Abstract
Lifetime childhood asthma prevalence (LCAP) percentages in Puerto Rico Health Regions (HR) are substantially higher in northeastern vs. southwestern HR. Higher average relative humidity in the northeast might promote mold and mite exposures and possibly asthma prevalence. To test this hypothesis, mold contamination, Environmental Relative Moldiness Index (ERMI) values were measured in floor dust (n = 26) and dust mite allergen concentrations in bed dust (n = 14). For this analysis, the eight HR were divided into those with LCAP > 30 % (n = 3) and < 30 % (n = 5). The average ERMI value was significantly greater (Wilcoxon Rank Sum, p < 0.001) in high than in low LCAP HR (14.5 vs. 9.3). The dust mite antigens Der p 1, Der f 1, and Blo t 5 were detected in 90 % of bed samples, but the concentrations were not significantly different in high vs. low LCAP HR. Mold exposures might partially explain the differences in LCAP HR in Puerto Rico.
Keywords: mold, dust, mites, ERMI, relative humidity
Introduction
The prevalence of asthma in Puerto Rico is approximately 16.5 % compared to 8.4 % for the US (CDC 2012). In some areas of Puerto Rico, 46 % of elementary children had asthma (Loyo-Berríos et al. 2006). In 2000, 30.6 % of the interviewed subjects reported having one to three visits to emergency departments as a result of asthma in the previous year (Pérez-Perdomo et al. 2003). However, the prevalence of asthma is not uniform across the island (Puerto Rico Department of Health 2010).
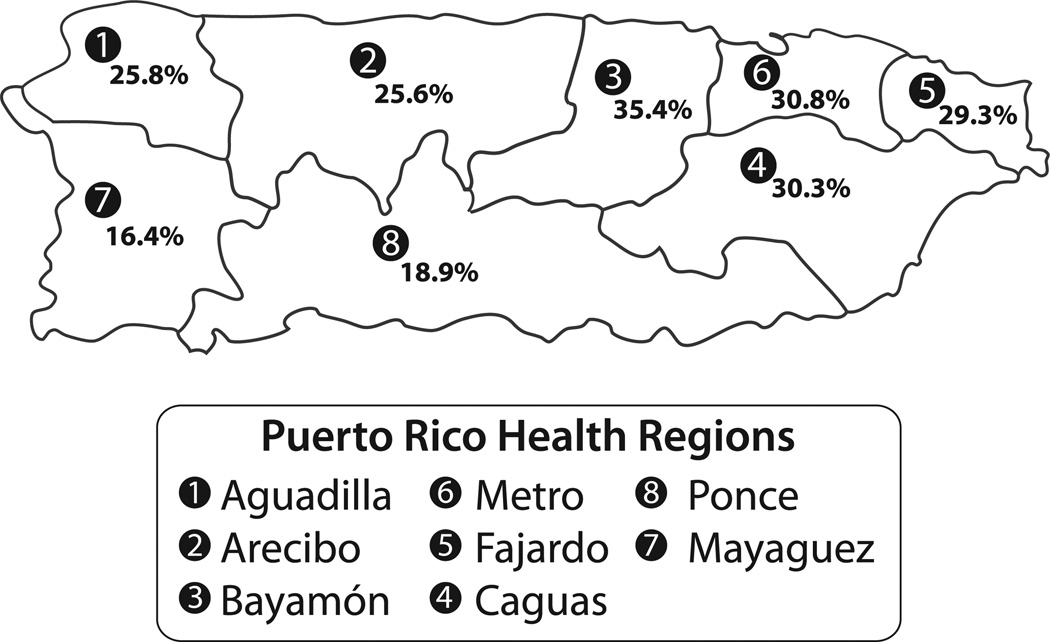
In 1993, the government of Puerto Rico divided its 78 municipalities into eight Health Regions (HR) (Figure 1) in order to centralize health care services. In some Puerto Rican HR, the lifetime childhood asthma prevalence (LCAP) was twice as high as in other HR (Figure 1). In general, the southwestern half of Puerto Rico has substantially lower incidence of asthma compared to the northeastern half of the island and this generally corresponds to differences in average relative humidities and rainfall in these areas (NOAA 2014). Moisture is critical for the life of both dust mites and molds.
Figure 1.
