed protoplasts (Supplemental Fig. S7C). Plasmolysis experiments on Arabidopsis epidermal cells showed a retraction of the fluorescence signal associated with the cell membrane (Fig. 6C), supporting the proper localization of ECD-TM at the plasma membrane, as reported previously for BAK1 (Li et al., 2002; Nam and Li, 2002; Bücherl et al., 2013). To determine if ECD-TM was able to interact with PRRs, it was coexpressed with myc-tagged FLS2 in N. benthamiana leaves. Coimmunoprecipitation experiments showed that ECD-TM interacts with FLS2 in an flg22-dependent manner (Fig. 6D), as reported previously for BAK1 (Chinchilla et al., 2007), while no FLS2 could be detected in immunoprecipitates from GFP-expressing tissue irrespective of the presence of flg22 (Fig. 6D). Our results demonstrate that the ectodomain and transmembrane domain of BAK1 are sufficient to form stable complexes with ligand-activated receptors like FLS2 treated with flg22 and possibly also with EFR activated by elf18. Since complex formation would not lead to the activation of cytoplasmic signaling, ECD-TM can act as a competitive inhibitor for intact and fully functional SERKs.
Arabidopsis Plants Expressing ECD-TM Show Growth Defects
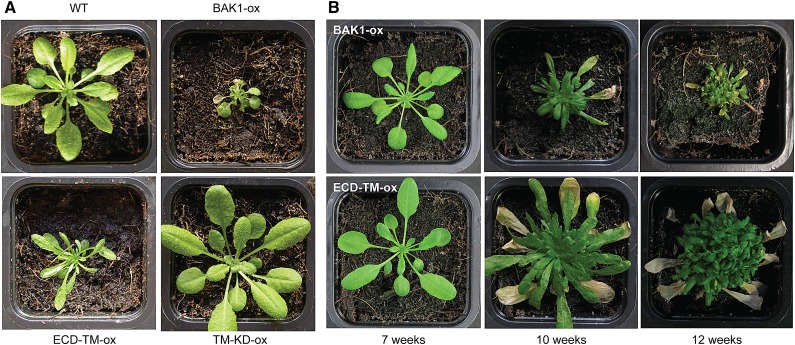
To complete this analysis, the impact of ECD-TM and TM-KD on plant development was investigated. Plants constitutively expressing TM-KD did not show any obvious phenotype when compared with wild-type plants (Fig. 7). In contrast, several independent lines expressing ECD-TM displayed dwarfism, although they seemed to develop somewhat better than the BAK1-ox plants. This difference may be explained by the two antagonistic effects of ECD-TM on immunity observed in plant cells (Figs. 5 and 6). A specific feature of ECD-TM plants was the presence of spatulate leaves in the rosette (Fig. 7), which could not be observed in BAK1-ox plants (Fig. 1). At later stages of development, more defects appeared, including leaf necrosis, meristematic disorganization, and loss of apical dominance during bolting, which resulted in a bushy phenotype comparable to the one exhibited by BAK1-ox plants (Fig. 7B; Supplemental Fig. S9). In some cases, plants developed flowers but seed production was reduced. Thus, expression of the membrane-anchored ectodomain of BAK1 strongly impaired Arabidopsis development, causing a phenotype partially overlapping with the BAK1-ox phenotype but also affecting leaf shape.
Figure 7.
Overexpression of ECD-TM, but not TM-KD, induces growth defects in Arabidopsis. A, Phenotypes of T1 transgenic plants overexpressing ECD-TM (ECD-TM-ox), BAK1 (BAK1-ox), or TM-KD (TM-KD-ox) under the control of the 2× 35S promoter compared with wild-type (WT) plants after 7 weeks of growth. Thirty-one and 16 independent T1 lines over five independent selections were examined for ECD-TM and TM-KD, respectively. B, Phenotypic changes for other T1 lines after 7, 10, and 12 weeks of growth.
Disruption of the SOBIR1 Gene Rescues the Stunted Growth of BAK1-ox Plants
In an effort to understand the molecular mechanisms underlying the immunostimulatory effect of BAK1 accumulation, the genetic determinants involved in this process were investigated. The classical approach of a suppressor screen could not be considered, because constitutive overexpression of BAK1 led to lethality and inducible expression was not stable over several plant generations. Therefore, the 2× 35S-BAK1 construct was used to generate transgenic lines from a set of well-characterized mutants affected in receptors and regulators functionally linked to BAK1 (Table I). Since the induction of ethylene production, MAPK phosphorylation, and defense gene expression are hallmarks of PTI and ETI (Jones and Dangl, 2006; Boller and Felix, 2009), BAK1 overexpression might trigger constitutive activation of one or both of these immune processes. Therefore, we transformed different mutants affected in known ETI regulator genes as well as others affected in PTI-related processes. The effect of mutations in the salicylic acid biosynthetic or signaling pathway on the growth of BAK1-overexpressing plants was also evaluated, as they partially rescued the phenotypes of bak1-4 bkk1 or bir1-1 mutants (He et al., 2007; Gao et al., 2009). However, all of these mutants remained as sensitive to BAK1 overexpression as the wild type, with the remarkable exception of sobir1. In seven independent selections of T1 plants from three different transformations of sobir1-13 plants, all BAK1-overexpressing seedlings grew like wild-type plants during the first 7 weeks of development (Fig. 8). BAK1 overexpression in wild-type plants severely affected the plant phenotype and also caused changes in the overall protein pattern (Fig. 1). Therefore, it was difficult to determine if BAK1 accumulated at the same levels in wild-type and sobir1-13 genotypes.
Table I. Candidate mutants transformed with 2× 35S-BAK1.
