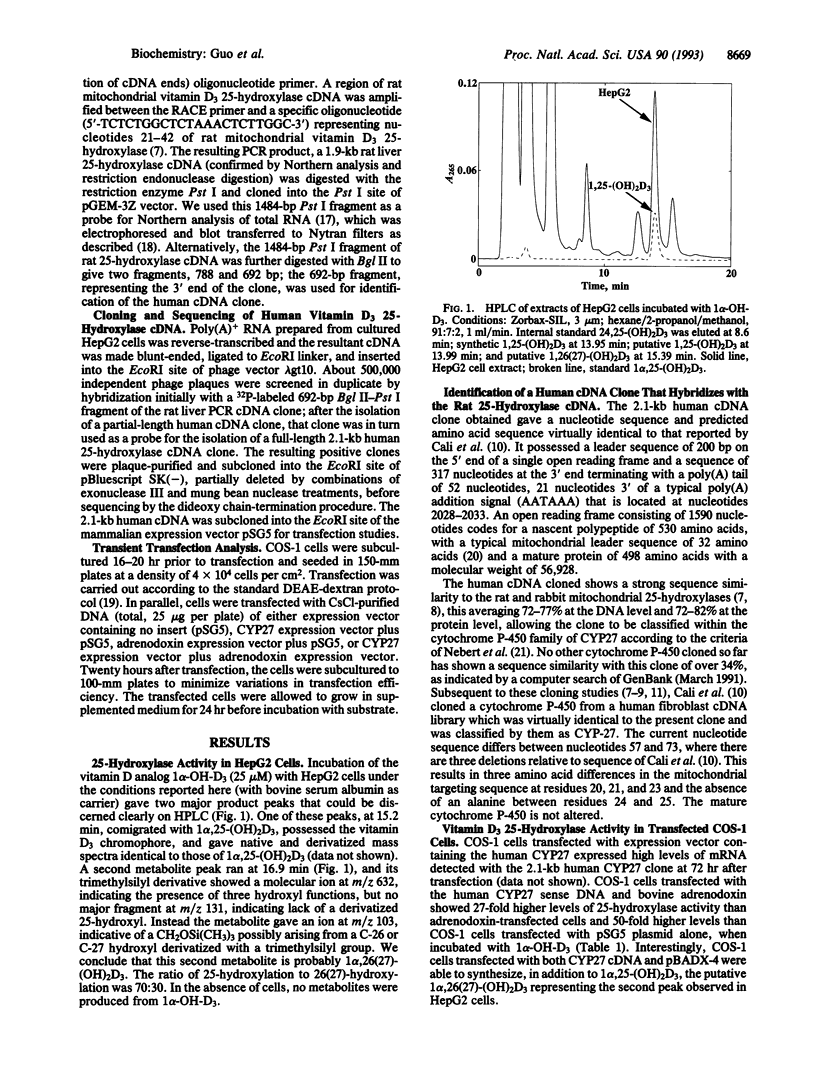
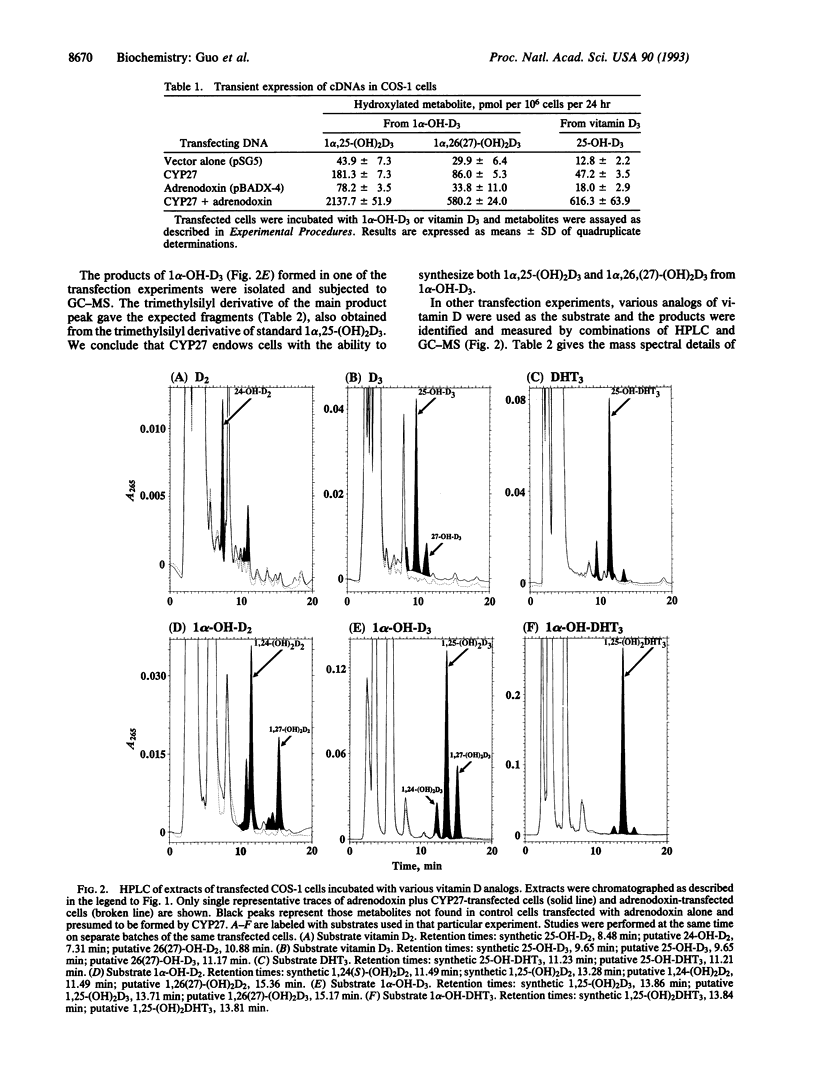
Abstract
A full-length cDNA for the human liver mitochondrial cytochrome P-450 CYP27 was cloned from a human hepatoma HepG2 cDNA library and then subcloned into the mammalian expression vector pSG5. When CYP27 cDNA was transfected into COS-1 transformed monkey kidney cells along with adrenodoxin cDNA, transfected cells revealed a 10- to 20-fold higher vitamin D3-25-hydroxylase activity than nontransfected cells. Transfected cells were capable of 25-hydroxylation of vitamin D3, 1 alpha-hydroxyvitamin D3 and 1 alpha-hydroxydihydrotachysterol3. In each case they also showed the ability to 26(27)-hydroxylate the cholesterol-like (D3) side chain. The relative rates of 25- and 26(27)-hydroxylation of 1 alpha-hydroxyvitamin D3 approximately mimicked the ratio of products observed in HepG2 cells. Vitamin D2 and 1 alpha-hydroxyvitamin D2, both with the ergosterol-like side chain, were 24- and 26(27)-hydroxylated by CYP27. The rate of side-chain 24-, 25-, or 26(27)-hydroxylation was greater for 1 alpha-hydroxylated vitamin D analogs than for their nonhydroxylated counterparts. We conclude that CYP27 is capable of 24-, 25-, and 26(27)-hydroxylation of vitamin D analogs and that the nature of products is partially dictated by the side chain of the substrate. This work has revealed that the cytochrome P-450 CYP27 may be important in the metabolism of vitamin D analogs used as drugs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Holmberg I., Wikvall K. 25-hydroxylation of C27-steroids and vitamin D3 by a constitutive cytochrome P-450 from rat liver microsomes. J Biol Chem. 1983 Jun 10;258(11):6777–6781. [PubMed] [Google Scholar]
- Bhattacharyya M. H., DeLuca H. F. The regulation of rat liver calciferol-25-hydroxylase. J Biol Chem. 1973 May 10;248(9):2969–2973. [PubMed] [Google Scholar]