The island of Puerto Rico as divided into the eight Health Regions.
Notes: The Health Regions number and name is followed by the number of samples from that Region in parentheses. 1 – Aguadilla (0 samples); 2 – Arecibo (3 samples); 3 – Bayamon (2 samples); 4 – Caguas (7 samples); 5 – Fajardo (1 sample); 6 – Mayaguez (2 samples); 7 – Metro (7 samples); 8 – Ponce (4 samples). In the box for each Health Region is the percent lifetime childhood asthma prevalence (LCAP). The LCAP data for each Health Region is the summary covering 1998–2008 (Puerto Rico Department of Health 2010).
Dust mites require moisture for respiration and dust mite populations are higher in the tropical regions than those in temperate climates due to higher humidity in the tropics (Chew et al. 2009). Dust mite sensitization is a risk factor for asthma development and morbidity (Platts-Mills et al. 1997; Chew et al. 2009). In Puerto Rico, more than 70 % of allergic asthmatics were sensitized to dust mite allergens (Montealegre et al. 1997). However, a recent meta-analysis suggests that dust mite allergen exposure is associated with serum-specific IgE but not with respiratory outcomes, including asthma (Bakolis et al. 2015).
On the other hand, exposures to damp indoor, moldy environments are linked to asthma in many scientific literature reviews (IOM 2004; WHO 2009; Quansah et al. 2012). In a prospective study of asthma development, Reponen et al. (2011, 2012) demonstrated that infants exposed to high concentrations of molds were significantly more likely to develop physician diagnosed asthma at age seven. The metric used to describe the mold contamination in these homes was the Environmental Relative Moldiness Index (ERMI) scale.
The ERMI scale was developed by the US Environmental Protection Agency in conjunction with the Department of Housing and Urban Development to standardize the assessment of the mold contamination in US homes (Vesper et al. 2007). For the ERMI analysis, a DNA-based technology, mold-specific quantitative PCR (MSQPCR), was used to measure the concentration of 36 indicator molds in settled-dust samples. This metric was previously applied in a pilot study of molds in settled dust inside and outside Puerto Rican homes (Bolaños-Rosero et al. 2013). The ERMI values inside Puerto Rican homes were found to be significantly greater than outside the homes. The objective of this study was to determine if the prevalence of asthma in the different HR was correlated with home mold contamination, as quantified by their ERMI values.
Materials and methods
Dust sample collection
Informed consent was obtained for this study in accordance with the Columbia University Medical Center institutional review board. When children in the New York City-Puerto Rico Asthma Project (Acosta et al. 2008) visited relatives in Puerto Rico, dust samples were collected in the Puerto Rican homes (n = 26) of the relative.
Bedroom floor dust was collected for 3 min onto 70-mm cellulose filters (Whatman International, Maidstone, UK) with a canister vacuum cleaner (Eureka Mighty Mite, Bloomington, IN) and a modified collection nozzle (ALK, Horshølm, Denmark) (Chew et al. 2003). The samples were returned to the laboratory and stored at −20 °C.
For the mold analysis, each of the dust samples was recovered from each of the filters and each then sieved through 300 µm pore, nylon mesh (Gilson Company, Lewis Center, OH). The DNA from each dust sample was then extracted and the DNA purified using the DNA-EZ kit, following the manufacturer’s instructions (GeneRite, Monmouth Junction, NJ). Each of the 36 molds in the ERMI panel (as listed in Table 1) was quantified in each of the bedroom floor samples from the Puerto Rican homes (n = 26) by MSQPCR. The quantification of each of the molds was based on standard curves generated for each mold species, as previously described (Haugland et al. 2004).
Table 1.
The percentage detection (% D) of each of the 36 Environmental Relative Moldiness Index (ERMI) molds and the median concentration (cells per mg dust) in the bedrooms in higher (n = 16) than in lower (n = 10) lifetime childhood asthma prevalence (LCAP) Health Regions in Puerto Rico.