A minimum of nine individual lines was examined for each genotype. The only mutation that rescued the autoimmunity phenotype was sobir1-13 (as indicated by the asterisk).
| Function | Name | References |
|---|---|---|
| PRRs | efr fls2 | Zipfel et al. (2006) |
| efr fls2 cerk1 | Gimenez-Ibanez et al. (2009) | |
| pep receptor1 pep receptor2 | Krol et al. (2010) | |
| PRR regulators | bak1-4 | Chinchilla et al. (2007) |
| Botrytis induced kinase1 AVR PPHB SUSCEPTIBLE1-like | Zhang et al. (2010) | |
| sobir1-13* | Katiyar-Agarwal et al. (2007) | |
| Gao et al. (2009) | ||
| R proteins | resistant to Pseudomonas syringae5-2 | Warren et al. (1999) |
| suppressor of npr1-1 constitutive1-11 | Yang and Hua (2004) | |
| resistance to P. syringae pv maculicola1 resistance to P. syringae2 | Mackey et al. (2003) | |
| R regulators | enhancer of TIR1-1 auxin resistance3 (suppressor of G2 allele of skp1) | Gray et al. (2003) |
| senescence-associated gene101-1 | Feys et al. (2005) | |
| phytoalexin deficient4-1 | Glazebrook et al. (1996) | |
| enhanced disease susceptibility1-2 | Falk et al. (1999) | |
| nonrace-specific disease resistance1 | Century et al. (1995) | |
| Regulators of hormone pathways | bri1-301 | Xu et al. (2008) |
| ethylene response1-1 | Wilkinson et al. (1997) | |
| salicylic acid induction deficient2-1 | Nawrath and Métraux (1999) | |
| enhanced disease susceptibility5-2 | Volko et al. (1998) | |
| Ecotypes | Col-0 Wassilewskija-2 (natural snc1 mutant) |
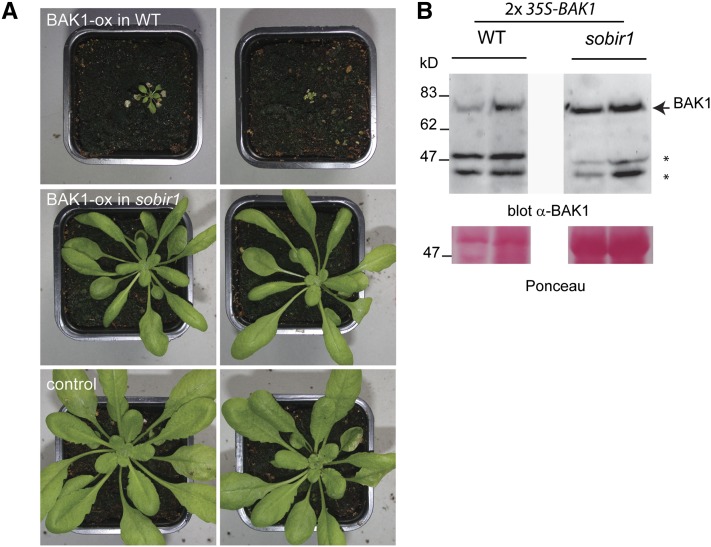
Figure 8.
A mutation in the SOBIR1 gene rescues the phenotype caused by BAK1 overexpression. A, Photographs from two T1 transgenic plants overexpressing BAK1 under the control of the 2× 35S promoter in wild-type (WT) Col-0 plants (top) or in the transfer DNA (T-DNA) insertional sobir1-13 mutant (bottom). Two lines of plants transformed with the empty vector are shown as controls. These results were reproduced in more than 20 independent lines. B, Western-blot analysis for BAK1 in crude extracts of the respective lines. Protein samples extracted from the same amount of leaf material were separated on the same gel.
Thus, we used transgenic seedlings prior to symptom development in order to assess BAK1 levels in wild-type and sobir1-13 T1 plants (Supplemental Fig. S10). We could conclude that these plants did not develop any of the phenotypes observed with wild-type overexpressing plants, although BAK1 accumulated to similar levels as in Col-0 BAK1-ox (Fig. 8; Supplemental Fig. S10). This rescue of the phenotype in sobir1-13 plants persisted in the T2 generation, where the accumulation of BAK1 was still clearly increased with respect to the wild-type levels, without causing severe symptoms (Supplemental Fig. S11). Similarly, the phenotype associated with ECD-TM overexpression was also rescued in sobir1-13 mutant plants (Supplemental Fig. S12), indicating that the receptor kinase SOBIR1 is important for the induction of the autoimmunity phenotype. Interestingly, recent work has shown that SOBIR1 acts as a critical regulator of many RLPs, including those involved in immunity (Liebrand et al., 2014).
DISCUSSION
In this work, we show that a well-balanced amount of BAK1, a coreceptor of many plant receptors, is crucial for Arabidopsis fitness, as its overexpression causes dwarfism, leaf necrosis, and premature death (Figs. 1 and 2). Overall, this phenotype is reminiscent of constitutive defense activation, a process previously referred to as autoimmunity in the case of the autoactivation of R proteins (Oldroyd and Staskawicz, 1998; Bendahmane et al., 2002; Zhang et al., 2003). As demonstrated in this report, the overexpression of BAK1 or its homologs SERK4 and SERK1 indeed triggers the rapid activation of common immune responses in the absence of microbes or MAMPs. These responses include MAPK phosphorylation, ethylene production, and the induction of defense gene expression and contribute to enhanced resistance against the virulent bacterial pathogen P. syringae pv tomato DC3000 (Figs. 3 and 4).
BAK1 Overexpression Drastically Impairs Arabidopsis Fitness
In a previous study, expression of the BAK1 rice ortholog was increased to enhance the BR pathway activity and, as a consequence, rice growth (Li et al., 2009). However, this overexpression leads, unexpectedly, to dwarfism and a loss of relevant agricultural traits such as seed weight and length. Besides these defects, transgenic plants also display increased resistance against the blast fungus Magnaporthe grisea. Thus, the rice stunted phenotype might derive from defense activation, which can negatively impact plant development in the absence of proper control mechanisms (Tian et al., 2003; Korves and Bergelson, 2004). This hypothesis is clearly supported by our new findings in Arabidopsis, showing that BAK1 overexpression (1) induces effects on plant growth different from those associated with activated or inhibited BR signaling (Choe et al., 2001; Wang et al., 2001; Kim et al., 2013), (2) triggers constitutive immunity, and (3) causes a dwarf phenotype that cannot be rescued by the bri1-301 mutation affecting BR perception (Table I).
