Abstract
Objective:
We aimed to demonstrate the effect of continuous or bolus nasogastric feeding on gastric emptying, small bowel water content, and splanchnic blood flow measured by magnetic resonance imaging (MRI) in the context of changes in plasma gastrointestinal hormone secretion.
Background:
Nasogastric/nasoenteral tube feeding is often complicated by diarrhea but the contribution of feeding strategy to the etiology is unclear.
Methods:
Twelve healthy adult male participants who underwent nasogastric intubation before a baseline MRI scan, received 400 mL of Resource Energy (Nestle) as a bolus over 5 minutes or continuously over 4 hours via pump in this randomized crossover study. Changes in gastric volume, small bowel water content, and superior mesenteric artery blood flow and velocity were measured over 4 hours using MRI and blood glucose and plasma concentrations of insulin, peptide YY, and ghrelin were assayed every 30 minutes.
Results:
Bolus nasogastric feeding led to significant elevations in gastric volume (P < 0.0001), superior mesenteric artery blood flow (P < 0.0001), and velocity (P = 0.0011) compared with continuous feeding. Both types of feeding reduced small bowel water content, although there was an increase in small bowel water content with bolus feeding after 90 minutes (P < 0.0068). Similarly, both types of feeding led to a fall in plasma ghrelin concentration although this fall was greater with bolus feeding (P < 0.0001). Bolus feeding also led to an increase in concentrations of insulin (P = 0.0024) and peptide YY (P < 0.0001), not seen with continuous feeding.
Conclusion:
Continuous nasogastric feeding does not increase small bowel water content, thus fluid flux within the small bowel is not a major contributor to the etiology of tube feeding-related diarrhea.
Keywords: bolus feeding, gastric emptying, mesenteric blood flow, nasogastric feeding, peptide YY
Undernourished patients have poorer hospital outcomes compared with those who are normally nourished,1,2 and nutritional support is often employed for patients who are unable to meet their nutritional requirements orally. Provision of nutritional support usually requires tube feeding, which is typically achieved by pump-assisted continuous feeding into the stomach at low rate (50–100 mL/h) spread out over 12 to 24 hours per day using a fine-bore nasogastric tube.2
Although tube feeding has been shown to reduce mortality in undernourished hospitalized patients,3 it can be associated with a number of gastrointestinal complications. The most common tube feeding-associated gastrointestinal symptom is diarrhea, occurring in up to 30% of enterally fed patients on hospital wards and over 60% of patients on intensive care units.4,5
Although tube feeding-related diarrhea can result from a number of factors, the mode of feed delivery may be a major contributor to the etiology. Some studies suggest that bolus feeding causes more abdominal bloating, nausea, and diarrhea6 than continuous feeding, but others have reported the converse.7
Bolus feeding, in contrast to continuous feeding, is considered a more physiological mode of delivery. In replicating normal patterns of feeding, bolus delivery allows the phased supply of hypertonic nutrient loads into the jejunum reducing the metabolic demand on the small intestine and possibly preventing excessive accumulation of jejunal fluid.8,9
The mode of delivery of feeds is also known to affect water absorption and motor responses in the colon via neurohumoral mechanisms involving peptide YY as well as affecting gastric emptying via the gastric hormone ghrelin. In human studies, polymeric diets infused either intragastrically or intraduodenally at low or high loads resulted in differing colonic responses with no change observed in small bowel motility or colonic in-flow, suggesting a predominant effect on the colon. Additional studies demonstrate that intragastric fluid loads increase fluid secretion in the ascending colon, indicating that intragastric bolus feeds may increase fluid delivery to the distal colon and possibly lead to diarrhea.10–12
It is possible that these changes and small bowel fluid flux may lead to excessive fluid loads reaching the colon. Although previous studies have indicated that small bowel motility remains unchanged in response to intragastric or intraduodenal feeding,10 small bowel water content in the context of bolus or continuous feeding remains poorly defined.
We hypothesized that small bowel water content is influenced by the mode of feed delivery and could contribute to the etiology of gastrointestinal symptoms experienced by tube fed patients. The aim of this study was, therefore, to establish the different effects of continuous and bolus nasogastric feeding on gastric emptying, small bowel water content, and splanchnic blood flow as measured by serial magnetic resonance imaging (MRI) in the context of changes in plasma gastrointestinal hormone secretion.
SUBJECTS AND METHODS
Study Design and Setting
This was a randomized, unblinded 2-way crossover study on healthy human volunteers. It was conducted at the Sir Peter Mansfield Magnetic Resonance Centre, University of Nottingham, United Kingdom.
Subjects and Ethics
We sought to recruit 12 healthy adult male volunteers with a body weight of 65 to 85 kg. Those with acute illness in the preceding 6 weeks, on regular medication, with a history of substance abuse or having factors precluding safe MRI were excluded. Additional exclusion criteria included a history of gastrointestinal motility disorders (eg, gastroesophageal reflux disease, irritable bowel syndrome, gastroparesis, sphincter of Oddi dysfunction, etc), previous thoracic or abdominal surgery. Participants were also excluded if they had 3 or less bowel movements per week or more than 2 per day. Ethical approval was granted by the University of Nottingham Medical School Research Ethics Committee, and the study protocol was registered at http://www.clinicaltrials.gov (NCT01557673).
