Abstract
Objectives:
To compare the effects of intraoperative goal-directed fluid therapy (GDFT) with conventional fluid therapy, and determine whether there was a difference in outcome between studies that did and did not use Enhanced Recovery After Surgery (ERAS) protocols.
Methods:
Meta-analysis of randomized controlled trials of adult patients undergoing elective major abdominal surgery comparing intraoperative GDFT versus conventional fluid therapy. The outcome measures were postoperative morbidity, length of stay, gastrointestinal function and 30-day mortality.
Results:
A total of 23 studies were included with 2099 patients: 1040 who underwent GDFT and 1059 who received conventional fluid therapy. GDFT was associated with a significant reduction in morbidity (risk ratio [RR] 0.76, 95% confidence interval [CI] 0.66–0.89, P = 0.0007), hospital length of stay (LOS; mean difference −1.55 days, 95% CI −2.73 to −0.36, P = 0.01), intensive care LOS (mean difference −0.63 days, 95% CI −1.18 to −0.09, P = 0.02), and time to passage of feces (mean difference −0.90 days, 95% CI −1.48 to −0.32 days, P = 0.002). However, no difference was seen in mortality, return of flatus, or risk of paralytic ileus. If patients were managed in an ERAS pathway, the only significant reductions were in intensive care LOS (mean difference −0.63 days, 95% CI −0.94 to −0.32, P < 0.0001) and time to passage of feces (mean difference −1.09 days, 95% CI −2.03 to −0.15, P = 0.02). If managed in a traditional care setting, a significant reduction was seen in both overall morbidity (RR 0.69, 95% CI 0.57 to −0.84, P = 0.0002) and total hospital LOS (mean difference −2.14, 95% CI −4.15 to −0.13, P = 0.04).
Conclusions:
GDFT may not be of benefit to all elective patients undergoing major abdominal surgery, particularly those managed in an ERAS setting.
Keywords: complications, goal-directed fluid therapy, intraoperative, meta-analysis, outcome
Intraoperative hypovolemia caused by loss of as little as 10% to 15% of blood volume can result in an appreciable fall in splanchnic perfusion, which often outlasts the period of hypovolemia.1 This results in an intramucosal acidosis of the gut,2 leading to a cascade of events that impair postoperative gastrointestinal function and cause complications.3 Postoperative gastrointestinal morbidity in the form of an inability to tolerate oral or enteral tube feeding, nausea, vomiting, and abdominal distension can be responsible for over half of delayed discharges.4 This concept led to the use of intraoperative goal-directed fluid therapy (GDFT) in which relatively small-volume (200–250 mL) boluses of fluid (usually a colloid) over background crystalloid infusions have been used to increase stroke volume and cardiac output, improve gut perfusion,1 and decrease gut mucosal acidosis.
A number of methods, including transesophageal Doppler (TED), lithium dilution, arterial pulse contour analysis, thoracic electrical bioimpedance, partial non-rebreathing systems, and transpulmonary thermodilution techniques have been used to measure intraoperative stroke volume and cardiac output and, thereby, help direct fluid therapy.5 The methods used most frequently in clinical practice are the TED and lithium dilution techniques. The commonest algorithm assesses the change in stroke volume in response to a fluid bolus of 200 to 250 mL infused over 5 to 10 minutes. An increase in stroke volume of more than 10% in response to this bolus signifies hypovolemia and indicates the need for a further bolus. An increase in stroke volume of 10% or less suggests adequate filling and continuation of the background crystalloid infusion without the need for another fluid bolus. A reduction in stroke volume by more than 10% during continued monitoring necessitates a further bolus and repetition of the cycle. Variations in this methodology include monitoring of stroke volume variation and corrected flow time (FTc).6,7
Randomized controlled trials and meta-analyses8–11 published in the first decade of the twenty-first century suggested that intraoperative GDFT resulted in a statistically significant reduction in postoperative complication rates and length of stay (LOS) when compared with patients receiving conventional intraoperative fluid therapy. This led to intraoperative GDFT being recommended as a standard of care by the UK National Institute for Health and Clinical Excellence (NICE).12 However, postoperative fluid therapy regimens were not clear in most of the early studies, and perioperative care was not standardized. Avoidance of postoperative salt and water overload and maintaining patients in as near a state to zero fluid balance as possible has been shown to reduce both complication rates and length of hospital stay even in patients not receiving GDFT.13–16 In addition, the use of fast-track or Enhanced Recovery After Surgery (ERAS) protocols,17,18 which are multimodal perioperative care pathways designed to reduce the metabolic stress of surgery and accelerate postoperative recovery, have resulted in fewer complications [risk ratio 0.5, 95% confidence interval (CI) 0.4–0.7] and reduction in LOS by 2.5 (95% CI −3.5 to −1.5) days after colorectal surgery when compared with patients managed with traditional care.19 More recent trials14,17,18 in which patients have been managed within ERAS protocols with avoidance of postoperative fluid overload have suggested that, although intraoperative GDFT resulted in improvement of cardiovascular variables when compared with conventional fluid therapy, there was no significant difference in clinical outcomes.14,20,21
The aims of this meta-analysis of randomized clinical trials of intraoperative GDFT versus conventional fluid therapy in patients undergoing elective major abdominal surgery were to
compare the effects of intraoperative GDFT with conventional fluid therapy on postoperative complications, length of hospital stay, gastrointestinal function, and mortality.
determine whether there was a difference in outcome between studies that used ERAS protocols for perioperative care and those that did not.
METHODS
Search Strategy
A search of the PubMed, MEDLINE, Web of Science, Google™ Scholar, and Cochrane Library databases was conducted to identify studies evaluating the impact of intraoperative goal-directed fluid therapy on postoperative elective surgical outcomes in all branches of surgery published in all languages between January 1995 and December 2014. Electronic search terms used were [“goal-directed fluid therapy” OR “flow-directed fluid therapy”] AND [“surgery” OR “intraoperative”] and the search was limited to adult patients undergoing elective surgery. The bibliography of studies that met the inclusion criteria were also searched for other relevant articles and conference abstracts to ensure study inclusion was as comprehensive as possible. The meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Selection of Articles
Full-text articles were screened after exclusion of citations on the basis of article title and abstract. We selected studies if they included adult patients undergoing elective major abdominal surgery who were randomized to receive either GDFT or conventional intraoperative fluid therapy and if the study reported at least 1 relevant postoperative outcome. “Major abdominal surgery” included general, vascular, gynecologic, and urologic procedures where the bowel was handled. We excluded studies if they involved patients undergoing non-abdominal surgery such as cardiac, orthopedic, or peripheral vascular surgery, included emergency surgical procedures, did not report any relevant clinical outcome measures, or if both groups received GDFT. One study23 was excluded due to retraction of a large number of articles by 1 of the authors. We discussed studies where the inclusion criteria were not clear and made a final decision.
