In an open-label, randomized controlled trial conducted to determine whether regular screening and treatment of asymptomatic bacterial vaginosis with oral metronidazole reduced the incidence of gonorrhea and/or chlamydia, no significant difference was found between treatment and observation alone.
Keywords: bacterial vaginosis, STD, home screening
Abstract
Background. Longitudinal studies have consistently found a significant association between bacterial vaginosis (BV) and acquisition of sexually transmitted diseases. However, there are limited prospective data to confirm these findings.
Methods. We conducted a prospective, randomized, open-label trial of home screening and treatment of young women with asymptomatic BV who were also at high risk for sexually transmitted diseases. These women were screened every 2 months for 12 months and randomized to treatment with oral metronidazole 500 mg twice daily for 7 days or observation alone. The primary outcome was the incidence of gonorrhea and/or chlamydia.
Results. A total of 1365 subjects were enrolled in the study across 10 sites. Adherence with mailing specimens obtained at home was excellent in both groups (84%–88%). The incidence of gonorrhea and/or chlamydia was 19.1 per 100 person-years (95% confidence interval, 15.1–22.1) for the treatment group and 18.5 per 100 person-years (15.1–22.8) for the observation arm, a difference that was not statistically significant.
Conclusions. Young women were very amenable to home screening for BV, gonorrhea, and chlamydia. Treatment of asymptomatic BV with 1 week of oral metronidazole did not decrease the incidence of gonorrhea and/or chlamydia.
Clinical Trials Registration. NCT00667368.
Bacterial vaginosis (BV) is characterized by a shift in the vaginal bacterial flora from lactobacillus predominant to lactobacilli depleted and high concentrations of bacteria such as Gardnerella vaginalis, and anaerobes [1]. Although BV may cause symptoms of vaginal discharge and odor, a significant number of women are asymptomatic [2, 3]. Currently, treatment is not recommended for the latter group [4]. BV is significantly associated with acquisition of sexually transmitted diseases (STDs), including chlamydia and gonorrhea [5–8], but there have been few published clinical trials to determine whether screening and treatment of asymptomatic BV will reduce this risk [9]. We conducted a clinical trial to determine whether regular screening and treatment for asymptomatic BV reduces the 1-year incidence of chlamydia and gonorrhea.
METHODS
Women aged 15–25 years were recruited from 10 sites (STD, family planning, obstetrics-gynecology, and clinical research clinics) in 6 geographic locations (Baltimore, Maryland; Birmingham, Alabama; Durham and Raleigh, North Carolina; Pittsburgh, Pennsylvania; and San Francisco, California). All sites were approved to conduct the study by their local institutional review boards. To qualify for participation, women must have had vaginal intercourse in the past 3 months and also had ≥2 of the following risk factors for STDs: age ≤20 years, African American race, Hispanic ethnicity, douching at least once per month, ≥2 sex partners in the past 12 months, and STD diagnosis in the past year (self-reported or from clinic notes) [10–12]. These criteria were designed to ensure that subjects were at increased risk for STDs. In addition, participants had to have asymptomatic BV, defined as having 2 of the 4 clinical criteria commonly used to diagnose BV (vaginal pH >4.5% and >20% clue cells on vaginal wet preparation microscopy) and no endorsement of abnormal vaginal discharge or odor. Compared with microbiological criteria for BV (Nugent criteria), elevated pH and clue cells have a specificity of 92%; thus, most women with these 2 criteria would meet criteria for BV [13]. Women were excluded if they were pregnant, used antibiotics daily, lived with their sexual partner (due to lower risk of future STDs), were homeless, were unwilling to abstain from alcohol during treatment, were allergic to metronidazole, had seizure disorder or liver disease, took cimetidine or warfarin, had undergone hysterectomy, were currently menstruating, or had trichomoniasis in addition to BV.
Recruitment
Women were informed about the study by contact with a recruiter working in the community, referral from a clinician, or a study brochure. Some participating clinics conducted wet mount screening of asymptomatic women as part of routine care, and women with asymptomatic BV were asked if they were interested in the study. At other sites, a screening informed consent was obtained to determine whether the woman had asymptomatic BV. Screening was conducted from vaginal swabs that were either self-obtained after receiving instruction from study staff or obtained by the clinician. For women with asymptomatic BV who were interested in the study, full informed consent was obtained, followed by assessment of all eligibility criteria.
