Transmitted human immunodeficiency virus drug resistance in Europe is stable at around 8%. The impact of baseline mutation patterns on susceptibility to antiretroviral drugs should be addressed using clinical guidelines. The impact on baseline susceptibility is largest for nonnucleoside reverse transcriptase inhibitors.
Keywords: HIV-1, drug resistance, transmission, antiretroviral therapy, Europe
Abstract
Background. Numerous studies have shown that baseline drug resistance patterns may influence the outcome of antiretroviral therapy. Therefore, guidelines recommend drug resistance testing to guide the choice of initial regimen. In addition to optimizing individual patient management, these baseline resistance data enable transmitted drug resistance (TDR) to be surveyed for public health purposes. The SPREAD program systematically collects data to gain insight into TDR occurring in Europe since 2001.
Methods. Demographic, clinical, and virological data from 4140 antiretroviral-naive human immunodeficiency virus (HIV)–infected individuals from 26 countries who were newly diagnosed between 2008 and 2010 were analyzed. Evidence of TDR was defined using the WHO list for surveillance of drug resistance mutations. Prevalence of TDR was assessed over time by comparing the results to SPREAD data from 2002 to 2007. Baseline susceptibility to antiretroviral drugs was predicted using the Stanford HIVdb program version 7.0.
Results. The overall prevalence of TDR did not change significantly over time and was 8.3% (95% confidence interval, 7.2%–9.5%) in 2008–2010. The most frequent indicators of TDR were nucleoside reverse transcriptase inhibitor (NRTI) mutations (4.5%), followed by nonnucleoside reverse transcriptase inhibitor (NNRTI) mutations (2.9%) and protease inhibitor mutations (2.0%). Baseline mutations were most predictive of reduced susceptibility to initial NNRTI-based regimens: 4.5% and 6.5% of patient isolates were predicted to have resistance to regimens containing efavirenz or rilpivirine, respectively, independent of current NRTI backbones.
Conclusions. Although TDR was highest for NRTIs, the impact of baseline drug resistance patterns on susceptibility was largest for NNRTIs. The prevalence of TDR assessed by epidemiological surveys does not clearly indicate to what degree susceptibility to different drug classes is affected.
Transmission of human immunodeficiency virus (HIV) harboring resistance mutations is associated with impaired outcomes from antiretroviral therapy (ART) [1–4]. In developed countries, guidelines therefore recommend baseline genotypic testing in patients newly diagnosed with HIV to guide the choice of the first line of ART [5, 6]. The International AIDS Society–USA (IAS-USA) continuously updates a chart of mutations that are associated with clinical resistance to HIV, based on biological data, that is, from in vitro experiments or susceptibility testing of clinical isolates [7]. Several algorithms have been developed to predict the susceptibility of HIV to antiretrovirals based on the detected resistance mutations.
Surveillance of the transmission of HIV drug resistance (TDR) is essential to inform treatment policy making and guidance. Previous studies assessing TDR have used different criteria to define the relevant resistance mutations, resulting in widely varying estimates. The World Health Organization (WHO) developed a consensus list of drug resistance mutations for surveillance purposes, which was updated in 2009 [8]. This list differs from the IAS-USA chart developed for clinical use [7]. Resistance mutations that can occur naturally as polymorphisms in the absence of drug pressure are not indicators of transmitted drug resistance. Although polymorphisms may be clinically relevant and are included on the IAS-USA chart, these mutations are excluded from the WHO list, to avoid falsely elevated estimates of TDR.
The European surveillance program SPREAD has been monitoring the transmission of HIV drug resistance in Europe since 2002. We have previously shown that the prevalence of TDR in Europe was stabilizing at just below 10% [9–11], although in 2006–2007 an increase in nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance mutations to 4% was detected [12], particularly in men who have sex with men (MSM) [13]. Over the years more countries have joined SPREAD, and by 2010, >9500 patients had been included. In this article we describe the prevalence of TDR from 2008 to 2010, the predicted baseline susceptibility to currently available nucleoside reverse transcriptase inhibitors (NRTIs), NNRTIs, and protease inhibitors (PIs), and the first-line regimens commonly used in Europe [5], based on all observed mutations at baseline (including polymorphisms). In addition, we assessed whether the prevalence of TDR changed over time from 2002 to 2010 by comparing the 2008–2010 data to earlier SPREAD data.
METHODS
Sampling Strategy
The SPREAD program has continuously collected data from newly diagnosed HIV type 1 (HIV-1)–infected patients since its start in 2002. For the 2008–2010 analysis, 26 countries contributed data: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Ireland, Israel, Italy, Latvia, Lithuania, Luxembourg, the Netherlands, Norway, Poland, Romania, Serbia, Slovakia, Slovenia, Spain, and Sweden. For every participating country, data were collected according to one of the following sampling strategies: (1) a random sample was drawn from a national program or national reference center, or (2) inclusion was stratified according to risk groups and geographical distribution within the country [10, 11]. Until 2008, the sample size for each country was determined based on its total number of newly infected individuals per year [10]. Since 2008, countries could increase their initially determined sample size. To correct for overrepresentation of data from certain countries, data were weighted at the time of the analysis.
Ethical Issues
Ethical approval was obtained for each participating country, according to national legislation. All data were anonymized and coded at a national level before submission.
Patient Inclusion and Data Collection
To be included, patients had to be at least 18 years old, naive to ART, and have a viral load >1000 copies/mL at the time of HIV-1 diagnosis. Clinical and virological data were collected using a standardized questionnaire. A blood sample was taken for resistance testing within 6 months of diagnosis. Population-based sequencing of reverse transcriptase and protease was performed locally. All countries were part of a quality control program for HIV sequencing. Additionally, the quality of all sequences was verified, checking for length, variability, conservative sites, frameshifts, and stop codons. Before data analysis, all collected data had passed a thorough data verification process.