Data Extraction
Data were extracted by 1 author (KER) and checked by another (DNL). The primary outcome measure was postoperative morbidity with secondary outcome measures being 30-day mortality, hospital and intensive care LOS, time to return of gastrointestinal function (flatus and feces), and incidence of paralytic ileus. Data were also collated on patient demographics (age, sex, American Society of Anesthesiology [ASA] grade), surgical variables (surgical procedure, number of laparoscopic cases, and estimated blood loss), and intraoperative fluid administration (overall, colloid and crystalloid, and inotrope administration). We noted the method of administration of GDFT and whether patients were managed using ERAS principles (eg, if stated by the authors or having a combination of 4 or more elements such as avoidance of prolonged preoperative starvation, provision of preoperative carbohydrate loading, use of thoracic epidural analgesia, avoidance of premedication, opioids and postoperative fluid overload, and early postoperative feeding and mobilization)17,18 or traditional care. We contacted the corresponding author on 3 occasions over a 6-week period if the data required were not available in the article. If the authors did not provide the data, the medians and interquartile ranges (IQR) were converted to means and standard deviations (SDs) using the technique described by Hozo et al.24 This technique uses the median as the best estimate of the mean, and the SD is calculated by the following formula:
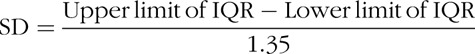 |
The risk of bias was assessed using the Cochrane Collaboration tool in RevMan 5.3,25 which focuses upon random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias).
Statistical Analysis
RevMan 5.3 software25 was used for data analysis. Dichotomous variables were quoted as a risk ratio (RR) with 95% CI and analyzed using the Mantel–Haenszel random effects model. Continuous variables were quoted as a mean difference using a random effects model with 95% CI and analyzed using the inverse-variance random effects model. Forest plots were constructed and a P value less than 0.05 on 2-tailed testing signified a statistically significant difference. Study heterogeneity and inconsistency was assessed using the I2 statistic26: less than 25%—low heterogeneity, 25% to 50%—moderate heterogeneity, and more than 50%—high heterogeneity. A predetermined secondary analysis was conducted on results obtained when the intervention was delivered within or without ERAS protocols. The quality of the evidence for each outcome was comprehensively assessed and graded using GRADEpro software.27
Protocol Registration
We registered the protocol for this meta-analysis with the PROSPERO database (www.crd.york.ac.uk/prospero)—registration no. CRD42014015595.
RESULTS
From 294 studies identified, 23 studies were eligible for inclusion (Fig. 1).6,7,21,28–47 There were 8 studies based in colorectal surgery,6,21,36–39,45,46 1 in upper gastrointestinal surgery,29 2 in urology,34,40 1 in abdominal vascular surgery,47 1 in gynecology,35 and 10 in a range of abdominal procedures.7,28,30–33,41–44 The risk of bias in the studies included was low and, in general, study quality was high (see Supplemental Digital Content Table 1, available at). The quality of the evidence for each outcome in the meta-analysis is summarized in Supplemental Digital Content Table 2, available at. Although there was no risk of bias or indirectness for all end-points, there was inconsistency and imprecision for hospital and intensive therapy unit (ITU) LOS.
FIGURE 1.
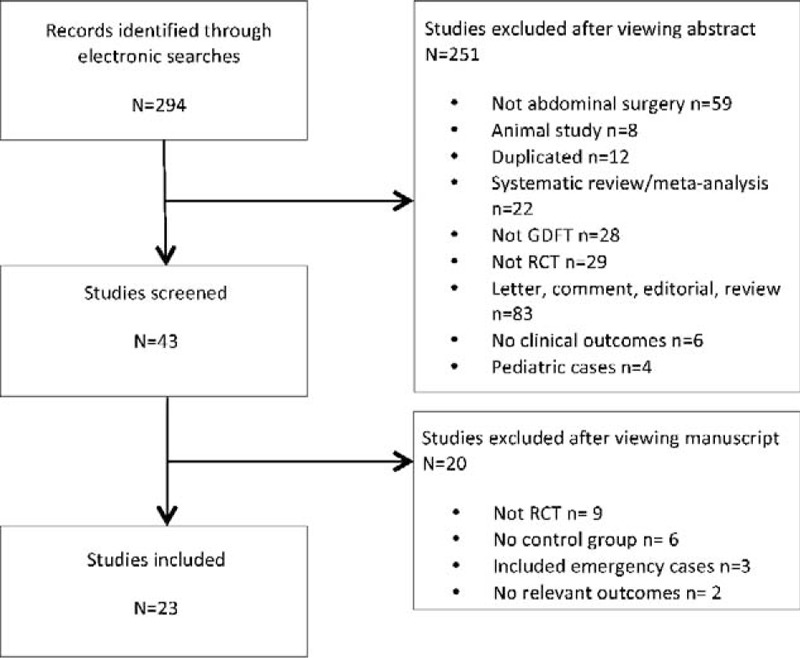
PRISMA diagram showing identification of relevant studies from initial search.
Demographics
The 23 randomized controlled trials included a total of 2099 patients, of whom 1040 had been randomized to intraoperative GDFT and 1059 to traditional intraoperative fluid management strategies. GDFT was administered as part of an ERAS program in 10 studies6,21,30,34,36–40,45 and as part of a traditional recovery pathway in 13.7,28,29,31–33,35,41–44,46,47 The method for administering GDFT in the studies was: TED in 12,6,7,21,34–40,45,46 hemodynamic parameters from radial arterial line (including lithium dilution) in 9,29–33,42–44,47 pleth variability index from the pulse oximeter in 1,41 and a noninvasive cardiac output monitoring device in 1.28 Patient demographics are detailed in Table 1.
TABLE 1.
Baseline Patient Demographics for All Included Studies
| Reference | ERAS or Traditional | Number of Patients | Type of Surgery | Laparoscopic Approach | ASA 1:2:3:4 | ||||
| GDFT | Control | GDFT | Control | GDFT | Control | GDFT | Control | ||
| Pestana 201428 | Traditional | 72 | 70 | 54 colonic, 2 abdominoperineal resection, 11 gastric surgery, 5 other | 50 colonic, 3 abdominoperineal, 11 gastric, 6 other | 0 | 0 | 2:31:37:2 | 2:34:34:0 |
| Phan 201421 | ERAS | 50 | 50 | 12 right hemicolectomy, 17 anterior resection, 21 other (29 cancer surgery) | 14 right hemicolectomy, 22 anterior resection, 1 abdominoperineal, 13 other (34 cancer surgery) | 31 (additional 8 converted to open) | 28 (additional 8 converted to open) | Median ASA class 2 (1–3) | Median ASA class 2 (1–3) |
