Abstract
Endoplasmic reticulum (ER) stress is a key link between obesity, insulin resistance, and type 2 diabetes. Here, we provide evidence that this mechanistic link can be exploited for therapeutic purposes with orally active chemical chaperones. 4-Phenyl butyric acid and taurine-conjugated ursodeoxycholic acid alleviated ER stress in cells and whole animals. Treatment of obese and diabetic mice with these compounds resulted in normalization of hyperglycemia, restoration of systemic insulin sensitivity, resolution of fatty liver disease, and enhancement of insulin action in liver, muscle, and adipose tissues. Our results demonstrate that chemical chaperones enhance the adaptive capacity of the ER and act as potent antidiabetic modalities with potential application in the treatment of type 2 diabetes.
Insulin resistance is a common feature of obesity and predisposes the affected individuals to a variety of pathologies, including hypertension, dyslipidemias, cardiovascular disease, and type 2 diabetes mellitus (1). Although considerable progress has been made in understanding the molecular mechanisms underlying the insulin resistance and type 2 diabetes, satisfactory treatment modalities remain limited.
Studies in the past decade have demonstrated that obesity is associated with inflammation and established a link between inflammatory responses, particularly through the c-Jun N-terminal kinase (JNK) and inhibitory kappa B kinase (IKK) signaling pathways, and abnormal insulin action (2). We have recently shown that obesity also induces ER stress, and this, in turn, plays a central role in the development of insulin resistance and diabetes by triggering JNK activity via inositol-requiring enzyme–1 (IRE-1) and inhibition of insulin receptor signaling (3). Subsequent independent studies have also verified the role of ER stress in insulin resistance in several experimental systems (4, 5). Taken together, in vitro and in vivo genetic evidence demonstrate a strong and causal relation between the functional capacity of the ER and insulin action, suggesting the possibility of exploiting this mechanism for therapeutic application.
Chemical or pharmaceutical chaperones, such as 4-phenyl butyric acid (PBA), trimethylamine N-oxide dihydrate (TMAO), and dimethyl sulfoxide, are a group of low molecular weight compounds known to stabilize protein conformation, improve ER folding capacity, and facilitate the trafficking of mutant proteins (6). Likewise, endogenous bile acids and derivatives such as ursodeoxycholic acid and its taurine-conjugated derivative (TUDCA) can also modulate ER function (7). In this study, we investigated whether pharmacologically active, small-molecule chemical chaperones could alleviate the increased ER stress seen in obesity and reverse insulin resistance and type 2 diabetes in experimental models.
To investigate the action of putative chemical chaperones, we first tested whether PBA and TUDCA protected against experimental ER stress in cultured cells. Pretreatment of Fao rat hepatoma cells with PBA suppressed tunicamycin-induced phosphorylation of double-stranded RNA-activated protein kinase–like endoplasmic reticulum kinase (PERK) (Thr-980) and eukaryotic initiation factor 2 alpha (eIF2α) (Ser-51) and JNK activation (fig. S1A). TUDCA pretreatment showed similar effects on tunicamycin-induced ER stress (fig. S1B). Pretreatment of liver cells with TUDCA reduced PERK and eIF2α phosphorylation and JNK activation upon exposure to tunicamycin (fig. S1B). Under these conditions, ER stress–induced splicing of X-box binding protein 1 (XBP-1) mRNA was also markedly reduced by both PBA and TUDCA (fig. S1, C and D). To exclude the possibility that PBA and TUDCA block general stress signaling without specificity for ER stress, we treated Fao cells with anisomycin, which activates JNK independent of ER stress. Neither PBA nor TUDCA prevented anisomycin-induced JNK activation (fig. S1, E and F).
XBP-1−/− mouse embryonic fibroblasts (MEFs) are hypersensitive to ER stress (3) because of the decreased ER folding capacity (8). Treatment of XBP-1−/− MEFs with PBA (fig. S2) also suppressed low-dose tunicamycin-induced phosphorylation of PERK and eIF2α, and activation of JNK, indicating that chemical chaperone treatment can reduce ER stress in multiple cell types and in a XBP-1–independent manner.