Interpretation of Sequences
The WHO list for surveillance of drug resistance mutations was used to estimate the prevalence of TDR [8]. The overall prevalence was defined as the percentage of patients infected with a virus carrying any mutation indicative of TDR. The prevalence of TDR for the different drug classes (NRTIs, NNRTIs, and PIs) was defined as the percentage of patients infected with a virus carrying any mutation indicative of TDR associated with each particular drug class. Patients with multiclass resistance (eg, a virus with mutations associated with both NRTIs and NNRTIs) were counted once in the overall prevalence, but were counted both in the analysis for NRTIs as well as for NNRTIs. We have performed a separate analysis in those patients known to have been recently infected. We identified patients as being recently infected, based on the availability of a last negative HIV-1 test not more than 1 year before the first positive HIV-1 test, or initial documented indeterminate HIV-1 serological results followed by seroconversion and confirmation of HIV-1 diagnosis by immunoblotting. Separate analyses were also performed for different risk groups and subtypes. We determined the prevalence of mutations for the most common HIV subtypes (A, B, C, CRF 01_AE, CRF 02_AG), based on the percentage of patients infected with a virus of this subtype carrying each particular mutation. HIV-1 subtypes were determined by use of the subtyping tool COMET version 0.5 [14]. To predict the susceptibility to available NRTIs, NNRTIs, and PIs, sequences were analyzed using the Stanford HIVdb algorithm version 7.0 [15]. This analysis determined the effect of all resistance-related mutations at baseline on the susceptibility of the virus to antiretroviral drugs, including polymorphisms that were not included in analyses for the prevalence of TDR. A genotypic susceptibility score (GSS) was calculated for the first-line regimens commonly used in Europe [5]. Integrase strand transfer inhibitor (INSTI) regimens were excluded from this analysis due to the unavailability of integrase sequence data. Each drug in the regimen was scored based on the results of the Stanford algorithm: High-level resistance was scored as 0, intermediate and low-level resistance as 0.5, and potential low-level resistance and susceptibility were scored as 1. The GSS for each 3-drug combination was the sum of the individual scores for the drugs in the combination.
Statistical Analysis
The prevalence of TDR was estimated in a weighted analysis. Each risk group (MSM, heterosexuals, injecting drug users [IDUs], and others) in each country formed a stratum. The weight of each individual was computed as the ratio between the proportion of his or her stratum in the sample and the proportion of this stratum in the population estimated using data from European Centre for Disease Prevention and Control reports [16]. A sensitivity analysis was performed to investigate the influence of each country on the prevalence of TDR by repeating the analysis after omitting that country from the computation. Trends over time were assessed using logistic regression, by comparing our 2008–2010 dataset to previous SPREAD datasets from 2002–2005 and 2006–2007. The dataset from 2006–2007 included data from the same 26 countries, whereas the dataset from 2002–2005 did not include data from Bulgaria, Croatia, France, Lithuania, or Romania, but included data from Portugal. The frequency of mutations at baseline and the predicted susceptibility analyses were computed using the same weighting scheme as in the analysis of the prevalence of TDR. All analyses were performed with SAS statistical software, version 9.3 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
From 2008 to 2010, 4140 patients were included for analysis, compared to 2990 and 2458 patients in previous SPREAD datasets from 2002–2005 and 2006–2007, respectively, resulting in a total of 9588 patients (Table 1). In 2008–2010, there was a significantly higher percentage of MSM compared with 2002–2005 and 2006–2007. Just under half of the patients originated from Western Europe, 15.1% from Central and Eastern Europe, and 4.8% from sub-Saharan Africa. Most patients were infected with subtype B, followed by subtypes 02_AG, A and C.
Table 1.
Baseline Characteristics of Newly Diagnosed Therapy-Naive Human Immunodeficiency Virus-Infected Patients in the SPREAD Program
| Characteristic | All (N = 9588) | 2002–2005 (n = 2990) | 2006–2007 (n = 2458) | 2008–2010 (n = 4140) | P Value |
|---|---|---|---|---|---|
| Male sex | 7695 (80.3) | 2337 (78.2) | 1952 (79.4) | 3406 (82.3) | .0001 |
| Age at diagnosis, y, median (IQR) | 37 (29–43) | 38 (29–42) | 36 (29–42) | 37 (29–43) | NS |
| Transmission risk group | <.0001 | ||||
| MSM | 4969 (51.8) | 1486 (49.7) | 1169 (47.6) | 2314 (55.9) | |
| Heterosexual | 3195 (33.3) | 1060 (35.5) | 812 (33.0) | 1323 (32.0) | |
| Injecting drug user | 607 (6.3) | 245 (8.2) | 174 (7.1) | 188 (4.5) | |
| Continent of origin | <.0001 | ||||
| Western Europe | 5072 (52.9) | 1823 (61.0) | 1270 (51.7) | 1979 (47.8) | |
| Central and Eastern Europe | 1719 (17.9) | 561 (18.8) | 534 (21.7) | 624 (15.1) | |
| Sub-Saharan Africa | 735 (7.7) | 377 (12.6) | 161 (6.6) | 197 (4.8) | |
| HIV RNA load, log copies/mL, median (IQR) | 4.8 (4.2–5.3) | 4.9 (4.3–5.3) | 4.8 (4.2–5.3) | 4.8 (4.2–5.3) | .002 |
| CD4 count, cells/µL, median (IQR) | 391 (193–544) | 355 (180–547) | 360 (192–535) | 393 (202–544) | NS |
| CDC stage at diagnosis | .0005 | ||||
| A or B | 6860 (71.5) | 2460 (82.3) | 1567 (63.8) | 2833 (68.4) | |
| C | 871 (9.1) | 360 (12.0) | 209 (8.5) | 302 (7.3) | |
| Subtype | <.0001 | ||||
| A | 632 (6.6) | 234 (7.8) | 174 (7.1) | 224 (5.4) | |
| B | 6310 (65.8) | 2010 (67.2) | 1519 (61.8) | 2781 (67.2) | |
| C | 552 (5.8) | 190 (6.4) | 141 (5.7) | 221 (5.3) | |
| 01_AE | 309 (3.2) | 100 (3.3) | 87 (3.5) | 122 (2.9) | |
| 02_AG | 484 (5.0) | 135 (4.5) | 104 (4.2) | 245 (5.9) | |
| G | 107 (1.1) | 49 (1.6) | 23 (0.9) | 35 (0.8) | |
| F | 212 (2.2) | 29 (1.0) | 72 (2.9) | 111 (2.7) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; NS, not significant (P value >.05).
