The range of species containing chlorinated auxin is extended to the genera Trifolium, Melilotus, and Medicago and is important in seed development.
Abstract
Auxin is a pivotal plant hormone, usually occurring in the form of indole-3-acetic acid (IAA). However, in maturing pea (Pisum sativum) seeds, the level of the chlorinated auxin, 4-chloroindole-3-acetic acid (4-Cl-IAA), greatly exceeds that of IAA. A key issue is how plants produce halogenated compounds such as 4-Cl-IAA. To better understand this topic, we investigated the distribution of the chlorinated auxin. We show for the first time, to our knowledge, that 4-Cl-IAA is found in the seeds of Medicago truncatula, Melilotus indicus, and three species of Trifolium. Furthermore, we found no evidence that Pinus spp. synthesize 4-Cl-IAA in seeds, contrary to a previous report. The evidence indicates a single evolutionary origin of 4-Cl-IAA synthesis in the Fabaceae, which may provide an ideal model system to further investigate the action and activity of halogenating enzymes in plants.
The chlorinated form of auxin, 4-chloroindole-3-acetic acid (4-Cl-IAA), is a highly active hormone that is thought to play a key role in early pericarp growth (Reinecke et al., 1995, 1999; Ozga et al., 2009). Exogenous 4-Cl-IAA, for example, has been shown to promote the pericarp elongation of deseeded pea (Pisum sativum) pods (Reinecke et al., 1999). Johnstone et al. (2005) reported that 4-Cl-IAA and bioactive GA (GA3 or GA1) act synergistically on pericarp growth when applied simultaneously, and a growth regulatory role has been proposed for 4-Cl-IAA through induction of GA biosynthesis and inhibition of ethylene action. In other species, e.g. tomato (Solanum lycopersicum), the nonchlorinated form of auxin, indole-3-acetic acid (IAA), also stimulates fruit growth via GAs (Serrani et al., 2008; Tang et al., 2015). The chlorinated auxin is mainly found in reproductive structures (Katayama et al., 1988), in which its levels often exceed those of the more widespread IAA (Tivendale et al., 2012). The chlorinated form is thought to be restricted to members of the leguminous tribe Fabeae (Reinecke 1999), which includes the genera Vicia, Pisum, Lathyrus, Lens, and Vavilovia (Schaefer et al., 2012). However, there is a curious exception: 4-Cl-IAA has been reported also from Scots pine (Pinus sylvestris; Ernstsen and Sandberg, 1986).
We previously published evidence that most 4-Cl-IAA in maturing pea seeds is synthesized from 4-Cl-tryptophan (4-Cl-Trp) via 4-Cl-indole-3-pyruvic acid (Tivendale et al., 2012, 2014). 4-Cl-Trp has been identified in extracts from pea and broad bean (Vicia faba) seeds (Kettner et al., 1992; Manabe et al., 1999), but whether the precursors of Trp can be chlorinated is unknown.
Virtually nothing is known about the enzymes that catalyze halogenation reactions in plants. In bacteria, fungi, and marine algae, there are six types of enzymes responsible for the addition of halogen atoms to organic molecules. These include heme haloperoxidases, vanadium-dependent haloperoxidases, mononuclear nonheme iron halogenases, flavin-dependent halogenases, S-adenosyl-l-Met-dependent chlorinases and fluorinases, and methyl halide transferases (Butler and Sandy, 2009; Wagner et al., 2009). However, in the genomes of angiosperms, the only type of halogenating enzyme that has been annotated are haloperoxidases, but very little is known about these enzymes. To further understand the activity and action of halogenating enzymes in plants, a comparative system is required.
In this study, we investigated the distribution of 4-Cl-IAA and 4-Cl-Trp in the Fabaceae by monitoring these compounds in the seeds of representative species spanning the phylogeny of this family. Most of these species have not been previously tested for the presence of the chlorinated compounds. In addition, we reexamined the reported occurrence of 4-Cl-IAA outside the Fabaceae, namely in Scots pine; several other Pinus species were investigated here as well. We also examined the endogenous levels of 4-Cl-IAA in both vegetative tissues and seeds of broad bean to address the question of whether 4-Cl-IAA is largely restricted to seeds (Pless et al., 1984; Katayama et al., 1988).
