Dalbavancin when delivered as a single 1500-mg infusion is noninferior to the same total dose given as a 2-dose regimen, removing the logistical constraints related to the second dose while improving compliance and patient convenience.
Keywords: dalbavancin, skin infection, efficacy, methicillin-resistant Staphylococcus aureus, clinical trial
Abstract
Background. Acute bacterial skin and skin structure infections (ABSSSIs) are a cause of significant morbidity and therapy can be a burden to the healthcare system. New antibiotics that simplify treatment and avoid hospitalization are needed. This study compared the safety and efficacy of a single intravenous infusion of 1500 mg of dalbavancin to the 2-dose regimen.
Methods. This study was a randomized, double-blind trial in patients aged >18 years with ABSSSIs. Patients were randomized to dalbavancin 1500 mg either as a single intravenous (IV) infusion or 1000 mg IV on day 1 followed 1 week later by 500 mg IV. The primary endpoint was a ≥20% reduction in the area of erythema at 48–72 hours in the intent-to-treat population. Noninferiority was to be declared if the lower limit of the 95% confidence interval (CI) on the difference in the outcomes was greater than −10%. Clinical outcome was also assessed at days 14 and 28.
Results. Six hundred ninety-eight patients were randomized. Demographic characteristics were similar on each regimen, although there were more patients with methicillin-resistant Staphylococcus aureus (MRSA) at baseline on the 2-dose regimen (36/210 [17.1%] vs 61/220 [27.7%]). Dalbavancin delivered as a single dose was noninferior to a 2-dose regimen (81.4% vs 84.2%; difference, −2.9% [95% CI, −8.5% to 2.8%]). Clinical outcomes were also similar at day 14 (84.0% vs 84.8%), day 28 (84.5% vs 85.1%), and day 14 in clinically evaluable patients with MRSA in a baseline culture (92.9% vs 95.3%) in the single- and 2-dose regimens, respectively. Treatment-emergent adverse events occurred in 20.1% of the single-dose patients and 19.9% on the 2-dose regimen.
Conclusions. A single 1500-mg infusion of dalbavancin is noninferior to a 2-dose regimen, has a similar safety profile, and removes logistical constraints related to delivery of the second dose.
Clinical Trials Registration. NCT02127970.
Acute bacterial skin and skin structure infection (ABSSSI) remains a significant cause of morbidity in both the hospital and outpatient settings [1]. Gram-positive pathogens are responsible for the majority of cases of ABSSSI, a significant proportion of which in the United States are methicillin-resistant Staphylococcus aureus (MRSA) [2, 3]. Therapy is largely empiric and must address the spectrum of possible pathogens, penetration into the affected tissue, safety, and efficacy outcomes with a route of administration dictated largely by the setting of patient care [4, 5].
Dalbavancin is a lipoglycopeptide antibiotic with activity against the gram-positive pathogens responsible for ABSSSI, including MRSA. Its pharmacokinetic/pharmacodynamic properties would predict that larger doses given early in the course of therapy would be most beneficial [6, 7]. Currently approved therapy is given as 2 doses 1 week apart, a regimen made possible by its terminal half-life of 15.5 days. Based on the principle that treatment for infection should be given early in the course of therapy while the bacterial burden is highest and the likelihood of compliance is greatest [8], further studies to optimize the treatment course were warranted. The objective of this clinical trial was to compare the efficacy and safety of the entire treatment course of dalbavancin given in a single infusion with the established 2-dose regimen.
METHODS
Study Conduct
This study was a double-blind, pharmacist-unblinded, randomized trial conducted between April 2014 and March 2015 at 60 centers in the United States, Eastern Europe, Russia, and South Africa. The protocol and informed consent form were reviewed by the institutional review boards at each center, and all patients provided written informed consent.