Randomization
Women were randomized into either the treatment group or the control group (observation), using a computer-generated block randomization scheme stratified by site. Assignments were concealed using sealed envelopes at each site. Women randomized to treatment were provided oral metronidazole at enrollment, 500 mg twice daily for 7 days. When a follow-up home-testing sample was positive for BV (Nugent score, 7–10 [14]), subjects in the treatment group were informed of their results and provided the same 7-day treatment for BV either by mail or by pick-up at the clinic. Women in the control group did not receive notification of BV status or treatment for BV, which is consistent with usual care for asymptomatic BV. Subjects in both groups were encouraged to see their healthcare provider if they developed genitourinary symptoms, and women were allowed to receive any recommended treatments received outside the study protocol. Women in both groups with positive chlamydia and/or gonorrhea results were notified and referred to a healthcare provider or a public STD clinic to receive treatment. This was an open-label study; however, the primary end point, positive chlamydia and/or gonorrhea results, was determined at central laboratories that were masked to randomization assignment.
Enrollment and Follow-up
At enrollment, subjects completed a baseline questionnaire to assess demographic factors, sexual risk behavior, contraceptive methods, and health history. Subjects provided 2 vaginal swabs (self-obtained or obtained by a clinician). One swab was rolled across a microscope slide, and another was placed in a transport tube for gonorrhea and chlamydia testing. Subjects received a home testing kit in the mail every 2 months for 12 months, including 2 vaginal swabs, an instruction sheet for swab collection, a microscope slide and holder, and a prelabeled, stamped return envelope. For BV evaluation, women obtained a vaginal swab sample and rolled the swab across a prelabeled glass slide in a prepackaged slide holder. Self-testing for BV with vaginal swabs has excellent reliability and validity compared with clinician-obtained swabs [15, 16]. Swabs for chlamydia and gonorrhea testing were provided at 4, 8, and 12 months after study entry. With each home testing kit, subjects completed a brief survey that assessed sexual risk behavior, contraceptive use, as well as any treatment for BV outside the study. We did not collect information about symptoms with the follow-up testing. Subjects mailed their specimens and questionnaires through the US mail to the study laboratory. In the laboratory, slides were interpreted for Nugent score by technicians blinded to treatment assignment, swabs were tested for chlamydia and gonorrhea using the BDProbeTec assay, and study questionnaires were sent to the central data management site. Study research staff regularly contacted subjects to remind them to send in the testing kits. Subjects received a financial incentive for each follow-up kit submitted. The laboratory notified research staff of results from BV, chlamydia, and gonorrhea testing.
Sample Size Calculations
The planned enrollment was 1500 eligible subjects, from which 1200 were expected to complete the study, reflecting an expected loss to follow-up rate of 20%. Sample size and power calculations were based on the primary study outcome: the 1-year STD infection rate which counted each occurrence of a positive chlamydia or gonorrhea infection during the follow-up period in estimating the infection rate. This approach allowed the distinction between women who had a single STD and those who had multiple positive STD tests. We considered a 2-sided significance level (α value) of .05; an estimated proportion of 15% and 20% of subjects in the treatment and observation groups, respectively, having any chlamydial and gonococcal infections during the 12-month follow-up; and an estimated 30% of subjects with 1 STD who would acquire an additional infection during the follow-up period. Assuming the STD incidence rate above and a Poisson distribution, the study had >80% power to detect an absolute difference of 7 infections per 100 person-years in STD incidence rates (13 vs 20 infections per 100 person-years). The cumulative proportion of subjects who had ≥1 STD detected was estimated for both groups using the Kaplan–Meier method, and its 95% confidence interval (CI) was estimated using Greenwood's formula and compared using log-rank tests. The sample size had >90% power to detect a 5% difference in the cumulative proportion of persons with an STD (10% vs 15%) at 12 months.