To investigate the in vivo effects of the chemical chaperones, we studied leptin-deficient (ob/ob) mice, a model of severe obesity and insulin resistance. Oral administration of PBA to ob/ob mice reduced ambient blood glucose to normoglycemic levels seen in the lean wild-type (WT) controls (434.2 ± 34.7 mg/dl versus 125.8 ± 12.6 mg/dl in vehicle versus PBA-treated ob/ob mice at 20 days, P < 0.001) (Fig. 1A). Normoglycemia in ob/ob mice was established within 4 days of PBA treatment, was maintained for up to 3 weeks, and was not associated with changes in body weight (Fig. 1B). PBA-treated ob/ob mice showed a more than twofold reduction (P < 0.001) in hyperinsulinemia (Fig. 1C), suggesting that the blood glucose–lowering effect of PBA is due to increased systemic insulin sensitivity. Neither of these parameters—blood glucose and insulin levels—were different between PBA- and vehicle-treated lean WT mice (Fig. 1, A to C).
Fig. 1.
Effect of PBA treatment on glucose metabolism and insulin sensitivity in ob/ob mice. PBA (1 g per kg of body weight) was orally administered to 7- to 8-week-old male ob/ob mice and age- and sex-matched WT controls (n ≥ 10 mice in each group) for 20 days. (A) Blood glucose concentrations (mg/dl) in vehicle- or PBA-treated ob/ob and WT mice at the fed state. (B) Body weights of ob/ob and WT mice treated with vehicle or PBA. (C) Plasma insulin concentrations (ng/ml) measured after a 6-hour fast at the onset of experiments and after 20 days of treatment with PBA or vehicle. (D) Glucose (0.5 g/kg) and (E) insulin (2 IU/kg) tolerance tests, performed after 15 and 28 days of vehicle or PBA treatment, respectively. Data are presented as the means ± SEM, and asterisks indicate statistical significance determined by student’s t test (*P < 0.05, **P < 0.001).
We next examined whole-body insulin sensitivity by performing glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) in PBA- and vehicle-treated animals. Vehicle-treated ob/ob mice exhibited severe hyperglycemia upon administration of glucose (0.5 g/kg) and exhibited impaired glucose tolerance. PBA treatment significantly improved glucose tolerance in ob/ob mice with glucose disposal curves comparable to those of lean animals (Fig. 1D). Similarly, the insulin-stimulated glucose disposal curves in PBA-treated ob/ob mice were markedly enhanced compared to those receiving vehicle (Fig. 1E).
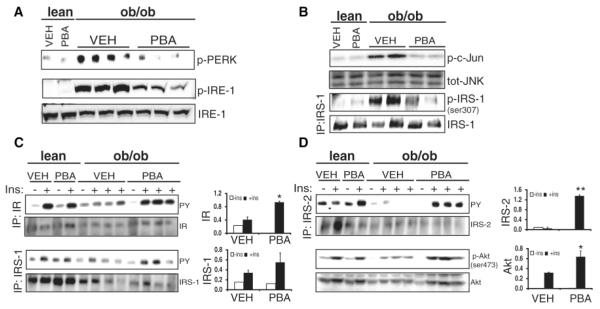
If the reversal of hyperglycemia, increased glucose tolerance, and insulin sensitivity are related to decreased ER stress, PBA-treated ob/ob mice should display a reduction in indicators of ER stress (3). Indeed, in PBA-treated ob/ob mice, PERK and IRE-1α phosphorylation in liver was markedly reduced in comparison with vehicle-treated ob/ob controls (Fig. 2A). Consistent with these results, JNK activation and insulin receptor substrate 1 (IRS-1) phosphorylation at Ser-307 was significantly suppressed in the liver of PBA-treated ob/ob mice (Fig. 2B). Similar results were also observed in adipose tissue of PBA-treated ob/ob mice (fig. S3, A and B). We next examined whether these biochemical alterations enhanced the signaling capacity of the insulin receptor (IR) in liver and adipose tissues of obese mice. Treatment with PBA led to improvements in insulin-induced IR (2.3-fold), IRS-1 (1.5-fold), and IRS-2 (19-fold) tyrosine phosphorylation and more distally Akt Ser-473 phosphorylation (3.2-fold) in liver tissue (Fig. 2, C and D). Similarly, insulin receptor signaling in obese mice was improved in adipose tissue after PBA treatment (fig. S3, C and D). Thus, PBA enhances insulin action at peripheral tissues in vivo.
Fig. 2.