Study Patients
Patients enrolled in the study had an ABSSSI described as either a major abscess, cellulitis, or traumatic wound/surgical site infection. All patients were required to have an area of erythema of at least 75 cm2 associated with the infection. A major abscess by definition required surgical incision and drainage and the erythema was to extend for at least 5 cm in all directions beyond the central area of induration. A traumatic wound/surgical site infection required 5 cm of erythema beyond the margin of the wound. In addition to erythema, patients were required to have other local signs or symptoms such as warmth, tenderness/pain, fluctuance, swelling, and drainage, as well as at least 1 systemic sign including a white blood cell count >12 000 cells/µL, ≥10% immature neutrophils on peripheral smear, or a temperature ≥38°C within the prior 24 hours. Patients could not have taken antibiotics in the prior 14 days except for a single dose of a short-half-life drug. There was no exclusion for patients with liver function abnormalities or renal insufficiency. Excluded were patients with catheter infection, infected devices, diabetic foot ulceration, perirectal abscess, or decubitus ulcer.
Randomization and Intervention
Patients were assigned to either of the study-drug regimens by an unblinded pharmacist through an interactive Web-based randomization system in a 1:1 fashion, with a block size of 4. Randomization was stratified by geographic location, subtype of ABSSSI (with <30% of patients having a major abscess), and administration in the previous 14 days of a single dose of a short-half-life antibiotic (capped at <25% of those enrolled). Patients received dalbavancin as either a single intravenous infusion of 1500 mg of dalbavancin over 30 minutes or in 2 doses as 1000 mg intravenously over 30 minutes followed 1 week later by 500 mg intravenously. Dosing was adjusted by the unblinded pharmacist for patients not on dialysis with a creatinine clearance of <30 mL/minute such that those randomized to the single-dose regimen received 1000 mg as a single infusion and those randomized to the 2-dose regimen received 750 mg intravenously followed 1 week later by 375 mg intravenously. Patients randomized to the 1500-mg single dose received a placebo infusion on day 8. Metronidazole 500 mg intravenously or orally every 8 hours was allowed in both treatment groups for infection with suspected anaerobic pathogens, and aztreonam was allowed for the empiric treatment of ABSSSI or for a gram-negative pathogen identified in culture postbaseline. Patients could receive therapy as inpatients or outpatients.
Endpoints
The primary endpoint was a comparison of the proportion of patients in the intent-to-treat (ITT) population who achieved a ≥20% reduction in the size of the erythema 48–72 hours (±3 hours) after initiation of study drug and did not receive rescue antibacterial therapy. Preplanned sensitivity analyses included an assessment at 36–72 hours (+3 hours), a modified ITT (mITT) analysis including only those receiving study drug, and adjustments to the confidence interval (CI) based on the stratification variables. Clinical status, defined as improvement in lesion size as well as resolution or improvement of clinical signs and symptoms, was performed at day 14 and day 28. Investigator assessments were also collected at these timepoints.
The clinically evaluable (CE) population includes patients who met all of the required inclusion criteria and did not meet any of the exclusion criteria, did not have a bacteremia at baseline with a gram-negative pathogen, received dalbavancin as randomized, received for a non-ABSSSI indication no more than 1 dose of another systemic antibacterial that has documented activity against the causative organism, had an assessment in the time window, and received appropriate adjunctive antibacterial coverage if the patient had a culture-documented ABSSSI with 1 or more gram-negative aerobic or anaerobic organisms.
Safety data were collected at each visit through day 28 and included adverse events as well as chemistry and hematology test results and physical examinations.
Statistical Analysis
The initial proposed sample size of 410 patients was based on the method of Farrington and Manning [9], assuming a noninferiority margin of 10%, power of 90%, a 1-sided α level of .025, and a 90% treatment response rate [10], estimated from the Dalbavancin for Infections of the Skin Compared to Vancomycin at an Early Response (DISCOVER) studies [11]. To test the assumption of a 90% response rate, a prespecified interim analysis was performed when approximately 240 patients had been enrolled, at which time the aggregate, blinded response rate at 48–72 hours was 82%, leading to a recommendation by the independent, blinded data monitoring committee to increase the sample size to 698 patients to maintain adequate power for noninferiority testing.