| Lifetime childhood asthma prevalence region | Wilcoxon Rank sum test |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % D | High Median | LIQ | UIQ | % D | Low Median | LIQ | UIQ | Value | p value | |
| Group 1 | ||||||||||
| Aspergillus flavus | 63 | 1 | 0 | 2 | 40 | 0 | 0 | 1 | 117 | 0.321 |
| Aspergillus fumigatus | 44 | 0 | 0 | 2 | 20 | 0 | 0 | 0 | 110 | 0.118 |
| Aspergillus niger | 94 | 12 | 4 | 105 | 100 | 8 | 5 | 17 | 127 | 0.688 |
| Aspergillus ochraceus | 38 | 0 | 0 | 2 | 60 | 1 | 0 | 2 | 150 | 0.397 |
| Aspergillus penicillioides | 100 | 5100 | 2550 | 17000 | 100 | 2850 | 530 | 9300 | 108 | 0.161 |
| Aspergillus restrictus | 81 | 15 | 3 | 55 | 80 | 4 | 1 | 8 | 103.5 | 0.099 |
| Aspergillus sclerotiorum | 94 | 4 | 1 | 74 | 60 | 1 | 0 | 12 | 106.5 | 0.135 |
| Aspergillus sydowii | 94 | 32 | 9 | 87 | 80 | 11 | 1 | 17 | 111.5 | 0.225 |
| Aspergillus unguis | 31 | 0 | 0 | 1 | 40 | 0 | 0 | 1 | 140 | 0.812 |
| Aspergillus versicolor | 94 | 52 | 19 | 155 | 70 | 6 | 0 | 20 | 86 | 0.008 |
| Aureobasidium pullulans | 100 | 23 | 4 | 145 | 90 | 17 | 7 | 54 | 131 | 0.846 |
| Chaetomium globosum | 50 | 1 | 0 | 2 | 50 | 1 | 0 | 3 | 144 | 0.621 |
| Cladosporium sphaerospermum | 100 | 32 | 10 | 110 | 100 | 64 | 6 | 110 | 141.5 | 0.746 |
| Eurotium group | 100 | 69 | 12 | 145 | 90 | 11 | 7 | 27 | 92.5 | 0.024 |
| Paecilomyces variotii | 50 | 1 | 0 | 3 | 40 | 0 | 0 | 3 | 129.5 | 0.767 |
| Penicillium brevicompactum | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 120 | 0.262 |
| Penicillium group 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 135 | 1.000 |
| Penicillium crustosum | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 130 | 1.000 |
| Penicillium purpurogenum | 19 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 127.5 | 0.545 |
| Penicillium spinulosum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 135 | 1.000 |
| Penicillium variabile | 44 | 0 | 0 | 2 | 10 | 0 | 0 | 0 | 105.5 | 0.064 |
| Scopulariopsis brevicaulis | 38 | 0 | 0 | 2 | 30 | 0 | 0 | 2 | 128.5 | 0.716 |
| Scopulariopsis chartarum | 44 | 0 | 0 | 1 | 60 | 2 | 0 | 19 | 162 | 0.132 |
| Stachybotrys chartarum | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 151 | 0.138 |
| Trichoderma viride | 31 | 0 | 0 | 1 | 30 | 0 | 0 | 3 | 139.5 | 0.745 |
| Wallemia sebi | 100 | 650 | 191 | 5200 | 100 | 200 | 44 | 750 | 116 | 0.336 |
| Group 2 | ||||||||||
| Acremonium strictum | 38 | 0 | 0 | 2 | 50 | 1 | 0 | 3 | 142 | 0.711 |
| Alternaria alternata | 38 | 0 | 0 | 1 | 60 | 2 | 0 | 3 | 158.5 | 0.186 |
| Aspergillus ustus | 31 | 0 | 0 | 1 | 50 | 1 | 0 | 6 | 159 | 0.165 |
| Cladosporium cladosporioides (Type 1) | 100 | 270 | 111 | 490 | 100 | 435 | 220 | 650 | 159 | 0.215 |
| Cladosporium cladosporioides (Type 2) | 50 | 1 | 0 | 5 | 70 | 2 | 0 | 6 | 151 | 0.391 |
| Cladosporium herbarum | 38 | 0 | 0 | 2 | 20 | 0 | 0 | 0 | 120.5 | 0.374 |
| Epicoccum nigrum | 81 | 3 | 1 | 19 | 90 | 18 | 6 | 46 | 167 | 0.094 |
| Mucor racemosus | 88 | 11 | 2 | 21 | 90 | 7 | 2 | 12 | 124.5 | 0.595 |
| Penicillium chrysogenum (Type2) | 6 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 138.5 | 0.754 |
| Rhizopus stolonifer | 44 | 0 | 0 | 1 | 40 | 0 | 0 | 4 | 137 | 0.949 |
(LIQ = lower interquartile range; UIQ = upper interquartile range.)