| Zeng 201429 | Traditional | 30 | 30 | All radical gastric cancer surgery | All radical gastric cancer surgery | Not stated | Not stated | 0:31:9:0 | 0:32:8:0 |
| Zheng 201330 | ERAS | 30 | 30 | 6 gastrectomy, 4 radical gastrectomy for cancer, 7 proctectomy, 9 partial small bowel resection, 4 radical colectomy for cancer | 5 gastrectomy, 6 radical gastrectomy for cancer, 6 proctectomy, 7 partial small bowel resection, 6 radical colectomy for cancer | 0 | 0 | 0:11:19:0 | 0:13:17:0 |
| Salzwedel 201331 | Traditional | 79 | 81 | 47 bowel, 32 non-bowel | 41 bowel, 40 non-bowel | 0 | 0 | 33 ASA III | 33 ASA III |
| Scheeren 201332 | Traditional | 26 | 26 | 11 major abdominal surgery, 15 radical cystectomy | 12 major abdominal surgery, 14 radical cystectomy | Not stated | Not stated | 0:0:24:2 | 0:0:26:0 |
| Ramsingh 201333 | Traditional | 18 | 20 | 8 gyneoncology including TAH/BSO, 5 GI surgery inc. small and large bowel, 3 urologic oncology inc. cystectomy with ileal conduit, 2 Whipple's procedures | 9 gyneoncology, 8 GI surgery, 0 urologic, 3 Whipple's procedures | 0 | 0 | Not stated | Not stated |
| Bundgaard-Nielsen 201334 | ERAS | 21 | 21 | All open radical prostatectomy | All open radical prostatectomy | 0 | 0 | 14:7:0:0 | 12:9:0:0 |
| McKenny 201335 | Traditional | 51 | 50 | 51 major gynecologic (36 malignant; 19 ovarian cancer) | 50 major gynecologic (36 malignant; 17 ovarian cancer) | 0 | 0 | Mean ASA 2.0 (0.6) | Mean ASA 2.0 (0.7) |
| Srinivasa 201336 | ERAS | 37 | 37 | 14 right hemicolectomy, 4 extended right hemicolectomy, 14 high anterior resection, 5 total/subtotal colectomy | 17 right hemicolectomy, 5 extended right hemicolectomy, 14 high anterior resection, 1 total/subtotal colectomy | 5 | 6 | 5:20:12:0 | 5:15:17:0 |
| Zakhaleva 201338 | ERAS | 32 | 40 | 24 colectomy, 7 proctectomy, 1 small bowel resection - 16 malignant, 11 benign, 5 inflammatory bowel disease | 30 colectomy, 6 proctectomy, 4 small bowel resection - 17 malignant, 19 benign, 4 inflammatory bowel disease | 16 + 2 converted to open | 19 + 5 converted to open | 0:7:26:0 | 0:7:32:0 |
| Brandstrup 201237 | ERAS | 71 | 79 | All elective colorectal | All elective colorectal | 32, additional 11 converted from laparoscopic to open | 38, additional 12 converted from laparoscopic to open | 26:37:8:0 | 20:43:16:0 |
| Challand 201239 | ERAS | 89 | 90 | 32 colonic, 57 rectal (65 carcinoma) | 37 colonic, 53 rectal (68 carcinoma) | 28 | 37 | 11:51:27 (3 + 4) | 11:52:27 (3 + 4) |
| Pillai 201140 | ERAS | 32 | 34 | All radical cystectomy for bladder cancer | All radical cystectomy for bladder cancer | 0 | 0 | Mean ASA class 1.87 (95% CI 1.77–2.04) | Mean ASA class 1.92 (1.08–2.04) |
| Forget 201041 | Traditional | 41 | 41 | 7 upper gastrointestinal, 11 hepatobiliary, 24 lower gastrointestinal | 5 upper gastrointestinal, 15 hepatobiliary, 22 lower gastrointestinal | 5 | 5 | 0:22:19:0 | 0:22:19:0 |
| Benes 201042 | Traditional | 60 | 60 | 17 colorectal, 5 pancreatic, 38 intraabdominal vascular | 16 colorectal, 3 pancreatic, 41 intraabdominal vascular | Not stated | Not stated | 0:14:37:9 | 0:11:40:9 |
| Buettner 200843 | Traditional | 40 | 40 | Elective major abdominal surgery | Elective major abdominal surgery | Not stated | Not stated | Median ASA class 2 (1–3) | Median ASA class 2 (1–3) |
| Lopes 200744 | Traditional | 17 | 16 | 4 upper gastrointestinal, 3 hepatobiliary, 10 colorectal | 4 upper gastrointestinal, 2 hepatobiliary, 8 colorectal, 1 urology, 1 other | Not stated | Not stated | 0:3:8:6 | 0:3:9:4 |
| Noblett 20066 | ERAS | 51 | 52 | 30 colonic, 24 rectal | 25 colonic, 29 rectal | 13 | 13 | Mean ASA class 2.1 (0.6) | Mean ASA class 2.2 (0.6) |
| Wakeling 200545 | ERAS | 64 | 64 | 31 anterior and abdominoperineal resection, 15 left hemi and sigmoid colectomy, 15 right hemicolectomy, 3 reversal of Hartmann's | 33 anterior and abdominoperineal resection, 15 left hemi and sigmoid colectomy, 9 right hemicolectomy, 4 subtotal colectomy, 2 reversal of Hartmann's, 1 Crohn's resection | Not stated | Not stated | Median ASA class 2 (1) | Median ASA class 2 (1) |
| Conway 200246 | Traditional | 29 | 28 | All major bowel surgery | All major bowel surgery | Not stated | Not stated | Median ASA class 1 (1–3) | Median ASA class 2 (1–3) |
| Gan 20027 | Traditional | 50 | 50 | 16 general, 13 gynecology, 21 urology | 15 general, 19 gynecology, 16 urology | 3:36:11:0 | 8:32:10:0 | ||
| Bonazzi 200247 | Traditional | 50 | 50 | All infrarenal abdominal aortic aneurysm repair | All infrarenal abdominal aortic aneurysm repair | 0 | 0 | Not stated | Not stated |
Fluid Therapy
There was some variation in fluid therapy over time (Table 2). One of the earliest studies7 infused 4405 ± 2650 mL lactated Ringer solution and 847 ± 373 mL 6% hydroxyethyl starch (HES) intraoperatively in the GDFT group versus 4375 ± 2452 mL Ringer and 282 ± 470 mL HES in the control group. In contrast, the most recently published study21 administered 1500 mL (1000–2000 mL) intraoperative crystalloid and 500 mL (250–750 mL) colloid in the GDFT group versus 1400 mL (1000–1900 mL) and 0 mL (0–300 mL) in the control group.
TABLE 2.