Study Protocol
The protocol was similar to that used in a previous study examining gastric emptying.13 Participants reported at 9 am after a fast from midnight and after having abstained from alcohol, nicotine, and caffeine from 6 pm the day before. Participants were not permitted to eat or drink for the duration of the study period. Before blood sampling and nasogastric intubation, they underwent an MRI scan to determine baseline values for superior mesenteric artery blood flow, small bowel water content, and gastric fluid volume. Subsequently, a 16 G venous cannula (BD Venflon, Franklin Lakes, NJ) was inserted into the right antecubital fossa for blood sampling. After cannulation, an 80-cm 8 F Freka (Fresenius Kabi, Runcorn, UK) nasogastric tube was inserted. After the tube had been sited and the subjects adapted to its presence a 20-mL blood sample was drawn for analysis of plasma concentrations of glucose, insulin, ghrelin, and peptide YY. The position of the tube and the physiological effect of cannulation and nasogastric intubation were confirmed by a second baseline MRI scan (Fig. 1).
FIGURE 1.
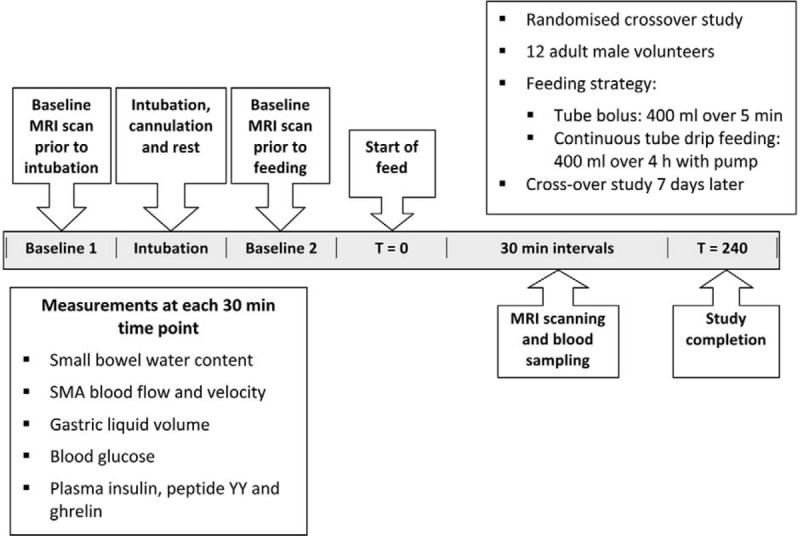
Summary of study protocol. SMA indicates superior mesenteric artery.
Two feeding strategies of 400 mL via the nasogastric tube were undertaken for each volunteer using Nestle Resource Energy (Nestlé Health Science, Liverpool, UK) (Table 1):
Bolus feeding via syringe delivered over 5 minutes.
Continuous feeding via pump delivered at 100 mL/h over 4 hours.
TABLE 1.
Composition of the Feed (Nestle Resource Energy)
| Typical Values | Per 100 mL | Per 400 mL |
| General | ||
| Energy, kJ/kcal | 637/151 | 2548/606 |
| Protein (15% kcal), g | 5.6 | 22.4 |
| Carbohydrates (55% kcal), g | 21 | 84 |
| of which sugars, g | 5.7 | 22.8 |
| of which lactose, g | <0.5 | <2.0 |
| Fat (30% kcal), g | 5 | 20 |
| of which saturates, g | 0.7 | 2.8 |
| of which monounsaturates, g | 1.9 | 7.6 |
| of which polyunsaturates, g | 2.3 | 9.2 |
| Fiber (0% kcal), g | 0 | 0 |
| Vitamins | ||
| A, μg retinol equivalents | 138 | 552 |
| D, μg | 1.8 | 7.2 |
| K, μg | 14 | 56 |
| C, mg | 15 | 60 |
| B1, (Thiamin) mg | 0.23 | 0.92 |
| B2, (Riboflavin) mg | 0.22 | 0.88 |
| B6, mg | 0.35 | 1.4 |
| Niacin, mg niacin equivalents | 2.5 | 10 |
| Folic acid, μg | 45 | 180 |
| B12, μg | 0.22 | 0.88 |
| Pantothenic acid, mg | 0.85 | 3.4 |
| Biotin, μg | 6.3 | 26 |
| E, mg α tocopherol equivalents | 3 | 12 |
| Electrolytes and minerals | ||
| Sodium, mg (mmol) | 80 (3.5) | 320 (14) |
| Chloride, mg (mmol) | 185 (5.2) | 740 (20.8) |
| Potassium, mg (mmol) | 170 (4.4) | 680 (17.6) |
| Calcium, mg (mmol) | 80 (2.0) | 320 (8.0) |
| Phosphorus, mg (mmol) | 80 (2.6) | 160 (10.4) |
| Magnesium, mg (mmol) | 28 (1.2) | 112 (4.8) |
| Iron, mg | 1.7 | 6.8 |
| Zinc, mg | 1.7 | 6.8 |
| Copper, μg | 190 | 760 |
| Iodine, μg | 16 | 64 |
| Selenium, μg | 8 | 32 |
| Manganese, mg | 0.35 | 1.4 |
| Chromium, μg | 7.5 | 30 |
| Molybdenum, μg | 13 | 52 |
| Fluoride, mg | 0.15 | 0.6 |
| Osmolarity, mOsm/L | 488 | |
| Osmolality, mOsm/kg | 636 |
Randomization and Blinding
The participants were allocated with equal probability to receive 1 of the 2 feeding strategies on their first study visit. Crossover studies using the alternate feeding strategy were performed 7 to 10 days after the index study. This treatment sequence was based on a computer-generated random code. Because of the differences in interventions, blinding was not feasible.