The population of Aspergillus versicolor (bolded) is significantly greater in high than in low LCAP Health Regions, even after Holms-Bonferroni correction for multiple comparisons.
The MSQPCR assay contained 12.5 µl of “Universal Master Mix” (Applied Biosystems Inc., Foster City, CA). To this was added 0.5 µl of a 25 µM solution of the forward primer and 0.5 µl of a 25 µM solution of the reverse primer. Then 2.5 µl of a 400 nM TaqMan probe solution (Applied Biosystems Inc.), 2.5 µl of 2 mg/ml fraction V bovine serum albumin solution (Sigma Chemical, St. Louis, MO), and 1.5 µl of DNA free water (Cepheid, Sunnyvale, CA) was added. To this mix was added 5 µl of the DNA extract from the sample to make a final volume of 25 µl for each MSQPCR analysis. All primer and probe sequences for these 36 ERMI molds (Table 1) are shown at the website: http://www.epa.gov/nerlcwww/moldtech.htm.
The ERMI value for each home was calculated according to Equation (1), by taking the “Sum of the Logs” of the concentrations of the Group 1 species (s1) and subtracting the “Sum of the Logs” of the concentrations of Group 2 species (s2) (Vesper et al. 2007).
| (1) |
The Group 1 molds contains 26 species and Group 2 molds contains 10 species (Table 1).
Dust mite allergen analysis
The dust mite allergen analysis was based on the dust collected from the mothers’ beds in the visited Puerto Rican homes (n = 14). Bed dust samples were collected by vacuuming the pillows, upper half of the bed, and upper half of all bed layers for 3 min onto 70-mm cellulose filters (Whatman International, Maidstone, UK) with a canister vacuum cleaner (Eureka Mighty Mite, Bloomington, IN) and a modified collection nozzle (ALK, Horshølm, Denmark) (Chew et al. 2003). The samples were returned to the laboratory and stored at −20 °C.
Dust samples were extracted with PBS with 0.05 % Tween 20 and analyzed for three mite allergens (Chew et al. 2003). Dust mite allergens from Dermatophagoides pteronyssinus (Der p 1) and Dermatophagoides farinae (Der f 1) were measured by ELISA (Indoor Biotechnologies, Charlottesville, VA) (Chew et al. 2003) and the dust mite allergen from Blomia tropicalis (Blo t 5) was measured as previously described (Yi et al. 2005). For the dust mite allergen analysis, only 14 homes, 8 from high and 6 from low LCAP HR, were available for testing.
Statistical analysis
The statistical differences in the average ERMI values and the average concentrations of dust mite allergens in high vs. low LCAP HR were determined using the Wilcoxon Rank Sum test (Dharmage et al. 1999) since this nonparametric test makes no assumptions about the probability distributions of the variables being assessed. The differences in concentrations of individual mold species between high vs. low LCAP HR were also evaluated by the Wilcoxon Rank Sum test and corrections for multiple comparisons were made using the Holms-Bonferroni test since a large number of comparisons were made and less stringent significance could have been a result of chance. Analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.14 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The number of homes sampled in each Health Region is shown in Figure 1. The average ERMI in these 26 homes was 12.5 ± 5.4 standard deviations (SD).
The average ERMI values in homes from HR with LCAP values above and below 30 % were 14.5 (SD ± 4.5) and 9.29 (SD ± 5.4), respectively. These values were statistically significantly different (Wilcoxon Rank Sum, p < 0.001).
The mean concentrations of each of the 36 molds in the high compared to low LCAP HR is shown in Table 1. Penicillium spinulosum and Penicillium group 2 were not detected in these samples. The population of Aspergillus versicolor was the only one of the 36 ERMI molds that was significantly greater in high than in low LCAP HR, after Holms-Bonferroni correction for multiple comparisons (Table 1).
The dust mite antigens, Der p 1, Der f 1, and Blo t 5, were detected in 100, 86, and 86 %, respectively, i.e. 38 of 42 bed dust samples (Table 2). Although the average concentration of each of the dust mite antigens was greater in the high asthma HR, the differences were not statistically significant (Table 2).