Intraoperative Fluid Infused in the Goal-directed and Control Groups
| Reference | Average Total Fluid Infused (mL) | Average Crystalloid (mL) | Average Colloid Bolus (mL) | Average Blood Loss (mL) | Requirement for Perioperative Inotropes | |||||
| GDFT | Control | GDFT | Control | GDFT | Control | GDFT | Control | GDFT | Control | |
| Pestana 201428 | 2500 (1625–3000) | 2325 (1600–3000) | Not stated | Not stated | 2.4 boluses ± 1.8* | 1.3 boluses ± 1.4* | 300 (200–500) | 250 (200–400) | 18 dobutamine, 5 noradrenaline, 25 ephedrine | 1 dobutamine, 4 noradrenaline, 22 ephedrine |
| Phan 201421 | 2190 (1350–2560)* | 1500 (1200–2000)* | 1500 (1000–2000) | 1400 (1000–1900) | 500 (250–750)* | 0 (0–300)* | Not stated | Not stated | Not stated | Not stated |
| Zeng 201429 | 2732 ± 488* | 3135 ± 346* | Not stated | Not stated | 1225 ± 360* | 760 ± 280* | 482 ± 168 | 473 ± 156 | Not stated | Not stated |
| Zheng 201330 | 2650 (2400–3200)* | 3950 (2875–4200)* | 1550 (1400–1925)* | 2350 (2000–2925)* | 1000 (900–1100)* | 800 (600–1000)* | 200 (100–362.5) | 200 (100–800) | 4 (13.3) | 6 (20) |
| Salzwedel 201331 | 3854.2 ± 1954.2 | 3770.8 ± 2827.5 | 2862 ± 1216 | 2680.2 ± 1153.8 | 773.7 ± 664.6 | 724.7 ± 720.2 | 668.2 ± 676.6 | 704.4 ± 889.6 | 33 dobutamine*, 26 norepinephrine, 0 phenylephrine, 11 ephedrine | 0 dobutamine*, 32 norepinephrine, 4 phenylephrine, 8 ephedrine |
| Scheeren 201332 | 4477 (2107) | 4528 (2387) | Not stated | Not stated | 1589 (1283)* | 927 (845)* | 984 (647) | 1118 (1057) | Norepinephrine dosage 0.04 μg/kg/min (0.06) | 0.05 μg/kg/min (0.05) |
| Ramsingh 201333 | 4082.2 ± 2044* | 6845.6 ± 3893.4* | 3343.3 ± 1563.7* | 5851.5 ± 3197.9* | 544.4 ± 493.5 | 422.5 ± 590.8 | Not stated | Not stated | Not stated | Not stated |
| Bundgaard-Nielsen 201334 | Not stated | Not stated | 1879 (1205–2052) | 1636 (1428–1843) | 1758 (1441–2076)* | 1057 (778–1336)* | 1285 (875–1696) | 1152 (774–1530) | Average 9 mg ephedrine (4–13) | Average 15 mg ephedrine (8–22) |
| McKenny 201335 | 2620 | 2881 | 1000 (787–1750)* | 2000 (1725–2500)* | 1000 (1000–1500)* | 500 (0–1000)* | 500 (311–745) | 600 (326–1000) | Not stated | Not stated |
| Srinivasa 201336 | 1994 (590)* | 1614 (420)* | Not stated | Not stated | 591 (471)* | 297 (275)* | Not stated | Not stated | 31 (83.7) | 34 (91.9) |
| Zakhaleva 201338 | 3100 (700–77000) | 4000 (900–6200) | 2700 (500–6500) | 3200 (500–5600) | 500 (0–2800)* | 300 (0–4500)* | 100 (10–650) | 100 (10–500) | Not stated | Not stated |
| Brandstrup 201237 | 1876* | 1491* | Saline and LR: 483 (419) | 443 (480) | 810 (543)* | 475 (598)* | Not stated | Not stated | Not stated | Not stated |
| Challand 201239 | Not stated | Not stated | 3479 (1181) | 3593 (1398) | 1718 (446)* | 336 (623)* | 500 (200–1000)* | 250 (100–500)* | Not stated | Not stated |
| Pillai 201140 | 0.23 mL/kg/min (0.21–0.25)* | 0.19 mL/kg/min (0.15–0.2)* | Not stated | Not stated | Not stated | Not stated | 9.82 mL/kg (95% CI 7.53–12.12) | 10.7 mL/kg (8.42–12.9) | Not stated | Not stated |
| Forget 201041 | 2394 (2097–2692)* | 2918 (2478–3358)* | 1363 (1185–1540)* | 1815 (1568–2064)* | 890 (709–1072) | 1003 (779–1227) | 349 (230–468) | 440 (242–637) | 9 continuous norepinephrine infusion | 9 continuous norepinephrine infusion |
| Benes 201042 | Not stated | Not stated | 2321 ± 681 | 2459 ± 930 | 1425 (1000–1500)* | 1000 (540–1250)* | 700 (500–1200) | 800 (400–1325) | 3 (5.88%) norepinephrine, 2 (3.92%) dobutamine | 11 (20.37%) norepinephrine, 0 dobutamine |
| Buettner 200843 | 6000 | 5250 | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | 30 | 29 |
| Lopes 200744 | 4618 ± 1557* | 1694 ± 705* | 2176 ± 1060 | 1563 ± 02 | 2247 ± 697* | 0* | Not stated | Not stated | Not stated | Not stated |
| Noblett 20066 | Not stated | Not stated | 2298 (863) | 2625 (1004) | 1340 (838) | 1209 (824) | 250 (40–2455) | 475 (100–2900) | 16 (31)* | 26 (50) * |
| Wakeling 200545 | Not stated | Not stated | 3000 | 3000 | 2000* | 1500* | 500 (700) | 500 (975) | Not stated | Not stated |
| Conway 200246 | 64.6 mL/kg (36.4) | 55.2 mL/kg (24) | Not stated | Not stated | 28 mL/kg (16) | 19.4 mL/kg (14.7) | Not stated | Not stated | Not stated | Not stated |
| Gan 20027 | Not stated | Not stated | 4405 ± 2650 | 4375 ± 2452 | 847 ± 373* | 282 ± 470* | 703 ± 649 | 624 ± 632 | 8 (16) | 13 (26) |
| Bonazzi 200247 | 4500 (3250–6500)* | 3250 (2500–4750)* | Not stated | Not stated | Not stated | Not stated | 1000 (450–2750) | 1100 (500–2500) | Not stated | Not stated |
*Indicates statistically significant difference between GDFT and control groups.
Morbidity
Eighteen studies6,7,21,28,31,32,35–39,41,42,44–47 on 899 patients managed with GDFT versus 914 patients with traditional fluid management reported morbidity rates (Fig. 2). These were further divided by whether the patients had been managed as part of an ERAS pathway (866 patients) or as part of a traditional care pathway (947 patients). One study30 focused on cardiac morbidity alone, but these data are included in the overall analysis. Overall morbidity was significantly lower in patients managed with GDFT versus those in the control group (RR 0.76, 95% CI 0.66–0.89, P = 0.0007). When just those managed with GDFT in a traditional care pathway setting were considered, morbidity rates were also significantly lower in the GDFT group when compared with controls (RR 0.69, 95% CI 0.57–0.84, P = 0.0002). However, when the GDFT was administered in conjunction with an ERAS pathway, it did not result in a reduction in morbidity risk (RR 0.86, 95% CI 0.70–1.05, P = 0.14). The funnel plot for the primary outcome measure of morbidity showed no major asymmetry to indicate a significant bias in either group.
FIGURE 2.

Forest plot comparing overall morbidity rate for patients receiving GDFT versus control, divided by those managed using ERAS or traditional principles. A Mantel–Haenszel random effects model was used to conduct the meta-analysis, and risk ratios are quoted including 95% confidence intervals. (Zheng et al., 201330 considered cardiac morbidity alone).
Mortality
Mortality rates were detailed in 18 studies6,7,21,28,29,32,35,37–47 that included 855 patients in the GDFT group and 870 in the traditional group (Fig. 3). Overall, there was no statistically significant difference in the incidence of mortality between GDFT and control patients, nor was there any difference in those managed with an ERAS pathway or traditional care.
FIGURE 3.
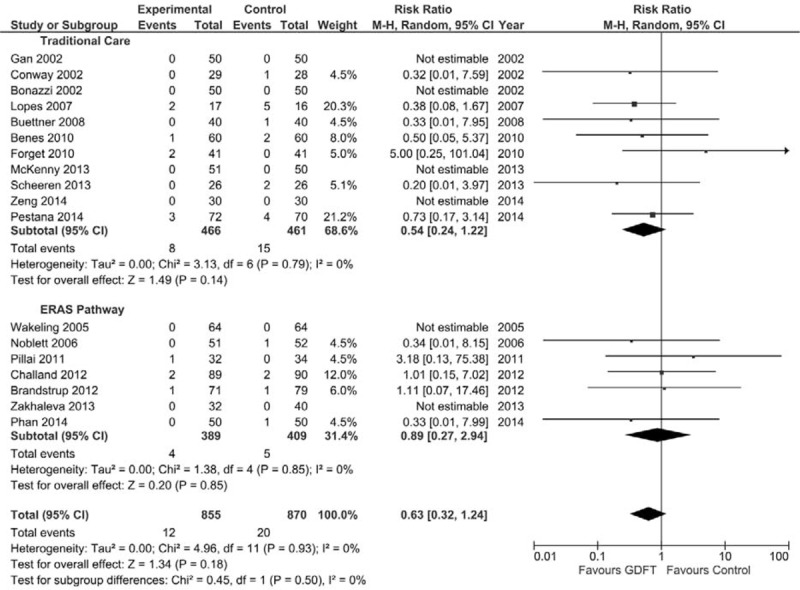
Forest plot comparing in-hospital or 30-day mortality rate for patients receiving GDFT versus control, divided by those managed using ERAS or traditional principles. A Mantel–Haenszel random effects model was used to conduct the meta-analysis, and risk ratios are quoted including 95% confidence intervals.