Prevalence of TDR
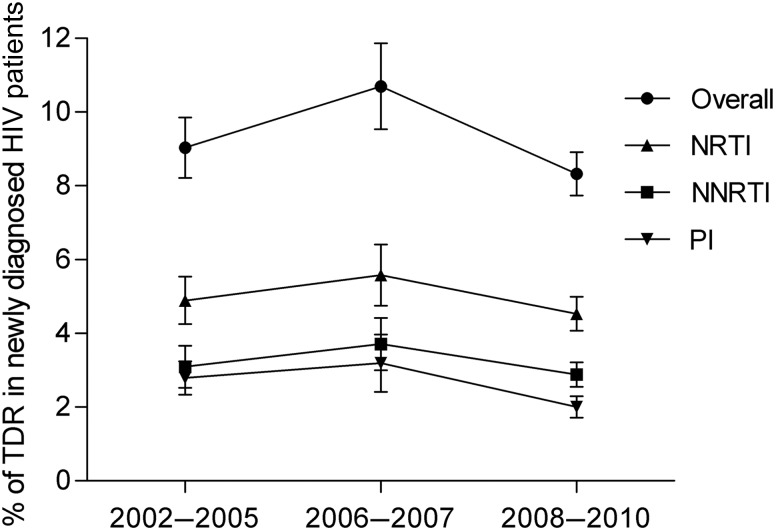
The overall prevalence of TDR in 2008–2010 was 8.3% (95% confidence interval [CI], 7.2%–9.5%), with mutations associated with NRTIs observed most frequently (4.5% [95% CI, 3.6%–5.4%]), followed by mutations associated with NNRTIs (2.9% [95% CI, 2.2%–3.5%]) and PIs (2.0% [95% CI, 1.4%–2.6%]). We did not observe a significant change in prevalence over time overall or for the different drug classes (Figure 1). Sensitivity analyses showed no significant difference when individual countries were excluded. A subset of patients from 2008–2010 (n = 534) was identified as recently infected. The overall percentage of TDR was somewhat higher among these patients (10.1%, P = .15) vs those with an indeterminate date of infection, with a slightly higher prevalence of TDR for all drug classes (Table 2). The K103N mutation was detected significantly more often in recently infected patients compared with patients with an unknown duration of infection (3.35% vs 1.49%, P = .0055). Recently infected patients were more often MSM, but the significant difference in prevalence of K103N was still observed when the analysis was limited to MSM only in both groups. The prevalence of K103N was slightly lower in patients recently infected in previous years and was not significantly different from those with an unknown duration of infection (2002–2005: 2.20% vs 1.45%, P = .22; 2006–2007: 2.07% vs 2.09%, P = .98).
Figure 1.
Overall weighted prevalence of transmitted drug resistance in patients with newly diagnosed human immunodeficiency virus (HIV) in Europe. The error bars indicate the standard error. Abbreviations: NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDR, transmitted drug resistance.
Table 2.
Prevalence of Transmitted Drug Resistance Mutations in Recently Infected Patients and in Patients With an Unknown Duration of Infection, Diagnosed During 2008–2010
| Mutation | Recently Infected Patients (n = 534), % | Patients With an Unknown Duration of Infection (n = 3606), % | P Value |
|---|---|---|---|
| TDR prevalence | |||
| TDR any class | 10.1 | 8.2 | NS |
| TDR NRTI | 4.7 | 4.4 | NS |
| TDR NNRTI | 3.8 | 2.9 | NS |
| TDR PI | 2.4 | 2.0 | NS |
| NRTI mutations | |||
| T215reva | 3 | 2.47 | NS |
| M41L | 1.57 | 1.47 | NS |
| D67N | 0.20 | 0.68 | NS |
| K219Q | 0.20 | 0.52 | NS |
| L210W | 0.79 | 0.35 | NS |
| M184V | 0 | 0.27 | NS |
| NNRTI mutations | |||
| E138Ab | 3.15 | 3.62 | NS |
| K103N | 3.35 | 1.49 | .0055 |
| G190A | 0 | 0.52 | NS |
| Y181C | 0.20 | 0.46 | NS |
| Y181V | 0 | 0.03 | NS |
| K101E | 0 | 0.27 | NS |
| K101P | 0.20 | 0.08 | NS |
| K103S | 0.39 | 0.19 | NS |
| P225H | 0 | 0.16 | NS |
| Y188L | 0 | 0.16 | NS |
| PI mutations | |||
| L90M | 0.38 | 0.58 | NS |
| M46I | 0.38 | 0.26 | NS |
| M46L | 0.38 | 0.24 | NS |
| V82A | 0.19 | 0.18 | NS |
Abbreviations: NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NS, not significant (P value >.05); PI, protease inhibitor; TDR, transmitted drug resistance.
a T215rev represent revertant mutations (S/D/C/E/I/V) that can occur at position 215.
b E138A is a polymorphic mutation. This mutation is for this reason not included in the World Health Organization list for surveillance of TDR mutations. As this list is used to determine the prevalence of TDR, this mutation is not included in the prevalence of TDR for NNRTIs.
Prevalence of TDR Among Risk Groups and Subtypes
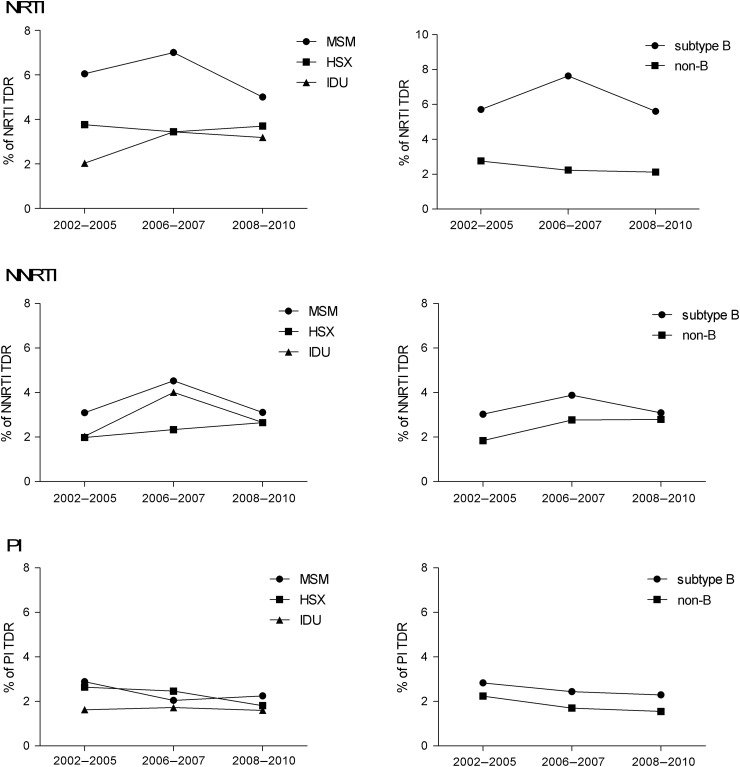
Resistance mutations associated with NRTIs were somewhat more prevalent among MSM than among heterosexuals and IDUs (5.0% vs 3.7% and 3.2%, respectively, P = .24) and significantly more prevalent in subtype B than in other subtypes (5.6% vs 2.1%, P < .0001; Figure 2). These differences were also observed previously. The observed mutations were mainly thymidine analogue mutations (TAMs) such as revertant mutations at position 215 (S/D/C/E/I/V) and M41L (Table 3). For resistance mutations associated with NNRTIs, no differences were observed between risk groups (MSM =3.1%, heterosexual = 2.7%, IDU = 2.7%, P = .69) or subtypes (B =3.1%, non-B = 2.8%, P = .81; Figure 2). For all investigated subtypes, E138A (a polymorphic mutation) and K103N were most frequently seen at baseline (Table 3). Resistance mutations associated with PIs were of similarly low prevalence among the different risk groups and subtypes. An overview of the prevalence of all mutations for the different subtypes is provided in Supplementary Table 1.