RESULTS AND DISCUSSION
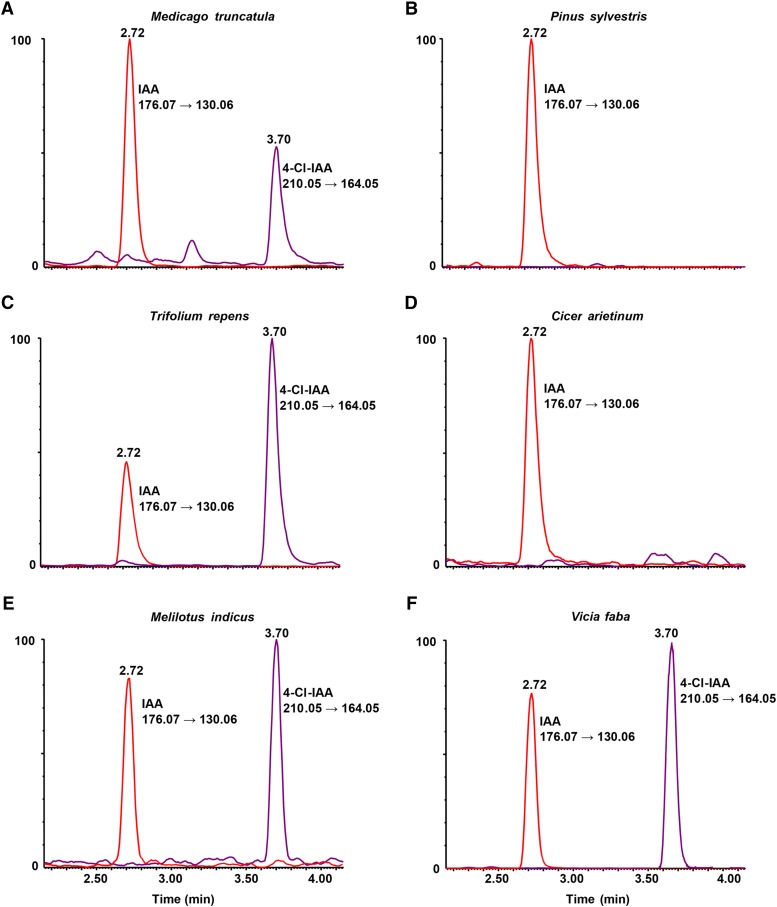
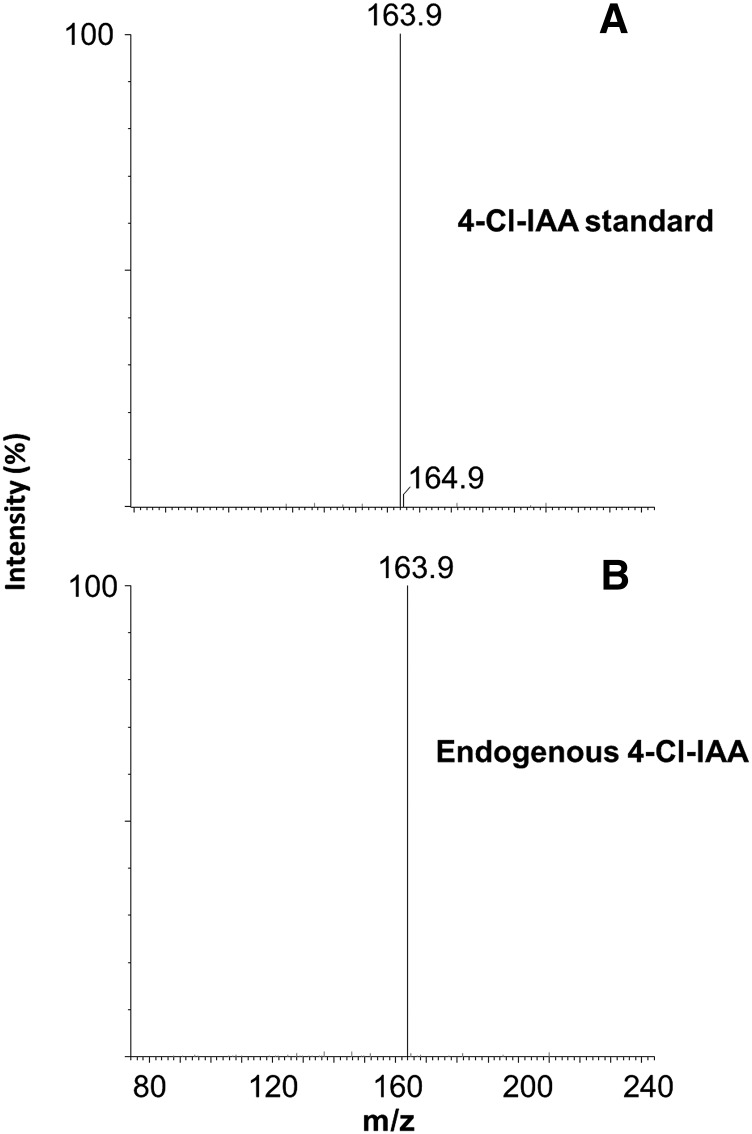
We screened 15 species from the Fabaceae for chlorinated auxin, selected to represent all major clades of the Papilionoid Fabaceae (Wojciechowski et al., 2004). We have extended the range of leguminous species that contain 4-Cl-IAA by detecting this compound in reproductive structures of Trifolium repens, Trifolium subterraneum, Trifolium micranthum, Melilotus indicus, and Medicago truncatula, all from outside the tribe Fabeae. Identification was based firstly on the correct liquid chromatography retention time (Fig. 1) and fragmentation patterns (Fig. 2), as indicated by comparison with standard forms of 4-Cl-IAA. Secondly, peaks for the expected mass transitions, 210 to 164 and 212 to 166, were observed, with the latter comprising approximately 33% of the former (data not shown). This ratio of peak areas is diagnostic for compounds containing one chlorine atom (Gross, 2011). The peak corresponding to the transition for 211 to 165 was only 10% of the 210 to 164 peak, as expected from naturally occurring isotopes. In some cases, dry seeds were analyzed (Table I), and in these cases, the extract was hydrolyzed to release 4-Cl-IAA and IAA from their conjugated forms.
Figure 1.
Ultra Performance Liquid Chromatrography-Tandem Mass Spectrometry (UPLC-MS/MS) chromatograms (Multiple Reaction Monitoring [MRM] mode) obtained from M. truncatula (A; dry seeds, after hydrolysis), Scots pine (B; dry seeds), T. repens (C; fresh pods, containing seeds), C. arietinum (D; young seeds), M. indicus (E; fresh pods, containing seeds), and broad bean (F; young seeds), showing the presence of endogenous IAA (red channel, MRM transition mass-to-charge ratio, 176.07–130.06; retention time = 2.72 min) and 4-Cl-IAA (purple channel, MRM transition mass-to-charge ratio, 210.05–164.05; retention time = 3.70 min). Retention times were subjected to minor adjustment to compensate for run-to-run variation.
Figure 2.
Product ion spectrum from 35Cl 4-Cl-IAA. A, Product ions of [M+H]+ (mass-to-charge ratio, 210) from 4-Cl-IAA standard. B, Endogenous 4-Cl-IAA from T. repens.
