Abstract
Background
Obstetric estimate (OE) of gestational age, recently added to the standard US birth certificate, has not been validated. Using early ultrasound-based gestational age (prior to 20 weeks gestation) as the criterion standard, we estimated the prevalence of preterm delivery and the sensitivity and positive predictive value (PPV) of gestational age estimates based on OE.
Methods
We analyzed 165 148 singleton livebirth records (38% of California livebirths during the study period) with linked early ultrasound information from a statewide California prenatal screening programme. OE of gestational age estimates was obtained from birth certificates.
Results
Prevalence of preterm delivery (<37 weeks gestation) was higher based on early ultrasound (8.1%) compared with preterm delivery based on OE (7.1%). Sensitivity for preterm birth when using OE for gestational age was 74.9% (95% confidence interval [CI] [74.1, 75.6]), and PPV was 85.1% (95% CI [84.4, 85.7]). Incongruence, defined as a ≥ 14-day difference between early-ultrasound-derived gestational age and OE, was 3.4%.
Conclusions
OE reported on the birth certificate may underestimate the prevalence of preterm delivery, particularly among women of non-Hispanic non-white race and ethnicity and women with lower educational attainment, public insurance at time of delivery, and missing prepregnancy BMI. Additional validation studies in other samples of births are needed.
Keywords: validity, gestational age, preterm
Introduction
Gestational age at delivery, as reported on birth certificates in the US, is used in multiple measures of infant health by public health practitioners to conduct surveillance and to develop policy to improve infant health. While two sources of gestational age at delivery – last menstrual period (LMP) and obstetric estimate (OE) – are included in the latest 2003 revised US standard birth certificate, health objectives and national statistics involving gestational age (e.g. percentage of births born preterm or <37 completed weeks of gestation) historically have used gestational age estimates mainly derived from LMP. However, LMP-based gestational age estimates are known to have systematic biases.1 Low sensitivity has also been reported. A previous study that compared gestational age based on LMP to that based on early ultrasound among 165 908 women who gave birth in California in 2002 found LMP to have low sensitivity (64.3%) and low positive predictive value (PPV) (58.7%) for gestational age <37 weeks.2
In 2003, the OE of gestational age replaced the clinical estimate (CE) on the US standard birth certificate.3 According to the instructions for completing the 2003 revised birth certificate, ‘The best obstetric estimate of infant’s gestation is completed weeks based on the birth attendant’s final estimate of gestation. This estimate of gestational age should be determined by all perinatal factors and assessments such as ultrasound, but not the neonatal exam. Ultrasound taken early in pregnancy is preferred.’4 Only one published study was found that assessed the validity of OE. The study compared mean infant birthweight for gestational age based on CE and OE to mean infant birth for gestational age based on a criterion standard (the gestational age for deliveries in which the LMP and CE or OE agreed within 1 week and in which the woman began prenatal care within the first 3 months of pregnancy).5 That study found mean birthweight for gestational age and cut points for the 10th and 90th percentiles for gestational week using OE and CE to be similar to those for each gestational week using the criterion standard. The birthweight distribution for gestational age when based on LMP, however, deviated from all of the other estimates. For example, the cut point for 90th percentile infant birthweight at 28 weeks gestation based on LMP differed from that based on the criterion standard by approximately 1700–1800 g, whereas OE differed from the criterion standard by only 113 g, and CE differed from the criterion standard by only 69 g. The American College of Obstetricians and Gynecologists (ACOG) recommends that ultrasound-based dates should take preference over LMP-based dates when there are discrepancies between dates.6 A limitation of the above study was that early ultrasound (conducted <20 weeks gestation), the more reliable method for dating the pregnancy, was not available.
Our study was undertaken to assess the validity of OE using early ultrasound as the criterion standard. To date, no studies have determined the sensitivity and PPV of OE-based gestational age or the prevalence of preterm delivery using early ultrasound as the criterion standard. A secondary purpose was to explore the characteristics of mothers whose infants’ OE differed substantially from the criterion standard.