Plasma Hormone and Glucose Assays
Blood samples were withdrawn via a 3-way tap; the first 5 mL was discarded to avoid contamination with saline. One 20-mL baseline blood sample was collected and then additional 20-mL samples were collected at 30-minute intervals during the 4-hour postprandial period. Immediately after samples were drawn, blood glucose was measured (YSI 2300 STATplus, Yellow Springs Instruments) and then the remainder of the sample was dispensed. Whole blood (2 mL) was collected into tubes containing 75-μL ethylene glycol tetra-acetic acid and after centrifugation at 1500 g for 10 minutes. The plasma was then removed and stored at −80°C. Whole blood (2 mL) was dispensed into tubes containing 50 μL trasylol and centrifuged at 1500 g for 10 minutes. The plasma was removed and stored at −80°C and later analyzed for the hormones peptide YY and ghrelin. Concentrations of peptide YY and ghrelin were measured in samples collected at 30-minute intervals during the postprandial period using assay methods that have been previously described.14 The remaining whole-blood sample was dispensed into a plain tube and left to clot for 30 minutes. Serum samples were then obtained after centrifugation at 1500 g for 10 minutes. Aliquots of serum were stored at −80°C and later analyzed for insulin by radioimmuno assay (Coat-A-Count Insulin, Diagnostic Products, Los Angeles, CA).
Magnetic Resonance Methods
MRI scanning was carried out on a 1.5T Philips Achieva MRI scanner (Philips Medical Systems, Best, The Netherlands). MRI compatible vector cardiogram (VCG, Philips Medical Systems, Best, The Netherlands) electrodes were placed on the subject's chest to allow cardiac gating for the superior mesenteric artery blood flow scans. The subjects were then positioned supine with a 16-element parallel imaging coil wrapped around the abdomen.
Gastric emptying was assessed using a balanced gradient echo (balanced turbo field echo) sequence acquiring 40 contiguous axial slices with reconstructed in-plane resolution 1.56 mm × 1.56 mm and slice thickness of 5 mm with no gaps between slices. The sequence parameters were: echo time TE = 1.5 milliseconds, repetition time TR = 3.0 milliseconds, flip angle 45°. The data were collected during an expiration breath hold of 13 seconds.
Small bowel water content was assessed as previously described15,16 using a coronal single shot fast spin echo sequence acquiring 24 contiguous coronal slices with reconstructed in-plane resolution 0.78 mm × 0.78 mm and slice thickness 7 mm with no gaps between slices, TE = 320 milliseconds and TR = 8000 milliseconds. The data were collected during an expiration breath hold of 24 seconds.
The superior mesenteric artery blood flow was measured using a cine phase contrast MRI scan. Axial and sagittal balanced gradient echo standard, bright blood localizer scans were used as needed to position the flow measurement plane perpendicular to the superior mesenteric artery. The 2-dimensional cine phase contrast MRI scan then used after localization was a turbo-field echo sequence with TE = 3.2 milliseconds, TR = 5.6 milliseconds, and flip angle 25°, with reconstructed in-plane resolution 1.17 mm × 1.17 mm and slice thickness 6 mm, velocity encoding 140 cm/s and flow encoding through plane. This was acquired in 14 seconds.
At each time point, the positioning of the participant on the scanner bed, set up, scout imaging, and data collection took approximately 8 to 10 minutes, after which the participants left the scanner and were kept sitting upright in a quiet room next to the scanner.
Data Analysis
Volumes of gastric contents were measured by a single, experienced operator using an intensity-based, semiautomatic method, which defined the bright liquid stomach contents on each image slice17 using in-house software on an IDL platform (IDL 6.4; Research Systems Inc, Boulder, CO). Small bowel water content was measured using in-house software written in IDL (Research Systems Inc, Boulder, CO).15 This technique was previously validated by intubation studies in which measured values were shown to closely parallel infused volumes.15 As previously reported,16 small bowel water content shows several distinct phases postprandially, which in this 4-hour study can be divided into an early phase dominated by gastric emptying, the “gastric phase” and a later phase influenced more by pancreaticobiliary and intestinal secretions, the “small bowel” phase. The flow measurements were carried out on the scanner using the manufacturer's software.18 Each cine phase contrast MRI scan was gated retrospectively using the vector cardiogram data generating a set of velocity-encoded images, uniformly spaced across the R to R wave of the cardiac cycle. A region of interest was drawn manually around the superior mesenteric artery on one image. After this, an automated contour propagation algorithm was applied across the entire data set. The scanner software then automatically calculated velocity and flow rate. The change in gastric liquid volume was calculated by subtracting the liquid volume present before commencement of feed.
Sample Size and Statistical Analysis
Previous work in healthy volunteers using glucose orally delivered indicated a mean postprandial small bowel water content at 40 minutes of 47 mL (SD: 15).18 This indicates that using 12 subjects we should be able to detect here a true difference in the mean response of matched pairs of −15 mL or 15 mL with 90% power with an associated type I error probability of 0.05.
The collected data were analyzed and graphs created with GraphPad Prism 6.0 for Mac OS X (GraphPad Software, La Jolla, CA, www.graphpad.com). Parametric data were expressed as mean (SEM). The Student paired t test was used to test for any significance of differences between groups. After this, to test mean values for any between-treatment effects (eg, bolus vs continuous), a repeated-measures analysis of variance with Bonferroni correction was employed. Differences were considered significant at P < 0.05.