Hospital Length of Stay
Overall hospital LOS was reported in all studies except one32 included in the meta-analysis (Fig. 4). However, 2 studies30,34 reported only median (IQR) data, and we were unable to obtain the mean ± SD from the authors. These data were estimated using the technique described by Hozo et al.24 and all data were included in the analysis of hospital LOS. There were 1043 patients managed in an ERAS setting and 1014 in a traditional setting (Fig. 4). GDFT resulted in a significant decrease in hospital length of stay in the overall group (mean difference −1.55 days, 95% CI −2.73 to −0.36, P = 0.01). If patients managed in a traditional care setting were specifically examined, GDFT again resulted in a significant reduction in overall hospital LOS (mean difference −2.14 days, 95% CI −4.15 to −0.13, P = 0.04). However, there was no significant difference in hospital LOS in those managed with an ERAS pathway (mean difference −0.71 days, 95% CI −1.91 to 0.49, P = 0.25).
FIGURE 4.
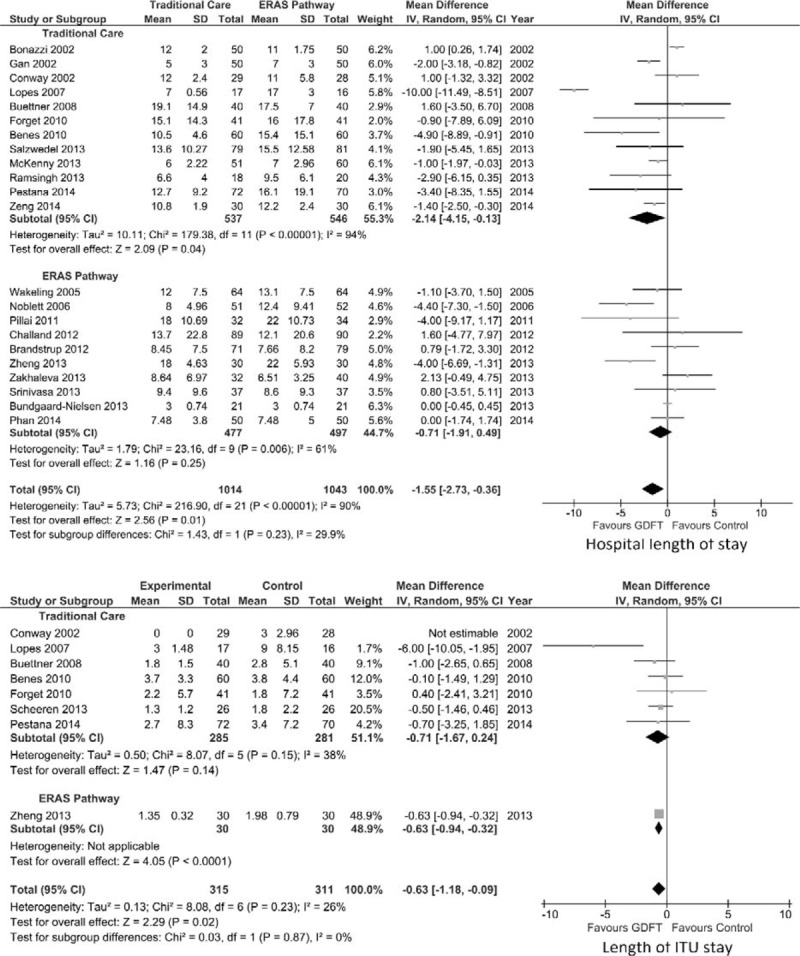
Forest plot comparing overall hospital LOS (top) and intensive treatment unit (ITU) LOS (bottom) for patients receiving GDFT versus control including studies with estimated data, divided by those managed using ERAS or traditional principles. An inverse-variance random effects model was used to conduct the meta-analysis, and mean differences are quoted including 95% confidence intervals.
Intensive Care Length of Stay
Postoperative LOS in the ITU was reported in 8 studies28,30,32,41–44,46 (Fig. 4). Again, 3 studies30,44,46 provided only median (IQR) data; therefore, estimated mean ± SD data were included for these studies. Only 1 study in an ERAS setting reported intensive care LOS,30 whereas 7 studies in a traditional setting reported this. GDFT resulted in a significant reduction in intensive care LOS (Fig. 4) in all patients (mean difference −0.63 days, 95% CI −1.18 to −0.09, P = 0.02) and in the 1 study in which patients were managed with an ERAS pathway (mean difference −0.63 days, 95% CI −0.94 to −0.32, P < 0.0001). GDFT, however, made no significant difference to intensive care LOS in those patients managed within a traditional care setting.
Return of Gastrointestinal Function
Eleven studies examined time to return of gastrointestinal function postoperatively, in the form of passage of flatus,28,31,38 feces,6,29,33,35,45 or both.30,39,40 First, considering time to passage flatus in all studies including those with calculated data (Fig. 5), there were 334 patients who were managed with GDFT and 345 in the control group. There was no significant difference in the time to passage of flatus in either the overall group or in those managed in combination with traditional care or an ERAS pathway.
FIGURE 5.
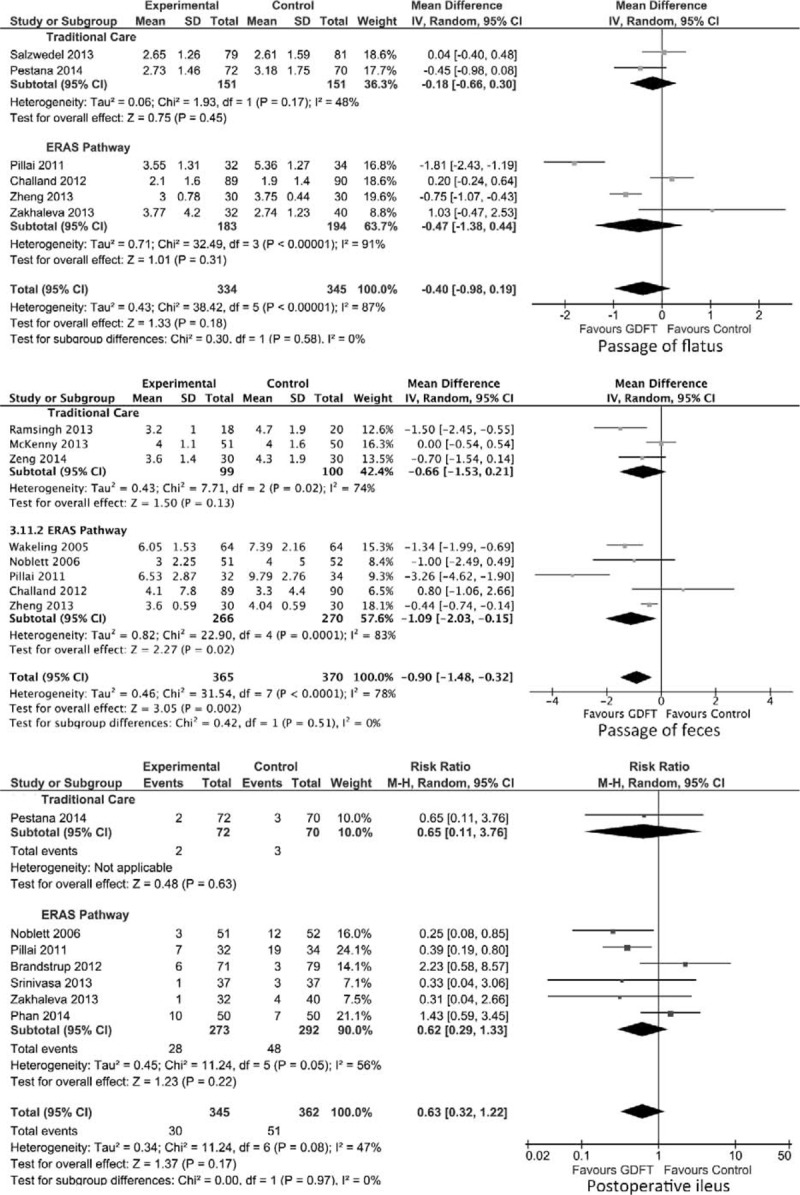
Forest plot comparing time to return of flatus and feces and incidence of paralytic ileus for patients receiving GDFT versus control including studies with estimated data, divided by those managed using ERAS or traditional principles. An inverse-variance random effects model was used to conduct the meta-analysis, and mean differences are quoted including 95% confidence intervals. The Mantel-Haenszel random effects model with risk ratios was used for postoperative ileus.