Table I. Endogenous levels of 4-Cl-IAA and IAA in 15 representative species from the family Fabaceae.
n.d., Not detected.
| Species | Phylogenetic Clade | Tissue Type | IAA | 4-Cl-IAA |
|---|---|---|---|---|
| ng g–1 | ||||
| M. truncatula | Trifoleae | Dry seeds | 26a | 85a |
| T. repens | Trifoleae | Fresh pods, containing seeds | 36 | 1,388 |
| T. micranthum | Trifoleae | Fresh pods, containing seeds | 47 | 569 |
| T. subterraneum | Trifoleae | Fresh pods, containing seeds | 100 | 243 |
| M. indicus | Trifoleae | Fresh pods, containing seeds | 7 | 39 |
| Broad bean | Fabeae | Young seeds | 175 | 885 |
| Old seeds | 73 | 91 | ||
| Young leaves | 16 | 3 | ||
| Old leaves | 9 | n.d. | ||
| C. arietinum | Cicereae | Young seeds | 14 | n.d. |
| C. puniceus | Galegeae | Young seeds | 343 | n.d. |
| I. australis | Indigoferoid | Fresh pods, containing seeds | 11 | n.d. |
| L. japonica | Robinioid | Dry seeds | 98a | n.d.a |
| G. clandestine | Millettioid | Young seeds | 340 | n.d. |
| H. comptoniana | Millettioid | Young seeds | 2,066 | n.d. |
| P. juniperina | Mirbelioid | Young seeds | 274 | n.d. |
| A. hypogaea | Dalbergioid | Dry seeds | 137a | n.d.a |
| L. angustifolius | Genistoid | Dry seeds | 30a | n.d.a |
Chlorinated auxin was not detected in representative species from the Galegeae tribe (Clianthus puniceus), the Robinioids (Lotus japonicus), the Indigoferoids (Indigofera australis), the Millettioids (Glycine clandestine and Hardenbergia comptoniana), the Mirbelioids (Pultenaea juniperina), the Dalbergioids (Arachis hypogaea), or the Genistoids (Lupinus angustifolius; Table I). Previously, 4-Cl-IAA was not detected in a number of Dalbergioid species, including Phaseolus vulgaris (Hofinger and Böttger, 1979), Glycine max, Vigna catiang, and Dolichos lablab (Katayama et al., 1987). Furthermore, we confirmed a previous finding that 4-Cl-IAA is not detectable in Cicer arietinum (Engvild, 1994; Fig. 1).
In additional experiments, chlorinated auxin was quantified using a deuterated internal standard. We found very high levels of 4-Cl-IAA in fruits of T. repens (which consisted mainly of seeds; Fig. 3) and in immature seeds of broad bean (Table I). In the case of M. truncatula, the most convincing evidence for chlorinated auxin was obtained after hydrolysis of dry seeds. For the five species shown, to our knowledge, for the first time here to contain 4-Cl-IAA (M. truncatula, M. indicus, T. repens, T. subterraneum, and T. micranthum), the level of the chlorinated compound in reproductive structures of these species exceeded that of IAA, as is the case for pea (Tivendale et al., 2012) and broad bean (Table I).
Figure 3.
Fruit of T. repens. A, Seeds in a bisected seed pod. B, Whole seed pod. C, Floret covering a single seed pod. Bar = 1 mm.