Methods
Study population
The study population was derived from pregnant women who enrolled in California’s statewide Expanded Alpha-Fetoprotein Prenatal Screening Program (XAFP) and who delivered a livebirth between April 2007 and December 2007. The XAFP is a voluntary, prenatal screening programme offered to all women entering prenatal care by 20 weeks gestation. Obtaining accurate gestational age data is a critical aspect of the programme in order to improve screening accuracy, and 68% of estimated dates of delivery for women who participate in the screening are based on early ultrasound. On average, 350 000 women enroll in this screening programme annually, representing approximately 70% of all women who deliver in California each year. Probabilistic matching was used to link records from the XAFP to birth certificates, using mother’s name, date of birth, social security number, delivery date, XAFP accession date, telephone number, street address, city, and zip code (IBM Web Sphere Quality Stage Version 7.5). Linkages were confirmed with post-match queries and clerical review.
Between 1 April 2007 and 31 December 2007, there were 418 471 singleton livebirths in California. During the study period, 264 077 (63%) births linked to one or more XAFP records. Mothers with deliveries at less than 20 weeks or greater than 42 weeks gestation (based on early-ultrasound estimates) (n = 81), an ultrasound performed after 20 weeks gestation (n = 3539), or missing ultrasound-based gestational age (n = 86 028) were excluded from this study. Of the remaining 175 325 women, we also excluded women with missing information on gestational age based on OE (n = 2534) and other key variables of interest (n = 9235), yielding an analytic sample of 164 158 (38% of the 9-month birth cohort). Median week of gestation at the time of ultrasound among the included women was 11.3 (interquartile range = 8.7–15.1).
Variables
Obstetric estimates of gestational age at delivery, in completed weeks, were obtained from the birth certificate;4 early-ultrasound-based estimates of gestational age, in completed weeks, were obtained from XAFP records. The two gestational age variables were categorised into the following cumulative groupings: <28, <32, <34, <36, and <37 weeks. Records for which the difference between the early-ultrasound-derived gestational age and OE was more than 14 days were deemed incongruent. We examined differences greater or less than 14 days in order to identify those births with gross differences in the two measures of gestational age.
Race and ethnicity data were obtained from the birth certificate; multiple race and Hispanic origin fields were recoded and classified according to the following hierarchy: Hispanic (any race), non-Hispanic black, Asian (including Hawaiian/Pacific Islander), non-Hispanic White, and Other (which included American Indian). This hierarchy was chosen to allow a distinction between Hispanic women and women of other races and ethnicities. Maternal age (<20, 20–24, 25–35, ≥35 years), education (<12, 12, 13–15, ≥16 years), delivery insurance status and source (public, private, other/none), parity (i.e. number of prior livebirths; 0, 1, ≥2), smoking during pregnancy (none, any), and infant birthweight (<1500, 1500–2499, 2500–4499, ≥4500 g) were also obtained from the birth certificate. Maternal prepregnancy body mass index (BMI) was calculated using prepregnancy weight and height as reported on the birth certificate. Prepregnancy BMI (in kg/m2) was categorised into missing, underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30). Missing data ranged from 0% (for maternal age) to 12% (for prepregnancy BMI).
Analysis
Statistical analyses were run in Statistical Analysis Software version 9.2 (SAS Institute, Cary, NC, USA). Distributions of maternal demographic and pregnancy-related characteristics were described for women who were included in the analytic sample compared with women in the 9-month birth cohort. Gestational age was considered in 1-week increments as well as in cumulative categories (<28, <32, <34, <36, and <37 weeks). Distributions and 95% confidence intervals [CI] of both OE and ultrasound-based estimates of gestational age are shown in Figure 1. Sensitivity and PPV were calculated for OE, using the early-ultrasound-based estimates of gestational age as the criterion standard. The prevalence of preterm delivery (<37 weeks gestation) was calculated using each of the gestational age estimates. Incongruence of OE-based estimates with ultrasound-based estimates was reported by selected maternal and infant characteristics. Bivariate and multivariable logistic regression analyses were used to estimate the odds ratios (OR) and associated 95% CI for obtaining incongruent estimates of gestational age using OE and ultrasound by the same maternal characteristics, which were selected a priori due to their associations with gestational age estimates based upon a review of previous literature.