Table 2.
Average dust mite antigen concentrations from Dermatophagoides pteronyssinus (Der p 1), D. farinae (Der f 1) and from Blomia tropicalis (Blo t 5) in bed samples collected from Puerto Rican homes in Health Regions with either lifetime childhood asthma prevalence (LCAP) above 30 % (high) or below 30 % (low).
| Type of mite allergen | |||
|---|---|---|---|
| Lifetime asthma prevalence region | Der p 1 µg/g | Der f 1 µg/g | Blo t 5 µg/g |
| High LCAP | 3.16 | 2.78 | 0.58 |
| Low LCAP | 2.45 | 2.36 | 0.35 |
| p-values | 0.75 | 0.66 | 0.95 |
The p values are based on a Wilcoxon Rank Sum test.
Discussion
Homes in Puerto Rican HR with higher LCAP values had higher levels of mold contamination, defined by their ERMI values. Recently, Blatter et al. (2014) demonstrated that Puerto Rican children with asthma, who were exposed to very high Beta-D-glucan concentrations (as a general estimate of mold contamination), had nearly a ninefold greater odds of needing hospital emergency department or urgent care for their asthma. Together, these results indicate that mold contamination is associated with asthma prevalence in Puerto Rico.
One specific mold, A. versicolor, was in significantly greater concentrations in high compared to low LCAP HR. A. versicolor has been associated with adverse health effects in humans, including pulmonary disease and lung infections (Hodgson et al. 1998; Pepeljnjak et al. 2004). Recently, Millien et al. (2013), in a mouse model of asthma, showed that some species of Aspergillus could induce airway surface mycotic infections (ASMI) which caused chronic lung damage like enhanced airway epithelial and vascular endothelial cell permeability which initiated the cascade of events that resulted in asthma-like conditions in mice.
Furthermore, Reponen et al. (2012) showed that infant exposures to two Aspergillus species (i.e. A. ochraceus and A. unguis) are significantly associated with development of physician-diagnosed asthma at age seven. It may be that A. versicolor can also increase the risk for these types of ASMI. Therefore, understanding the factor(s) controlling mold growth in Puerto Rican homes should improve our understanding of asthma prevalence in the HR of Puerto Rico.
Most Puerto Rican families keep their homes’ windows open throughout the year since this is a tropical environment. Therefore, the partial pressure of water in the air, i.e. the relative humidity, appears to be a major source of water to support mold growth. Generally, northeastern Puerto Rico has higher RH than southwestern Puerto Rico (Montealegre et al. 1997). The HR with higher relative humidity are generally those with the higher LCAP percentages and higher concentration of mold, i.e. significantly higher ERMI values. Higher relative humidity is also favorable for the survival of dust mites.
Larger dust mite populations are found when the absolute indoor air humidity is above 45 % relative humidity at 20 °C (Hart 1995). Montealegre et al. (1997) reported the rate of detection of the common mites D. pteronyssinus, B. tropicalis, and D. farinae across Puerto Rico was 45.6, 31.6, and 17.5 %, respectively. However, they did not observe significant differences in the total mite counts between the northern and southern regions of Puerto Rico. Only B. tropicalis was more frequent (43 %) in the northern areas of Puerto Rico than in the southern (19 %).
Although the average concentration of each type of mite allergen was greater in the high LCAP HR compared to the low LCAP HR, this did not reach statistical significance (p < 0.05). This lack of statistical significance could be the result of the small number (n = 16) of bed samples.
It appears that the differences in relative humidity in Puerto Rican HR may be very important for both the mold and dust mite populations. It is known that the presence of both mold and dust mites increases the frequency and severity of asthma attacks (Prescott 2003). Su et al. (2005) showed that the level of total IgE was significantly higher in children exposed to both molds and dust mites. However, dust mites are also involved in both mold spore consumption and dispersion (Naegele et al. 2013). Therefore, the interactions between mold and dust mites and the development of asthma in Puerto Rico will need further exploration.