When time to passage of feces was considered, 365 patients were managed with GDFT and 370 with control intraoperative fluid (Fig. 5). GDFT resulted in a significant reduction in time to passage of feces in the overall group (mean difference −0.90 days, 95% CI −1.48 to −0.32 days, P = 0.002) as well as those managed with GDFT in combination with an ERAS pathway (mean difference −1.09 days, 95% CI −2.03 to −0.15, P = 0.02). However, this difference was not significant in patients managed in a traditional care setting.
Incidence of Postoperative Ileus
Seven studies (707 patients) included data on the incidence of postoperative ileus in 345 patients managed with intraoperative GDFT versus 362 patients in the control group6,21,28,36–38,40 (Fig. 5). The use of GDFT did not affect the incidence of postoperative ileus significantly in either the overall group or in those managed in combination with either traditional care or an ERAS pathway.
DISCUSSION
This meta-analysis of 23 randomized controlled trials including 2099 patients has demonstrated that, in patients undergoing elective major abdominal surgery, GDFT was associated with a significant reduction in overall morbidity, LOS (both hospital and intensive care), and time to passage of feces when compared with conventional intraoperative fluid therapy when all studies were considered. However, there were no significant differences in short-term mortality, time to passage of flatus, or risk of paralytic ileus.
When the effect of GDFT was considered in the setting of ERAS pathways, which are being implemented increasingly internationally, there was no statistically significant impact on morbidity and mortality, hospital LOS, time to passage of flatus, or incidence of paralytic ileus. A significant reduction in intensive care LOS with GDFT was seen, but this was based on a single study.30 When the impact of GDFT was considered in the setting of a traditional care pathway, a significant reduction in morbidity and overall hospital LOS was seen when compared with controls, but there was no significant difference in any other outcome considered.
The studies included in this meta-analysis were conducted over a 12-year period during which significant advances have been made in the concept and implementation of ERAS principles and there is evidence that ERAS programs are associated with reduced hospital LOS,19,48,49 decreased morbidity, and improved cost-effectiveness.50 The studies were conducted in a variety of surgical specialties which have differing expected LOS; however, if the studies examining colorectal surgery alone are analyzed,6,21,36–39,45 LOS has declined progressively over a temporal scale from 12.0 ± 7.5 days in 200545 to 7.48 ± 3.8 days in 2014.21 With the ongoing push for decreasing LOS, reinforced by recent reports of 2-day51 and 23-hour52 hospital stays for laparoscopic colorectal resection, the margin for overall improvement in LOS provided by GDFT may decrease. Overall heterogeneity was high for LOS (90%) and, although it reduced to 61% for the ERAS group, it was still high. Therefore, it is not certain whether the lack of difference in the LOS in the ERAS subgroup was a time-dependent effect or a reflection of the effect of ERAS pathways.
The other issue raised by the temporal spread of the results is that of the volume of fluid infused intraoperatively. This volume has changed drastically from the earliest to more recent papers, with a progressively greater difference in volume infused between GDFT and conventional fluid management groups, suggesting that the concept and impact of GDFT may have changed during this period. It is possible that, in the early phase of introduction of GDFT, patients were being frequently fluid overloaded intraoperatively. Given that postoperative morbidity is associated in a U-shaped manner with the volume of intraoperative fluid infused,51 excessive fluid administration in some of these studies may have attenuated some of the potential benefits of GDFT. Further to this, the majority of early studies did not consider the importance of postoperative salt and water overload, which may also have impacted negatively on outcome. In contrast, near-zero fluid balance is considered more carefully in recent studies due to advancing knowledge of the importance of these factors52 in the perioperative setting. The provision of high–chloride-containing fluids, with the resultant undesirable hyperchloremic acidosis,53–55 may also have masked some benefits provided by GDFT. Worldwide, there is now a move away from 0.9% saline-based fluids to balanced crystalloids and colloids, and this may lead to a further improvement in outcomes.56 One further factor to consider is that different studies have employed different goals for GDFT, and the emphasis of this has evolved over time. In the earlier studies included in this meta-analysis, patients were given fluid boluses if they were fluid responsive, regardless of their hemodynamic status, to maximize stroke volume by pushing patients to the top of their Frank–Starling curve. This approach is likely to result in fluid overload by “optimizing” patients to a point where they are no longer fluid responsive rather than assessing “good enough” resuscitation. In contrast, more contemporary studies administer bolus fluid only if patients were fluid responsive and had evidence of hemodynamic compromise, which may be reflected in the overall smaller volumes administered in more recent studies where a target of near-zero fluid balance was employed.
The present study was conducted using rigorous methodology and represents the largest meta-analysis examining the role of GDFT versus conventional intraoperative fluid management in patients undergoing elective major abdominal surgery. Not only did we set out to establish the difference in clinical outcome measures but also at the outset a secondary outcome of comparing those managed within ERAS pathways with those who were managed in traditional care setting was specified. This secondary analysis has resulted in some interesting observations in outcomes between the 2 settings, which appear to differ considerably. A further strength was that to ensure the data were as complete as possible for all studies included, most importantly the mean ± SD data for continuous variables, all authors were contacted on 3 separate occasions requesting the necessary raw data rather than the median (IQR). Unfortunately, not all authors responded to the request for information, and data for several studies30,34,40,44 were estimated for inclusion in the meta-analysis. This estimation was done using an established method24 that has been employed in other meta-analyses.