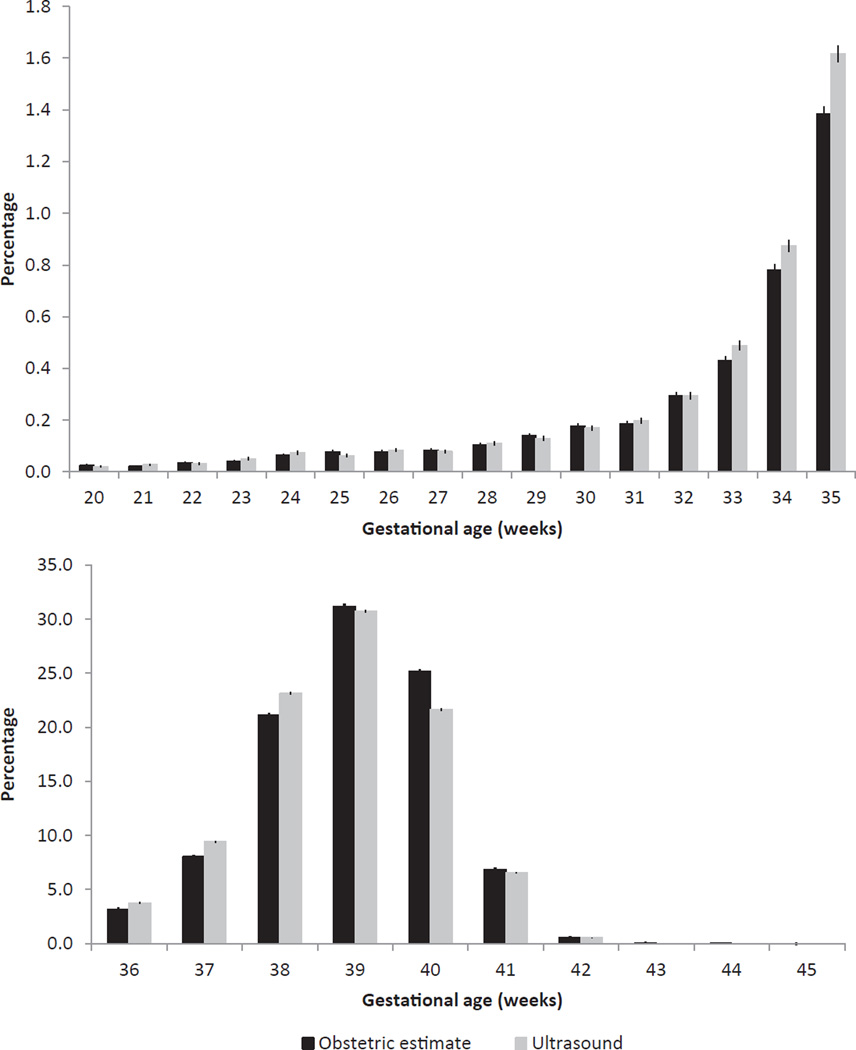
Figure 1.
Distribution of obstetric-estimate- and ultrasound-based measures of gestational age at birth by week: California Statewide Expanded Alpha Fetoprotein Prenatal Screening Program, 1 April–31 December 2007, n = 164 158.
Results
The population was racially and ethnically diverse. Approximately 54% of the study subjects were Hispanic, 6% non-Hispanic black, 14% Asian, 26% non-Hispanic white, and less than 1% other races/ethnicities (Table 1). Nearly 8% of the study subjects were less than 20 years of age and 15% were at least 35 years old; however, the majority of included women were between 25 and 34 years of age. The distribution of years of education was evenly distributed across the categories. The majority of women had private insurance at delivery (53%). Approximately 41% of included women were overweight or obese prior to pregnancy. About 41% of women were primiparous. Less than 2% of included women reported any smoking during pregnancy. Compared with the birth cohort during the specified period, the analytic sample included fewer teens, women 35 years and older, women with less than 12 years of education, and women whose deliveries were paid for by public insurance.