Noninferiority of the single-dose regimen compared to the 2-dose regimen was to be declared if the lower limit of the 95% CI for the difference in response rates for the primary endpoint in the ITT population was greater than −10%. The ITT population is defined as all randomized patients. Other patient populations included a mITT population of randomized patients who received a dose of study drug in whom all safety analyses were performed; a CE population of patients who met all the inclusion criteria and none of the exclusion criteria, received the correct study drug, received appropriate additional antibacterial therapy if culture confirmed a gram-negative pathogen, and met minimum dosing requirements; and a microbiologic ITT and a microbiologically evaluable population in which patients had a pathogen identified at baseline and met either ITT or CE requirements, respectively. P values for the analysis of demographic characteristics and safety analyses were performed using Fisher's exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Cochran–Mantel–Haenszel weights were used for the stratum weights in the calculation of the 95% CI. In the ITT analyses, patients with missing data were considered failures whereas in the CE analyses, patients with missing data were not included. Safety data were coded using version 17.1 of the Medical Dictionary for Regulatory Activities (MedDRA Maintenance and Support Services Organization). Version 9.3 of the SAS statistical software package was used for statistical analyses.
RESULTS
Patients
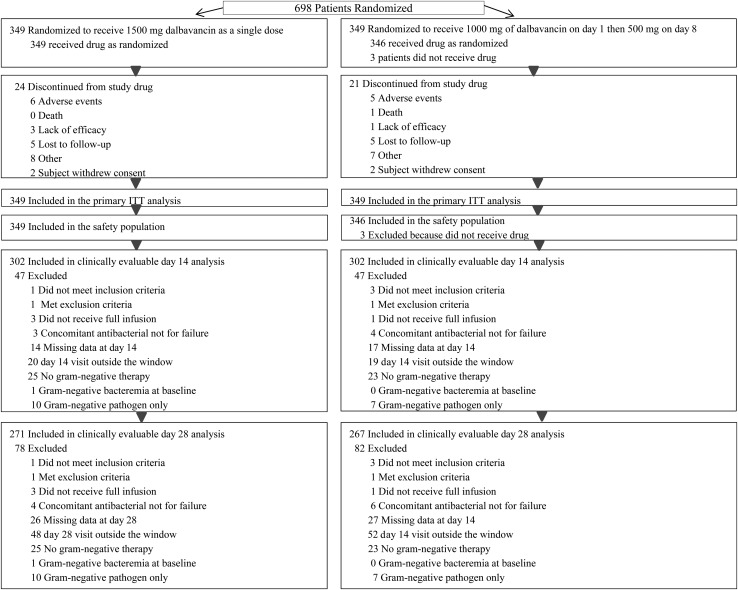
Six hundred ninety-eight patients were randomized to either a single dose of dalbavancin or the 2-dose regimen (Figure 1); 695 patients received study drug and are included in the safety analyses. A total of 99.6% (695/698) of patients received the first dose on day 1 and 93.1% (650/698) on day 8 (P < .0001), with the same number receiving a day 8 dose of active or placebo (325/349 [93.1%] on each regimen). Eighty-seven percent were included in the CE population at day 14.
Figure 1.
Disposition of patients and analysis sets. Abbreviation: ITT, intent to treat.
The treatment regimens were well balanced with regard to demographic and other baseline characteristics (Table 1). Diabetes mellitus was present in 11.5% of patients, and an additional 5.0% of patients without a history of diabetes had a random glucose level at baseline >11.1 mmol/L (200 mg/dL). A total of 30.4% of patients were intravenous drug users and 16.2% had hepatitis C. Cellulitis was the subtype of infection in 48% of patients, a major abscess in 25%, and a traumatic wound/surgical site infection in 27%, similarly distributed among regimens. Systemic signs of infection at baseline included temperature >38°C in 82% of patients, elevated white blood cell count in 37%, and immature neutrophils in 15%. Forty-three percent of patients had at least 2 signs required for a diagnosis of systemic inflammatory response syndrome (SIRS). A pathogen was isolated from a baseline culture in 61.6% of patients overall, with more patients having MRSA in the 2-dose regimen (36/210 [17.1%] vs 61/220 [27.7%] in the single-dose and 2-dose regimens, respectively; P = .011). Based on follow-up cultures taken after baseline, no patient developed a resistant organism on therapy. Twenty-two patients (6.3%) in the single-dose group and 19 patients (5.4%) in the 2-dose group received a systemic antibacterial agent in the 14 days prior to study drug assignment. A similar number of patients received concomitant aztreonam on the single-dose (12 [3.4%]) and 2-dose regimens (23 [6.6%]). Metronidazole was used by 6.6% and 4.3% of patients through day 14 on the single- and 2-dose regimens, respectively.