RESULTS
Thirteen male subjects were approached and 12, who were all nonsmokers, were enrolled and their baseline characteristics are summarized in Table 2. All participants completed both arms of the study and were included in the analysis. None reported adverse effects.
TABLE 2.
Baseline Characteristics of the Participants
| Number | 12 |
| M:F ratio | 12:0 |
| Age, mean (SE), yr | 22.7 (0.7) |
| Body weight, mean (SE), kg | 75.9 (2.5) |
| Body mass index, mean (SE), kg/m2 | 22.5 (0.6) |
Gastric Liquid Volume
Significant differences were observed between feeding strategies in measured gastric liquid volume during the course of the study (P < 0.0001) (Fig. 2). There was a marked elevation in mean gastric liquid volumes immediately after feeding, reaching a peak at 30 minute after bolus. Mean gastric liquid volume in this group then fell reaching fasted values by 180 minutes. In contrast, the continuous strategy led to only modest elevations in mean gastric liquid volume, with a peak volume of 133 ± 15 mL recorded at 90 minutes (Fig. 2) and returning to fasted values at 240 minutes. Bolus feeding provided a total intragastric carbohydrate load of 333 kcal (1.39 MJ). As total gastric emptying in the bolus group was achieved by 180 minutes, this indicates a carbohydrate-emptying rate of 1.9 kcal/min (7.95 kJ/min). Gastric volumes using the continuous feeding strategy reached fasted values at 240 minutes, thus giving a gastric emptying rate of carbohydrate of 1.4 kcal/min (5.86 kJ/min).
FIGURE 2.
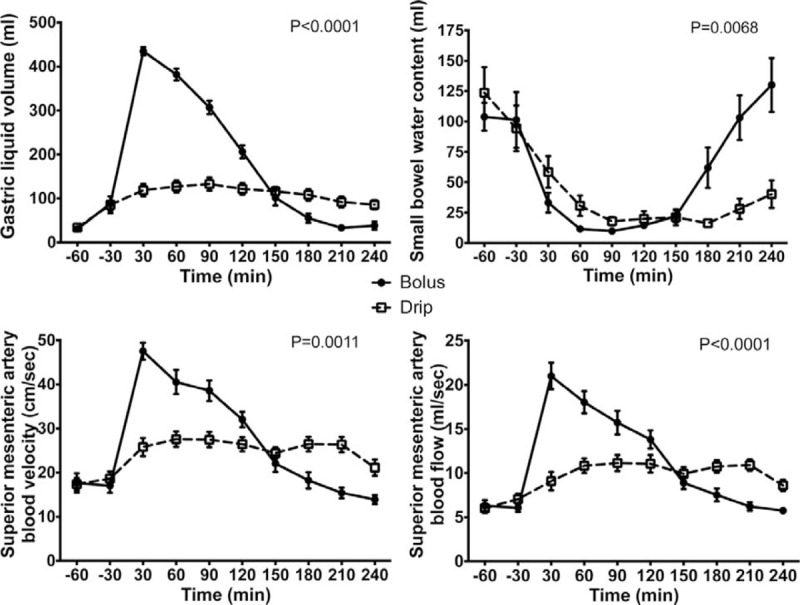
Changes in gastric liquid volume, small bowel water content, superior mesenteric artery blood velocity, and superior mesenteric artery blood flow after nasogastric infusion of 400 ml of Nestle Resource Energy over 4 hours (continuous) or 5 minutes (bolus). All values are mean ± SEM. The P values are for the test of continuous drip versus bolus feeding using the analysis of variances and a repeated measures model.
Small Bowel Water Content
During the first 2 hours, the responses to both feeds were similar with a decline in mean small bowel water content of around 90 mL (Fig. 2) but with differing responses following this time point. After 90 minutes in the bolus fed group, mean small bowel water content was seen to increase leading to a net efflux of small bowel water of 30 mL by the end of the study. This was reflected in a between treatment difference (P < 0.0068).
Superior Mesenteric Artery Blood Velocity and Flow
Measurements of superior mesenteric artery blood velocity and flow produced responses mirroring the gastric emptying plot (Fig. 2). For bolus feeding, mean values for both superior mesenteric artery velocity and flow increased significantly after feeding (baseline vs velocity and flow at 30 minutes, P < 0.0001). Superior mesenteric artery velocity was increased by 30 cm/s and flow by 15 mL/s above fasted values before falling after the 30-minute time point. There were significant differences between feeding strategies over the course of the study in the responses observed for both superior mesenteric artery velocity and superior mesenteric artery flow (bolus vs continuous, P = 0.0011 and P < 0.0001, respectively).
Plasma Glucose and Hormone Concentrations
Glucose
Initiation of feeding led to an increase in plasma glucose concentrations in both bolus and continuous feeding groups of 0.8 and 0.6 mmol/L, respectively (Fig. 3). Peak venous glucose concentrations were obtained at 30 minutes with a subsequent decline.
FIGURE 3.