Table 1.
Selected maternal and pregnancy characteristics by study eligibility and inclusion status among livebirths: California Statewide Expanded Alpha Fetoprotein Prenatal Screening Program, April–December 2007
| Total sample (n = 420 544) |
Analytic samplea (n = 164 158) |
|
|---|---|---|
| Race/ethnicity | ||
| Black, non-Hispanic | 6.1 | 5.7 |
| White, non-Hispanic | 27.2 | 26.4 |
| Hispanic | 53.5 | 53.5 |
| Asian, non-Hispanic | 12.8 | 14.0 |
| Other, non-Hispanic | 0.4 | 0.4 |
| Maternal age (years) | ||
| <20 | 9.7 | 7.9 |
| 20–24 | 22.8 | 21.2 |
| 25–34 | 50.6 | 56.3 |
| ≥35 | 16.9 | 14.6 |
| Maternal education (highest year completed) | ||
| <12 | 27.9 | 24.9 |
| 12 | 27.5 | 27.1 |
| 13–15 | 21.9 | 23.5 |
| ≥16 | 22.8 | 24.6 |
| Delivery insurance | ||
| Public | 49.5 | 43.2 |
| Private | 45.8 | 53.2 |
| Other/none | 4.7 | 3.6 |
| Prepregnancy BMI | ||
| Underweight (<18.5 kg/m2) | 3.8 | 3.8 |
| Normal (18.5–24.9 kg/m2) | 45.0 | 45.3 |
| Overweight (25.0–29.9 kg/m2) | 22.4 | 23.1 |
| Obese (≥30.0 kg/m2) | 16.8 | 18.0 |
| Missing | 12.0 | 9.9 |
| Parity | ||
| 0 | 39.8 | 40.8 |
| 1 | 31.1 | 31.9 |
| ≥2 | 29.1 | 27.3 |
| Smoking during pregnancy | ||
| None | 97.3 | 98.1 |
| Any | 2.7 | 1.9 |
| Birthweight (g) | ||
| <1500 | 0.9 | 0.9 |
| 1500–2499 | 4.4 | 4.2 |
| 2500–4499 | 93.5 | 93.7 |
| ≥4500 | 1.2 | 1.2 |
| Obstetric-based gestational age (weeks) | ||
| <37 | 7.4 | 7.1 |
| 37–41 | 91.8 | 92.2 |
| 42–44 | 0.7 | 0.7 |
| >44 | <0.1 | <0.1 |
Subjects were excluded if records indicated multiple birth, mother-pregnancy was not linked to Expanded Alpha-Fetoprotein Prenatal Screening Program (XAFP) record, birth certificate was missing obstetric estimate or early ultrasound was not recorded in the XAFP record, or ultrasound was conducted after 20 weeks.