Figure 2.
Prevalence of transmitted drug resistance (TDR) among different transmission risk groups (men who have sex with men [MSM], heterosexuals [HSX], and injecting drug users [IDUs]) and subtypes, for nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs).
Table 3.
Prevalence of Most Frequently Detected Mutations at Time of Diagnosis for Subtypes A, B, C, CRF 01_AE, and CRF 02_AG in Newly Diagnosed Antiretroviral-Naive Human Immunodeficiency Virus-Infected Patients in Europe, 2008–2010
| Subtype | A (n = 224), % | B (n = 2781), % | C (n = 221), % | CRF 01_AE (n = 122), % | CRF 02_AG, % (n = 245) |
|---|---|---|---|---|---|
| NRTI mutations | |||||
| T215rev | 0.88 | 3.44 | 0.88 | 0.80 | 0 |
| M41L | 0.88 | 1.99 | 0.44 | 0.80 | 0 |
| D67N | 0 | 0.80 | 0.88 | 0 | 0 |
| K219Q | 0 | 0.62 | 0.44 | 0 | 0.72 |
| L210W | 0 | 0.58 | 0 | 0 | 0 |
| M184V | 0 | 0.29 | 0 | 0 | 0.36 |
| NNRTI mutations | |||||
| E138A | 7.89 | 3.48 | 4.82 | 2.40 | 1.81 |
| K103N | 1.32 | 1.81 | 0.88 | 1.60 | 2.17 |
| Y181C | 1.32 | 0.40 | 0 | 0 | 0.36 |
| G190A | 0 | 0.54 | 0.44 | 0 | 0 |
| K101E | 0 | 0.18 | 0.88 | 0 | 0 |
| K103S | 0 | 0.25 | 0.44 | 0 | 0 |
| PI mutations | |||||
| L90M | 0 | 0.76 | 0.44 | 0 | 0.35 |
| M46I | 0.43 | 0.24 | 0 | 0.80 | 0.70 |
| M46L | 0.87 | 0.21 | 0 | 1.60 | 0 |
| V82A | 0 | 0.17 | 0.44 | 0 | 0 |
| I54V | 0 | 0 | 0.44 | 0 | 0.70 |
The prevalence of all mutations at baseline can be found in Supplementary Table 1.
Abbreviations: CRF, circulating recombinant form; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Baseline Susceptibility
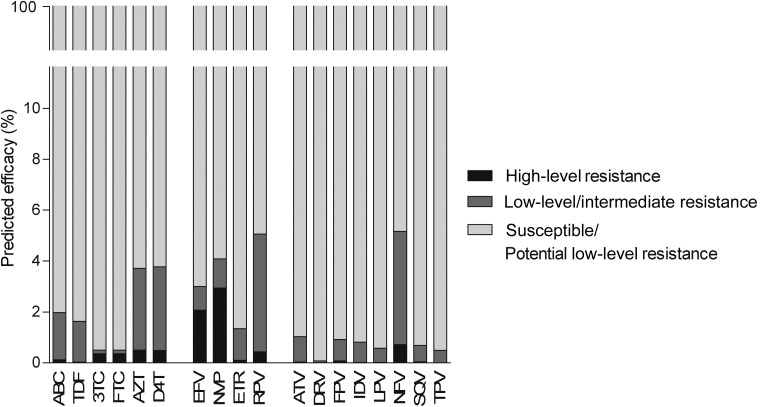
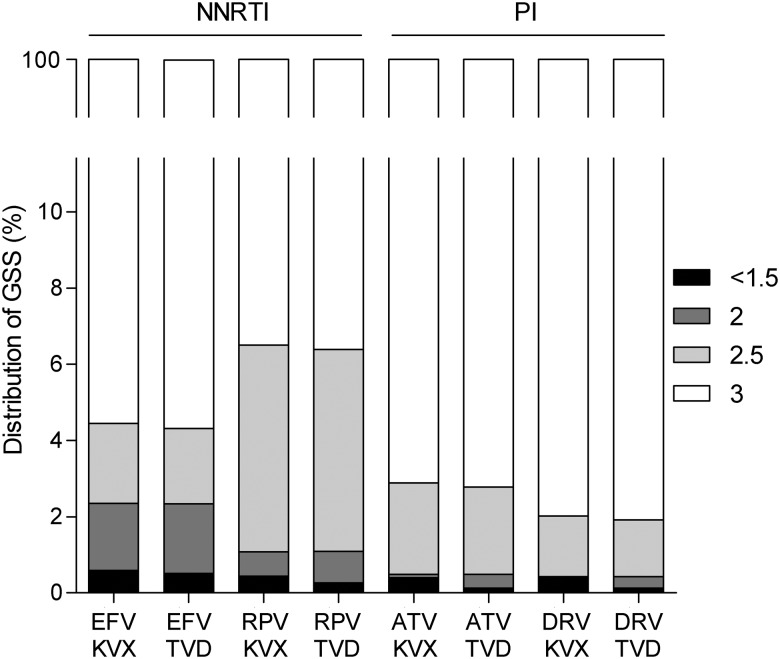
The observed NRTI-associated mutations had the largest effect on susceptibility to zidovudine and stavudine, for which 3.7% and 3.8% of viruses were predicted to have some resistance, followed by abacavir (2.0%), tenofovir (1.6%), lamivudine, and emtricitabine (both 0.5%) (Figure 3). For NNRTIs, 3.0% and 4.1% of viruses were predicted to be resistant to efavirenz and nevirapine, which is mostly attributable to the presence of K103N. This single mutation causes high-level resistance to both efavirenz and nevirapine, but not to etravirine and rilpivirine. Nevertheless, 5.0% of viruses were predicted to have some resistance to rilpivirine, largely due to polymorphic mutation E138A, which was observed in 3.9% of patients. Less than 1% of the viruses were predicted to be resistant to any of the PIs, except nelfinavir (5.1%, caused by minor mutations that are not included in the WHO list). HIV-infected patients are treated with a combination of at least 3 different compounds. In our study, regimens based on boosted atazanavir or boosted darunavir had a GSS of 3, indicative of susceptibility to all 3 compounds, in 97% and 98% of patients, respectively (Figure 4). This did not depend on whether the combination of NRTIs chosen for the backbone was abacavir/lamivudine or tenofovir/emtricitabine. Regarding NNRTI-based regimens, 4.5% and 6.5% of patients scored a GSS <3 for regimens based on efavirenz or rilpivirine, respectively. Again, this was independent of the NRTI backbone.