Surprisingly, the observed phenotype has not been described in most studies using BAK1 overexpression in Arabidopsis (Li et al., 2002; Nam and Li, 2002; Kemmerling et al., 2007; Gou et al., 2012). The only exception was the study by Belkhadir et al. (2012), which shows that an increased dosage of BAK1 causes growth inhibition and moderate cell death and defense responses in wild-type plants. In our work, we further demonstrate that strong overexpression of BAK1 correlates with a general induction of immune responses in a dose-dependent manner, leading to extreme dwarfism and extensive necrosis often associated with seedling premature death. Moreover, as evidenced by our experiments using inducible BAK1 overexpression, immune responses are triggered very quickly, within the first hours of BAK1 overaccumulation, both in seedlings and adult plants and occur in the absence of MAMPs or microbes.
BAK1 Overdosage May Overwhelm Normal PRR Regulation
To prevent inappropriate signaling in the absence of microbes, plant immunity is under tight control involving negative and positive molecular mechanisms that regulate PRR activity. For example, BAK1 forms a stable complex with FLS2 only when flagellin is present (Chinchilla et al., 2007; Sun et al., 2013). Nevertheless, the sheer number of BAK1 molecules in the overexpressing plants might lead to defense signaling initiation by direct PRR activation, even in the absence of microbes or microbial patterns (model 1 in Supplemental Fig. S13). However, PRR activation requires BAK1 kinase activity (Schulze et al., 2010; Schwessinger et al., 2011). Therefore, our original finding that the BAK1 ectodomain (lacking the kinase domain) is sufficient to trigger constitutive immune responses (Figs. 5 and 7) is incompatible with the straightforward hypothesis of direct PRR activation. On the contrary, we find that, at lower expression levels, ECD-TM inhibits the activation of FLS2 and EFR by flg22 and elf18, respectively (Fig. 6). Since ECD-TM associates with FLS2 in an flg22-dependent manner, it could outcompete endogenous BAK1 and other SERKs, thus affecting FLS2 function (Fig. 6). According to our results, it is well possible that ECD-TM causes competitive inhibition of different BAK1-dependent pathways within the cell affecting not only MAMP-mediated PTI activation but also other processes, including BR signaling.
On the other hand, BAK1 has been reported to interact constitutively with several structurally related LRR-RLKs, termed BIRs. Interestingly, BIR2 was recently shown to inhibit PRRs, possibly by sequestering BAK1 in membrane complexes to prevent its inappropriate association with PRRs (Halter et al., 2014b). So far, by purifying BAK1 immunocomplexes from Arabidopsis seedlings, we have not been able to identify BIR2 but two close homologs, BIR1 and BIR3 (Supplemental Tables S1 and S2), which may share a similar negative function. Intriguingly, bir1-1 knockout mutant plants resemble BAK1-ox plants in that they exhibit strong dwarfism and extensive cell death, associated with defense induction (Gao et al., 2009). When searching for mutants suppressing the BAK1-ox phenotype, we identified a known bir1-1 suppressor called sobir1 (Fig. 8; Gao et al., 2009). This interesting finding supports our model of constitutive immunity activation, since the LRR-RLK SOBIR1 emerges as a positive regulator and partner for many LRR-RLP receptors, including some PRRs regulated by BAK1 (Liebrand et al., 2014). The exact mechanism of BAK1-ox phenotype suppression by sobir1-13 remains to be discovered, but BAK1 accumulation does not seem to be affected in this mutant. Similarly, the role of SOBIR1 in the BIR1 pathway is poorly understood. The bir1-1 phenotype can be somewhat reverted by mutants in ETI regulation or in the salicylic acid pathway (Gao et al., 2009), but these mutants did not affect the overexpression phenotype of the BAK1-ox plants (Table I). Thus, despite sharing some similarities, these two phenotypes may overlap only partially. It is possible that SOBIR1 acts as a direct activator of BAK1, inducing immune responses in a PRR-independent manner. On the other hand, BAK1 has been described extensively as an interactor of different PRRs, but it has never been observed to induce immunity on its own (Kim et al., 2013; Böhm et al., 2014). Overall, based on our results, we suggest a second scenario, in which ECD-TM or extra BAK1 may induce PRR derepression by withdrawing negative regulators, such as BIRs, thus increasing the pool of active free BAK1/SERKs able to activate PRRs (notably SOBIR1-dependent RLPs) in the absence of MAMP (model 2 in Supplemental Fig. S13).
Increasing BAK1 Dosage May Activate Guarding Systems by R Proteins
Our finding that BAK1 overexpression causes extensive cell death in Arabidopsis corroborates its function in cell death control (Kim et al., 2013). Yet, the molecular mechanisms by which BAK1 exerts this regulation are unclear. Recent observations reinforce the idea that the integrity or activity of multimeric SERK-BIR complexes is guarded by R proteins (Gao et al., 2009; Wang et al., 2011; Halter et al., 2014a). In line with this hypothesis, several microbial effectors have been identified that target BAK1 to defeat plant immunity (Shan et al., 2008; Cheng et al., 2011; Zhou et al., 2014).
The constitutive immunity observed in Arabidopsis after BAK1 overexpression shares some common characteristics with the ETI responses (Jones and Dangl, 2006; Tsuda et al., 2013), like the prolonged MAPK activation and the presence of cell death (Figs. 1–3). Therefore, the increased levels of BAK1 or ECD-TM may be detected by specific guarding systems, resulting in immunity induction (Jones and Dangl, 2006; model 3 in Supplemental Fig. S13). However, mutants affected in known ETI components still presented the BAK1-ox phenotype when overexpressing BAK1 (Table I), indicating that R protein activity may be only one of several factors responsible for the observed autoimmunity. Alternatively, single mutations in the selected genes may be insufficient, because BAK1 overexpression induces several redundant ETI pathways or some nontested pathway(s).
In our hands, the only mutant tested able to rescue the BAK1-ox phenotype is affected in SOBIR1. As mentioned previously, SOBIR1 interacts with and regulates some RLPs known as PRRs (i.e. RECEPTOR OF enigmatic MAMP of Xanthomonas; Jehle et al., 2013) but also RLPs like the tomato (Solanum lycopersicum) Cf proteins or the Ve1 receptor that are considered classical R proteins (Thomma et al., 2011; Liebrand et al., 2013). Thus, the involvement of SOBIR1 in the autoimmunity caused by BAK1 accumulation would also be consistent with the induction of ETI pathways in the transgenic plants (Supplemental Fig. S13).