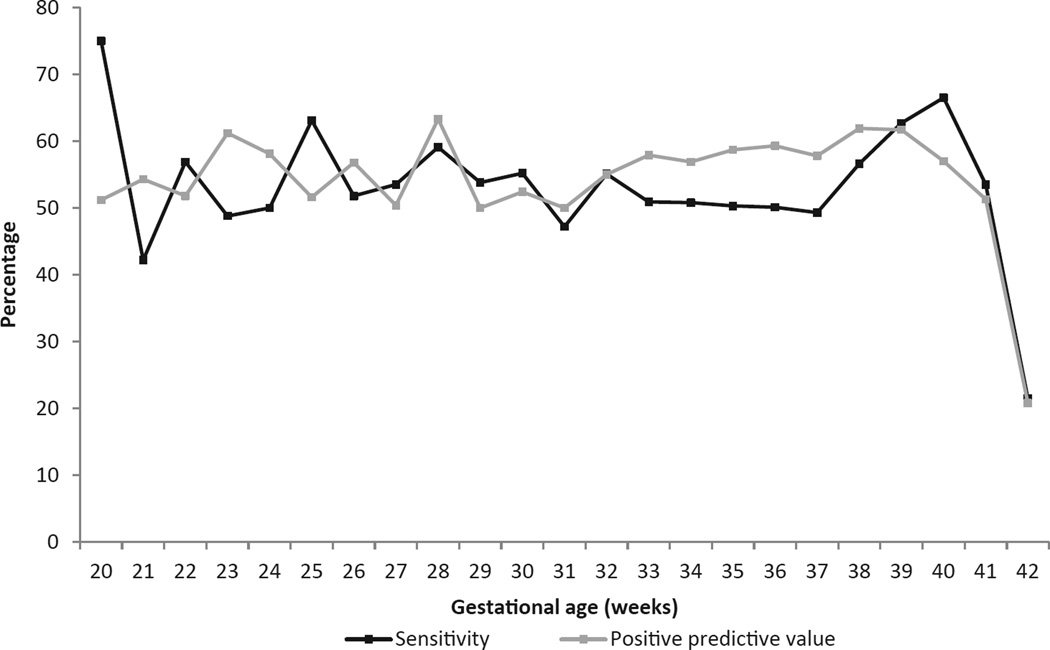
The distributions of OE and ultrasound-based gestational age estimates are shown in Figure 1. Compared with ultrasound, OE was slightly lower in weeks 33–38 and in excess in week 40 compared with ultrasound-based estimates (P < 0.001). When examining gestational age in 1-week increments, the week-specific sensitivity for OE ranged from 21.5% for week 42 to 75.0% for week 20 (Figure 2). PPV for OE ranged from 20.8% for week 42 to 63.3% for week 28. For the cumulative gestational age grouping <37 weeks, the sensitivity and PPV of OE was 74.9% (95% CI [74.1, 75.6]) and 85.1% (95% CI [84.4, 85.7]), respectively (Table 2).
Figure 2.
Sensitivity and positive predictive value of obstetric estimate of gestational age by week using early ultrasound as the criterion standard: California Statewide Expanded Alpha Fetoprotein Prenatal Screening Program, April–31 December 2007, n = 164 158.
Table 2.
Cumulative sensitivity and positive predictive value of obstetric estimate of gestational age using early ultrasound as the criterion standard: California Statewide Expanded Alpha Fetoprotein Prenatal Screening Program, April–December 2007 (n = 164 158)
| Gestational age (weeks) |
Sensitivity [95% CI] |
Positive predictive value [95% CI] |
|---|---|---|
| <28 | 89.4 [87.1, 91.7] | 89.5 [87.3, 91.8] |
| <32 | 89.0 [87.5, 90.5] | 89.0 [87.5, 90.5] |
| <34 | 85.8 [84.5, 87.0] | 88.6 [87.5, 89.8] |
| <36 | 79.5 [78.5, 80.4] | 87.3 [86.4, 88.1] |
| <37 | 74.9 [74.1, 75.6] | 85.1 [84.4, 85.7] |
The source of gestational age estimates affected the calculated prevalence of preterm delivery. The prevalence of preterm delivery was 8.1% (95% CI [7.9, 8.2]) using early ultrasound and 7.1% (95% CI [7.0, 7.2]) using OE (Table 3). Regarding preterm delivery, false negatives (2.0%) were nearly twice as prevalent as false positives (1.1%) when using OE, resulting in a lower preterm delivery prevalence estimate.
Table 3.
Preterm (20–36 weeks gestation) and term births by obstetric- and ultrasound-based gestational age: California Statewide Expanded Alpha Fetoprotein Prenatal Screening Program, April–December 2007
| Ultrasound (20–36 weeks) |
Ultrasound (37–42 weeks) |
Total | |
|---|---|---|---|
| OE (≤36 weeks), n (%) | 9 928 (6.0) | 1 745a (1.1) | 11 673 (7.1) |
| OE (≥37 weeks), n (%) | 3 324b (2.0) | 149 151 (90.9) | 152 485 (92.9) |
| Total | 13 262 (8.1) | 150 896 (91.9) | 164 158 (100) |
Column percentage totals may not equal the sum of cell percentages because of rounding error.