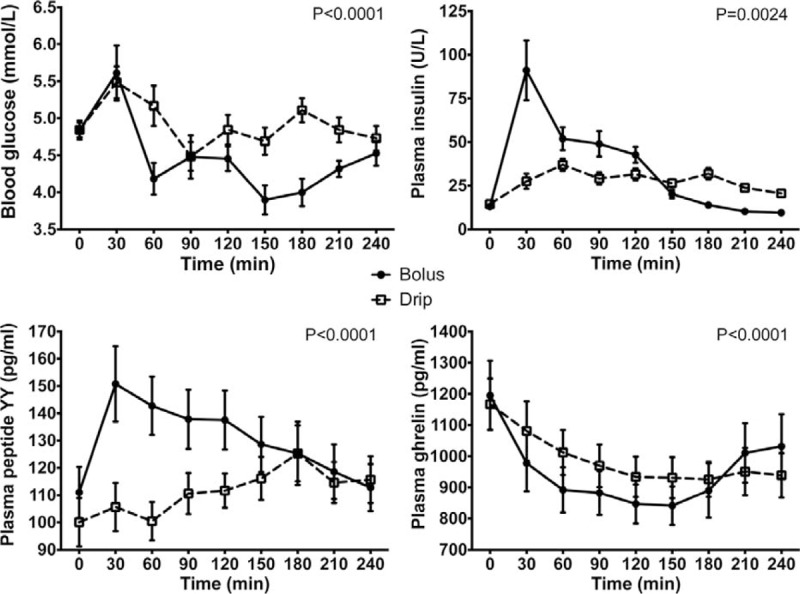
Changes in concentrations of blood glucose, and plasma insulin, peptide YY, and ghrelin after nasogastric infusion of 400 mL of Nestle Resource Energy over 4 hours (continuous) or 5 minutes (bolus). All values are mean ± SEM. The P values are for the test of continuous drip versus bolus feeding using the analysis of variances and a repeated measures model.
Insulin
Bolus feeding led to a significant rise in plasma insulin concentrations compared with the fasted state (baseline vs insulin concentration at 30 minutes, P = 0.0008). Insulin concentrations then declined to reach fasted values by 180 minutes (Fig. 3). In comparison with bolus feeding, there was only a modest increase in insulin concentrations with continuous feeding (P = 0.0024).
Peptide YY
Differences in plasma concentrations of peptide YY were observed between bolus and continuous feeding groups (P < 0.0001) (Fig. 3). In the bolus group, feeding led to an increase in peptide YY concentrations of 39.8 pg/mL compared with the fasted state. After this initial elevation, there was a steady decline in peptide YY concentrations to approach fasted concentrations by the end of the study. In comparison, peptide YY concentrations in the continuous feeding group did not increase to the same magnitude as the bolus fed group with a maximum change of 25.2 pg/mL.
Ghrelin
Plasma concentrations of ghrelin fell in both groups after the initiation of feeding (Fig. 3). The fall in ghrelin of 354 pg/mL was greatest in the bolus fed group with recovery of concentrations toward fasting values after 150 minutes. In contrast, ghrelin concentrations remained depressed in the continuous fed group throughout the 4-hour feeding phase with a maximum reduction in concentrations of 228 pg/mL.
DISCUSSION
This is the first study to demonstrate changes in postprandial small bowel water content, superior mesenteric artery blood velocity and flow, and plasma gut hormones in response to 2 different nasogastric feeding strategies using a validated MRI method.15 Previous isotope studies have indicated differences in gastric emptying in response to different methods of feeding13 and the findings of this study add to those results.
We have observed that continuous nasogastric feeding did not elevate gastric liquid volume, small bowel water content, or superior mesenteric artery blood flow or velocity to any great extent, whereas the converse was true for an equivalent volume given via bolus.
It is known that the principal factor governing the rate of gastric emptying is total carbohydrate load19 mediated by glucose receptors located within the small bowel providing negative feedback to ensure a duodenal glucose delivery rate of approximately 1.5 to 3 kcal/min (6.28 to 12.55 kJ/min).20,21 Although the same total carbohydrate load was delivered with both modes of feeding, the rate of carbohydrate emptying was lower with the continuous strategy. Other nutrients that stimulate cholecystokinin release are likely to play a part in gastric emptying but probably to a lesser extent than glucose as many complex proteins and fats require enzymatic hydrolysis in the small bowel facilitated by pancreatic exocrine secretion. More important perhaps is the rate of delivery with bolus feeding causing gastric distension. Animal studies using isotonic saline indicate that elevations in gastric pressure increase the rate of gastric emptying of fluids22,23 and this may have contributed to faster gastric emptying seen with the bolus strategy in this study. It has also been reported previously that increased gastric filling increases intragastric pressure as well as gastric emptying rate.24,25
The rate of gastric emptying is known to affect subsequent plasma glucose concentrations, although hyperglycemia is tightly controlled by regulatory hormones, insulin, glucagon-like peptide-1, and gastric inhibitory polypeptide. In this study, insulin responses corresponded closely with gastric liquid volumes. By the time gastric emptying was complete in the bolus group at 180 minutes, the insulin concentrations had returned toward fasted values. In contrast, elevated plasma insulin concentration was observed beyond 180 minutes with the continuous strategy, probably as a result of sustained duodenal glucose delivery. The maximal capacity of glucose absorption from the small intestine into the systemic circulation is reported to be in the region of 2 kcal/min (8.37 kJ/min) per 30 cm.26 Thus delivery of carbohydrate at rates of 1.9 kcal/min (7.95 kJ/min) in the bolus and 1.4 kcal/min (5.86 kJ/min) in the continuous group are adequate to facilitate complete absorption within the small bowel and avoid excessive fluid efflux due to high osmotic loads.