- Björkhem I., Holmberg I., Oftebro H., Pedersen J. I. Properties of a reconstituted vitamin D3 25-hydroxylase from rat liver mitochondria. J Biol Chem. 1980 Jun 10;255(11):5244–5249. [PubMed] [Google Scholar]
- Cali J. J., Hsieh C. L., Francke U., Russell D. W. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991 Apr 25;266(12):7779–7783. [PMC free article] [PubMed] [Google Scholar]
- Cali J. J., Russell D. W. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991 Apr 25;266(12):7774–7778. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fukushima M., Suzuki Y., Tohira Y., Nishii Y., Suzuki M. 25-Hydroxylation of 1alpha-hydroxyvitamin D3 in vivo and in perfused rat liver. FEBS Lett. 1976 Jun 1;65(2):211–214. doi: 10.1016/0014-5793(76)80482-6. [DOI] [PubMed] [Google Scholar]
- Holmberg I., Berlin T., Ewerth S., Björkhem I. 25-Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin D2 and D3. Scand J Clin Lab Invest. 1986 Dec;46(8):785–790. doi: 10.3109/00365518609084051. [DOI] [PubMed] [Google Scholar]
- Horst R. L., Koszewski N. J., Reinhardt T. A. 1 alpha-hydroxylation of 24-hydroxyvitamin D2 represents a minor physiological pathway for the activation of vitamin D2 in mammals. Biochemistry. 1990 Jan 16;29(2):578–582. doi: 10.1021/bi00454a035. [DOI] [PubMed] [Google Scholar]
- Jones G. A new pathway of 25-hydroxyvitamin D3 metabolism. Methods Enzymol. 1986;123:141–154. doi: 10.1016/s0076-6879(86)23017-7. [DOI] [PubMed] [Google Scholar]
- Jones G., Schnoes H. K., Levan L., Deluca H. F. Isolation and identification of 24-hydroxyvitamin D2 and 24,25-dihydroxyvitamin D2. Arch Biochem Biophys. 1980 Jul;202(2):450–457. doi: 10.1016/0003-9861(80)90449-x. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Qaw F., Calverley M. J., Schroeder N. J., Trafford D. J., Makin H. L., Jones G. In vivo metabolism of the vitamin D analog, dihydrotachysterol. Evidence for formation of 1 alpha,25- and 1 beta,25-dihydroxy-dihydrotachysterol metabolites and studies of their biological activity. J Biol Chem. 1993 Jan 5;268(1):282–292. [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Saarem K., Bergseth S., Oftebro H., Pedersen J. I. Subcellular localization of vitamin D3 25-hydroxylase in human liver. J Biol Chem. 1984 Sep 10;259(17):10936–10940. [PubMed] [Google Scholar]
- Su P., Rennert H., Shayiq R. M., Yamamoto R., Zheng Y. M., Addya S., Strauss J. F., 3rd, Avadhani N. G. A cDNA encoding a rat mitochondrial cytochrome P450 catalyzing both the 26-hydroxylation of cholesterol and 25-hydroxylation of vitamin D3: gonadotropic regulation of the cognate mRNA in ovaries. DNA Cell Biol. 1990 Nov;9(9):657–667. doi: 10.1089/dna.1990.9.657. [DOI] [PubMed] [Google Scholar]
- Tam S. P., Strugnell S., Deeley R. G., Jones G. 25-Hydroxylation of vitamin D3 in the human hepatoma cell lines Hep G2 and Hep 3B. J Lipid Res. 1988 Dec;29(12):1637–1642. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui E., Noshiro M., Okuda K. Molecular cloning of cDNA for vitamin D3 25-hydroxylase from rat liver mitochondria. FEBS Lett. 1990 Mar 12;262(1):135–138. doi: 10.1016/0014-5793(90)80172-f. [DOI] [PubMed] [Google Scholar]
- Wichmann J., Schnoes H. K., DeLuca H. F. Isolation of identification of 24(R)-hydroxyvitamin D3 from chicks give large doses of vitamin D3. Biochemistry. 1981 Apr 14;20(8):2350–2353. doi: 10.1021/bi00511a043. [DOI] [PubMed] [Google Scholar]
- Yoon P. S., Deluca H. F. Resolution and reconstitution of soluble components of rat liver microsomal vitamin D3-25-hydroxylase. Arch Biochem Biophys. 1980 Sep;203(2):529–541. doi: 10.1016/0003-9861(80)90210-6. [DOI] [PubMed] [Google Scholar]