In our study, the small number of homes sampled is a major limitation. We also realize that there are many other environmental exposures that we did not measure in these homes. However, the novel aspect of our preliminary investigation is that we were able to see differences in mold profile over a small geographic range, based upon regional humidity differentials alone. Heretofore, we only expected to see difference in mold profiles over much more extreme humidity differentials, (e.g. tropical Florida vs. arid Arizona). Our observation of a different mold profile over an island that only extends about 178 km by 65 km (i.e. 110 miles by 40 miles) was surprising. Thus, further research is required to confirm whether the regional relative humidity on the island contributes to mold growth and increased dust mite allergen concentrations.
These results suggest that the higher children’s lifetime prevalence of asthma in certain HR in Puerto Rico might be associated with the exposure to high levels of mold contamination within homes. In another tropical country, Taiwan, asthmatic children were found to be living in homes with significantly higher ERMI values than non-asthmatic children (Chen et al. 2015). As the ERMI is a standardized test, it provides a metric to quantify the link between mold contamination (including some specific molds) and asthma.
Acknowledgments
Our thanks go to the individuals and families who allowed us to sample their residences.
Disclosure statement
The US Environmental Protection Agency (EPA), through its Office of Research and Development, collaborated in the research described here. Although this work was reviewed by EPA and approved for publication, it may not necessarily reflect official EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use. Since MSQPCR technology is patented by the US EPA, the Agency has a financial interest in its commercial use.
Funding
This work was supported by the National Institutes of Health [grant number P30 ES 009089], [grant number R01 ES 10922].
References
- Acosta LM, Acevedo-García D, Perzanowski MS, Mellins R, Rosenfeld L, Cortés D, Gelman A, Fagan JK, Bracero LA, Correa JC, Reardon AM, Chew GL. The New York City Puerto Rican asthma project: study design, methods, and baseline results. J Asthma. 2008;45:51–57. doi: 10.1080/02770900701815784. [DOI] [PubMed] [Google Scholar]
- Bakolis I, Heinrich J, Zock JP, Norbäck D, Svanes C, Chen CM, Accordini S, Verlato G, Olivieri M, Jarvis D. House dust-mite allergen exposure is associated with serum specific IgE but not with respiratory outcomes. Indoor Air. 2015;25:235–244. doi: 10.1111/ina.12137. [DOI] [PubMed] [Google Scholar]
- Blatter J, Forno E, Brehm J, Acosta-Pérez E, Alvarez M, Colón-Semidey A, Thorne PS, Metwali N, Canino G, Celedón JC. Fungal exposure, atopy, and asthma exacerbations in Puerto Rican children. Ann Am Thoracic Soc. 2014;11:925–932. doi: 10.1513/AnnalsATS.201402-077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños-Rosero B, Betancourt D, Dean T, Vesper S. Pilot study of mold populations inside and outside of Puerto Rican residences. Aerobiologia. 2013;29:537–543. [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. 2012 Aug; Available from: http://www.cdc.gov/nchs/data/databriefs/db94.pdf.
- Chen C-H, Chao HJ, Shen W-C, Chen B-Y, Lin K-T, Guo YL, Vesper S. Pilot study of mold in homes of asthmatic children in Taipei, Taiwan using the environmental relative moldiness index. Aerobiologia. 2015;31:213–218. [Google Scholar]
- Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, Becker MG, Kinney PL. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111:1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GL, Reardon AM, Correa JC, Young M, Acosta L, Mellins R, Chew FT, Perzanowski M. Mite sensitization among Latina women in New York, where dust mite allergen levels are typically low. Indoor Air. 2009;19:193–197. doi: 10.1111/j.1600-0668.2008.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmage S, Bailey M, Raven J, Mitakakis T, Guest D, Cheng A, Rolland J, Thien F, Abramson M, Walters EH. A reliable and valid home visit report for studies of asthma in young adults. Indoor Air. 1999;9:188–192. doi: 10.1111/j.1600-0668.1999.t01-1-00005.x. [DOI] [PubMed] [Google Scholar]