Figure 3.
Predicted efficacy to antiretroviral compounds of patients in Europe newly diagnosed with human immunodeficiency virus in 2008–2010. Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; AZT, zidovudine; D4T, stavudine; DRV, darunavir; EFV, efavirenz; ETR, etravirine; FPV, fosamprenavir; FTC, emtricitabine; IDV, indinavir; LPV, lopinavir; NFV, nelfinavir; NVP, nevirapine; RPV, rilpivirine; SQV, saquinavir; TDF, tenofovir; TPV, tipranavir.
Figure 4.
Genotypic sensitivity scores (GSSs) of 8 recommended first-line regimens in patients in Europe newly diagnosed with human immunodeficiency virus in 2008–2010. Abbreviations: ATV, atazanavir; DRV, darunavir; EFV, efavirenz; KVX, Kivexa (abacavir + lamivudine); NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RPV, rilpivirine; TVD, Truvada (tenofovir +emtricitabine).
DISCUSSION
These results indicate that the prevalence of TDR in Europe has remained fairly stable from 2002 to 2010. Our 2008–2010 dataset included significantly more MSM than in previous years, reflecting the increasing number of HIV cases among this risk group in many of the countries included in our analysis [16]. NRTI mutations were significantly more prevalent in subtype B viruses compared with other subtypes. Subtype B has been the most prevalent subtype in Europe from the start of the epidemic, whereas non-B subtypes dominate in sub-Saharan Africa and Asia. It is likely that this difference in NRTI-related TDR is a result of the longer exposure of subtype B to NRTIs, including suboptimal monotherapy before the introduction of combination ART, and not due to specific viral characteristics. African countries with a longer period of ART rollout reported higher rates of TDR, showing the potential for TDR in viruses of non-B subtypes [17].
TAMs were the most frequently observed but were not predicted to affect susceptibility to those NRTIs in most frequent use to any great extent. When we compared 8 first-line regimens, the percentage of patients infected with a virus that was predicted to be fully susceptible to the regimen did not depend on the NRTI backbone chosen (tenofovir/emtricitabine or abacavir/lamivudine). Instead, it depended on the third component added to the backbone—that is, an NNRTI or PI.
The previously reported increase in transmitted NNRTI resistance among MSM did not persist in more recent years. We only observed a higher prevalence of NNRTI-mutation K103N in recently infected patients vs patients with an unknown duration of infection. One could argue that this results from reversion of the mutation to wild type in the absence of drug pressure in chronically infected individuals. However, this difference between the patient groups was not observed in previous years. Moreover, it has been well described that K103N can persist for a prolonged period after transmission due to its limited effect on the replicative capacity of the virus [18]. As such, we think it is most likely that the difference reflects a recent increase in transmitted K103N.
The prevalence of TDR associated with NNRTIs is reported to be 2-fold higher in cohorts in the United States and Canada compared with European data. The prevalence of NNRTI-mutation K103N quadrupled between 1995 and 2010 in a cohort of recently infected MSM in New York [19], and a nearly 8-fold rise in prevalence of TDR associated with NNRTIs between 2002 and 2009 was observed in a Canadian cohort [20]. The higher rates of NNRTI resistance may be partly due to differences in sampling strategies, as sampling in recently infected patients, high-risk groups, and larger cities may result in higher estimates of TDR.
The predicted reduced susceptibility to NNRTIs is concerning. Although we did not see a further increase in TDR for NNRTIs over time, the NNRTI resistance mutations observed at baseline were predicted to cause high-level or intermediate resistance to first-line NNRTIs. A GSS <3 was predicted for 1 in 22 patients for efavirenz-based regimens and for 1 in 15 patients for rilpivirine-based regimens. The high rate of predicted resistance to rilpivirine is partly due to mutation E138A. This mutation is not included in the WHO surveillance list due to its polymorphic nature, but it has recently been included in algorithms as generating low-level resistance to rilpivirine and is present in 1 in 25 patients in our study. In vitro data from site-directed mutagenesis indicate that E138A causes a 2-fold reduction in sensitivity to rilpivirine [21, 22]. Although this is generally considered a small change in vitro, there are limited clinical data on its effect in vivo. In phase 3 clinical trials of rilpivirine, patients infected with a virus that harbored E138A were excluded [23]. Patients who failed rilpivirine-based regimens commonly developed E138K and/or M184I mutations [24]. Considering the relatively low dose and drug levels of rilpivirine, it cannot be excluded that the E138A mutation may be relevant in vivo.
In general, the prevalence of TDR to PIs was low, which was reflected in almost all patients being predicted to be fully susceptible to regimens including boosted atazanavir or boosted darunavir. The low and stable prevalence of TDR to PIs may be a result of the change in treatment guidelines to NNRTIs and INSTIs as preferred first-line regimens and the high genetic barrier of boosted PIs. The GSS does not take into account the potency and genetic barrier of a drug, as the same score is given to all drugs to which the virus is predicted to be susceptible. A score of 1 for a fully susceptible PI is likely to be an underestimation of its potency compared with drugs from other classes.
The strength of this study is the longitudinal collection of data from a large number of countries. By using the same sampling strategy over the years, analyses over time can be performed. The large sample size also allows for analyses within risk groups or subtypes, which may not be possible at a country level. Unlike some other cohorts, we did not limit our analyses to recently infected patients. This could be considered a limitation as mutations may revert over time and could therefore be missed in a cohort that also includes chronically infected patients. On the other hand, newly diagnosed patients represent those coming into care. Recently infected patients, from our data, are more often MSM (possibly due to higher frequency of HIV testing in this group), so only including recently infected patients would not be representative of the entire HIV-infected population. Another limitation is the unavailability of integrase sequence data. As INSTIs are more frequently used in first-line regimens, surveillance of TDR associated with INSTIs becomes more relevant. However, available studies indicate that the prevalence is still low [25, 26].