Previous studies have indicated that tube feeding-related diarrhea is likely to be a disorder of colonic function rather than small intestinal motility10,12,27 although the small bowel response to different feeds and strategies remains ill-defined.16,28,29 Both feeding strategies were characterized by a fall in small bowel water content, an effect which has been demonstrated previously and is likely due to net intestinal absorption.16,28–30 Small molecule carbohydrates are hydrolyzed by enzymes in the upper gastrointestinal tract, which increases their solubility and hence their rate of delivery to the duodenum where they are absorbed rapidly along with water. Complex molecules, on the other hand, such as fat, protein, and undigested polysaccharides, have delayed entry into the small bowel where their osmotic potential results in the efflux of water into the lumen.16,29,31 This may account for the increase in small bowel water content after 90 minutes with bolus feeding, mediated by a combination of gastric emptying and small bowel fluid secretion.
Postprandial gastric emptying and the absorption of fluid in the small bowel and colon have been shown to be under the influence of a number of gastrointestinal hormones released in response to nutrient intake.21,32–35 In particular, the relationship between cholecystokinin and peptide YY has been highlighted as providing humoral enterogastric feedback to slow gastric emptying and small intestinal transit in what has been termed the “ileal brake.”36 Disturbances of gastric emptying and small bowel motility are often a feature of patients receiving tube feeding particularly in the setting of critical care, and it is possible that aberrant release of these regulatory hormones could contribute to tube feeding-related diarrhea. Postprandial peptide YY concentrations are known to increase, but there is a calorie threshold of at least 530 kcal (2.22 MJ) below which no rise is observed.37 This is consistent with the results of this study with elevations in postprandial peptide YY concentrations seen with bolus feeding [total 606 kcal (2.54 MJ)] but a slower response obtained with continuous feeding [total 151 kcal/h (632 kJ/h)] requiring 3.5 hours to exceed the caloric threshold. It follows, therefore, that maximal peptide YY concentrations using the continuous strategy would only be obtained after 3 hours of feeding have elapsed. Complete gastric emptying and transit of nutrients to the distal small bowel to facilitate nutrient stimulated release of peptide YY probably occurs only after 180 minutes. The primary effect of peptide YY is to increase absorption in the small bowel, and although this could account for the reduction in small bowel water content seen initially with bolus feeding, the same cannot be said for continuous feeding where peptide YY concentrations remain modest until much later. This discrepancy is not simple to explain, although it is possible that the relative contribution to fluid absorption of regulatory hormones in different areas of the gut could differ. In human surgical specimens, peptide YY concentrations are found to be highest in the sigmoid colon, suggesting that this may be the site of maximal activity.38 It is also possible that drip feeding failed to switch on the complete fed response, which, in the absence of ileal brakes, could lead to malabsorbed fat entering the colon, triggering diarrhea and other symptoms.
Another hormone implicated in regulation of gastric emptying and the intake of nutrients is ghrelin, produced by endocrine cells located within gastric mucosa. In this study, plasma ghrelin concentrations demonstrated a typical decrease after ingestion of carbohydrate and fat. It has previously been reported that nutrient-stimulated effects on ghrelin secretion reaches maximal suppression by 1 hour,39 although in this study, concentrations continued to fall after 2 hours. Previous studies have indicated that gastric distension does not play a significant role in the control of ghrelin secretion and this would be consistent with the results in this study. Although suppression of ghrelin after both feeding strategies was of a similar magnitude, ghrelin concentrations were lower after bolus feeding and is likely to be due to the greater nutrient delivery encountered by the small bowel. Very little is known regarding the action of ghrelin on small intestinal fluid flux, although studies have indicated that ghrelin increases pancreatic exocrine secretion and may increase small bowel water content in this way. In the critical care setting, plasma concentrations of ghrelin are suppressed with return to normal plasma levels on recovery. It may be that the influence of ghrelin on intestinal fluid flux is different in patients receiving tube feeding although this is yet to be studied in detail.
Both splanchnic blood volume and flow is known to increase in response to feeding and may be due to the vasoactive properties of carbohydrate, fat, and protein.40 In addition, studies in both animals and humans have suggested that gastric distension can be a stimulus for changes in superior mesenteric artery flow although the mechanisms underlying this effect are unclear.41,42 In this study, both superior mesenteric artery velocity and flow were seen to increase significantly, mainly in response to bolus feeding. It is not clear whether the differences in superior mesenteric artery blood flow are the result of variations in nutrient load, gastric distension, or the vasoactive properties of insulin, although the responses of gastric fluid volume and insulin concentration seem to follow a similar temporal pattern. Physiological parameters such as systemic blood pressure, heart rate, and vasoactive sympathetic mediators are known to influence superior mesenteric artery flow although the relative contributions of these parameters in response to bolus or continuous feeding have not previously been studied. Although it is difficult to draw conclusions about tube feeding–induced changes in splanchnic blood flow that might occur in the clinical setting, it is clear at least in healthy individuals, the primary effect of bolus feeding is to increase superior mesenteric artery flow.
Some limitations of this study should be clarified. It was not possible to determine the relative contributions to gastric liquid volume of gastric secretions or saliva and it may be possible that production of both differed between the 2 groups. In addition, this study was conducted in healthy younger subjects and the findings may not be directly applicable to those in whom tube feeding is most widely employed. By including a noncaloric-fed control group, it may have been possible to distinguish the effects that were directly attributable to the nutrients of the feed rather than those of volume. The choice of the polymeric feed containing protein, carbohydrate, and fat was based on what is used commonly in the clinical situation, and it is possible that the other feeds such as elemental, monomeric, or fiber-containing ones may have yielded different results because of the way in which they modify the digestive and absorptive process. However, testing of several different types of feed would have necessitated a much larger sample size or multiple visits from the same participants, thereby increasing the complexity of the study.
