Abstract
Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24) encodes a tumor suppressor gene implicated in the growth of various tumor types including breast cancer. We previously demonstrated that recombinant adenovirus-mediated mda-7/IL-24 expression in the mammary glands of carcinogen-treated (methylnitrosourea, MNU) rats suppressed mammary tumor development. Since most MNU-induced tumors in rats contain activating mutations in Ha-ras, which arenot frequently detected in humans, we presently examined the effect of MDA-7/IL-24 on Her2/Neu-induced mammary tumors, in which the RAS pathway is induced. We generated tet-inducible MDA-7/IL-24 transgenic mice and crossed them with Her2/Neu transgenic mice. Triple compound transgenic mice treated with doxycycline exhibited a strong inhibition of tumor development, demonstrating tumor suppressor activity by MDA-7/IL-24 in immune-competent mice. MDA-7/IL-24 induction also inhibited growth of tumors generated following injection of Her2/Neu tumor cells isolated from triple compound transgenic mice that had not been treated with doxycycline, into the mammary fat pads of isogenic FVB mice. Despite initial growth suppression, tumors in triple compound transgenic mice lost mda-7/IL-24 expression and grew, albeit after longer latency, indicating that continuous presence of this cytokine within tumor microenvironment is crucial to sustain tumor inhibitory activity. Mechanistically, MDA-7/IL-24 exerted its tumor suppression effect on HER2+ breast cancer cells, at least in part, through PERP, a member of PMP-22 family with growth arrest and apoptosis-inducing capacity. Overall, our results establish mda-7/IL-24 as a suppressor of mammary tumor development and provide a rationale for using this cytokine in the prevention/treatment of human breast cancer.
Keywords: mda-7/IL-24, HER2, breast cancer, prevention, mouse model
INTRODUCTION
Breast cancer is a devastating disease, constituting more than 25% of the total number of new cancer cases diagnosed annually among women throughout the world [1]. Amplification or over-expression of the receptor tyrosine kinase HER2 induces one of the most aggressive forms of breast cancer [2]. Although anti-HER2 therapy provides some improvement in disease free survival, drug-refractory metastatic disease is almost invariably fatal [3]. The development of new treatments or prophylactic means to reduce the incidence of this disease is, therefore, of great interest.
Melanoma differentiation associated gene-7 was originally identified and cloned using subtraction hybridization from metastatic human melanoma cells that were induced to undergo terminal differentiation and lose proliferative and tumorigenic properties [4]. mda-7/IL-24 is a member of the IL-10 family of cytokines, and named IL-24 (mda-7/IL-24) [5-8]. It encodes a secreted tumor suppressor protein of 206 amino acids, which inhibits the growth of diverse human cancers, without harming normal cells or tissues [4, 9-11]. mda-7/IL-24 displays restricted expression in normal and cancer cells, observed in melanocytes and subsets of T-cells, but not in the majority of normal or cancer cells [8, 11, 12]. Expression of this cytokine can be induced in both immune and non-immune cells through interaction of the secreted protein with IL-20R1/IL-20R2 and IL-22R1/IL-20R2 cell surface receptors inducing autocrine and paracrine secretion of this cytokine and cancer-specific apoptosis [8, 13-15]. mda-7/IL-24 displays multiple properties that support its ability to serve as a cancer suppressor gene, including an ability to selectively induce apoptosis and toxic authophagy in a broad-spectrum of cancer cells, potent “bystander” antitumor activity, anti-angiogenic activity, immune modulatory properties and synergy with conventional therapeutics (radiation, chemotherapy and antibody-based therapies) [rev. 7, 8, 15, 16]. mda-7/IL-24 has shown significant anti-tumor activity in vivo in pre-clinical animal models including multiple human tumor xenografts in nude mice [17-24] and recently in prostate cancer genetically engineered mouse (GEM) models [25, 26]. When mda-7/IL-24 was administered to patients with advanced cancers by repeat intratumoral injection using a replication incompetent adenovirus (Ad.mda-7; INGN 241) it was shown to be safe and induced a 44% response rate, promoting cancer apoptosis in injected lesions [27-32]. A potential link between mda-7/IL-24 and specific autoimmune diseases such as psoriasis and rheumatoid arthritis has been suggested [33].