Table 1.
Demographics and Baseline Patient and Disease Characteristics in the Intent-to-Treat Population
| Characteristic | Dalbavancin Treatment |
|
|---|---|---|
| Single-Dose (n = 349) | 2-Dose (n = 349) | |
| Age, y, mean (SD) | 48.0 (14.8) | 48.3 (14.7) |
| Female sex | 145 (41.5) | 146 (41.8) |
| Race | ||
| White | 312 (89.4) | 311 (89.1) |
| Black or African American | 28 (8.0) | 31 (8.9) |
| Other | 9 (2.6) | 7 (2.0) |
| Hepatitis C | 49 (14.0) | 64 (18.3) |
| Current or former intravenous drug use, % | 105 (30.1) | 107 (30.7) |
| Diabetes | 38 (10.9) | 42 (12.0) |
| BMI, kg/m2 | ||
| Mean (SD) | 28.7 (7.5) | 29.0 (7.3) |
| Median (Min, Max) | 26.9 (15.9, 70.6) | 27.8 (17.9, 65.5) |
| BMI distribution | ||
| <25 kg/m2 | 115 (33.0) | 122 (35.0) |
| 25–30 kg/m2 | 123 (35.2) | 99 (28.4) |
| >30 kg/m2 | 111 (31.8) | 128 (36.7) |
| Location of trial center | ||
| North America | 158 (45.3) | 160 (45.8) |
| Rest of world | 191 (54.7) | 189 (54.2) |
| Cellulitis | 167 (47.9) | 166 (47.6) |
| Major abscess | 86 (24.6) | 89 (25.5) |
| Traumatic wound/surgical site infection | 96 (27.5) | 94 (26.9) |
| White blood cell count >12 000 cells/µL | 132 (37.9) | 126 (36.8) |
| Temperature ≥38°C at baseline | 290 (83.1) | 283 (81.8) |
| Immature neutrophils ≥10% | 56 (21.3) | 46 (17.2) |
| Median infection area, cm2 (range) | 296.1 (56.0–4235.0) | 293.3 (76.5–2668.0) |
| Systemic inflammatory response syndrome | 148 (42.4) | 154 (44.4) |
| Pathogen at baseline | 210 (60.2) | 220 (63.0) |
| MRSA, no./No. (%)* | 36/210 (17.1) | 61/220 (27.7) |
| MSSA, no./No. (%) | 103/210 (49.0) | 96/220 (43.6) |
| Gram-negative aerobic organism, no./No. (%) | 19/210 (9.0) | 28/220 (12.7) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; SD, standard deviation.
* P = .011.
Efficacy Outcomes
Clinical response at 48–72 hours was demonstrated in 81.4% of those randomized to the single-dose regimen vs 84.2% in the 2-dose regimen. The absolute difference was −2.9% and the lower limit of the CI on that difference was greater than −10%, allowing the single-dose regimen to be declared noninferior to the 2-dose regimen (Table 2). The most common reason for failure was a result of not achieving a 20% reduction in lesion area followed by missing data in the 48- to 72-hour window. Expanding the window of observation earlier to 36 hours narrowed the treatment difference.
Table 2.