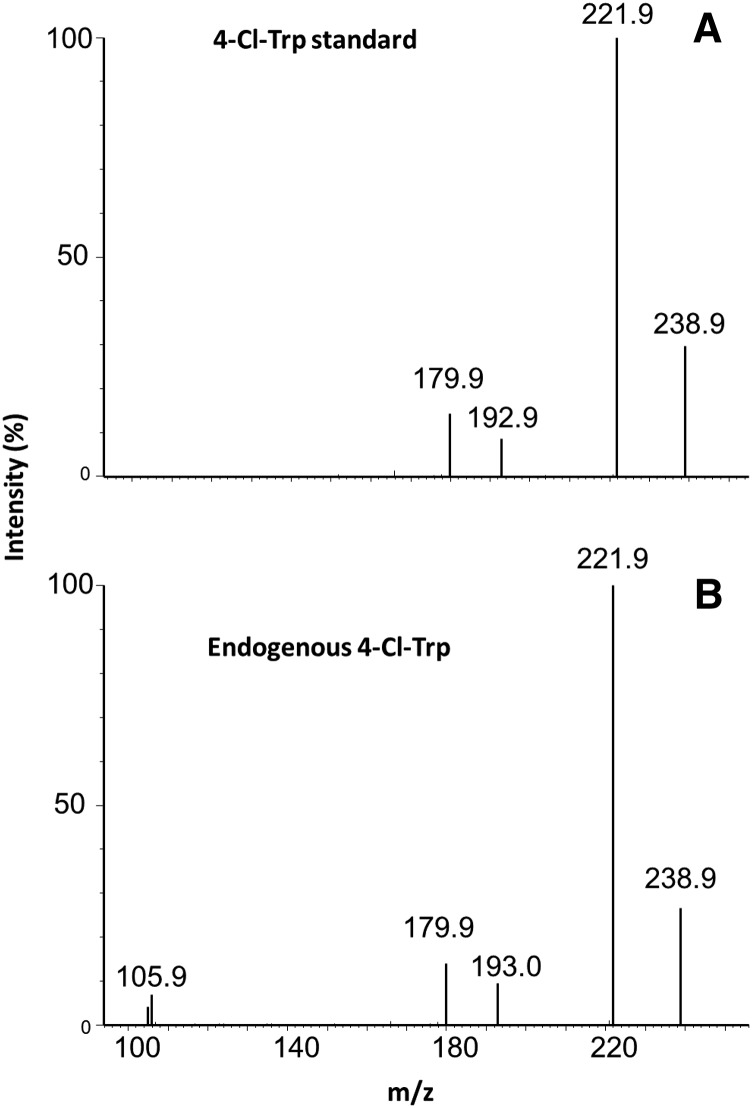
Endogenous 4-Cl-Trp was also detected in M. truncatula burrs as well as the fresh pods (containing seeds) of T. repens and M. indicus, providing further evidence that Trp or a Trp precursor can be chlorinated (Tivendale et al., 2012; Fig. 4). Again, chlorinated Trp was identified on the basis of retention times and fragmentation pattern (Fig. 4), as well as the diagnostic ratio (approximately 3 to 1) of peak areas for the chlorine isotopes of the [M+H]+ cluster (Gross, 2011). Chlorinated Trp was not detected in the Fabaceae spp. C. arietinum, C. puniceus, I. australis, G. clandestine, and H. comptoniana or the Pinus spp. Pinus flexilis or Pinus parviflora.
Figure 4.
Product ion spectrum from 35Cl 4-Cl-Trp. A, Product ions of [M+H]+ (mass-to-charge ratio, 239) from 4-Cl-Trp standard. B, Endogenous 4-Cl-Trp from T. repens.
Scots pine is the only species outside the Fabaceae previously reported to contain chlorinated auxin, although the levels reported (only from seeds) were low (Ernstsen and Sandberg, 1986). In our investigation, UPLC-MS/MS failed to detect any trace of 4-Cl-IAA in dry seeds of Scots pine (Fig. 1; Table II). Furthermore, we did not detect 4-Cl-IAA in young seeds of a diversity of species from the genus Pinus, including P. flexilis, P. parviflora ‘Glauca,’ and P. parviflora ‘Shikoku-goyo,’ or in mature seeds of Pinus pinea or Pinus radiata (Table II). Our results cast serious doubt over the reported presence of 4-Cl-IAA in Scots pine seeds.
Table II. Endogenous levels of 4-Cl-IAA and IAA of six Pinus spp.
n.d., Not detected.
In another intriguing report, very high levels (16,000 ng g–1 fresh weight [FW]) of 4-Cl-IAA were documented for young but fully developed leaves of field-grown broad bean plants (Pless et al., 1984). This is inconsistent with the hypothesis that 4-Cl-IAA is largely restricted to seeds (Katayama et al., 1988). Moreover, the figure reported by Pless et al. (1984) exceeds their reported seed content (up to 15,000 ng g–1 FW). To investigate these findings, we determined the distribution of 4-Cl-IAA in broad bean. We detected only very low levels in young leaves (approximately 3 ng g–1 FW), but none in mature leaves. We found that the levels of 4-Cl-IAA in young and mature seeds of broad bean were 886 and 90 ng g–1 FW, respectively. These data do not support the reported levels of Pless et al. (1984) and confirm, instead, previous evidence (Katayama et al., 1988) that seeds contain much higher levels of chlorinated auxin than vegetative tissues.