- Hart BJ. The biology of allergenic domestic mites. An update. Clin Rev Allergy Immunol. 1995;13:115–133. doi: 10.1007/BF02758097. [DOI] [PubMed] [Google Scholar]
- Haugland RA, Varma M, Wymer LJ, Vesper SJ. Quantitative PCR analysis of selected Aspergillus, Penicillium and Paecilomyces species. Syst Appl Microbiol. 2004;27:198–210. doi: 10.1078/072320204322881826. [DOI] [PubMed] [Google Scholar]
- Hodgson MJ, Morey P, Leung WY, Morrow L, Miller D, Jarvis BB, Robbins H, Halsey JF, Storey E. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J Occup Environ Med. 1998;40:241–249. doi: 10.1097/00043764-199803000-00006. [DOI] [PubMed] [Google Scholar]
- [IOM] Institute of Medicine. National academies of science damp indoor spaces and health. Washington (DC): The National Academies Press; 2004. [PubMed] [Google Scholar]
- Loyo-Berríos NI, Orengo JC, Serrano-Rodríguez RA. Childhood asthma prevalence in northern Puerto Rico, the Rio Grande, and Loíza experience. J Asthma. 2006;43:619–624. doi: 10.1080/02770900600878693. [DOI] [PubMed] [Google Scholar]
- Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, Song LZ, Knight JM, Creighton CJ, Luong A, Kheradmand F, Corry DB. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montealegre F, Sepulveda A, Bayona M, Quiñones C, Fernández-Caldas E. Identification of the domestic mite fauna of Puerto Rico. P R Health Sci J. 1997;16:109–116. [PubMed] [Google Scholar]
- Naegele A, Reboux G, Scherer E, Roussel S, Millon L. Fungal food choices of Dermatophagoides farinae affect indoor fungi selection and dispersal. Int J Environ Health Res. 2013;23:91–95. doi: 10.1080/09603123.2012.699029. [DOI] [PubMed] [Google Scholar]
- [NOAA] National Oceanic and Atmospheric Administration. Mean annual rainfall, 1981–2010. [cited 2014 June 1];2014 Available from: http://www.srh.noaa.gov/sju/?n=mean_annual_precipitation2.
- Pepeljnjak S, Slobodnjak Z, Šegvić M, Peraica M, Pavlović M. The ability of fungal isolates from human lung aspergilloma to produce mycotoxins. Hum Exp Toxicol. 2004;23:15–19. doi: 10.1191/0960327104ht409oa. [DOI] [PubMed] [Google Scholar]
- Pérez-Perdomo R, Pérez-Cardona C, Disdier-Flores O, Cintrón Y. Prevalence and correlates of asthma in the Puerto Rican population: Behavioral Risk Factor Surveillance System, 2000. J Asthma. 2003;40:465–474. doi: 10.1081/jas-120018713. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S2–S24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- Prescott SL. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. 2003;3:125–132. doi: 10.1097/00130832-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Puerto Rico Department of Health. Puerto Rico asthma prevalence and mortality fact sheet. 2010 Available from: http://www.proyectoasmapr.org/uploads/GeoInfo_Mayo_6_2010.pdf. [Google Scholar]
- Quansah R, Jaakkola MS, Hugg TT, Heikkinen SA, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS One. 2012;7:e47526. doi: 10.1371/journal.pone.0047526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana-Hershey GK, Zheng S, Ryan P, Grinshpun SA, Villareal M, LeMasters G. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol. 2012;130:639.e5–644.e5. doi: 10.1016/j.jaci.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, Grinshpun SA, Zheng S, Bernstein DI, Lockey J, et al. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Ann Allergy Asthma Immunol. 2011;107:120–126. doi: 10.1016/j.anai.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HJ, Wu PC, Lei HY, Wang JY. Domestic exposure to fungi and total serum IgE levels in asthmatic children. Mediators Inflamm. 2005;2005:167–170. doi: 10.1155/MI.2005.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper SJ, McKinstry C, Haugland R, Wymer L, Bradham K, Ashley P, Cox D, Dewalt G, Friedman W. Development of an Environmental Relative Moldiness Index for US homes. J Occup Environ Med. 2007;49:829–833. doi: 10.1097/JOM.0b013e3181255e98. [DOI] [PubMed] [Google Scholar]
- [WHO Europe] World Health Organization Europe. WHO guidelines for indoor air quality: dampness and mold. Copenhagen: World Health Organization; 2009. [PubMed] [Google Scholar]
- Yi FC, Lee BW, Cheong N, Chua KY. Quantification of Blo t 5 in mite and dust extracts by two-site ELISA. Allergy. 2005;60:108–112. doi: 10.1111/j.1398-9995.2004.00597.x. [DOI] [PubMed] [Google Scholar]