Clinical Response at Early and Late Timepoints
| Timepoint | Dalbavancin Treatment Group |
||
|---|---|---|---|
| Single-Dose, no./No. (%) | 2-Dose, no./No. (%) | Differencea (95% CI) | |
| 48–72 h | |||
| Treatment response (ITT) | 284/349 (81.4) | 294/349 (84.2) | −2.9 (−8.5, 2.8) |
| Treatment nonresponder or indeterminate | 65/349 (18.6) | 55/349 (15.8) | |
| Death | 0 | 1/55 (1.8) | |
| Antibacterial therapy for ABSSSI | 4 /65 (6.2) | 4/55 (7.3) | |
| Decrease of <20% in lesion area | 41/65 (63.1) | 34/55 (61.8) | |
| Missing lesion data | 22/65 (33.8) | 18/55 (32.7) | |
| Lesion area data outside window | 15/65 (23.1) | 11/55 (20.0) | |
| Treatment response (mITT) | 284/349 (81.4) | 294/346 (85.0) | −3.6 (−9.2, 2.0) |
| Treatment response at 36–75 hours (ITT) | 293/349 (84.0) | 298/349 (85.4) | −1.4 (−6.8, 4.0) |
| Day 14 | |||
| Clinical success (ITT) | 293/349 (84.0) | 296/349 (84.8) | −0.9 (−6.3, 4.6) |
| Clinical success (CE) | 267/302 (88.4) | 270/302 (89.4) | −1.0 (−6.1, 4.1) |
| Day 28 | |||
| Clinical success (ITT) | 295/349 (84.5) | 297/349 (85.1) | −0.6 (−6.0, 4.8) |
| Clinical success (CE) | 250/271 (92.3) | 247/267 (92.5) | −0.3 (−4.9, 4.4) |
| Investigator assessment of cure | |||
| Clinical response, day 14 (CE) | 292/302 (96.7) | 292/301 (97.0) | −0.3 (−3.4, 2.7) |
| Clinical response, day 28 (CE) | 263/271 (97.0) | 258/266 (97.0) | 0.1 (−3.1, 3.2) |
Data are presented as No. of patients with the respective observation/No. of patients in the respective population.
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; CE, clinically evaluable; CI, confidence interval; ITT, intent to treat; mITT, modified intent-to-treat.
a For the difference in clinical response rates (single-dose group minus 2-dose group).
Clinical success rates as well as the investigator assessment of response at day 14 and day 28 were similar between regimens, and adjustments based on stratification variables did not change the CI. Subset analyses in the CE population at day 28 did not demonstrate any difference between the single- and 2-dose regimens by SIRS at baseline (104/114 [91.2%] vs 108/117 [92.3%], respectively). In the ITT subpopulation of patients with a history of intravenous drug use, clinical success at 48–72 hours on the single- and 2-dose regimens was seen in 94 of 105 (89.5%) and 92 of 107 (86.0%) (difference, 3.5% [95% CI, −5.6% to 12.7%]) patients, respectively, and in the CE population at end of treatment in 78 of 87 (89.7%) and 78 of 85 (91.8%) (difference, −2.1% [95% CI, −11.5% to 7.1%) patients, respectively.
Overall, 318 of 698 (45.6%) of patients enrolled in the trial were hospitalized and 379 of 698 (54.3%) were treated as outpatients. The median duration of hospitalization was 8.0 days for patients randomized to either the single- or 2-dose regimen. Outcome rates for those patients hospitalized were similar to those treated completely as outpatients (260/312 [83.3%] vs 318/386 [82.4%], respectively), and outcome rates for the outpatients treated with the single- and 2-dose regimens were similar (156/190 [82.1%] and 162/196 [82.7%], respectively). Similar findings in these populations were observed at the day 14 and day 28 visits.
Of the 49 patients receiving either the single- or 2-dose regimen who had a gram-negative pathogen identified at baseline that did not receive additional gram-negative antibacterial coverage, the success rate was 89.7% at 48–72 hours in the ITT population and 88.9% at day 14 in the CE population.
Clinical success by baseline pathogen was also similar for each regimen (Table 3). The dalbavancin mean inhibitory concentration to inhibit 90% of organisms (MIC90) for S. aureus was 0.06 µg/mL. No organisms had an MIC to vancomycin >2 µg/mL. Methicillin resistance in S. aureus did not affect the outcome of patients treated with dalbavancin. At 48–72 hours, 7 of 8 patients on the single-dose regimen and all 7 patients on the 2-dose regimen with S. aureus bacteremia were clinical responders and all had documented clearance of their bacteremia.
Table 3.