False positives.
False negatives.
OE, obstetric estimate.
The incongruence of OE with early-ultrasound-based estimates was 3.4% (Table 4). The strongest independent predictors of an incongruent OE-based gestational age were non-Hispanic non-white race/ethnicity, low maternal education, public insurance at time of delivery, and missing prepregnancy BMI information; however, the differences were small and adjusted ORs were all ≤1.7.
Table 4.
Maternal demographic and pregnancy characteristics associated with incongruenta obstetric estimate of gestational age compared with ultrasound-based estimates: California Statewide Expanded Alpha Fetoprotein Prenatal Screening Program, April–December 2007
| % Underb | % Overc | OR [95% CI] | Adjusted OR [95% CI]d | |
|---|---|---|---|---|
| Race/ethnicity | ||||
| White, non-Hispanic | 1.2 | 0.9 | 1.0 [Reference] | 1.0 [Reference] |
| Black, non-Hispanic | 2.1 | 1.6 | 1.7 [1.5, 1.9] | 1.4 [1.3, 1.6] |
| Hispanic | 2.1 | 2.0 | 2.0 [1.8, 2.1] | 1.4 [1.3, 1.5] |
| Asian | 1.5 | 1.1 | 1.2 [1.1, 1.4] | 1.3 [1.2, 1.4] |
| Other | 1.6 | 1.2 | 1.3 [0.8, 2.1] | 1.1 [0.6, 1.7] |
| Maternal age (years) | ||||
| <20 | 2.7 | 1.7 | 1.5 [1.3, 1.6] | 1.1 [1.0, 1.2] |
| 20–24 | 2.0 | 1.9 | 1.3 [1.2, 1.4] | 1.1 [1.0, 1.1] |
| 25–34 | 1.7 | 1.4 | 1.0 [Reference] | 1.0 [Reference] |
| ≥35 years | 1.8 | 1.6 | 1.1 [1.0, 1.2] | 1.1 [1.0, 1.2] |
| Maternal education (years) | ||||
| <12 | 2.5 | 2.2 | 2.4 [2.2, 2.7] | 1.7 [1.5, 1.9] |
| 12 | 2.0 | 1.8 | 2.0 [1.8, 2.1] | 1.5 [1.4, 1.7] |
| 13–15 | 1.5 | 1.3 | 1.4 [1.3, 1.6] | 1.2 [1.1, 1.4] |
| ≥16 | 1.2 | 0.8 | 1.0 [Reference] | 1.0 [Reference] |
| Delivery insurance | ||||
| Public | 2.3 | 2.1 | 1.7 [1.6, 1.8] | 1.2 [1.1, 1.3] |
| Private | 1.5 | 1.1 | 1.0 [Reference] | 1.0 [Reference] |
| None/other | 1.6 | 1.4 | 1.2 [1.0, 1.4] | 1.1 [0.9, 1.3] |
| Prepregnancy BMI | ||||
| Underweight (<18.5 kg/m2) | 1.9 | 1.1 | 1.0 [0.9, 1.2] | 1.1 [0.9, 1.2] |
| Normal (18.5–24.9 kg/m2) | 1.8 | 1.2 | 1.0 [Reference] | 1.0 [Reference] |
| Overweight (25.0–29.9 kg/m2) | 1.8 | 1.6 | 1.1 [1.0, 1.2] | 1.0 [0.9, 1.1] |
| Obese (≥30.0 kg/m2) | 1.7 | 2.0 | 1.3 [1.2, 1.4] | 1.1 [1.0, 1.2] |
| Missing | 2.4 | 2.4 | 1.6 [1.5, 1.8] | 1.4 [1.3, 1.5] |
| Parity | ||||
| 0 | 1.9 | 1.3 | 1.0 [Reference] | 1.0 [Reference] |
| 1 | 1.6 | 1.6 | 1.0 [1.0, 1.1] | 1.0 [0.9, 1.1] |
| ≥2 | 2.0 | 2.0 | 1.3 [1.2, 1.4] | 1.1 [1.0, 1.2] |
| Smoking during pregnancy | ||||
| None | 1.8 | 1.6 | 1.0 [Reference] | 1.0 [Reference] |
| Any | 2.0 | 1.2 | 0.9 [0.8, 1.1] | 1.0 [0.8, 1.2] |
>14 days absolute difference between obstetric-based and ultrasound-based estimates of gestational age.