This meta-analysis had several weaknesses inherent in its design and conduct. The methodology for conducting GDFT differed greatly between studies, including TED,6,7,21,34–40,45,46 hemodynamic parameters from an arterial line,29–33,42–44,47 pleth variability index from the pulse oximeter,41 and a noninvasive cardiac output monitoring device.28 Inclusion of all techniques for conducting GDFT was chosen purposefully to ensure that the conclusions of this meta-analysis were generalizable to different GDFT methods. However, subgroup analyses comparing the various methods was not feasible because of the small numbers of patients who were managed with techniques other than TED or monitoring of hemodynamic parameters from arterial lines. One factor that was not measured consistently between the studies was that of postoperative fluid administration and overall balance, which may significantly impact upon some of the postoperative outcomes. The use of rescue therapy such as diuretics and inotropes is also difficult to discern from the studies. None of the ERAS pathway studies included an assessment of compliance with the ERAS standards, which is particularly important because of the correlation between compliance with the standards and clinical outcomes.57–59
There was a large degree of heterogeneity in the studies included in this review. Using the I2 statistic26 for the 7 clinical outcomes, 1 outcome had low (I2 < 25%), 3 had moderate (I2 25%–50%), and 3 had high heterogeneity (I2 > 50%). This great variation may have impacted upon the significance of the results. In addition, to improve generalizability, we included all studies that included patients who had major abdominal surgery where the bowel was handled. It was also not possible to differentiate the effects of temporal changes in perioperative management algorithms and other treatment interventions such as the use of vasopressors from the effect of GDFT.
NICE guidance12 released in 2011 on the use of TED-guided fluid therapy has recommended its use “in patients undergoing major or high-risk surgery or other surgical patients in whom a clinician would consider using invasive cardiovascular monitoring.” However, this guidance was made mainly on the findings of older studies, some of which were on patients undergoing cardiac and hip fracture surgery and most of which were conducted within a traditional setting of perioperative care. All studies included in the present meta-analysis focused on patients who would meet the criteria for major or high-risk surgery, making this meta-analysis an excellent setting in which to examine the potential benefits of this technique. By comparing the older studies with newer studies that have been conducted using multimodal enhanced recovery perioperative care pathways, we have shown in our meta-analysis that modern perioperative care reduces the impact of GDFT on outcome. This could help inform healthcare providers better and facilitate a more rational decision-making process before recommending GDFT as “standard of care.” Given the unclear benefits of GDFT found in this study, particularly in those managed within an ERAS pathway, it is uncertain whether this recommendation ought to be adopted for all patients undergoing elective major abdominal surgery.
The bolus fluid administered as part of the GDFT protocol in the included studies was variable, with HES being the documented fluid administered in 14 studies.7,21,28,29,32,34,35,37,39,41–44,46 However, there has recently been a moratorium in Europe on the use of HES due to concerns of increased risk of acute kidney injury requiring renal replacement therapy,60–62 as well as mortality60,62 based on recent randomized controlled trials in critically ill patients. Given that much of the evidence in this study, as well as other meta-analyses, are based upon the use of HES as the bolus fluid administered for GDFT, the impact of GDFT using gelatin (or other colloid)-based fluid may differ from current evidence. Further literature63 has examined the role of balanced crystalloid (Hartmann solution) versus colloid (6% HES) as the bolus agent for GDFT, demonstrating no clinical benefit from colloid in terms of morbidity or coagulopathy. Only 2 of the studies included in this meta-analysis30,47 administered crystalloid as the bolus agent. Crystalloids may be increasingly utilized in future studies regarding GDFT due to suggested therapeutic equivalence of colloid and crystalloid in combination with concerns with regard to some forms of colloid.
An updated meta-analysis on perioperative administration of fluids, with or without inotropes/vasoactive drugs, targeted to increase blood flow (relative to control) against measured goals in patients undergoing abdominal and extra-abdominal surgery, including emergency procedures showed that patients randomized to a hemodynamic therapy algorithm, had fewer complications and shorter LOS than controls.64 Nevertheless, the findings of the present meta-analysis for patients managed within ERAS pathways are in agreement with a previous meta-analysis of 6 trials of 691 patients undergoing elective colorectal surgery in which it was shown that TED-guided GDFT did not influence LOS or complications.65
However, although the benefits of GDFT on clinical outcomes may be marginal, the presence of an important benefit such as cost savings cannot be ruled out on the basis of this meta-analysis. Further large-scale randomized trials addressing all the issues that we have highlighted, including a cost-effectiveness analysis, are necessary before the real impact of GDFT in elective abdominal surgery is known.
CONCLUSIONS
This meta-analysis has shown that the benefits of GDFT may not be as clear as has been suggested historically. The overall perioperative management of patients has changed during the period of inclusion of studies in this meta-analysis, including decreasing expected hospital LOS, overall decreasing volumes of intraoperative fluid infusion, avoidance of postoperative salt and water overload, and introduction and compliance with ERAS programs. Despite the NICE Guidance12 which recommends that GDFT technology should be used “in patients undergoing major or high-risk surgery,” this study suggests that GDFT may not be of use to all elective patients undergoing major abdominal surgery. The benefit conveyed by GDFT is particularly attenuated by its combination with ERAS pathways that are being increasingly implemented internationally. GDFT may be more of use in the intraoperative care of high-risk patients; however, as yet, there are no definitive data to support this belief.
Supplementary Material
Supplementary Material
Footnotes
Disclosure: Supported by a Research Fellowship awarded by the European Society for Clinical Nutrition and Metabolism (ESPEN; to KER). The sponsors had no role in the design, execution, and reporting of the study. DNL has received unrestricted research funding and speaker's honoraria from Fresenius Kabi, B. Braun Medical, and Baxter Healthcare for unrelated work. DNL is Chairman of the Scientific Committee of the Enhanced Recovery After Surgery (ERAS®) Society. KER declares no conflicts of interest.