Clinical Status at Day 14 by Pathogen in Clinically Evaluable Patients With a Monomicrobial Infection at Baseline
| Pathogen | Dalbavancin Treatment Group |
|
|---|---|---|
| Single-Dose, no./No. (%) | 2-Dose, no./No. (%) | |
| All target pathogens | 109/121 (90.1) | 128/134 (95.5) |
| Gram-positive aerobe | ||
| Staphylococcus aureus | 85/94 (90.4) | 101/105 (96.2) |
| MRSA | 26/28 (92.9) | 41/43 (95.3) |
| MSSA | 59/66 (89.4) | 60/62 (96.8) |
| Streptococcus agalactiae | 2/2 (100.0) | 1/2 (50.0) |
| Streptococcus anginosus group | 14/17 (82.4) | 9/9 (100.0) |
| Streptococcus anginosus | 2/3 (66.7) | 1/1 (100.0) |
| Streptococcus constellatus | 3/3 (100.0) | 2/2 (100.0) |
| Streptococcus intermedius | 9/11 (81.8) | 6/6 (100.0) |
| Streptococcus dysgalactiae | 0 | 2/2 (100.0) |
| Streptococcus pyogenes | 6/6 (100.0) | 11/12 (91.7) |
| Streptococcus mitis | 4/4 (100.0) | 4/4 (100.0) |
| Enterococcus faecalis | 2/2 (100.0) | 4/4 (100.0) |
Data are presented as no. of clinical responders/No. of patients with the specified organism.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Safety
The number of patients with a treatment-emergent adverse event (TEAE), drug-related TEAE, serious TEAE, a TEAE leading to premature discontinuation of study drug, or the number of deaths was similar on each regimen (Table 4). Nausea was the only event reported in >2% of patients in either arm. Headache and vomiting were the only other events seen in >1% of the pooled population. No significant difference was observed in the rate of a TEAE occurring in the first 12 hours after infusion of either the 1500-mg or the 1000-mg dose (24/349 [6.9%] vs 15/346 [4.3%], respectively; P = .14) or the type of TEAE observed. The number of patients with laboratory abnormalities of serum chemistry or hematology was similar on each treatment regimen.
Table 4.
Patients With Treatment-Emergent Adverse Events
| Adverse Event | Dalbavancin Treatment Group |
|
|---|---|---|
| Single-Dose, No. (%) (n = 349) | 2-Dose, No. (%) (n = 346) | |
| Patients experiencing ≥1 of: | ||
| TEAE | 70 (20.1) | 69 (19.9) |
| Drug-related TEAE | 25 (7.2) | 26 (7.5) |
| Serious TEAE | 7 (2.0) | 5 (1.4) |
| Death | 1 (0.3) | 1 (0.3) |
| TEAE leading to premature discontinuation of study drug | 6 (1.7) | 5 (1.4) |
| TEAE >1% | ||
| Nausea | 12 (3.4) | 7 (2.0) |
| Headache | 6 (1.7) | 4 (1.2) |
| Vomiting | 6 (1.7) | 3 (0.9) |
| Diarrhea | 4 (1.1) | 2 (0.6) |
| Dizziness | 4 (1.1) | 0 (0.0) |
| Cellulitis | 1 (0.3) | 5 (1.4) |
| Chills | 0 (0.0) | 4 (1.2) |
| Localized infection | 0 (0.0) | 5 (1.4) |
Abbreviation: TEAE, treatment-emergent adverse event.
DISCUSSION
The primary purpose of this study was to determine whether a 1500-mg dose of dalbavancin delivered as a single infusion would result in safety and efficacy outcomes similar to the same total dose given as 1000 mg on day 1 and 500 mg on day 8. An analysis of the primary endpoint of ≥20% reduction at 48–72 hours confirmed that the single dose is noninferior to the 2-dose regimen and that the early activity of each regimen was sustained at day 14 and day 28, confirming results from prior studies [11, 12]. By day 14, patients in both regimens had received 1500 mg of drug and the additional increase in serum levels on day 8, as a consequence of the 500 mg intravenous infusion, was not found to be essential to a sustained clinical response.