Interim Analyses
Interim analyses were prespecified and conducted after one-third and two-thirds of the study subjects had completed study participation, with stopping rules for superiority based on the Lan-DeMets spending function with the O'Brien-Fleming boundary to maintain an overall .05 significance level. In July 2013, an ad hoc meeting of the Data and Safety Monitoring Board was held to review accrual and to discuss results from a futility analysis based on 1265 subjects. The Board recommended that recruitment be terminated because further accrual would not significantly contribute to the outcome of the study.
Statistical Analysis Plan
To assess the primary study outcome, the incidence of chlamydia and/or gonorrhea in the 2 groups was estimated as Poisson rates, and the difference between them tested for statistical significance at the .05 significance level. In addition, the Cox proportional hazards model was used to compare the time to first STD. The event time was considered the midpoint between the date of the positive test collection and the date of prior test collection, with the exception of baseline. Baseline STDs were not included in the incidence rate calculation. Subject characteristics and the proportion of subjects experiencing adverse events were compared according to arm using the Cochran–Mantel–Haenszel test stratified by site (categorical variables) or analysis of variance with adjustment for site (continuous variables). The primary analysis population for efficacy and safety was the intent-to-treat (ITT) population, which included all subjects randomized. Confirmatory analyses was performed with the per-protocol (PP) population, which was defined as all randomized subjects who completed 12 months of follow-up and returned ≥4 of the 6 home testing kits. This definition was based on the assumption that 100% adherence with kits was unlikely; thus, it was decided to include those who adhered the majority of the time.
RESULTS
Enrollment Data
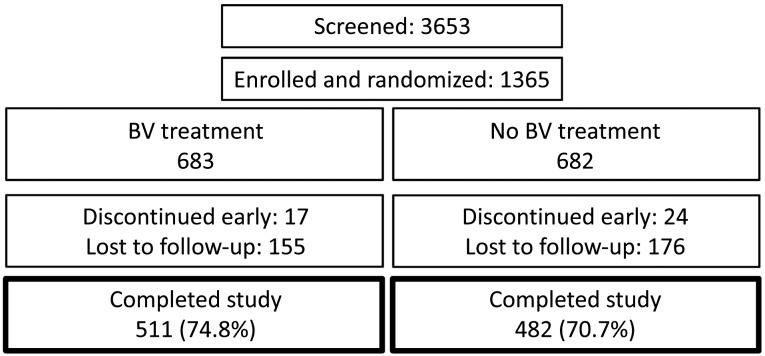
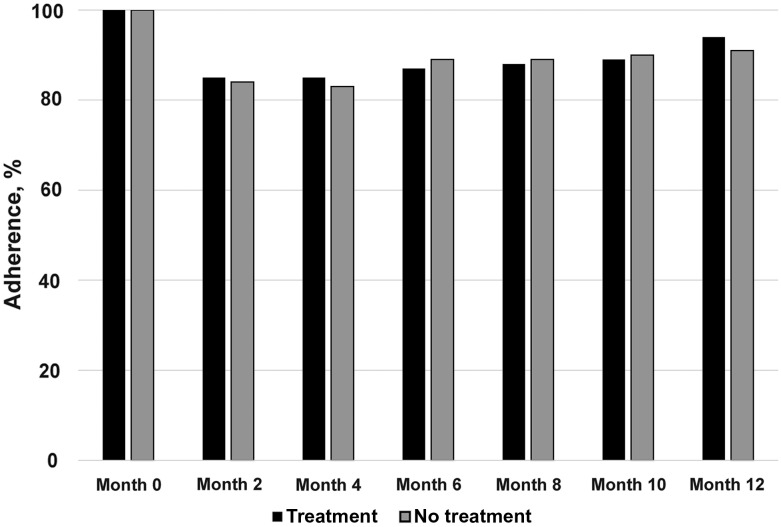
There were 1370 enrollments to the study; however, 5 subjects were enrolled twice and data from their second enrollment was removed from both ITT and PP populations, leaving 1365 subjects in the ITT population. Of these, 986 subjects met criteria for the PP population. Of 1365 subjects enrolled in the trial (Figure 1), 683 were randomized to treatment and 682 to observation. Owing to early discontinuation or loss to follow-up of 172 treatment subjects and 200 observation subjects, 511 (74.8%) and 482 (70.7%) completed the study, respectively. The early discontinuation or loss to follow-up rate was 25% for the treatment arm and 29% for the observation arm (P = .09). The median number of visits was 7 for both arms (P = .76). Comparison of women who completed the study with those who discontinued early showed the latter to be slightly younger (20.8 vs 21.4 years; P = .04) and to have significantly higher rates of STD at the baseline visit (1% vs 3%; P = .004). Regardless of completion status, data from all evaluable visits for all randomized subjects were analyzed in the ITT analysis. Table 1 shows the baseline characteristics of the ITT population stratified by study arm. The 2 arms did not significantly differ in terms of baseline subject characteristics. At follow-up visits, kit completion rate ranged from 84% to 88% across arms, with no difference in adherence between treatment arms (Figure 2).