REFERENCES
- 1.Giglio MT, Marucci M, Testini M, et al. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 2009; 103:637–646. [DOI] [PubMed] [Google Scholar]
- 2.Holland J, Carey M, Hughes N, et al. Intraoperative splanchnic hypoperfusion, increased intestinal permeability, down-regulation of monocyte class II major histocompatibility complex expression, exaggerated acute phase response, and sepsis. Am J Surg 2005; 190:393–400. [DOI] [PubMed] [Google Scholar]
- 3.Filsoufi F, Rahmanian PB, Castillo JG, et al. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg 2007; 246:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett-Guerrero E, Welsby I, Dunn TJ, et al. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg 1999; 89:514–519. [DOI] [PubMed] [Google Scholar]
- 5.Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg 2009; 108:887–897. [DOI] [PubMed] [Google Scholar]
- 6.Noblett SE, Snowden CP, Shenton BK, et al. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg 2006; 93:1069–1076. [DOI] [PubMed] [Google Scholar]
- 7.Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002; 97:820–826. [DOI] [PubMed] [Google Scholar]
- 8.Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia 2008; 63:44–51. [DOI] [PubMed] [Google Scholar]
- 9.Harten J, Crozier JE, McCreath B, et al. Effect of intraoperative fluid optimisation on renal function in patients undergoing emergency abdominal surgery: a randomised controlled pilot study (ISRCTN 11799696). Int J Surg 2008; 6:197–204. [DOI] [PubMed] [Google Scholar]
- 10.Pearse R, Dawson D, Fawcett J, et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial (ISRCTN38797445). Crit Care 2005; 9:R687–R693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senagore AJ, Emery T, Luchtefeld M, et al. Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis Colon Rectum 2009; 52:1935–1940. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Clinical Excellence. CardioQ-ODM Oesophageal Doppler Monitor. London: National Institute for Health and Clinical Excellence; 2011. Available at: https://www.nice.org.uk/guidance(mtg3/resources/guidance-cardioqodm-oesophageal-doppler-monitor.pdf Accessed December 12, 2014. [Google Scholar]
- 13.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002; 359:1812–1818. [DOI] [PubMed] [Google Scholar]
- 14.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003; 238:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005; 103:25–32. [DOI] [PubMed] [Google Scholar]
- 16.Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 2010; 69:488–498. [DOI] [PubMed] [Google Scholar]
- 17.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005; 24:466–477. [DOI] [PubMed] [Google Scholar]
- 18.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144:961–969. [DOI] [PubMed] [Google Scholar]
- 19.Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010; 29:434–440. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasa S, Taylor MH, Sammour T, et al. Oesophageal Doppler-guided fluid administration in colorectal surgery: critical appraisal of published clinical trials. Acta Anaesthesiol Scand 2011; 55:4–13. [DOI] [PubMed] [Google Scholar]
- 21.Phan TD, D'Souza B, Rattray MJ, et al. A randomised controlled trial of fluid restriction compared to oesophageal Doppler-guided goal-directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Anaesth Intensive Care 2014; 42:752–760. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336–341. [DOI] [PubMed] [Google Scholar]
- 23.Mayer J, Boldt J, Mengistu AM, et al. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care 2010; 14:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Review Manager (Version, 5.3). Oxford, UK: Cochrane Collaboration; 2014. [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 27.GRADEpro. Version 3.6 for Windows. 2015. Available at: http://tech.cochrane.org/revman/other-resources/gradepro/download Accessed April 2, 2015. [Google Scholar]
- 28.Pestana D, Espinosa E, Eden A, et al. Perioperative goal-directed hemodynamic optimization using noninvasive cardiac output monitoring in major abdominal surgery: a prospective, randomized, multicenter, pragmatic trial:POEMAS Study (PeriOperative goal-directed thErapy in Major Abdominal Surgery). Anesth Analg 2014; 119:579–587. [DOI] [PubMed] [Google Scholar]
- 29.Zeng K, Li Y, Liang M, et al. The influence of goal-directed fluid therapy on the prognosis of elderly patients with hypertension and gastric cancer surgery. Drug Des Devel Ther 2014; 8:2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Zheng H, Guo H, Ye JR, et al. Goal-directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: randomized trial. World J Surg 2013; 37:2820–2829. [DOI] [PubMed] [Google Scholar]
- 31.Salzwedel C, Puig J, Carstens A, et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Crit Care 2013; 17:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheeren TW, Wiesenack C, Gerlach H, et al. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput 2013; 27:225–233. [DOI] [PubMed] [Google Scholar]
- 33.Ramsingh DS, Sanghvi C, Gamboa J, et al. Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: a randomized controlled trial. J Clin Monit Comput 2013; 27:249–257. [DOI] [PubMed] [Google Scholar]
- 34.Bundgaard-Nielsen M, Jans O, Muller RG, et al. Does goal-directed fluid therapy affect postoperative orthostatic intolerance? A randomized trial. Anesthesiology 2013; 119:813–823. [DOI] [PubMed] [Google Scholar]
- 35.McKenny M, Conroy P, Wong A, et al. A randomised prospective trial of intra-operative oesophageal Doppler-guided fluid administration in major gynaecological surgery. Anaesthesia 2013; 68:1224–1231. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasa S, Taylor MH, Singh PP, et al. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg 2013; 100:66–74. [DOI] [PubMed] [Google Scholar]
- 37.Brandstrup B, Svendsen PE, Rasmussen M, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth 2012; 109:191–199. [DOI] [PubMed] [Google Scholar]
- 38.Zakhaleva J, Tam J, Denoya PI, Bishawi M, et al. The impact of intravenous fluid administration on complication rates in bowel surgery within an enhanced recovery protocol: a randomized controlled trial. Colorectal Dis 2013; 15:892–899. [DOI] [PubMed] [Google Scholar]
- 39.Challand C, Struthers R, Sneyd JR, et al. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth 2012; 108:53–62. [DOI] [PubMed] [Google Scholar]
- 40.Pillai P, McEleavy I, Gaughan M, et al. A double-blind randomized controlled clinical trial to assess the effect of Doppler optimized intraoperative fluid management on outcome following radical cystectomy. J Urol 2011; 186:2201–2206. [DOI] [PubMed] [Google Scholar]
- 41.Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg 2010; 111:910–914. [DOI] [PubMed] [Google Scholar]
- 42.Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010; 14:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buettner M, Schummer W, Huettemann E, et al. Influence of systolic-pressure-variation-guided intraoperative fluid management on organ function and oxygen transport. Br J Anaesth 2008; 101:194–199. [DOI] [PubMed] [Google Scholar]
- 44.Lopes MR, Oliveira MA, Pereira VO, et al. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care 2007; 11:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95:634–642. [DOI] [PubMed] [Google Scholar]
- 46.Conway DH, Mayall R, Abdul-Latif MS, et al. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia 2002; 57:845–849. [DOI] [PubMed] [Google Scholar]
- 47.Bonazzi M, Gentile F, Biasi GM, et al. Impact of perioperative haemodynamic monitoring on cardiac morbidity after major vascular surgery in low risk patients. A randomised pilot trial. Eur J Vasc Endovasc Surg 2002; 23:445–451. [DOI] [PubMed] [Google Scholar]
- 48.Miller TE, Thacker JK, White WD, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg 2014; 118:1052–1061. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014; 101:172–188. [DOI] [PubMed] [Google Scholar]
- 50.Lee L, Mata J, Ghitulescu GA, et al. Cost-effectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg 2014. [DOI] [PubMed] [Google Scholar]
- 51.Bellamy MC. Wet, dry or something else? Br J Anaesth 2006; 97:755–757. [DOI] [PubMed] [Google Scholar]
- 52.Doherty M, Buggy DJ. Intraoperative fluids: how much is too much? Br J Anaesth 2012; 109:69–79. [DOI] [PubMed] [Google Scholar]
- 53.Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr 2008; 27:179–188. [DOI] [PubMed] [Google Scholar]
- 54.Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012; 256:18–24. [DOI] [PubMed] [Google Scholar]
- 55.Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent “pre-renal” acute kidney injury? Kidney Int 2014; 86:1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krajewski ML, Raghunathan K, Paluszkiewicz SM, et al. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg 2015; 102:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cakir H, van Stijn MF, Lopes Cardozo AM, et al. Adherence to enhanced recovery after surgery and length of stay after colonic resection. Colorectal Dis 2013; 15:1019–1025. [DOI] [PubMed] [Google Scholar]
- 58.Alcantara-Moral M, Serra-Aracil X, et al. Observational cross-sectional study of compliance with the fast track protocol in elective surgery for colon cancer in Spain. Int J Colorectal Dis 2014; 29:477–483. [DOI] [PubMed] [Google Scholar]
- 59.Gustafsson UO, Hausel J, Thorell A, et al. Enhanced Recovery After Surgery Study Group. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer. Arch Surg 2011; 146:571–577. [DOI] [PubMed] [Google Scholar]
- 60.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139. [DOI] [PubMed] [Google Scholar]
- 61.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 62.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130(0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012; 367:124–134. [DOI] [PubMed] [Google Scholar]
- 63.Yates DR, Davies SJ, Milner HE, et al. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth 2014; 112:281–289. [DOI] [PubMed] [Google Scholar]
- 64.Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014; 311:2181–2190. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasa S, Lemanu DP, Singh PP, et al. Systematic review and meta-analysis of oesophageal Doppler-guided fluid management in colorectal surgery. Br J Surg 2013; 100:1701–1708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