The BAK1 Ectodomain Influences Regulatory Processes in Plant Immunity
In animal cells, modification of receptor ectodomains (e.g. by proteolytic cleavage) is sometimes necessary for full receptor activation (Bauer, 2013; Carpenter and Liao, 2013). For example, cleavage of the TOLL-LIKE RECEPTOR9 ectodomain allows a key conformational change required for activation (Li et al., 2012). The epidermal growth factor receptor is modified at the transmembrane domain to release an active intracellular domain: the latter is translocated to the nucleus, where it may regulate gene expression (Carpenter and Liao, 2013). In plants, cleavage at the ectodomain has been proposed for several RLKs, like the rice PRR Xa21, the Arabidopsis regulator CHITIN ELICITOR RECEPTOR KINASE1, and the symbiotic receptor kinase from Lotus japonicus (Park and Ronald, 2012; Antolín-Llovera et al., 2014; Petutschnig et al., 2014). However, the biological significance of these observations needs to be further shown experimentally.
One of the most interesting open questions raised by our study is certainly the biological relevance of BAK1-derived proteins. We show the presence of several BAK1 polypeptides in BAK1-ox but also in wild-type plant extracts (Supplemental Tables S1 and S2) that do not derive from alternative splicing variants but rather originate from in vivo proteolytic cleavage. Moreover, we report that the overexpression of constructs containing the cytoplasmic domain of BAK1, either soluble or anchored to the membrane, does not induce autoimmunity or affect MAMP responses. These data refute a model where the cytoplasmic domain of BAK1 transfers to the nucleus to modulate gene expression. However, the presence of N-terminally truncated BAK1 forms, as detected in our analysis, indicates the simultaneous production of BAK1 derivatives carrying the ectodomain. Precisely, we demonstrate that the BAK1 ectodomain, associated with the membrane, can modulate the immune response induced by PRRs, even if the exact mechanism remains to be investigated. In the future, it will be interesting to find out whether the BAK1 ectodomain emerges from a proteolytic cleavage in planta, whether its abundance varies in response to developmental or environmental cues, and whether this process has any biological relevance in the control of plant immunity.
MATERIALS AND METHODS
Plant Material
The Arabidopsis (Arabidopsis thaliana) mutants and ecotypes used in this study are listed in Table I. The SOBIR1 insertion line SALK_009453 (also known as small RNA-generating RLK-2 [Katiyar-Agarwal et al., 2007] and sobir1-13 [Gao et al., 2009]) was obtained from the Nottingham Arabidopsis Stock Centre. SOBIR1-specific (SOBIR1-For, 5′-GGTGATTGGGAAGCTTCC-3′; SOBIR1-Rev, 5′-CTGGGACAACATGGTCCTG-3′) and T-DNA-specific primers (Microsynth) were used to select for plant homozygosity. To study the effect of BAK1 and BAK1 derivatives on MAMP responses and for coimmunoprecipitation experiments, Nicotiana benthamiana plants were used after 4 weeks of growth in a climate chamber (14-h day at 25°C/10-h night at 20°C, 75% humidity).
Growth Assays
Arabidopsis plants were grown at 18°C/21°C with a 10-h photoperiod either in soil as one plant per pot or in sterile pots containing Murashige and Skoog (MS) solid medium (Duchefa) supplemented with 1% (w/v) Suc and 0.8% (w/v) agar. To monitor the effect of BAK1/BAK1 derivative overexpression under sterile conditions at the seedling stage, seeds were germinated under continuous light for 5 to 6 d on solid MS medium and transferred to liquid MS medium supplemented with 5 μL of β-estradiol (final concentration of 1 or 10 μm) or the same volume of 100% (v/v) ethanol (negative control). Seedling growth in the presence of MAMPs was determined according to Gómez-Gómez and Boller (2000) and used as a positive control of growth inhibition. The treatment effect was analyzed after 6 to 10 d by imaging and determining fresh weight and root length.
Peptides and Solutions
Peptides of flg22 (QRLSTGSRINSAKDDAAGLQIA) and elf18 (ac-SKEKFERTKPHVNVG-TIG) obtained from EZBiolabs were dissolved to a 1 mm final concentration in a water solution containing 1% (w/v) bovine serum albumin and 0.1 m NaCl. Chitin was purchased from Sigma-Aldrich (C9752) and dissolved in water. β-Estradiol (Sigma-Aldrich; E2758) was prepared as a stock solution at a final concentration of 10 mm in ethanol (100%).
Plasmids and Primers
For this study, plasmids were engineered according to standard methods (Gateway; Invitrogen) using pDONR207 as donor plasmid and pMDC7, pMDC83, or pMDC32 as acceptor plasmid; corresponding PCR primers are listed in Supplemental Table S3. The pMDC7 plasmid containing the chimeric transactivator XVE was kindly provided by Dr. Nam-Hai Chua. XVE is a fusion of the DNA-binding domain of the bacterial repressor LexA (X), the acidic transactivating domain of VP16 (V), and the regulatory region of the human estrogen receptor (E; Zuo et al., 2000). The transactivating activity of the chimeric XVE factor is strictly regulated by estrogens, allowing rapid induction of gene expression in response to estradiol addition. pK7WGF2 containing the GFP coding sequence and pFRK1-Luc containing the Luc gene under the control of the FRK1 promoter were used for protoplast experiments.
Trypan Blue Assay
To detect cell death development, Trypan blue staining was performed on sterile seedlings as described previously (Hann and Rathjen, 2007). Briefly, 6-d-old Arabidopsis seedlings grown on MS agar plates were transferred to liquid MS medium for treatment with estradiol (1 μm), elf18 (1 μm), or both solvents as a control. After 3 d of treatment, 12 seedlings per condition were incubated with 200 μL of 0.067% (w/v) Trypan blue solution at 95°C for 1 min and cooled at room temperature for 2 h. Samples were washed in clearing solution (2.5 g mL−1 chloral hydrate) for 3 d. The stained seedlings were transferred to 60% (v/v) glycerol and examined by light microscopy (Zeiss Axioplan microscope and Olympus DP70 camera).