Figure 1.
Participant flow. BV, bacterial vaginosis.
Table 1.
Baseline Characteristics
| Characteristic | All Subjects (N = 1365) | BV Treatment (n = 683) | Observation (n = 682) | P Valuea |
|---|---|---|---|---|
| Age, mean (SD), y | 21 (2) | 21 (2) | 21(2) | .93 |
| Race, No. (%) | ||||
| Black | 1065 (78) | 530 (78) | 535 (78) | .09 |
| White | 110 (8) | 65 (10) | 45 (7) | |
| Otherb | 190 (14) | 88 (13) | 102 (15) | |
| Times ever treated for BV, No. (%) | ||||
| 0 | 756 (55) | 385 (56) | 371 (54) | .12 |
| 1 | 272 (20) | 123 (18) | 149 (22) | |
| 2–4 | 242 (18) | 123 (18) | 119 (17) | |
| ≥5 | 75 (5) | 45 (7) | 30 (4) | |
| Unknown | 20 (1) | 7 (1) | 13 (2) | |
| No. of vaginal sex partners, No. (%) | ||||
| 0 | 86 (6) | 42 (6) | 44 (6) | .73 |
| 1 | 646 (47) | 328 (48) | 318 (47) | |
| ≥2 | 633 (46) | 313 (46) | 320 (47) | |
| New sexual partners in past year, No. (%) | ||||
| No | 680 (50) | 335 (49) | 345 (51) | .56 |
| Yes | 685 (50) | 348 (51) | 337 (49) | |
| BV results, No. (%)c | ||||
| BV present (NS, 7–10) | 1192 (87) | 598 (88) | 594 (87) | .37 |
| Intermediate flora (NS, 4–6) | 140 (10) | 74 (11) | 66 (10) | |
| Normal flora (NS, 0–3) | 24 (2) | 9 (1) | 15 (2) | |
| Missing | 8 (1) | 2 (<1) | 6 (1) | |
| Gonorrhea, No. (%)c | ||||
| Positive | 63 (5) | 37 (5) | 26 (4) | .15 |
| Negative | 1301 (95) | 646 (95) | 655 (96) | |
| Chlamydia, No. (%)c | .81 | |||
| Positive | 191 (14) | 94 (14) | 97 (14) | |
| Negative | 1173 (86) | 589 (86) | 584 (86) | |
| STD, No. (%)c | ||||
| Both gonorrhea and chlamydia | 22 (2) | 12 (2) | 10 (1) | .86 |
| Gonorrhea or chlamydia | 210 (15) | 107 (16) | 103 (15) | |
| Neither | 1132 (83) | 564 (83) | 568 (83) | |
Abbreviations: BV, bacterial vaginosis; NS, Nugent score; SD, standard deviation; STD, sexually transmitted disease.
a Analysis of variance adjusting for site (age) or stratified (by site) Cochran–Mantel–Haenszel test.
b The “Other” category comprised multiracial (n = 102), missing race (n = 55), Hawaiian/Pacific Islander (n = 13), Asian (n = 16), and Native American (n = 4).
c The total for these categories is 1364 instead of 1365 because baseline laboratory data were lost for 1 participant.
Figure 2.
Adherence to kit collection.