In conclusion, TDR is stable at around 8% in Europe. Although TDR was highest for NRTIs, the impact on drug susceptibility of the mutations detected was greatest for NNRTIs. The WHO list for surveillance of drug resistance mutations is specifically designed to identify mutations that provide clear evidence of drug exposure in a previous host and are therefore indicators of TDR. However, caution is warranted as relevant polymorphic resistance–related mutations are not included and some mutations that are listed may have only limited clinical impact. Prevalence figures from epidemiological surveys therefore may not directly translate to susceptibility of HIV to antiretroviral drugs in clinical practice. Our data underline that the impact of baseline mutation patterns on drug susceptibility should be assessed using clinical algorithms or guidelines.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the patients and doctors throughout Europe for their consent and support for the study.
Financial support. This work was supported by a CORE grant of Fond National de la Recherche Luxembourg (grant number C12/BM/4011111–HIV molecular epidemiology in Europe). This work has been partially supported by the European Commission (fifth framework, grant number QLK2-CT-2001-01344; sixth framework, grant number LSHP-CT-2006-518211, DynaNets grant number 233847; seventh framework, CHAIN grant number 223131); Belgium: Belgian AIDS Reference Laboratory Fund, Belgian Fonds voor Wetenschappelijk Onderzoek (grant number G.0692.14); Cyprus: Cyprus Research Promotion Foundation (grant number Health/0104/22); Denmark: Danish AIDS Foundation; France: Agence Nationale de Recherches sur le SIDA et les Hepatites Virales; Germany: Ministry of Health (grant number 1502-686-18), Ministry of Education and Research (grant number 01KI501); Italy: Fifth National Program on HIV/AIDS, Instituto Superiore di Sanità (grant numbers 40F.56 and 20D.1.6); Luxembourg: Fondation Recherche sur le SiDA and Ministry of Health; Republic of Serbia: Ministry of Education and Science (grant number 175024); Slovakia: project “Center of Excellence of Environmental Health,” ITMS number 26240120033, based on supporting operational research and development program financed from the European Regional Development Fund; and Sweden: Swedish Research Council and Swedish Civil Contingencies Agency.
SPREAD program investigators. Austria: E. Puchhammer-Stockl, M. Sarcletti, B. Schmied, M. Geit, and G. Balluch. Belgium: A.-M. Vandamme, J. Vercauteren, I. Derdelinckx, A. Sasse, M. Bogaert, H. Ceunen, A. De Roo, S. De Wit, F. Echahidi, K. Fransen, J.-C. Goffard, P. Goubau, E. Goudeseune, J.-C. Yombi, P. Lacor, C. Liesnard, M. Moutschen, D. Pierard, R. Rens, Y. Schrooten, D. Vaira, L. P. R. Vandekerckhove, A. Van den Heuvel, B. Van Der Gucht, M. Van Ranst, E. Van Wijngaerden, B. Vandercam, M. Vekemans, C. Verhofstede, N. Clumeck, and K. Van Laethem. Bulgaria: D. Beshkov and I. Alexiev. Croatia: S. Zidovec Lepej and J. Begovac. Cyprus: L. Kostrikis, I. Demetriades, I. Kousiappa, V. Demetriou, and J. Hezka. Czech Republic: M. Linka, M. Maly, and L. Machala. Denmark: C. Nielsen, L. B. Jørgensen, J. Gerstoft, L. Mathiesen, C. Pedersen, H. Nielsen, A. Laursen, and B. Kvinesdal. Finland: K. Liitsola, M. Ristola, J. Suni, and J. Sutinen. France: D. Descamps, L. Assoumou, G. Castor, M. Grude, P. Flandre, and A. Storto. Germany: O. Hamouda, C. Kücherer, T. Berg, P. Braun, G. Poggensee, M. Däumer, J. Eberle, H. Heiken, R. Kaiser, H. Knechten, K. Korn, H. Müller, S. Neifer, B. Schmidt, H. Walter, B. Gunsenheimer-Bartmeyer, and T. Harrer. Greece: D. Paraskevis, A. Hatzakis, A. Zavitsanou, A. Vassilakis, M. Lazanas, M. Chini, A. Lioni, V. Sakka, S. Kourkounti, V. Paparizos, A. Antoniadou, A. Papadopoulos, G. Poulakou, I. Katsarolis, K. Protopapas, G. Chryssos, S. Drimis, P. Gargalianos, G. Xylomenos, G. Lourida, M. Psichogiou, G. L. Daikos, Ν. V. Sipsas, A. Kontos, M. N. Gamaletsou, G. Koratzanis, Η. Sambatakou, H. Mariolis, A. Skoutelis, V. Papastamopoulos, O. Georgiou, P. Panagopoulos, and E. Maltezos. Ireland: S. Coughlan, C. De Gascun, C. Byrne, M. Duffy, C. Bergin, D. Reidy, G. Farrell, J. Lambert, E. O'Connor, A. Rochford, J. Low, P. Coakely, S. O'Dea, and W. Hall. Israel: O. Mor, I. Levi, D. Chemtob, and Z. Grossman. Italy: M. Zazzi, A. de Luca, C. Balotta, C. Riva, C. Mussini, I. Caramma, A. Capetti, M. C. Colombo, C. Rossi, F. Prati, F. Tramuto, F. Vitale, M. Ciccozzi, G. Angarano, and G. Rezza. Latvia: T. Kolupajeva and O. Vasins. Lithuania: A. Griskevicius and V. Lipnickiene. Luxembourg: J. C. Schmit, D. Struck, N. Sauvageot, R. Hemmer, V. Arendt, C. Michaux, T. Staub, and C. Sequin-Devaux. The Netherlands: A. M. J. Wensing, C. A. B. Boucher, D. A. M. C. van de Vijver, A. van Kessel, P. H. M. van Bentum, K. Brinkman, B. J. Connell, M. E. van der Ende, I. M. Hoepelman, M. van Kasteren, M. Kuipers, N. Langebeek, C. Richter, R. M. W. J. Santegoets, L. Schrijnders-Gudde, R. Schuurman, and B. J. M. van de Ven. Norway: B. Åsjö, A.