Immunological Techniques
Plant material was ground into liquid nitrogen and immediately extracted into SDS buffer, except for seedlings used for the kinetic experiment. In that case, ground powder (50 mg) was first resuspended in 100 μL of cold extraction buffer (50 mm Tris-HCl, pH 8, and 50 mm NaCl) and 1 μL of protease inhibitor cocktail (Sigma-Aldrich; P9599) before denaturation with SDS buffer. Proteins were separated using SDS-PAGE (7%–12% [w/v] acrylamide) and analyzed by western blot using anti-BAK1 antibodies (Schulze et al., 2010), anti-FLS2 antibodies (Chinchilla et al., 2006), anti-myc antibodies (06-549; Upstate), or anti-GFP antibodies (11814460001; Roche).
Immunoprecipitation experiments with membrane proteins from N. benthamiana leaves or Arabidopsis seedlings were performed as described (Schulze et al., 2010). Briefly, proteins were solubilized from plant powder using detergent buffer (25 mm Tris-HCl, pH 8, 150 mm NaCl, and 1% [w/v] Nonidet P-40 and protease inhibitor mixture). Anti-GFP tag agarose beads (MBL; D153-8) were used to immunoprecipitate ECD-TM from N. benthamiana extracts.
To identify potential partners of BAK1, wild-type or XVE-BAK1 seedlings were germinated for 5 d on MS plates, further grown in liquid MS medium for 5 d, and treated for 6 h with 1 μm β-estradiol. Total proteins were solubilized from 7 g of ground material using detergent buffer. After removal of insoluble material by centrifugation (10,000g, three times for 10 min), supernatants were incubated with anti-BAK1 antibodies preadsorbed to protein A magnetic beads (Milteniy Biotec) at 4°C on a rotary shaker for 1 h. The beads were washed according to the manufacturer’s instructions, and proteins were eluted and denatured in hot SDS buffer. Immunoprecipitated proteins were separated on an 8% (w/v) acrylamide SDS gel and stained with colloidal Coomassie Blue.
Liquid Chromatography-MS/MS Analysis
The protein bands were excised and reduced prior to digestion with 10 mm dithiothreitol for 2 h at 37°C and alkylated with iodoacetamide at 50 mm final concentration for 15 min at room temperature in the dark. Each gel piece was digested with 125 ng of trypsin (sequencing grade; Promega) for 18 h at 37°C. The peptides in the supernatant were collected, and the gel piece was extracted with 0.1% (v/v) acetic acid/50% (v/v) acetonitrile, and the extract was pooled with the tryptic peptides. The digest was dried in a SpeedVac and redissolved in 0.1% (v/v) acetic acid. Ten microliters was used for mass spectrometric analysis. The trypsin-digested proteins were analyzed by capillary liquid chromatography-MS/MS using a homemade separating column (0.075 mm × 15 cm) packed with Reprosil C18 reverse-phase material (2.4 μm particle size; Dr. Maisch). The column was connected on line to an Orbitrap FT hybrid instrument (Thermo Scientific). The solvents used for peptide separation were 0.1% (v/v) acetic acid in water/0.005% (v/v) trifluoroacetic acid (solvent A) and 0.1% (v/v) acetic acid/0.005% (v/v) trifluoroacetic acid and 80% (v/v) acetonitrile in water (solvent B). Two microliters of peptide digest was injected with a Proxeon nLC capillary pump (Thermo Scientific) set to 0.3 μL min−1. A linear gradient from 0% to 40% solvent B in solvent A in 95 min was delivered with the nano pump at a flow rate of 300 nL min−1. After 95 min, the percentage of solvent B was increased to 75% in 10 min. The eluting peptides were ionized at 2.5 kV. The mass spectrometer was operated in data-dependent mode. The precursor scan was done in the Orbitrap set to 60,000 resolution, while the fragment ions were mass analyzed in the LTQ instrument. A top 10 method was run so that the 10 most intense precursors were selected for fragmentation. The MS/MS spectra were then searched against an Arabidopsis data bank extracted from the National Center for Biotechnology Information data bank, and the liquid chromatography-MS/MS data were searched with Proteome Discoverer 1.4 (Thermo Scientific) set to Mascot and Sequest HT search engines with 10-ppm precursor ion tolerance, while the fragment ions were set to 0.5-D tolerance. The following modifications were used during the search: carbamidomethyl-Cys and oxidized Met as variable modifications. The peptide search matches were set to high confidence.
Ethylene Measurement
Ethylene measurements were performed as described previously (Felix et al., 1999). Briefly, leaf material from 6- to 7-week-old Arabidopsis plants or 4-week-old N. benthamiana plants was floated overnight on water or treated for 16 to 18 h with estradiol (1 or 10 μm) or ethanol before incubation with MAMPs. For the kinetic experiment, 6-d-old seedlings (n = 5 per tube) were pretreated with solvent (control) or 1 μm estradiol with different incubation times (3–27 h). During the last 3 h of incubation, an additional treatment with buffer (control) or flg22 (1 μm) was performed. For all types of samples, ethylene was measured by gas chromatography (GC-14A; Shimadzu) after incubation for 3 to 4 h with MAMPs or buffer used as a negative control.
MAPK Activation
MAPK activation was analyzed in seedling protein samples similar to those prepared for the kinetic experiment described above. MAPK activation was revealed after western blotting of the extracted proteins in the presence of anti-p42/44-phospho-ERK antibody (4370S; Cell Signaling Technology) to detect phosphorylated (activated) MPK3 and MPK6.