1-Year Incidence of Chlamydia and Gonorrhea
The 1-year incidence rate of gonorrhea or chlamydia was 18.3 (95% CI, 15.1–22.1) per 100 person-years for the BV treatment arm and 19.2 (15.9–23.2) per 100 person-years for the observation arm in the ITT population (Table 2). This risk difference of −0.9 (95% CI, −12.0 to 10.1) was not statistically significant (P = .75). The cumulative proportion of subjects with a diagnosis of gonorrhea and/or chlamydia infection by 12 months was 11.7 (95% CI, 9.3–14.7) for the BV treatment arm and 12.2 (9.7–15.3) for the observation arm (P = .80). Twenty-three subjects (2%) had multiple visits with a positive STD test. There was no difference in reported regular use of condoms during the course of the study, with 22% reporting regular use in the treatment arm versus 24% in the control arm.
Table 2.
Sexually Transmitted Disease Follow-up Event Rates and Intent-to-Treat Population Total Follow-up for the Cohort (1137.4 Person-years in 1365 Subjects)
| Randomization Arm | Gonorrhea |
Chlamydia |
Gonorrhea or Chlamydia |
|||
|---|---|---|---|---|---|---|
| BV Treatment (n = 683) | No BV Treatment (n = 682) | BV Treatment (n = 683) | No BV Treatment (n = 682) | BV Treatment (n = 683) | No BV Treatment (n = 682) | |
| Total follow-up, person-years | 580.0 | 557.4 | 580.0 | 557.4 | 580.0 | 557.4 |
| Total visits with any STD, No. | 26 | 19 | 80 | 88 | 106 | 107 |
| Rate per 100 person-years (95% CI) | 4.5 (3.1–6.6) | 3.4 (2.2–5.3) | 13.8 (11.1–17.2) | 15.8 (12.8–19.5) | 18.3 (15.1–22.1) | 19.2 (15.9–23.2) |
| Rate difference (95% CI) | 1.1 (−4.0 to 6.1) (P = .46) | −2.0 (−11.8 to 7.8) (P = .47) | −0.9 (−12.0 to 10.1) (P = .75) | |||
Abbreviations: BV, bacterial vaginosis; CI, confidence interval; STD, sexually transmitted disease.
Prevalence of BV
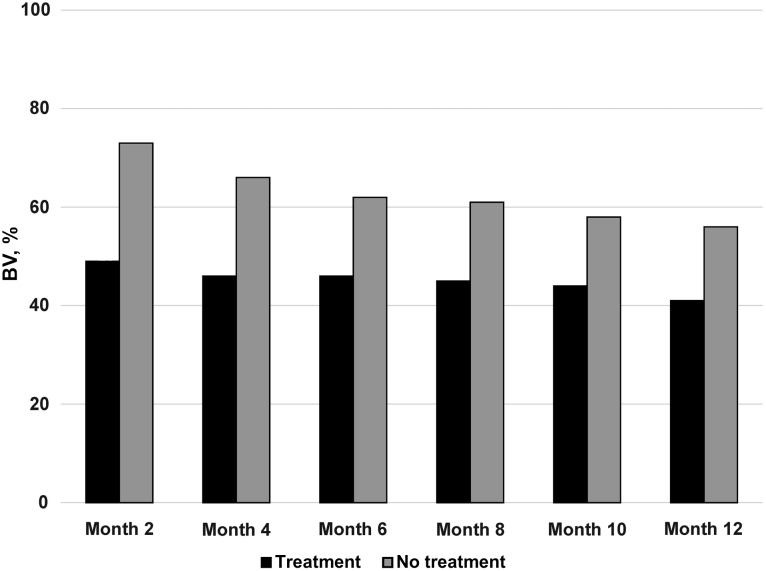
The proportion of subjects with BV at any follow-up visit differed significantly according to study arm, with ≥1 diagnosis of BV occurring in 83% of subjects in the BV treatment arm and 93% in the observation arm (P < .001). Considering the presence of BV across all visits, after adjustment for the prior visit's BV test result, age, race/ethnicity, and sexual history, the odds of BV being diagnosed at a follow-up visit in the treatment arm was nearly half that in the observation arm (odds ratio, 0.53; 95% CI, .46–.61; P < .001) (Figure 3). There was no significant difference in the percentage of women who reported treatment for BV outside the study protocol (11% vs 13% in the treatment and observation arms, respectively; P = .15).
Figure 3.
Acquisition, recurrence, and persistence of bacterial vaginosis (BV).