The results of this study indicate that there are distinct physiological differences between continuous and bolus intragastric tube feeding in gastrointestinal emptying, small intestinal fluid flux, splanchnic blood flow, and gastrointestinal hormone secretion. Continuously infused feeds, the most widely used approach of tube feeding, does not increase small bowel water content above volumes observed during fasting.
The extrapolation of these findings from healthy young volunteers to older sick patients is always open to challenge. Study design to investigate the gastrointestinal responses to tube feeding in ill patients is fraught with challenge and difficult to justify both ethically and financially. The findings described in this study provide further evidence that continuous intragastric feeding is unphysiological as it does not stimulate the gastrointestinal tract into a fed response and that the main source of enteral feeding–related diarrhea is colonic rather than small intestinal. This knowledge will allow researchers and clinicians more confidence to target studies and therapy at the colon.
ACKNOWLEDGMENTS
The authors thank the Nottingham Digestive Diseases Centre Biomedical Research Unit, National Institute for Health Research, for help with this study. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. The author contributions can be listed as follows: Study design—A.H.C., K.M., C.L.H., C.C., L.M., I.A.M., T.E.B., D.N.L.; Data collection and analysis—A.H.C., K.M., C.L.H., C.C., L.M.; Data interpretation—A.H.C., K.M., C.L.H., C.C., L.M., I.A.M., T.E.B., D.N.L.; Writing of manuscript—A.H.C., K.M., C.L.H., C.C., L.M., D.N.L.; Critical review—L.M., I.A.M., T.E.B., D.N.L.; and Final approval of manuscript—A.H.C., K.M., C.L.H., C.C., L.M., I.A.M., T.E.B., D.N.L.
Footnotes
Disclosure: A.H.C. was a recipient of Research Fellowships from the Nottingham Digestive Diseases Centre National Institute for Health Research Biomedical Research Unit and the Royal College of Surgeons of England. The running costs were funded by charitable donations made to the Clinical Nutrition Unit, Nottingham University Hospitals, Nottingham, UK. A.H.C. received an unrestricted research grant and travel grants from Baxter Healthcare. I.A.M. received unrestricted research grants from LighterLife, Mars Inc, and Unilever and served on the advisory boards for Coca-Cola and Mars Inc. T.E.B. served on advisory boards for Abbott and Calea. D.N.L. received unrestricted research grants, speaker's honoraria, and travel grants from Fresenius Kabi, BBraun, and Baxter Healthcare and served on the advisory boards of Baxter Healthcare and AbbVie. But none of the authors had any direct conflicts of interest.
REFERENCES
- 1.Dempsey DT, Mullen JL, Buzby GP. The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J Clin Nutr 1988; 47:352–356. [DOI] [PubMed] [Google Scholar]
- 2.Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut 2003; 52 suppl 7:vii1–vii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter J, Langhorne P, Roberts M. Routine protein energy supplementation in adults: systematic review. BMJ 1998; 317:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keohane PP, Attrill H, Love M, et al. Relation between osmolality of diet and gastrointestinal side effects in enteral nutrition. Br Med J (Clin Res Ed) 1984; 288:678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benya R, Layden TJ, Mobarhan S. Diarrhea associated with tube feeding: the importance of using objective criteria. J Clin Gastroenterol 1991; 13:167–172. [DOI] [PubMed] [Google Scholar]
- 6.Edes TE, Walk BE, Austin JL. Diarrhea in tube-fed patients: feeding formula not necessarily the cause. Am J Med 1990; 88:91–93. [DOI] [PubMed] [Google Scholar]
- 7.Hiebert JM, Brown A, Anderson RG, et al. Comparison of continuous vs intermittent tube feedings in adult burn patients. JPEN J Parenter Enteral Nutr 1981; 5:73–75. [DOI] [PubMed] [Google Scholar]
- 8.Seidl H, Schmidt T, Gundling F, et al. The effect of osmolarity and caloric load on small bowel motility. Neurogastroenterol Motil 2013; 25:e11–e16. [DOI] [PubMed] [Google Scholar]
- 9.Spiller RC, Trotman IF, Adrian TE, et al. Further characterisation of the “ileal brake” reflex in man–effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut 1988; 29:1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowling TE, Raimundo AH, Grimble GK, et al. Colonic secretory effect in response to enteral feeding in humans. Gut 1994; 35:1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowling TE, Silk DB. Intestinal responses induced by enteral feeding. Nutrition 1995; 11:304–307. [PubMed] [Google Scholar]
- 12.Bowling TE, Silk DB. Colonic responses to enteral tube feeding. Gut 1998; 42:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowling TE, Cliff B, Wright JW, et al. The effects of bolus and continuous nasogastric feeding on gastro-oesophageal reflux and gastric emptying in healthy volunteers: a randomised three-way crossover pilot study. Clin Nutr 2008; 27:608–613. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson EJ, Astbury NM, Simpson EJ, et al. Fat oxidation during exercise and satiety during recovery are increased following a low-glycemic index breakfast in sedentary women. J Nutr 2009; 139:890–897. [DOI] [PubMed] [Google Scholar]
- 15.Hoad CL, Marciani L, Foley S, et al. Non-invasive quantification of small bowel water content by MRI: a validation study. Phys Med Biol 2007; 52:6909–6922. [DOI] [PubMed] [Google Scholar]