In conclusion, our evidence extends the range of species that produce chlorinated auxin beyond the Fabeae but restricts it to the Fabaceae. We have obtained unequivocal evidence for 4-Cl-IAA and its precursor, 4-Cl-Trp, from M. truncatula and from genera between Medicago and the Fabeae in phylogenetic terms. However, we were not able to repeat the finding that chlorinated auxin occurs in Scots pine. Hence, there is no longer an indication that the capacity to chlorinate arose more than once in the evolutionary history of plants.
We suggest that the capacity of plants to produce chlorinated auxin evolved only once and that that event occurred in the Fabaceae. As with previous researchers (Engvild, 1994), we did not detect 4-Cl-IAA in C. arietinum. Also, Lulsdorf et al. (2013) did not report this compound when they studied endogenous hormone profiles during early seed development in C. arietinum and Cicer anatolicum. Therefore, there is no evidence from this or previous studies that species of Cicer produce chlorinated auxin. If that is the case, we can suggest that the chlorination capacity arose approximately 25 million years ago, after the divergence of the genus Cicer from the common ancestor of the Fabeae and Trifoleae tribes (which include the genera Trifolium, Melilotus, and Medicago; Fig. 5; Choi et al., 2004; Wojciechowski et al., 2004; Lavin et al., 2005; Schaefer et al., 2012).
Figure 5.
Phylogeny of the major lineages of the Meso-Papilionoideae clade of the family Fabaceae and closest lineages to the tribe Fabeae. The ability to produce 4-Cl-IAA appears to have evolved after the divergence of the genus Cicer, approximately 25 million years ago (indicated by arrow and red branches). Species with detectable 4-Cl-IAA are shown in red; species that do not have detectable 4-Cl-IAA are shown in black. Species investigated in this study are indicated by an asterisk. Phylogenetic relationships and divergence dates are taken from Choi et al. (2004), Lavin et al. (2005), Schaefer et al. (2012), and Wojciechowski et al. (2004). s.l., Sensu lato.
Our results may provide the basis for a unique model system to investigate halogenating enzymes in plants. A comparison of haloperoxidase sequences across the genera Pisum, Trifolium, Medicago, and Cicer may well be instructive with regard to discovering the genes encoding the halogenating enzymes.
MATERIALS AND METHODS
Plant Material
Medicago truncatula ‘Jemalong,’ broad bean (Vicia faba), and Cicer arietinum were grown in greenhouse conditions as described previously (Jager et al., 2007). Young seeds or fresh seed pods of other legume species, including Trifolium repens, Trifolium micranthum, Trifolium subterraneum, Melilotus indicus, Indigofera australis, Clianthus puniceus, Glycine clandestine, Hardenbergia comptoniana, and Pultenaea juniperina were collected from plants grown in the field (Hobart and Kingston, Tasmania). Dry seeds of M. truncatula ‘Jemalong,’ Lotus japonica, Arachis hypogaea, Lupinus angustifolius, and Scots pine (Pinus sylvestris) were obtained from commercial sources. Mature seeds of Pinus radiata were harvested from cones collected from wild plants (Kingston, Tasmania). Immature seeds were extracted from young cones of Pinus flexilis, Pinus pinea, Pinus parviflora ‘Glauca,’ and P. parviflora ‘Shikoku-goyo’ grown in the Royal Tasmanian Botanical Gardens (Hobart, Tasmania).