Analysis of Safety Data
There were 9 and 3 serious adverse events in the BV treatment and observation arms, respectively. None of these was deemed to be associated with use of metronidazole. The most common adverse event was vulvovaginal candidiasis, reported by 3% of subjects in both arms, and urinary tract infections, reported by 2% in the treatment arm and 3% in the observation arm. Thirty subjects (4.4%) experienced gastrointestinal events in the treatment arm and 12 (1.8%) in the observation arm (P = .005).
DISCUSSION
BV is the most common form of vaginitis and is linked to serious sequelae, such as preterm birth and acquisition/transmission of STD [8, 17, 18]. Although treatment recommendations differentiate between women who report symptoms and those who do not [4], this differentiation is probably somewhat arbitrary. In a large study of vaginal flora patterns, 63% of women with BV denied having vaginal discharge and/or odor and the difference in reported vaginal symptoms between women with and without BV was small [3]. To our knowledge, there are no published studies on differences in sequelae between asymptomatic and symptomatic BV. However, adverse outcomes linked to BV are probably caused by alterations in the vaginal flora that are seen in both [18, 19]. Current recommendations for withholding therapy for asymptomatic BV allowed us to ethically randomize women to observation alone [4].
We hypothesized that screening and treatment for asymptomatic BV would prevent STDs by restoring optimal vaginal flora, thus reducing susceptibility to STDs. This hypothesis is supported by studies consistently demonstrating an association between BV and an increased prevalence and incidence of STDs and human immunodeficiency virus (HIV) infection [6–8, 20, 21].
We are aware of only 2 prospective studies addressing whether treatment of asymptomatic BV prevents STD acquisition [9]. In one trial, treatment of BV with metronidazole gel followed by twice-weekly gel as BV prophylaxis reduced the incidence of chlamydia [9]. A recent, secondary analysis from a trial of intravaginal metronidazole and miconazole [22], showed a trend toward lower incidence of chlamydia and gonorrhea in the treatment arm compared with the placebo arm [23].
Although STDs were common in our study, (nearly 20 infections/100 woman-years), we found no difference in the incidence of chlamydia or gonorrhea during 1 year of follow-up. Women randomized to treatment for asymptomatic BV had lower rates of future BV, an expected finding. The lack of reduction in the incidence of chlamydia and gonorrhea suggests either that asymptomatic BV is not causally linked to a woman's increased risk of STDs or that cure rates with metronidazole or duration of cure were insufficient to protect against STDs. In terms of risk associated with treatment of asymptomatic BV, we found that the overall rate of adverse events was low and did not differ substantially between treatment and observation arms, except for gastrointestinal side effects.
Several limitations of our study warrant mention. It was not possible to blind participants or local research staff to randomization arm. However, we do not believe that this knowledge would affect a participant's risk of acquiring an STD. Laboratory staff evaluating the main outcome were blind to study assignment. We do not have information on adherence to treatment with metronidazole among women in the treatment arm. Lack of adherence to a treatment regimen is an issue on clinical trials as demonstrated in a study of preexposure prophylaxis for HIV infection [24]. Adherence in our study may have been affected by the fact that these women had no symptoms to alleviate as well as by the unpleasant taste of metronidazole. Although the study conducted testing for chlamydia and gonorrhea every 4 months, we did not obtain information regarding chlamydial or gonococcal infections diagnosed outside the trial. In another trial of home screening for chlamydia and gonorrhea, subjects received an average of 1 to 2 STD tests per year outside the testing protocol [25]. However, the nearly identical rates of STD diagnoses within our trial make it unlikely that a significant difference in overall STD rates would be found, even if these additional test results were known and included. Furthermore, this analysis only considered the effect of BV treatment on chlamydial and gonococcal infections and did not include other STDs, such as trichomonas or HIV infection.
In summary, this is the largest study to ever evaluate the impact of treatment of BV on STD outcomes and demonstrated that women were very compliant with the submission of self-collected vaginal specimens mailed every 2 months. However, based on this study, treatment of asymptomatic BV with oral metronidazole does not affect the incidence of gonorrhea or chlamydia. If more effective therapies for BV become available, consideration should be given to revisiting this approach.
Notes
Acknowledgments. We gratefully acknowledge the support of Jonathan Glock, National Institute of Allergy and Infectious Diseases (NIAID) Clinical Project Manager.