-M. Bakken Kran, V. Ormaasen, and P. Aavitsland. Poland: A. Horban, J. J. Stanczak, G. P. Stanczak, E. Firlag-Burkacka, A. Wiercinska-Drapalo, E. Jablonowska, E. Maolepsza, M. Leszczyszyn-Pynka, and W. Szata. Portugal: R. Camacho, C. Palma, F. Borges, T. Paixão, V. Duque, and F. Araújo on behalf of the Portuguese SPREAD Network. Romania: D. Otelea, S. Paraschiv, A. M. Tudor, R. Cernat, C. Chiriac, F. Dumitrescu, and L. J. Prisecariu. Republic of Serbia: M. Stanojevic, Dj. Jevtovic, and D. Salemovic. Slovakia: D. Stanekova, M. Habekova, Z. Chabadová, T. Drobkova, P. Bukovinova, A. Shunnar, and P. Truska. Slovenia: M. Poljak, M. Lunar, D. Babic, J. Tomazic, L. Vidmar, T. Vovko, and P. Karner. Spain: F. Garcia, R. Paredes, S. Monge, S. Moreno, J. del Amo, V. Asensi, J. L. Sirvent, C. de Mendoza, R. Delgado, F. Gutiérrez, J. Berenguer, S. Garcia-Bujalance, N. Stella, I. de los Santos, J. R. Blanco, D. Dalmau, M. Rivero, F. Segura, M. J. Pérez Elías, M. Alvarez, N. Chueca, C. Rodríguez-Martín, C. Vidal, J. C. Palomares, I. Viciana, P. Viciana, J. Cordoba, A. Aguilera, P. Domingo, M. J. Galindo, C. Miralles, M. A. del Pozo, E. Ribera, J. A. Iribarren, L. Ruiz, J. de la Torre, F. Vidal, and B. Clotet. Sweden: J. Albert, A. Heidarian, K. Aperia-Peipke, M. Axelsson, M. Mild, A. Karlsson, A. Sönnerborg, A. Thalme, L. Navér, G. Bratt, A. Karlsson, A. Blaxhult, M. Gisslén, B. Svennerholm, I. Bergbrant, P. Björkman, C. Säll, Å. Mellgren, A. Lindholm, N. Kuylenstierna, R. Montelius, F. Azimi, B. Johansson, M. Carlsson, E. Johansson, B. Ljungberg, H. Ekvall, A. Strand, S. Mäkitalo, S. Öberg, P. Holmblad, M. Höfer, H. Holmberg, P. Josefson, and U. Ryding.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the SPREAD Program, E. Puchhammer-Stockl, M. Sarcletti, B. Schmied, M. Geit, G. Balluch, A.-M. Vandamme, J. Vercauteren, I. Derdelinckx, A. Sasse, M. Bogaert, H. Ceunen, A. De Roo, S. De Wit, F. Echahidi, K. Fransen, J.-C. Goffard, P. Goubau, E. Goudeseune, J.-C. Yombi, P. Lacor, C. Liesnard, M. Moutschen, D. Pierard, R. Rens, Y. Schrooten, D. Vaira, L. P. R. Vandekerckhove, A. Van den Heuvel, B. Van Der Gucht, M. Van Ranst, E. Van Wijngaerden, B. Vandercam, M. Vekemans, C. Verhofstede, N. Clumeck, K. Van Laethem, D. Beshkov, I. Alexiev, S. Zidovec Lepej, J. Begovac, L. Kostrikis, I. Demetriades, I. Kousiappa, V. Demetriou, J. Hezka, M. Linka, M. Maly, L. Machala, C. Nielsen, L. B. Jørgensen, J. Gerstoft, L. Mathiesen, C. Pedersen, H. Nielsen, A. Laursen, B. Kvinesdal, K. Liitsola, M. Ristola, J. Suni, J. Sutinen, D. Descamps, L. Assoumou, G. Castor, M. Grude, P. Flandre, A. Storto, O. Hamouda, C. Kücherer, T. Berg, P. Braun, G. Poggensee, M. Däumer, J. Eberle, H. Heiken, R. Kaiser, H. Knechten, K. Korn, H. Müller, S. Neifer, B. Schmidt, H. Walter, B. Gunsenheimer-Bartmeyer, T. Harrer, D. Paraskevis, A. Hatzakis, A. Zavitsanou, A. Vassilakis, M. Lazanas, M. Chini, A. Lioni, V. Sakka, S. Kourkounti, V. Paparizos, A. Antoniadou, A. Papadopoulos, G. Poulakou, I. Katsarolis, K. Protopapas, G. Chryssos, S. Drimis, P. Gargalianos, G. Xylomenos, G. Lourida, M. Psichogiou, G. L. Daikos, N. V. Sipsas, A. Kontos, M. N. Gamaletsou, G. Koratzanis, H. Sambatakou, H. Mariolis, A. Skoutelis, V. Papastamopoulos, O. Georgiou, P. Panagopoulos, E. Maltezos, S. Coughlan, C. De Gascun, C. Byrne, M. Duffy, C. Bergin, D. Reidy, G. Farrell, J. Lambert, E. O'Connor, A. Rochford, J. Low, P. Coakely, S. O'Dea, W. Hall, O. Mor, I. Levi, D. Chemtob, Z. Grossman, M. Zazzi, A. de Luca, C. Balotta, C. Riva, C. Mussini, I. Caramma, A. Capetti, M. C. Colombo, C. Rossi, F. Prati, F. Tramuto, F. Vitale, M. Ciccozzi, G. Angarano, G. Rezza, T. Kolupajeva, O. Vasins, A. Griskevicius, V. Lipnickiene, J. C. Schmit, D. Struck, N. Sauvageot, R. Hemmer, V. Arendt, C. Michaux, T. Staub, C. Sequin-Devaux, A. M. J. Wensing, C. A. B. Boucher, D. A. M. C. van de Vijver, A. van Kessel, P. H. M. van Bentum, K. Brinkman, B. J. Connell, M. E. van der Ende, I. M. Hoepelman, M. van Kasteren, M. Kuipers, N. Langebeek, C. Richter, R. M. W. J. Santegoets, L. Schrijnders-Gudde, R. Schuurman, B. J. M. van de Ven, B. Åsjö, A.-M. Bakken Kran, V. Ormaasen, P. Aavitsland, A. Horban, J. J. Stanczak, G. P. Stanczak, E. Firlag-Burkacka, A. Wiercinska-Drapalo, E. Jablonowska, E. Maolepsza, M. Leszczyszyn-Pynka, W. Szata, R. Camacho, C. Palma, F. Borges, T. Paixão, V. Duque, F. Araújo, D. Otelea, S. Paraschiv, A.M. Tudor, R. Cernat, C. Chiriac, F. Dumitrescu, L. J. Prisecariu, M. Stanojevic, Dj. Jevtovic, D. Salemovic, D. Stanekova, M. Habekova, Z. Chabadová, T. Drobkova, P. Bukovinova, A. Shunnar, P. Truska, M. Poljak, M. Lunar, D. Babic, J. Tomazic, L. Vidmar, T. Vovko, P. Karner, F. Garcia, R. Paredes, S. Monge, S. Moreno, J. del Amo, V. Asensi, J. L. Sirvent, C. de Mendoza, R. Delgado, F. Gutiérrez, J. Berenguer, S. Garcia-Bujalance, N. Stella, I. de los Santos, J. R. Blanco, D. Dalmau, M. Rivero, F. Segura, M. J. Pérez Elías, M. Alvarez, N. Chueca, C. Rodríguez-Martín, C. Vidal, J. C. Palomares, I. Viciana, P. Viciana, J. Cordoba, A. Aguilera, P. Domingo, M.J. Galindo, C. Miralles, M. A. del Pozo, E. Ribera, J. A. Iribarren, L. Ruiz, J. de la Torre, F. Vidal, B. Clotet, J. Albert, A. Heidarian, K. Aperia-Peipke, M. Axelsson, M. Mild, A. Karlsson, A. Sönnerborg, A. Thalme, L. Navér, G. Bratt, A. Karlsson, A. Blaxhult, M. Gisslén, B. Svennerholm, I. Bergbrant, P. Björkman, C. Säll, Å. Mellgren, A. Lindholm, N. Kuylenstierna, R. Montelius, F. Azimi, B. Johansson, M. Carlsson, E. Johansson, B. Ljungberg, H. Ekvall, A. Strand, S. Mäkitalo, S. Öberg, P. Holmblad, M. Höfer, H. Holmberg, P. Josefson, and U. Ryding