Extract Preparation for the Detection and Quantification of Compounds
For the extraction and quantification of IAA and 4-Cl-IAA from young, fresh tissues, 0.3 to 2.5 g of tissue was weighed (±0.0001 g FW) and placed into a falcon tube with 4 volumes of cold (–20°C) extraction solvent (80% [v/v] methanol in water with butylated hydroxytoluene [BHT; 250 mg L–1]). The tissue was then homogenized and held at 4°C overnight to extract. For each species, the supernatant was then divided in half to conduct two separate analyses: one for detection of the compounds of interest and one for quantification of these compounds. For the former, no labeled internal standards were added; and for the latter, [13C6] IAA (Cambridge Isotope Laboratories) and [2H4] 4-Cl-IAA (supplied by Jerry Cohen, Department of Horticultural Science, University of Minnesota) were added as internal standards. The samples were reduced under vacuum at 35°C, taken up in 2% (v/v) acetic acid in water, and partitioned twice against diethyl ether (2/3 volumes). After drying the ether, samples were taken up in 1% (v/v) acetic acid in water and centrifuged for 5 min at 13,000g. Aliquots were then taken for analysis by UPLC-MS/MS as described previously (Tivendale et al., 2012).
In viable, dry seeds, the total levels of each of IAA and 4-Cl-IAA were monitored, including both free acids and conjugated forms. IAA and 4-Cl-IAA were extracted as described above, but 65% (v/v) isopropanol in water with BHT (250 mg L–1) was used as the extraction solvent. From the supernatant, hydrolysis of conjugated IAA and 4-Cl-IAA was carried out using the method described by Symons et al. (2002).
For the extraction of Trp and 4-Cl-Trp, tissue was weighed, homogenized, and extracted as described above, but distilled water with BHT (250 mg L–1) was the usual extraction solvent.
Acknowledgments
We thank Jenny Smith, Laura Quittenden, Sam Cook, Michelle Lang, Tracey Winterbottom, David Nichols, Noel Davies, Christine Howells, and Hou Ket Tang for technical assistance and advice and the Royal Tasmanian Botanical Gardens for access to plants of Pinus spp. and C. puniceus.
Glossary
- IAA
indole-3-acetic acid
- 4-Cl-IAA
4-chloroindole-3-acetic acid
- FW
fresh weight
- BHT
butylated hydroxytoluene
- 4-Cl-Trp
4-chloroindole-3-tryptophan
Footnotes
This work was supported by the Australian Research Council (grant nos. DP130103357 to J.J.R. and DE140100946 to S.A.M.M.).
Articles can be viewed without a subscription.
References
- Butler A, Sandy M (2009) Mechanistic considerations of halogenating enzymes. Nature 460: 848–854 [DOI] [PubMed] [Google Scholar]
- Choi HK, Mun JH, Kim DJ, Zhu H, Baek JM, Mudge J, Roe B, Ellis N, Doyle J, Kiss GB, et al. (2004) Estimating genome conservation between crop and model legume species. Proc Natl Acad Sci USA 101: 15289–15294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvild KC. (1994) The Chloroindole Auxins of Pea, Strong Plant Growth Hormones, or Endogenous Herbicides? Risø National Laboratory Denmark, Roskilde, Denmark [Google Scholar]
- Ernstsen A, Sandberg G (1986) Identification of 4‐chloroindole‐3‐acetic acid and indole‐3‐aldehyde in seeds of Pinus sylvestris. Physiol Plant 68: 511–518 [Google Scholar]
- Gross JH. (2011) Mass Spectrometry: A Textbook. Springer, Heidelberg [Google Scholar]
- Hofinger M, Böttger M (1979) Identification by GC-MS of 4-chloroindolylacetic acid and its methyl ester in immature Vicia faba seeds. Phytochemistry 18: 653–654 [Google Scholar]
- Jager CE, Symons GM, Nomura T, Yamada Y, Smith JJ, Yamaguchi S, Kamiya Y, Weller JL, Yokota T, Reid JB (2007) Characterization of two brassinosteroid C-6 oxidase genes in pea. Plant Physiol 143: 1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone MM, Reinecke DM, Ozga JA (2005) The auxins IAA and 4-Cl-IAA differentially modify gibberellin action via ethylene response in developing pea fruit. J Plant Growth Regul 24: 214–225 [Google Scholar]