Effect of PBA treatment on markers of ER stress in the liver tissue of ob/ob mice. (A) Phosphorylation of PERK (Thr980) and IRE-1α in liver tissues of PBA- or vehicle-treated ob/ob mice and lean controls. (B) Liver tissue total JNK activity, JNK protein levels, serine phosphorylation of IRS-1 (Ser-307), and total JNK and IRS-1 protein levels in the same group of mice. (C) Insulin-stimulated insulin receptor (IR), insulin receptor substrate 1 (IRS-1), insulin receptor substrate 2 (IRS-2), and (D) Akt (Ser-473) phosphorylation in liver tissues of PBA- and vehicle-treated lean and ob/ob mice upon insulin (2 IU/kg) infusion through the portal vein. The graphs on the right of each blot show the quantitation of phosphorylation for each protein. Data are presented as the means ± SEM, and asterisks indicate statistical significance determined by student’s t test (*P < 0.05, **P < 0.001).
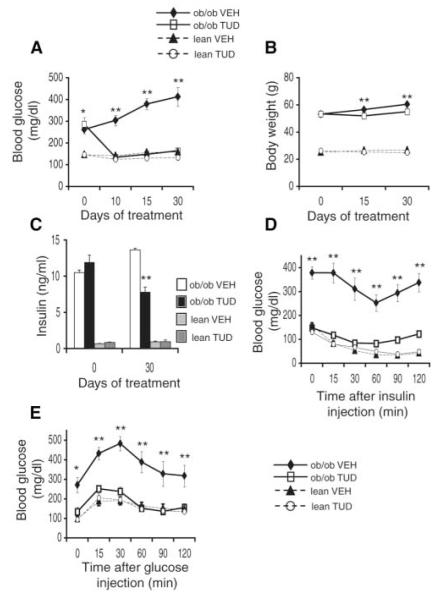
TUDCA is a hydrophilic bile acid derivative that, like PBA, diminishes ER stress responses in cultured liver cells (fig. S1B). When administered to ob/ob mice, TUDCA exhibited a potent antidiabetic activity with essentially complete normalization of blood glucose levels within 1 week of treatment (Fig. 3A). In the TUDCA-treated groups, there was a small but statistically significant decrease in body weight (1.3 g in the lean and 4.7 g in the ob/ob mice) (Fig. 3B). Normoglycemia in TUDCA-treated mice was maintained at significantly lower levels of blood insulin compared with vehicle-treated ob/ob mice (twofold reduction in TUDCA-treated mice, P < 0.001) (Fig. 3C). TUDCA treatment had no apparent effect on blood glucose or insulin levels in lean WT controls (Fig. 3, A to E). We next performed ITT and GTT experiments to examine systemic insulin sensitivity. The ability of insulin to decrease blood glucose concentration was markedly reduced in vehicle-treated ob/ob mice compared with lean controls (Fig. 3D). TUDCA administration significantly improved the in vivo responses to insulin in the ob/ob mice (Fig. 3D). The impaired glucose tolerance seen in vehicle-treated ob/ob mice was also corrected upon treatment with TUDCA (Fig. 3E).
Fig. 3.
Effect of TUDCA treatment on systemic glucose metabolism and insulin sensitivity in ob/ob mice. (A) Blood glucose concentrations (mg/dl) in the fed state in ob/ob and WT mice, treated with TUDCA or vehicle. (B) Body weights of ob/ob and WT mice treated with TUDCA or vehicle. (C) Plasma insulin concentrations (ng/ml) measured after a 6-hour fast at the onset of experiments and after 30 days of treatment with TUDCA or vehicle. (D) Insulin (2 IU/kg) tolerance and (E) glucose (0.5 g/kg) tolerance tests performed after 15 days of treatment with TUDCA or vehicle. The insulin and glucose tolerance tests were performed on different groups of mice. Data are presented as the means ± SEM, and asterisks indicate statistical significance determined by Student’s t test (*P < 0.05, **P < 0.001).
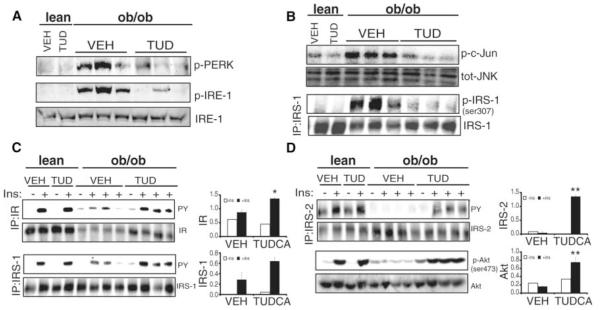
Biochemical indicators of ER stress and major elements of IR signal transduction pathway were examined in liver and adipose tissues of TUDCA-treated mice and controls. TUDCA-treated ob/ob mice showed suppression of obesity-induced PERK and IRE-1α phosphorylation and JNK activation in the liver (Fig. 4, A and B) and adipose (fig. S4A) tissues. Serine phosphorylation of IRS-1, which negatively regulates insulin receptor signaling, was also suppressed in liver tissues of TUDCA-treated ob/ob mice compared to vehicle-treated controls (Fig. 4B). Consistent with these changes, there was a marked recovery of insulin receptor signaling capacity in TUDCA-treated ob/ob mice. In liver and adipose tissues, acute insulin stimulation failed to induce IR, IRS-1, IRS-2, and Akt phosphorylations in vehicle-treated ob/ob mice compared to their lean controls, and TUDCA treatment restored the insulin signaling capacity in both tissues (Fig. 4, C and D; fig. S4B).