- 16.Marciani L, Cox EF, Hoad CL, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology 2010; 138:469–477. [DOI] [PubMed] [Google Scholar]
- 17.Parker HL, Hoad CL, Hudders N, et al. Validation of a novel, non-invasive assessment of gastric function and gastric emptying (GE) after a large liquid nutrient meal by magnetic resonance imaging (MRI). Gastroenterology 2012; 142:S610. [Google Scholar]
- 18.Totman JJ, Marciani L, Foley S, et al. Characterization of the time course of the superior mesenteric, abdominal aorta, internal carotid and vertebral arteries blood flow response to the oral glucose challenge test using magnetic resonance imaging. Physiol Meas 2009; 30:1117–1136. [DOI] [PubMed] [Google Scholar]
- 19.Gentilcore D, Nair NS, Vanis L, et al. Comparative effects of oral and intraduodenal glucose on blood pressure, heart rate, and splanchnic blood flow in healthy older subjects. Am J Physiol Regul Integr Comp Physiol 2009; 297:R716–R722. [DOI] [PubMed] [Google Scholar]
- 20.Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology 1983; 85:76–82. [PubMed] [Google Scholar]
- 21.Lin HC, Doty JE, Reedy TJ, et al. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol 1989; 256:G404–G411. [DOI] [PubMed] [Google Scholar]
- 22.Strunz UT, Grossman MI. Effect of intragastric pressure on gastric emptying and secretion. Am J Physiol 1978; 235:E552–E555. [DOI] [PubMed] [Google Scholar]
- 23.Kelly KA. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am J Physiol 1980; 239:G71–G76. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatek MA, Menne D, Steingoetter A, et al. Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber-optic pressure measurement and MRI. Am J Physiol Gastrointest Liver Physiol 2009; 297:G894–G901. [DOI] [PubMed] [Google Scholar]
- 25.Noakes TD, Rehrer NJ, Maughan RJ. The importance of volume in regulating gastric emptying. Med Sci Sports Exerc 1991; 23:307–313. [PubMed] [Google Scholar]
- 26.Duchman SM, Ryan AJ, Schedl HP, et al. Upper limit for intestinal absorption of a dilute glucose solution in men at rest. Med Sci Sports Exerc 1997; 29:482–488. [DOI] [PubMed] [Google Scholar]
- 27.Bowling TE. The Sir David Cuthbertson Medal Lecture. Enteral-feeding-related diarrhoea: proposed causes and possible solutions. Proc Nutr Soc 1995; 54:579–590. [DOI] [PubMed] [Google Scholar]
- 28.Marciani L, Wright J, Foley S, et al. Effects of a 5-HT(3) antagonist, ondansetron, on fasting and postprandial small bowel water content assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2010; 32:655–663. [DOI] [PubMed] [Google Scholar]
- 29.Marciani L, Pritchard SE, Hellier-Woods C, et al. Delayed gastric emptying and reduced postprandial small bowel water content of equicaloric whole meal bread versus rice meals in healthy subjects: novel MRI insights. Eur J Clin Nutr 2013; 67:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiller RC, Jones BJ, Silk DB. Jejunal water and electrolyte absorption from two proprietary enteral feeds in man: importance of sodium content. Gut 1987; 28:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marciani L, Hall N, Pritchard SE, et al. Preventing gastric sieving by blending a solid(water meal enhances satiation in healthy humans. J Nutr 2012; 142:1253–1258. [DOI] [PubMed] [Google Scholar]
- 32.Wishart JM, Horowitz M, Morris HA, et al. Relation between gastric emptying of glucose and plasma concentrations of glucagon-like peptide-1. Peptides 1998; 19:1049–1053. [DOI] [PubMed] [Google Scholar]
- 33.Schirra J, Katschinski M, Weidmann C, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 1996; 97:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham KM, Horowitz M, Read NW. The effect of short-term dietary supplementation with glucose on gastric emptying in humans. Br J Nutr 1991; 65:15–19. [DOI] [PubMed] [Google Scholar]
- 35.Savage AP, Adrian TE, Carolan G, et al. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987; 28:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiller RC, Trotman IF, Higgins BE, et al. The ileal brake-inhibition of jejunal motility after ileal fat perfusion in man. Gut 1984; 25:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985; 89:1070–1077. [DOI] [PubMed] [Google Scholar]
- 38.Ferri GL, Adrian TE, Allen JM, et al. Intramural distribution of regulatory peptides in the sigmoid-recto-anal region of the human gut. Gut 1988; 29:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai K, Hammond AJ, Wishart JM, et al. Carbohydrate and fat digestion is necessary for maximal suppression of total plasma ghrelin in healthy adults. Appetite 2010; 55:407–412. [DOI] [PubMed] [Google Scholar]
- 40.Gentilcore D, Hausken T, Meyer JH, et al. Effects of intraduodenal glucose, fat, and protein on blood pressure, heart rate, and splanchnic blood flow in healthy older subjects. Am J Clin Nutr 2008; 87:156–161. [DOI] [PubMed] [Google Scholar]
- 41.Vanis L, Gentilcore D, Hausken T, et al. Effects of gastric distension on blood pressure and superior mesenteric artery blood flow responses to intraduodenal glucose in healthy older subjects. Am J Physiol Regul Integr Comp Physiol 2010; 299:R960–R967. [DOI] [PubMed] [Google Scholar]
- 42.Vanis L, Gentilcore D, Lange K, et al. Effects of variations in intragastric volume on blood pressure and splanchnic blood flow during intraduodenal glucose infusion in healthy older subjects. Am J Physiol Regul Integr Comp Physiol 2012; 302:R391–R399. [DOI] [PubMed] [Google Scholar]