- Katayama M, Thiruvikraman SV, Marumo S (1987) Identification of 4-chloroindole-3-acetic acid and its methyl ester in immature seeds of Vicia amurensis (the tribe Vicieae), and their absence from three species of Phaseoleae. Plant Cell Physiol 28: 383–386 [Google Scholar]
- Katayama M, Thiruvikraman SV, Marumo S (1988) Localization of 4-chloroindole-3-acetic acid in seeds of Pisum sativum and its absence from all other organs. Plant Cell Physiol 29: 889–891 [Google Scholar]
- Kettner J, Böttger M, Fock A (1992) The occurrence of 4-chlorotryptophane in Vicia faba. Phytochemistry 31: 2327–2328 [Google Scholar]
- Lavin M, Herendeen PS, Wojciechowski MF (2005) Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol 54: 575–594 [DOI] [PubMed] [Google Scholar]
- Lulsdorf MM, Yuan HY, Slater SM, Vandenberg A, Han X, Zaharia LI, Abrams SR (2013) Endogenous hormone profiles during early seed development of C. arietinum and C. anatolicum. Plant Growth Regul 71: 191–198 [Google Scholar]
- Manabe K, Higashi M, Hatori M, Sakagami Y (1999) Biosynthetic studies on chlorinated indoles in Vicia faba. Plant Growth Regul 27: 15–19 [Google Scholar]
- Ozga JA, Reinecke DM, Ayele BT, Ngo P, Nadeau C, Wickramarathna AD (2009) Developmental and hormonal regulation of gibberellin biosynthesis and catabolism in pea fruit. Plant Physiol 150: 448–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless T, Böttger M, Hedden P, Graebe J (1984) Occurrence of 4-Cl-indoleacetic acid in broad beans and correlation of its levels with seed development. Plant Physiol 74: 320–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke DM. (1999) 4-Chloroindole-3-acetic acid and plant growth. Plant Growth Regul 27: 3–13 [Google Scholar]
- Reinecke DM, Ozga JA, Ilić N, Magnus V (1999) Molecular properties of 4-substituted indole-3-acetic acids affecting pea pericarp elongation. Plant Growth Regul 27: 39–48 [Google Scholar]
- Reinecke DM, Ozga JA, Magnus V (1995) Effect of halogen substitution of indole-3-acetic acid on biological activity in pea fruit. Phytochemistry 40: 1361–1366 [Google Scholar]
- Schaefer H, Hechenleitner P, Santos-Guerra A, Menezes de Sequeira M, Pennington RT, Kenicer G, Carine MA (2012) Systematics, biogeography, and character evolution of the legume tribe Fabeae with special focus on the middle-Atlantic island lineages. BMC Evol Biol 12: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56: 922–934 [DOI] [PubMed] [Google Scholar]
- Symons GM, Ross JJ, Murfet IC (2002) The bushy pea mutant is IAA‐deficient. Physiol Plant 116: 389–397 [Google Scholar]
- Tang N, Deng W, Hu G, Hu N, Li Z (2015) Transcriptome profiling reveals the regulatory mechanism underlying pollination dependent and parthenocarpic fruit set mainly mediated by auxin and gibberellin. PLoS One 10: e0125355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivendale ND, Davidson SE, Davies NW, Smith JA, Dalmais M, Bendahmane AI, Quittenden LJ, Sutton L, Bala RK, Le Signor C, et al. (2012) Biosynthesis of the halogenated auxin, 4-chloroindole-3-acetic acid. Plant Physiol 159: 1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivendale ND, Ross JJ, Cohen JD (2014) The shifting paradigms of auxin biosynthesis. Trends Plant Sci 19: 44–51 [DOI] [PubMed] [Google Scholar]
- Wagner C, El Omari M, König GM (2009) Biohalogenation: nature’s way to synthesize halogenated metabolites. J Nat Prod 72: 540–553 [DOI] [PubMed] [Google Scholar]
- Wojciechowski MF, Lavin M, Sanderson MJ (2004) A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. Am J Bot 91: 1846–1862 [DOI] [PubMed] [Google Scholar]