Fig. 4.
Effect of TUDCA treatment on ER stress parameters, JNK activation, and insulin receptor signal transduction pathway in the liver of ob/ob mice. (A) PERK (Thr-980) and IRE-1α phosphorylation, and total IRE-1α levels in liver tissues of TUDCA- or vehicle-treated ob/ob mice and lean controls. (B) JNK activity and IRS-1 (Ser-307) phosphorylation in liver tissues of ob/ob mice after TUDCA administration. (C) Insulin-stimulated tyrosine phosphorylation of insulin receptor and IRS-1. (D) Insulin-stimulated IRS-2 tyrosine, and Akt serine (Ser-473) phosphorylation in the liver tissues of TUDCA- and vehicle-treated ob/ob mice and lean WT controls. The graphs on the right of each blot demonstrate the quantitation of phosphorylation of each molecule. Data are presented as the means ± SEM, and asterisks indicate statistical significance determined by student’s t test (*P < 0.05, **P < 0.001).
To analyze the action of the chemical chaperones on systemic glucose metabolism and insulin action in more detail, we performed hyperinsulinemic-euglycemic clamps in ob/ob mice that had been treated for 20 days with these drugs (Fig. 5). We found that administration of both PBA and TUDCA significantly suppressed hepatic glucose production (HGP) at baseline and during the clamp studies (Fig. 5, A and B). Glucose infusion rates (GIRs) to maintain euglycemia were also higher in PBA- and TUDCA-treated ob/ob mice compared to vehicle-treated controls (Fig. 5C). Consistent with this result, whole-body glucose disposal rates (Rd) during the clamp were significantly increased after PBA and TUDCA treatments (Fig. 5D). We also performed hyperinsulinemic-euglycemic clamps in lean mice, in order to compare the improvement in glucose homeostasis in PBA- and TUDCA-treated ob/ob mice with lean mice. The HGP during clamps in lean mice was 3.9 ± 2.6 mg kg−1 min−1, indicating that the HGP is totally normalized in drug-treated ob/ob mice. However, Rd and GIR were partially normalized in this protocol because the Rd in lean mice was 52.2 ± 9.5 mg kg−1 min−1, and the GIR was 48.3 ± 8.1.
Fig. 5.
Hyperinsulinemic-euglycemic clamp studies in ob/ob mice treated with PBA or TUDCA. Hyperinsulinemic-euglycemic clamp studies were performed after 20 days of treatment with vehicle, PBA, or TUDCA. (A) Basal hepatic glucose production (HGP). (B) HGP during the clamp. (C) Glucose infusion rates (GIR). (D) Glucose disposal rates (Rd). (E) Glucose uptake into muscle tissue during the clamp. (F) Glucose uptake into white adipose tissue (WAT). (G) Glucose levels during clamp procedure. (H) Glucose infusion rates during the clamp procedure. Data are presented as the means ± SEM, and asterisks indicate statistical significance determined by analysis of variance, post hoc test–Fisher’s Protected Least Significant Difference (*P < 0.05, **P < 0.001).
These data indicate that, in addition to suppression of hepatic glucose output, PBA and TUDCA treatment also enhances insulin-stimulated glucose disposal in peripheral tissues, which principally include muscle and adipose tissues. To explore this further, we also determined the rate of glucose uptake in muscle and epididymal adipose tissue during the clamp procedure. In both PBA- and TUDCA-treated mice, glucose uptake in muscle and adipose tissue was increased compared to that in tissues from vehicle-treated control animals (Fig. 5, E and F). These results demonstrate that PBA and TUDCA improve systemic insulin resistance by influencing both hepatic glucose output and glucose disposal in muscle and adipose tissues. Consistent with this conclusion, we found no evidence for glucose storage in liver in the form of glycogen (fig. S5, A and B) or triglyceride (fig. S6, A and B; also see below) in PBA- or TUDCA-treated ob/ob mice.
