Figure 1. The engineered PrAg-PCIS targets tumor cell serine proteases.
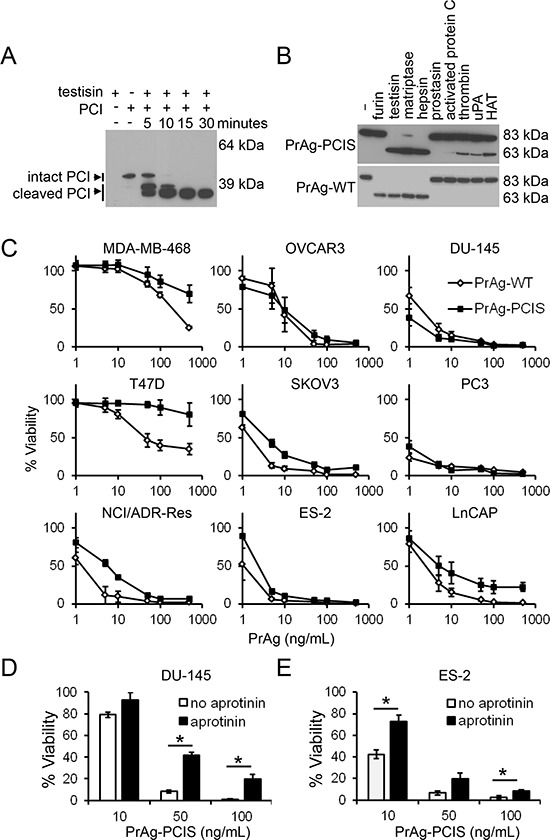
A. PCI is a testisin substrate. Recombinant testisin was incubated with recombinant PCI for various times up to 30 minutes. Individual reactions were stopped at indicated times and immunoblotted using anti-PCI antibody. The blot is representative of two independent experiments. B. PrAg-PCIS is resistant to furin cleavage, while PrAg-PCIS and PrAg-WT are susceptible to proteolytic cleavage by various recombinant serine proteases. PrAg-PCIS and PrAg-WT were incubated with furin, the recombinant catalytic domains of membrane-anchored serine proteases, or recombinant pericellular serine proteases for 2.5 hours. Reactions were immunoblotted using anti-PrAg antibody to detect PrAg activation cleavage. The blot is representative of two independent experiments. C. PrAg-PCIS and PrAg-WT toxin-induced human tumor cell cytotoxicity. The indicated tumor cell lines were incubated with PrAg proteins (0–500 ng/mL) and FP59 (50 ng/mL) for 48 hours, after which cell viability was evaluated by MTT assay. Values are the means calculated from two independent experiments performed in triplicate. D. and E. PrAg-PCIS toxin targets serine proteases on the surface of ES-2 and DU-145 tumor cells. Cells were pre-incubated in the presence of a final concentration of 100 μM aprotinin for 30 minutes prior to treatment with the indicated concentrations of PrAg-PCIS and FP59 (50 ng/mL) for 2 hours. Cell viability was evaluated by MTT assay 48 hours later. Values are the means calculated from two independent experiments performed in triplicate. *p < 0.05.