Quantitative Reverse Transcription-PCR
Six-day-old Arabidopsis seedlings (XVE-BAK1 and the wild type) grown on MS agar plates were transferred to water overnight and subsequently treated with 1 μm β-estradiol or an equivalent volume of ethanol as a control for 6 h before freezing in liquid nitrogen. As a positive control of defense gene induction, seedlings treated for 1 h with 1 μm flg22 were used. Total RNA was extracted using the NucleoSpin RNA Plant Extraction Kit (Macherey-Nagel) from 80 mg of plant tissue. After DNase treatment, first-strand cDNA was synthesized from 1 μg of RNA using Avian myeoloblastosis virus reverse transcriptase (Promega) and an oligo(dT) primer (Microsynth) according to each manufacturer’s instructions. For quantitative PCR, 5 μL of a 1:100 dilution of cDNA was combined with SYBR master mix. PCR was performed in triplicate with the 7500 Real Time PCR system (Applied Biosystems) using the primers listed in Supplemental Table S4. Data were collected and analyzed with the respective ABI PRISM (R) analyzing program. Data obtained for the selected genes were normalized to the housekeeping gene EUKARYOTIC INITIATION FACTOR4α (Boudsocq et al., 2010) and referred to the values for wild-type Col-0 in control conditions. Data are derived from three biological replicates.
Pathogen Growth Assay
To assess disease resistance in BAK1-overexpressing lines, the virulent bacterial pathogen Pseudomonas syringae pv tomato DC3000 was used to infect Arabidopsis plants (Zipfel et al., 2004). Since induction of BAK1 expression upon spraying or infiltration of estradiol was not reproducible in adult leaves, 6-d-old Arabidopsis seedlings grown on MS agar plates were used. Seedlings were transferred to aqueous solutions containing 1 µm β-estradiol, 1 µm flg22, or a control solution containing the corresponding solvents (including ethanol). After 24 h of treatment, the seedlings were submerged in a suspension of P. syringae pv tomato DC3000 (optical density at 600 nm = 0.01) and incubated for 1.5 h at 22°C. Seedlings were then washed three times with water to remove the excess bacteria and transferred again to aqueous solutions containing the treatments until measurement. For bacterial growth assessment, the seedlings were surface sterilized with 70% (v/v) ethanol and ground in water. The number of colony-forming units in the suspension was determined 4 h after infection (0 dpi) and 48 h later (2 dpi) by performing serial dilutions and plating them on yeast broth extract with selective antibiotics. A minimum of three replicates was prepared for each condition and time. The experiment was repeated three times with similar results.
Functional Assay in Arabidopsis Protoplasts
Transient expression in leaf mesophyll protoplasts of bak1-4 mutant plants was performed using a polyethylene glycol transformation method as described previously (Yoo et al., 2007). For individual transformations, aliquots with 20,000 protoplasts were cotransformed with 5 μg of plasmid DNA encoding pFRK1-Luc and 0.5 to 5 μg of plasmid DNA encoding BAK1, BAK1 derivatives, or GFP used as a negative control. The protoplasts were resuspended in 100 μL of W5 solution with 200 μm d-luciferin (L1349; Duchefa) and transferred to 96-well plates. After incubation for 17 h, the cells were treated with 100 nm flg22 or elf18 (which corresponds to time 0 in Supplemental Figs. S6 and S7). The luminescence of protoplast samples was quantified in vivo using a luminometer (Mithras LB940 [Berthold Technologies] for Supplemental Fig. S7 and MicroLumat LB96P plate reader [Berthold Technologies] for Supplemental Fig. S8). Fold induction of luciferase at time 0 was calculated as the ratio of luciferase activities obtained with the diverse BAK1 constructs normalized to the values obtained with GFP at time 0. Fold induction of luciferase after MAMP treatment was calculated as the ratio of activities measured at 3 and 0 h (Supplemental Fig. S6) or 4 and 0 h (Fig. 6) of the experimental treatment.
Transient Expression in N. benthamiana
Plasmids containing the different BAK1 constructs under the control of the 2× 35S promoter, FLS2-myc, or FLS2-3xmyc-GFP downstream of the FLS2 promoter (Robatzek et al., 2006) were transformed into Agrobacterium tumefaciens strain GV3101. Leaves from 4-week-old N. benthamiana plants were pressure infiltrated with a suspension of the bacteria (optical density at 600 nm = 0.2) in infiltration buffer (10 mm MgCl2 and 10 mm MES, pH 5.6). To test the activity of BAK1 constructs on ethylene production, various optical densities of the specific constructs were used (0.1, 0.01, and 0.001), but the final optical density was adjusted with the empty vector strain to the constant value of 0.2. Accumulation of the proteins of interest and defense responses were examined 2 d after infection. For coimmunoprecipitation experiments, N. benthamiana leaves transiently expressing FLS2-Myc and ECD-TM were treated with 1 μm flg22 or buffer (control) for 15 min.
Confocal Microscopy
Confocal microscopy was performed on a Zeiss point scanning confocal LSM700 Upright device with a 488-nm excitation mirror, and fluorescence emissions were captured between 505 and 530 nm. Autofluorescence was observed at 540 to 700 nm. For plasmolysis, leaves were mounted in 0.5 m sorbitol before microscopy. Images were processed using the LSM image browser and Photoshop CS5 software packages.
Statistical Analysis
Results from growth assays, quantitative PCR, ethylene production, bacterial growth, and protoplast assays were statistically analyzed by one-way ANOVA considering P ≤ 0.05 as significantly different.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phenotype of BAK1-ox Arabidopsis plants grown under axenic conditions.
Supplemental Figure S2. Seedling growth of XVE-BAK1 lines.
Supplemental Figure S3. BAK1 overexpression is required for constitutive defense activation in XVE-BAK1 seedlings.
Supplemental Figure S4. Overexpression of SERK4 or SERK1 induces defense responses in Arabidopsis.
Supplemental Figure S5. BAK1 truncated forms can be immunoprecipitated from Arabidopsis seedling extracts and detected by mass spectrometry.
Supplemental Figure S6. Effect of BAK1-derived constructs on the FRK1 promoter activity in Arabidopsis cells.
Supplemental Figure S7. Effect of BAK1-derived constructs on the FRK1 promoter activity in Arabidopsis cells.
Supplemental Figure S8. Defense phenotype in N. benthamiana leaves expressing BAK1 variants.
Supplemental Figure S9. ECD-TM expression causes leaf necrosis at late stages of development.
Supplemental Figure S10. Western-blot analysis of whole-seedling extracts showing BAK1 accumulation.
Supplemental Figure S11. Analysis of the T2 lines from sobir1-13 mutant plants overexpressing BAK1 under the control of the 2× 35S promoter.