Percent of population for which obstetric estimate was earlier than ultrasound-based estimate of gestational age.
Percent of population for which obstetric estimate was later than ultrasound-based estimate of gestational age.
Adjusted for all variables simultaneously.
Discussion
This study, the first to evaluate the OE-based gestational age on the US birth certificate using early ultrasound as the criterion standard, estimated that OE had a sensitivity of 74.9% and a PPV of 85.1%. The results of this study in conjunction with the results of a previous study that validated LMP against early ultrasound using the same California XAFP sample from a previous birth cohort2 suggest that OE may be a more valid source for gestational age than LMP-based estimates on the birth certificate. However, additional validation studies are needed to assess whether this finding is reaffirmed in other birth cohorts. In addition, while OE may perform better than LMP as a measure of gestational age on birth certificates, OE’s sensitivity is still lower than desired. In this sample OE missed one-quarter of preterm deliveries. Higher incongruence between ultrasound and OE was observed among population subgroups that have typically been associated with social disadvantage. As OE is the physician’s ‘best guess’, it may still be based on LMP, which has been shown to be less accurate among these same segments of the population.
This study includes the largest known population-based sample in the U.S. with validation of OE-based gestational age using early ultrasound as the criterion standard. The socio-economic and ethnic diversity of the sample allows identification of population subgroups in whom OE may be measured with less reliability or validity. For example, 53.5% of our sample was Hispanic mothers, who had 1.4 times the adjusted odds of 14-or-more-day differences between the two sources of gestational age estimates compared with white non-Hispanic mothers. In addition, the large sample size in this study permits evaluation of the sensitivity of OE for births <28 and <32 weeks gestation. However, the sample excluded 62% of livebirths. In our sample, only women who were enrolled in XAFP and who had early ultrasound dating of pregnancy were included. Early ultrasounds are recommended by ACOG for the diagnosis of major fetal anomalies, for determining accurate gestational age, and for detecting fetal growth disturbances or abnormalities in amniotic fluid volume.6 To the extent that our sample received an early ultrasound to get a more accurate gestational age, the OE in our sample may perform better than the OE in a sample of women who did not get an early ultrasound. However, it is possible that a certain percentage of women in our sample received an early ultrasound for reasons other than dating. Thus, the level of bias in our sample is unknown, and the quality of OE-based gestational age in the absence of early ultrasound is worthy of further investigation. The findings of this sample may not be generalisable to the US population, as California tends to have a greater proportion of births to Hispanic and Asian/Pacific Islander women and fewer births among non-Hispanic Black women and teenagers, compared with the U.S. population.7 California’s preterm birth rate is also lower than the national preterm birth rate – potentially further impacting the generalisability of these findings.
Early ultrasound has been established clinically as the criterion standard; studies have found that early ultrasound-based formulas are fairly accurate for gestational age dating, with random errors of ±10 days.8 However, some inherent biases exist with this source of measurement. When dating gestational age using early ultrasound, fetuses with characteristics associated with small fetal size, such as first births and female sex, were found to be systematically dated 1–2 days younger than second or later births and male-sex fetuses,9,10 whereas fetuses of obese women were more likely to be dated older.11,12 However, one large study of singleton pregnancies with ultrasound examinations conducted between 12 and 22 weeks found no evidence that growth-restricted fetuses were systematically classified incorrectly at these gestational ages, and found that the discrepancy between the LMP-based gestational age and the ultrasound-based gestational age was primarily related to ovulation occurring later in the cycle than at the assumed 14 days.13
Gestational age data from the US standard birth certificate are used to estimate US rates of preterm delivery and to establish fetal growth curves on which to base small and large for gestational age classifications. These public health measures are important indicators of infant health and thus should be based on the most accurate data sources available. Because OE is by definition rounded down to completed weeks of gestation, precision to the level of the day is not possible, which may explain the low week-specific sensitivity observed in this study. Nonetheless, the results from this study and others provide preliminary evidence to suggest that OE may be a more valid source of gestational age than LMP on birth certificates. Additional research assessing the validity of OE in other populations is needed.