The pooled point estimate of success was somewhat lower at the interim analysis than projected, requiring an increase in the sample size to maintain 90% power. The pooled point estimate in the DISCOVER studies [11] was 88% and the response rate at the interim was 82%. Some of the reason for this difference was that 3% of patients had their primary endpoint assessment slightly earlier than the 48- to 72-hour window specified in the protocol and were subsequently considered ITT failures due to missing data in the window. Almost all of these patients were a clinical success, however, and a broadened window to assess response at 36–72 hours, as seen in Table 1, increased the response rate to 85%, closer to outcomes in the previous trials.
Thirty percent of the patients in this study had a history of intravenous drug abuse. The treatment outcome for these patients was similar on each regimen and slightly higher than the overall population, likely a result of a higher incidence of major abscess in this subpopulation, which tends to resolve more quickly than cellulitis [11]. Given these encouraging clinical success rates and the absence of a requirement for an indwelling peripheral or central line, dalbavancin may be an attractive option for treatment of skin infections in this patient population.
No significant increase in the adverse event rate, including adverse events within the first 12 hours, was observed when the total dose was delivered at one time, consistent with studies in phase 1 volunteers [13], even though the concentration of dalbavancin in the infusate is higher in patients receiving the 1500-mg dose.
The option to use a single- or 2-dose regimen of dalbavancin introduces flexibility into the treatment decision for ABSSSIs. Some patients are less likely to return for follow-up than others, and this study documents that compliance with a single-dose regimen is superior to that of even a very simple weekly, 2-dose regimen. For other patients, especially those with a history of intravenous drug use, prolonged placement of an intravenous catheter is associated with the potential for abuse [14]; the robust clinical response rates for intravenous drug users in this study would support the single-dose treatment approach and ease the logistical burden associated with their care. Physicians can now make a rational choice between a single-dose regimen and a weekly regimen that recognizes a patient's social circumstance and tailors the regimen for that individual patient's needs. Delivery over 30 minutes is also a significant advantage that simplifies implementation in busy emergency rooms.
This study once again documents that 90% of patients with culturable material associated with an ABSSSI have an infection due to a gram-positive pathogen with only 5% of patients receiving concomitant gram-negative therapy, significantly lower than the 60%–80% of patients getting broad-spectrum gram-negative coverage in clinical practice [15, 16]. Patients with a gram-negative pathogen isolated at baseline who did not receive gram-negative therapy had outcome rates similar to the group overall. These data provide support for antibiotic stewardship programs focused on more judicious use of broad-spectrum therapy, even for those patients seriously ill with ABSSSI.
There are limitations to this study. Although the sample size provided >90% power to determine noninferiority, it was not powered to detect superiority if the difference in successful outcomes was small. The noninferiority margins established by this study, however, do provide confidence that any difference in efficacy between the 1500-mg and 1000-mg doses at day 3 is likely to be of limited clinical significance. Also, whereas the definitions of cellulitis, major abscess, and wound infection used in this study are consistent with US Food and Drug Administration guidance, clinicians may define these subtypes of skin infection differently in clinical practice. In addition, as selected by these definitions, the patients in this study were significantly ill and may not fully reflect the cross-section of illness severity encountered outside of a clinical trial.
In conclusion, when delivered as a single 1500-mg infusion for treatment of patients with ABSSSI, dalbavancin is noninferior to the same total dose delivered as 2 infusions 1 week apart and is associated with a similar adverse event profile.