Supplemental Figure S12. A mutation in the SOBIR1 gene rescues the phenotype caused by ECD-TM overexpression.
Supplemental Figure S13. Model of the activation of defense responses triggered by BAK1 overexpression.
Supplemental Table S1. Protein and peptide identifications obtained by searching the mass spectrometry data with Mascot and Sequest HT in immunoprecipitates from estradiol-treated wild-type seedlings.
Supplemental Table S2. Protein and peptide identifications obtained by searching the mass spectrometry data with Mascot and Sequest HT in immunoprecipitates from estradiol-treated XVE-BAK1 seedlings.
Supplemental Table S3. Oligonucleotides used for cloning.
Supplemental Table S4. Primers used for quantitative reverse transcription-PCR analysis.
Supplementary Material
Acknowledgments
We thank Dr. Paul Jenoe and Suzanne Moss for MS/MS analysis; Dr. Alexia Ferrand for technical assistance with confocal microscopy; Dr. Dagmar Hann and Dr. Thomas Boller for critically reading the article; Dr. Nam Hai Chua for providing the pMDC7 vector; Dr. Jian-Min Zhou, Jean-Pierre Metraux, Dr. John Rathjen, Sacco de Vries, Dr. David Mackey, Jane Parker, Roger Innes, Dr. Franck Vazquez, and Dr. Sebastian Bartels for mutant seeds; the Nottingham Arabidopsis Stock Centre for the T-DNA insertion line; and Dr. Thomas Boller for professional support.
Glossary
- PRR
pattern-recognition receptor
- MAMP
microbe-associated molecular pattern
- PTI
pattern-triggered immunity
- ETI
effector-triggered immunity
- RLP
receptor-like protein
- RLK
receptor-like kinase
- LRR
leucine-rich repeat
- BR
brassinosteroid
- MAPK
mitogen-activated protein kinase
- dpi
days post inoculation
- MS/MS
tandem mass spectrometry
- cDNA
complementary DNA
- Col-0
Columbia-0
- T-DNA
transfer DNA
- MS
Murashige and Skoog
Footnotes
This work was supported by the Swiss National Science Foundation (grant no. 31003A–120655 to D.C.).
References
- Albert M, Jehle AK, Fürst U, Chinchilla D, Boller T, Felix G (2013) A two-hybrid-receptor assay demonstrates heteromer formation as switch-on for plant immune receptors. Plant Physiol 163: 1504–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolín-Llovera M, Ried MK, Parniske M (2014) Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr Biol 24: 422–427 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gómez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bauer S. (2013) Toll-like receptor 9 processing: the key event in Toll-like receptor 9 activation? Immunol Lett 149: 85–87 [DOI] [PubMed] [Google Scholar]
- Belkhadir Y, Jaillais Y, Epple P, Balsemão-Pires E, Dangl JL, Chory J (2012) Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci USA 109: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahmane A, Farnham G, Moffett P, Baulcombe DC (2002) Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J 32: 195–204 [DOI] [PubMed] [Google Scholar]
- Böhm H, Albert I, Fan L, Reinhard A, Nürnberger T (2014) Immune receptor complexes at the plant cell surface. Curr Opin Plant Biol 20: 47–54 [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücherl CA, van Esse GW, Kruis A, Luchtenberg J, Westphal AH, Aker J, van Hoek A, Albrecht C, Borst JW, de Vries SC (2013) Visualization of BRI1 and BAK1(SERK3) membrane receptor heterooligomers during brassinosteroid signaling. Plant Physiol 162: 1911–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Liang Y, Tanaka K, Nguyen CT, Jedrzejczak RP, Joachimiak A, Stacey G (2014) The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G, Liao HJ (2013) Receptor tyrosine kinases in the nucleus. Cold Spring Harb Perspect Biol 5: a008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century KS, Holub EB, Staskawicz BJ (1995) NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc Natl Acad Sci USA 92: 6597–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Gao X, Feng B, Sheen J, Shan L, He P (2013) Plant immune response to pathogens differs with changing temperatures. Nat Commun 4: 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Munkvold KR, Gao H, Mathieu J, Schwizer S, Wang S, Yan YB, Wang J, Martin GB, Chai J (2011) Structural analysis of Pseudomonas syringae AvrPtoB bound to host BAK1 reveals two similar kinase-interacting domains in a type III effector. Cell Host Microbe 10: 616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18: 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, Feldmann KA (2001) Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J 26: 573–582 [DOI] [PubMed] [Google Scholar]
- Chung Y, Choe V, Fujioka S, Takatsuto S, Han M, Jeon JS, Park YI, Lee KO, Choe S (2012) Constitutive activation of brassinosteroid signaling in the Arabidopsis elongated-D/bak1 mutant. Plant Mol Biol 80: 489–501 [DOI] [PubMed] [Google Scholar]
- Falk A, Feys BJ, Frost LN, Jones JD, Daniels MJ, Parker JE (1999) EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA 96: 3292–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T (1994) The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci USA 91: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Wiermer M, Bhat RA, Moisan LJ, Medina-Escobar N, Neu C, Cabral A, Parker JE (2005) Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17: 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54: 137–164 [DOI] [PubMed] [Google Scholar]
- Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, et al. (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44 [DOI] [PubMed] [Google Scholar]
- Gao X, Li F, Li M, Kianinejad AS, Dever JK, Wheeler TA, Li Z, He P, Shan L (2013) Cotton GhBAK1 mediates Verticillium wilt resistance and cell death. J Integr Plant Biol 55: 586–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP (2009) AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol 19: 423–429 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Bauer Z, Boller T (2001) Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13: 1155–1163 [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gou X, Yin H, He K, Du J, Yi J, Xu S, Lin H, Clouse SD, Li J (2012) Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet 8: e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Muskett PR, Chuang HW, Parker JE (2003) Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 15: 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter T, Imkampe J, Blaum BS, Stehle T, Kemmerling B (2014a) BIR2 affects complex formation of BAK1 with ligand binding receptors in plant defense. Plant Signal Behav 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter T, Imkampe J, Mazzotta S, Wierzba M, Postel S, Bücherl C, Kiefer C, Stahl M, Chinchilla D, Wang X, et al. (2014b) The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr Biol 24: 134–143 [DOI] [PubMed] [Google Scholar]