Financial support. This work was supported by the Division of Microbiology and Infectious Diseases of the NIAID (contract HHSN26620040073C).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hillier SL. Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol 1993; 169:455–9. [DOI] [PubMed] [Google Scholar]
- 2.Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988; 158:819–28. [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff MA, Schwebke JR, Zhang J, Nansel TR, Yu KF, Andrews WW. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol 2004; 104:267–72. [DOI] [PubMed] [Google Scholar]
- 4.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Ness RB, Kip KE, Soper DE et al. Bacterial vaginosis (BV) and the risk of incident gonococcal or chlamydial genital infection in a predominantly black population. Sex Transm Dis 2005; 32:413–7. [DOI] [PubMed] [Google Scholar]
- 6.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003; 36:663–8. [DOI] [PubMed] [Google Scholar]
- 7.Martin HL, Richardson BA, Nyange PM et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. [DOI] [PubMed] [Google Scholar]
- 8.Brotman RM, Klebanoff MA, Nansel TR et al. Bacterial vaginosis assessed by Gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 2010; 202:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol 2007; 196:517.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaydos CA, Howell MR, Pare B et al. Chlamydia trachomatis infections in female military recruits. N Engl J Med 1998; 339:739–44. [DOI] [PubMed] [Google Scholar]
- 11.Stergachis A, Scholes D, Heidrich FE, Sherer DM, Holmes KK, Stamm WE. Selective screening for Chlamydia trachomatis infection in a primary care population of women. Am J Epidemiol 1993; 138:143–53. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CS, Shepherd BE, Vermund SH. Does douching increase risk for sexually transmitted infections? a prospective study in high-risk adolescents. Am J Obstet Gynecol 2009; 200:38.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutman RE, Peipert JF, Weitzen S, Blume J. Evaluation of clinical methods for diagnosing bacterial vaginosis. Obstet Gynecol 2005; 105:551–6. [DOI] [PubMed] [Google Scholar]
- 14.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson DB, Bellamy S, Gray TS, Nachamkin I. Self-collected versus provider-collected vaginal swabs for the diagnosis of bacterial vaginosis: an assessment of validity and reliability. J Clin Epidemiol 2003; 56:862–6. [DOI] [PubMed] [Google Scholar]
- 16.Strauss RA, Eucker B, Savitz DA, Thorp JM Jr. Diagnosis of bacterial vaginosis from self-obtained vaginal swabs. Infect Dis Obstet Gynecol 2005; 13:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eschenbach DA. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity—a review. Arch Gynecol Obstet 1993; 16:S282–7. [DOI] [PubMed] [Google Scholar]
- 18.Hillier S. The vaginal microbiol ecosystem and resistance to HIV. AIDS 1998; 14:17–21. [PubMed] [Google Scholar]
- 19.Eschenbach DA. Bacterial vaginosis and anaerobes in obstetric-gynecologic infection. Clin Infect Dis 1993; 16(suppl 4):S282–7. [DOI] [PubMed] [Google Scholar]
- 20.Balkus JE, Richardson BA, Rabe LK et al. Bacterial vaginosis and the risk of trichomonas vaginalis acquisition among HIV-1-negative women. Sex Transm Dis 2014; 41:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo MF, Macaluso M, Warner L et al. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol 2012; 22:213–20. [DOI] [PubMed] [Google Scholar]
- 22.McClelland RS, Balkus JE, Lee J et al. Randomized trial of periodic presumptive treatment with high-dose intravaginal metronidazole and miconazole to prevent vaginal infections in HIV-negative women. J Infect Dis 2015; 211:1875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balkus J, Anzala O, Kimani J et al. Periodic presumptive treatment for vaginal infections may reduce chlamydia and gonorrhea incidence: a secondary analysis from the Preventing Vaginal Infections trial. In: World STI and HIV Congress, Brisbane, Australia, 13–16 September 2015. [Google Scholar]
- 24.Marrazzo JM, Ramjee G, Richardson BA et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook RL, Ostergaard L, Hillier SL et al. Home screening for sexually transmitted diseases in high-risk young women: randomised controlled trial. Sex Transm Infect 2007; 83:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]