Notes
Acknowledgments. The authors recognize the contributions of the patients who volunteered to participate in this study, as well as the investigators: Igor Abramov, Konstantin Apartsin, Anna Barvinska, Sadia Dar, Oleksiy Datsenko, Douwe de Jong, Fareed Dindar, Mirjana Djordjevic, Hermanus JC du Plessis, Olga Dulovic, Robert Eyzaguirre, Brett Farley, Jan Fourie, Bruce Friedman, Mashra Gani, Philip Giordano, Carmen Giuglea, Sergey Goryunov, Erekle Gotsadze, Sinikka Green, Barry Heller, Luis Jauregui Peredo, Heidi Kabler, Aleksandar Karanikolic, Richard Keech, Lajos Kemeny, Jeff Kingsley, Kirill Korkorin, Sergii Kosulnykov, Bojan Kovacevic, Ivars Krastins, Dainis Krievins, Liudmila Lenskaya, Nodar Lomidze, Paul Manos, Silviu Marinescu, Elena Matevosyan, Jurijs Miscuks, Essack Mitha, Jeanne Nel, Nikolajs Novikovs, Steven O'Mara, Remus Orasan, Scott Overcash, Harald Plaudis, John Pullman, Oleksandr Pyptiuk, Shaukat Shah, Sergiy Shapoval, Vadym Shevchenko, Lubov Shpagina, Visnja Skerk, Mohammed Tayob, Juri Teras, Gia Tomadze, Tiit Vaasna, Jano Vashadze, Sergiy Vasylyuk, David Ware, Marcus Zervos. We also acknowledge the contribution of the Data Management Committee: Thomas F. Patterson, David Talan, and Chitra Lele.
Author contributions. M. W. D., S. P., and M. Z. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M. W. D.: design and conduct of the trial; interpretation of the data; preparation of the first draft of the manuscript. S. P.: conduct of the trial; interpretation of the data; participation in the drafting of the manuscript. J. B.: design and conduct of the trial; interpretation of the data; participation in the drafting of the manuscript. M. Z.: design of statistical methodologies of the trial; interpretation of the data; participation in the drafting of the manuscript. P. G. and D. K.: investigators in the study; contributed to the review and interpretation of the data and preparation of the manuscript. The employee-authors of the study were responsible for the design and conduct of the clinical trial as well as the data analysis and interpretation; all also contributed to the review and preparation of the manuscript.
Financial support. This work was supported by Durata Therapeutics and Allergan plc.
Potential conflicts of interest. M. W. D., S. P., and M. Z. report holding stock in Durata Therapeutics and were employees of Actavis at the time of publication; P. G. held stock and has been on the speakers’ bureau for Durata; J. B. was an employee of Durata Therapeutics and held stock in the company; D. K. has received research support from Durata. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pallin DJ, Egan DJ, Pelletier AJ et al. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:292–8. [DOI] [PubMed] [Google Scholar]
- 2.David MZ, Medvedev S, Hohmann SF, Ewigman B, Daum RS. Increasing burden of methicillin-resistant Staphylococcus aureus hospitalizations at US academic medical centers, 2003–2008. Infect Control Hosp Epidemiol 2012; 33:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David MZ, Daum RS, Bayer AS et al. Staphylococcus aureus bacteremia at five U.S. academic medical centers, 2008–2011: significant geographic variation in community-onset infections. Clin Infect Dis 2014; 15:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 5.Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357:380–90. [DOI] [PubMed] [Google Scholar]
- 6.Andes D, Craig WA. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother 2007; 51:1633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorr MB, Jabes D, Cavaleri M et al. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J Antimicrob Chemother 2005; 55(suppl 2):ii25–30. [DOI] [PubMed] [Google Scholar]
- 8.Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother 2012; 56:2795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis or non-zero risk difference or non-unity relative risk. Stat Med 1990; 9:1447–54. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4:213–26. [DOI] [PubMed] [Google Scholar]
- 11.Boucher HW, Wilcox M, Talbot GH et al. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 2014; 370:2169–78. [DOI] [PubMed] [Google Scholar]
- 12.Jauregui LE, Babazadeh S, Seltzer E et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 2005; 41:1407–15. [DOI] [PubMed] [Google Scholar]
- 13.Dunne MW, Zhou M, Borje Darpo B. A thorough QT study with dalbavancin: a novel lipoglycopeptide antibiotic for the treatment of acute bacterial skin and skin-structure infections. Int J Antimicrob Agents 2015; 45:393–8. [DOI] [PubMed] [Google Scholar]
- 14.Tice AD, Rehm SJ, Dalvasio JR et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis 2004; 38:1651–72. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins TC, Sabel AL, Sarcone EE, Price CS, Mehler PS, Burman WJ. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis 2010; 51:895–903. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins TC, Knepper BC, Sabel AL et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171:1072–9. [DOI] [PubMed] [Google Scholar]