Acknowledgments
The California Department of Public Health, Genetic Disease Screening Program collected the Expanded XAFP programme records, and the Health Information and Research Section was the original source of the livebirth files. Oren Bergman and Steve Graham of the Sequoia Foundation conducted the record linkage.
Footnotes
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the California Department of Public Health.
References
- 1.Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13:668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Dietz PM, England L, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California live birth and prenatal screening records. Paediatric and Perinatal Epidemiology. 2007;21(Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Report of the Panel to Evaluate the U.S. Standard Certificates and Reports. [last accessed December 2012];2001 Nov; Available at: http://www.cdc.gov/nchs/data/dvs/panelreport_acc.pdf.
- 4.National Center for Health Statistics. Guide to Completing the Facility Worksheets for the Certificate of Live Birth and Report of Fetal Death (2003 Revision) Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2003. Mar, [last accessed February 2012]. Updated May 2006. Available at: http://www.cdc.gov/nchs/data/dvs/GuidetoCompleteFacilityWks.pdf. [Google Scholar]
- 5.Callaghan W, Dietz P. Differences in birth weight for gestational age distributions according to the measures used to assign gestational age. American Journal of Epidemiology. 2010;171:826–836. doi: 10.1093/aje/kwp468. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists. Practice Bulletin No. 58. Ultrasonography in pregnancy. Obstetrics and Gynecology. 2009;101:1–11. [Google Scholar]
- 7.Hamilton BE, Martin JA, Ventura SJ. National Vital Statistics Reports. 5. Vol. 61. Hyattsville, MD: National Center for Health Statistics; 2012. Births: Preliminary Data for 2011. [PubMed] [Google Scholar]
- 8.Chervenak FA, Skupski DW, Romero R, Myers MK, Smith-Levitin M, Rosenwaks Z, et al. How accurate is fetal biometry in the assessment of fetal age? American Journal of Obstetrics and Gynecology. 1998;178:678–687. doi: 10.1016/s0002-9378(98)70477-6. [DOI] [PubMed] [Google Scholar]
- 9.Johnsen SL, Rasmussen S, Sollien R, Kiserud T. Fetal age assessment based on ultrasound head biometry and the effect of maternal and fetal factors. Acta Obstetricia et Gynecologica Scandinavica. 2004;83:716–723. doi: 10.1111/j.0001-6349.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 10.Kallen K. Mid-trimester ultrasound prediction of gestational age: advantages and systematic errors. Ultrasound in Obstetrics and Gynecology. 2002;20:558–563. doi: 10.1046/j.1469-0705.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- 11.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatric and Perinatal Epidemiology. 2007;21(Suppl. 2):86–96. doi: 10.1111/j.1365-3016.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 12.Simic M, Wahlin IA, Marsal K, Kallen K. Maternal obesity is a potential source of error in mid-trimester ultrasound estimation of gestational age. Ultrasound Obstetrics and Gynecology. 2010;35:48–53. doi: 10.1002/uog.7502. [DOI] [PubMed] [Google Scholar]
- 13.Larsen T, Nguyen TH, Greisen G, Engholm G, Møller H. Does a discrepancy between gestational age determined by biparietal and last menstrual period sometimes signify early intrauterine growth retardation? British Journal of Obstetrics and Gynaecology. 2000;107:238–244. doi: 10.1111/j.1471-0528.2000.tb11695.x. [DOI] [PubMed] [Google Scholar]