- Hann DR, Rathjen JP (2007) Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. Plant J 49: 607–618 [DOI] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17: 1109–1115 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Belkhadir Y, Balsemão-Pires E, Dangl JL, Chory J (2011) Extracellular leucine-rich repeats as a platform for receptor/coreceptor complex formation. Proc Natl Acad Sci USA 108: 8503–8507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle AK, Fürst U, Lipschis M, Albert M, Felix G (2013) Perception of the novel MAMP eMax from different Xanthomonas species requires the Arabidopsis receptor-like protein ReMAX and the receptor kinase SOBIR. Plant Signal Behav 8: e27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YJ, Shang Y, Kim BH, Kim SY, Song JH, Lee JS, Lee MM, Li J, Nam KH (2010) BAK7 displays unequal genetic redundancy with BAK1 in brassinosteroid signaling and early senescence in Arabidopsis. Mol Cells 29: 259–266 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Gao S, Vivian-Smith A, Jin H (2007) A novel class of bacteria-induced small RNAs in Arabidopsis. Genes Dev 21: 3123–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Müssig C, et al. (2007) The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Kim BH, Kim SY, Nam KH (2013) Assessing the diverse functions of BAK1 and its homologs in Arabidopsis, beyond BR signaling and PTI responses. Mol Cells 35: 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korves T, Bergelson J (2004) A novel cost of R gene resistance in the presence of disease. Am Nat 163: 489–504 [DOI] [PubMed] [Google Scholar]
- Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, Postel S, Arents M, Jeworutzki E, Al-Rasheid KA, et al. (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 285: 13471–13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang L, Wang M, Xu YY, Luo W, Liu YJ, Xu ZH, Li J, Chong K (2009) Engineering OsBAK1 gene as a molecular tool to improve rice architecture for high yield. Plant Biotechnol J 7: 791–806 [DOI] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Li Y, Berke IC, Modis Y (2012) DNA binding to proteolytically activated TLR9 is sequence-independent and enhanced by DNA curvature. EMBO J 31: 919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand TW, van den Berg GC, Zhang Z, Smit P, Cordewener JH, America AH, Sklenar J, Jones AM, Tameling WI, Robatzek S, et al. (2013) Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc Natl Acad Sci USA 110: 10010–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand TW, van den Burg HA, Joosten MH (2014) Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci 19: 123–132 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balagué C, Roby D (2003) Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci 8: 263–271 [DOI] [PubMed] [Google Scholar]
- Macho AP, Zipfel C (2014) Plant PRRs and the activation of innate immune signaling. Mol Cell 54: 263–272 [DOI] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112: 379–389 [DOI] [PubMed] [Google Scholar]
- Meindl T, Boller T, Felix G (2000) The bacterial elicitor flagellin activates its receptor in tomato cells according to the address-message concept. Plant Cell 12: 1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Bittel P, Chinchilla D, Jehle AK, Albert M, Boller T, Felix G (2012) Chimeric FLS2 receptors reveal the basis for differential flagellin perception in Arabidopsis and tomato. Plant Cell 24: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GE, Staskawicz BJ (1998) Genetically engineered broad-spectrum disease resistance in tomato. Proc Natl Acad Sci USA 95: 10300–10305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CJ, Ronald PC (2012) Cleavage and nuclear localization of the rice XA21 immune receptor. Nat Commun 3: 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Ryu HY, Kim BH, Kim SY, Yoon IS, Nam KH (2011) A subset of OsSERK genes, including OsBAK1, affects normal growth and leaf development of rice. Mol Cells 32: 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petutschnig EK, Stolze M, Lipka U, Kopischke M, Horlacher J, Valerius O, Rozhon W, Gust AA, Kemmerling B, Poppenberger B, et al. (2014) A novel Arabidopsis CHITIN ELICITOR RECEPTOR KINASE 1 (CERK1) mutant with enhanced pathogen-induced cell death and altered receptor processing. New Phytol 204: 955–967 [DOI] [PubMed] [Google Scholar]
- Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C (2011) Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Macho AP, Sanmartín M, Ntoukakis V, Sánchez-Serrano JJ, Zipfel C (2014) Negative control of BAK1 by protein phosphatase 2A during plant innate immunity. EMBO J 33: 2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nürnberger T, Martin GB, Sheen J (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, Zhang S, Bent AF, Krysan PJ (2007) MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol 143: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J (2013) Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628 [DOI] [PubMed] [Google Scholar]
- Thomma BP, Nürnberger T, Joosten MH (2011) Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23: 4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Traw MB, Chen JQ, Kreitman M, Bergelson J (2003) Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423: 74–77 [DOI] [PubMed] [Google Scholar]
- Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F (2013) Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet 9: e1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volko SM, Boller T, Ausubel FM (1998) Isolation of new Arabidopsis mutants with enhanced disease susceptibility to Pseudomonas syringae by direct screening. Genetics 149: 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Meng P, Zhang X, Ren D, Yang S (2011) BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J 67: 1081–1093 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380–383 [DOI] [PubMed] [Google Scholar]
- Warren RF, Merritt PM, Holub E, Innes RW (1999) Identification of three putative signal transduction genes involved in R gene-specified disease resistance in Arabidopsis. Genetics 152: 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ (1997) A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15: 444–447 [DOI] [PubMed] [Google Scholar]
- Xu W, Huang J, Li B, Li J, Wang Y (2008) Is kinase activity essential for biological functions of BRI1? Cell Res 18: 472–478 [DOI] [PubMed] [Google Scholar]
- Yang S, Hua J (2004) A haplotype-specific Resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 16: 1060–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. (2010) Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goritschnig S, Dong X, Li X (2003) A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15: 2636–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Thomma BP (2013) Structure-function aspects of extracellular leucine-rich repeat-containing cell surface receptors in plants. J Integr Plant Biol 55: 1212–1223 [DOI] [PubMed] [Google Scholar]
- Zhou J, Wu S, Chen X, Liu C, Sheen J, Shan L, He P (2014) The Pseudomonas syringae effector HopF2 suppresses Arabidopsis immunity by targeting BAK1. Plant J 77: 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua NH (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.