References
- 1.Little SJ, Holte S, Routy JP et al. . Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347:385–94. [DOI] [PubMed] [Google Scholar]
- 2.Violin M, Cozzi-Lepri A, Velleca R et al. . Risk of failure in patients with 215 HIV-1 revertants starting their first thymidine analog-containing highly active antiretroviral therapy. AIDS 2004; 18:227–35. [DOI] [PubMed] [Google Scholar]
- 3.Kuritzkes DR, Lalama CM, Ribaudo HJ et al. . Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis 2008; 197:867–70. [DOI] [PubMed] [Google Scholar]
- 4.Wittkop L, Gunthard HF, de Wolf F et al. . Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11:363–71. [DOI] [PubMed] [Google Scholar]
- 5.European AIDS Clinical Society. EACS guidelines version 8.0. Available at: http://www.eacsociety.org/files/guidelines_8_0-english_web.pdf. Accessed 29 Octobre 2015.
- 6.Gunthard HF, Aberg JA, Eron JJ et al. . Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society–USA Panel. JAMA 2014; 312:410–25. [DOI] [PubMed] [Google Scholar]
- 7.Wensing AM, Calvez V, Gunthard HF et al. . 2014 Update of the drug resistance mutations in HIV-1. Top Antivir Med 2014; 22:642–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DE, Camacho RJ, Otelea D et al. . Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009; 4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wensing AM, van de Vijver DA, Angarano G et al. . Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. J Infect Dis 2005; 192:958–66. [DOI] [PubMed] [Google Scholar]
- 10.Wensing AM, Vercauteren J, van de Vijver D et al. . Transmission of drug-resistant HIV-1 in Europe remains limited to single classes. AIDS 2008; 22:625–35. [DOI] [PubMed] [Google Scholar]
- 11.Vercauteren J, Wensing AM, van de Vijver DA et al. . Transmission of drug-resistant HIV-1 is stabilizing in Europe. J Infect Dis 2009; 200:1503–8. [DOI] [PubMed] [Google Scholar]
- 12.Frentz D, Van de Vijver DA, Abecasis AB et al. . Increase in transmitted resistance to non-nucleoside reverse transcriptase inhibitors among newly diagnosed HIV-1 infections in Europe. BMC Infect Dis 2014; 14:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frentz D, van de Vijver D, Abecasis A et al. . Patterns of transmitted HIV drug resistance in Europe vary by risk group. PLoS One 2014; 9:e94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Struck D, Lawyer G, Ternes AM, Schmit JC, Perez Bercoff D. COMET: adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res 2014; 42:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang MW, Liu TF, Shafer RW. The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology 2012; 55:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control/ WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2010. Available at: http://ecdc.europa.eu/en/publications/Publications/20121130-Annual-HIV-Surveillance-Report.pdf. Accessed 17 September 2015. [Google Scholar]
- 17.Gupta RK, Jordan MR, Sultan BJ et al. . Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little SJ, Frost SD, Wong JK et al. . Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J Virol 2008; 82:5510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castor D, Low A, Evering T et al. . Transmitted drug resistance and phylogenetic relationships among acute and early HIV-1-infected individuals in New York City. J Acquir Immune Defic Syndr 2012; 61:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burchell AN, Bayoumi AM, Rourke SB et al. . Increase in transmitted HIV drug resistance among persons undergoing genotypic resistance testing in Ontario, Canada, 2002–09. J Antimicrob Chemother 2012; 67:2755–65. [DOI] [PubMed] [Google Scholar]
- 21.Xu HT, Colby-Germinario SP, Asahchop EL et al. . Effect of mutations at position E138 in HIV-1 reverse transcriptase and their interactions with the M184I mutation on defining patterns of resistance to nonnucleoside reverse transcriptase inhibitors rilpivirine and etravirine. Antimicrob Agents Chemother 2013; 57:3100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picchio GR, Rimsky LT, Van Eygen V, Haddad M, Napolitano LA, Vingerhoets J. Prevalence in the USA of rilpivirine resistance-associated mutations in clinical samples and effects on phenotypic susceptibility to rilpivirine and etravirine. Antivir Ther 2014; 19:819–23. [DOI] [PubMed] [Google Scholar]
- 23.Molina JM, Cahn P, Grinsztejn B et al. . Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet 2011; 378:238–46. [DOI] [PubMed] [Google Scholar]
- 24.Rimsky L, Van Eygen V, Hoogstoel A et al. . 96-week resistance analyses of rilpivirine in treatment-naive, HIV-1-infected adults from the ECHO and THRIVE phase III trials. Antivir Ther 2013; 18:967–77. [DOI] [PubMed] [Google Scholar]
- 25.Cossarini F, Boeri E, Canducci F et al. . Integrase and fusion inhibitors transmitted drug resistance in naive patients with recent diagnosis of HIV-1 infection. J Acquir Immune Defic Syndr 2011; 56:e51–4. [DOI] [PubMed] [Google Scholar]
- 26.Stekler JD, McKernan J, Milne R et al. . Lack of resistance to integrase inhibitors among antiretroviral-naive subjects with primary HIV-1 infection, 2007–2013. Antivir Ther 2015; 20:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.