Obesity in mice and humans is associated with alterations in liver lipid metabolism and fatty liver disease. As shown in fig. S6, TUDCA and PBA treatment in ob/ob mice resulted in resolution of the obesity-induced lipid accumulation in liver. Analysis of liver triglyceride content showed a significant reduction in both PBA- and TUDCA-treated ob/ob mice compared to control animals (fig. S6, A and B). Consistent with the resolution of fatty liver infiltration, liver functional enzymes alanine aminotransferase and aspartate aminotransferase were normalized in both PBA- and TUDCA-treated ob/ob mice (fig. S7, A and B).
In summary, we have shown that small-molecule agents that modulate ER and increase folding capacity improve systemic insulin action and may have therapeutic potential for the treatment of insulin resistance and type 2 diabetes. The exact mechanisms triggering ER stress in obesity and type 2 diabetes are unclear but likely involve multiple signals, including chronically increased demand on synthetic machinery along with profound alterations in energy fluxes in metabolically active tissues. Chemical enhancement of ER function to cope with these alterations therefore provides a unique approach to manage metabolic abnormalities associated with obesity and diabetes.
Several lines of evidence suggest that chemical chaperones enhance ER functional capacity. For example, PBA increases the trafficking of the cystic fibrosis transmembrane regulator with Δ508 mutation (CFTRΔ508) and enhances the secretion of the mutant α1-ATZ protein in α1-antitrypsin deficiency (9). Although data on the chaperone activity of TUDCA are sparse, recent studies demonstrated this compound’s ability to prevent ER stress–induced apoptosis (7). Our data indicate that both PBA and TUDCA can alleviate ER stress in vitro and in vivo.
The chemical chaperones we studied, PBA and TUDCA, have outstanding in vivo safety profiles. PBA, for example, has been approved by the U.S. Food and Drug Administration for clinical use in urea-cycle disorders as an ammonia scavenger and has been in clinical trials for the treatment of other diseases such as thalassemia and cystic fibrosis (10–12). TUDCA is a derivative of an endogenous bile acid, and it has been safely used as a hepatoprotective agent in humans with cholestatic liver diseases (13, 14). The ability of these chemical chaperones to alleviate ER stress, establish normoglycemia, and rescue insulin action in mice provides strong support to the recently proposed ER stress–based mechanistic model of type 2 diabetes (3) and demonstrates the feasibility of targeting ER function for therapeutic gain. We suggest that chemical chaperones in general, and PBA and TUDCA in particular, may warrant clinical investigation as treatments for type 2 diabetes.
Supplementary Material
Acknowledgments
We thank J. Kim and G. I. Shulman for training and advice on the hyperinsulinemic-euglycemic clamp experiments and R. J. Foote for administrative assistance. Supported by NIH grant DK52539 to G.S.H. M.F is supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science. L.O. is supported by the American Diabetes Association mentor-based postdoctoral fellowship.
Footnotes
www.sciencemag.org/cgi/content/full/313/5790/1137/DC1
Materials and Methods
Figs. S1 to S7
References
References and Notes
- 1.Permutt MA, Wasson J, Cox N. J. Clin. Invest. 2005;115:1431. doi: 10.1172/JCI24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS. J. Clin. Invest. 2005;115:1111. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozcan U, et al. Science. 2004;306:457. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 4.Ozawa K, et al. Diabetes. 2005;54:657. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 5.Nakatani Y, et al. J. Biol. Chem. 2005;280:847. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 6.Welch WJ, Brown CR. Cell Stress Chaperones. 1996;1:109. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Q, et al. Hepatology. 2002;36:592. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 8.Shaffer AL, et al. Immunity. 2004;21:81. [Google Scholar]
- 9.Burrows JA, Willis LK, Perlmutter DH. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1796. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maestri NE, Brusilow SW, Clissold DB, Bassett SS. N. Engl. J. Med. 1996;335:855. doi: 10.1056/NEJM199609193351204. [DOI] [PubMed] [Google Scholar]
- 11.Collins AF, et al. Blood. 1995;85:43. [PubMed] [Google Scholar]
- 12.Chen WY, Bailey EC, McCune SL, Dong JY, Townes TM. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5798. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poupon RE, Bonnand AM, Chretien Y, Poupon R. Hepatology. 1999;29:1668. doi: 10.1002/hep.510290603. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MM, Gershwin ME. N. Engl. J. Med. 2005;353:1261. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.