The mechanism by which mda-7/IL-24 exerts its tumor suppressor activity has been extensively studied in the past decade [rev. in 7, 8, 15, 16, 28]. Several studies indicate that mda-7/IL-24 selectively induces apoptosis in cancer cells without harming normal cells, by promoting an endoplasmic reticulum (ER) stress response [34-37]. Recent studies also demonstrate selective apoptosis by Suppressor of AP-1, induced by an IFN (SARI)-dependent mechanism [14]. Although the majority of studies have emphasized solid tumors, mda-7/IL-24 also induces ER stress and mitochondrial apoptosis pathway in human acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL) [38, 39]. A potential role for ceramide production (ceramide synthase, PP2A) and generation of reactive oxygen species as a consequence of induction of ER stress by mda-7/IL-24 in tumor cells has also been demonstrated [7, 8, 40-42]. As emphasized in numerous reviews, mda-7/IL-24 can elicit cancer-selective killing through multiple pathways, including those involving modification of signaling pathways and molecules (including BiP/GRP78, GRP94, P-PKR, PERK, P-p38 MAPK, CD95, Bax, Bak) that can lead to activation of caspase 9/3 resulting in mitochondrial-mediated apoptosis, through death-receptor mediated killing or toxic authophagy [rev. in 7, 8, 15, 16, 28]. Despite this progress, there is only limited direct evidence for tumor suppressor activity by MDA-7/IL-24 in immune-competent transgenic mice [25, 26].
We have previously shown that growth suppression by mda-7/IL-24 is associated with transcriptional up-regulation of p27Kip1 via Stat3 activation in breast cancer cells [43]. Furthermore, we showed that β4 integrin is a downstream target of mda-7/IL-24 [43]. More recently, we identified the growth arrest-specific gene 3 (gas3) as a downstream target of mda-7/IL-24 [44]. We further demonstrated that the induction of gas3 by mda-7/IL-24 inhibits attachment and proliferation of tumor cells in vitro and in vivo by blocking interaction of β1 integrin with fibronectin [44].
We, and others, have shown that delivery of mda-7/IL-24 via an adenovirus vector can efficiently inhibit growth of diverse cancer cells in vivo and in vitro [rev. in 7, 8], including breast cancer [10, 17, 43-49]. Specifically, we demonstrated significant inhibition of tumor development following injection of an adenovirus carrying mda-7/IL-24 into the main mammary ducts of rats induced to develop breast cancer by treatment with methylnitrosourea (MNU) [44]. Most MNU-induced tumors in rats contain activating mutations in the Ha-ras oncogene [50]. Since Ha-ras mutations are not frequently detected in humans, in the present study we investigated whether mda-7/IL-24 could inhibit development of Her2/Neu-induced breast cancer. We generated tet-inducible mda-7/IL-24 transgenic mice to investigate whether mda-7/IL-24 expression could suppress development of Her2/Neu mammary tumors in compound immunocompetent transgenic mice. Our results provide a rationale for the prophylactic use of mda-7/IL-24 in the prevention as well as applications for therapy of HER2+ breast cancer.
RESULTS
Generation of doxycycline-inducible mda-7/IL-24 transgenic mice
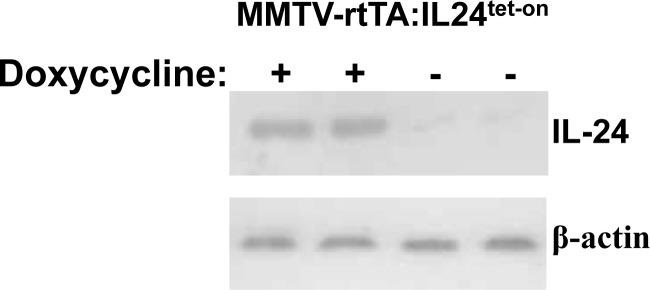
To generate transgenic mice that can be induced to over-express MDA-7/IL-24 specifically in the mammary gland, we placed the mda-7/IL-24 gene under control of a tet-inducible PtetOS-4264 promoter [51]. The plasmid was injected into pronuclei of FVB mouse oocytes, and transgenic mice, referred to as IL24tet-on, were identified by PCR. To induce expression of mda-7/IL-24 specifically in mammary epithelium, IL24tet-on mice were mated with MMTV-rtTA mice (Figure 1A). The latter mice express the reverse tetracycline-dependent transactivator rtTA under control of the mouse mammary tumor long terminal repeat (MMTV-LTR) [52]. Transgene expression in this system can be rapidly induced by feeding mice doxycycline-containing chow (625 mg/kg), is highly mammary specific, and is essentially undetectable in the un-induced state [52].
Figure 1.
A. Schematic of generation of IL24tet-on transgenic mice and genetic cross to create tet-inducible MMTV-rtTA:IL24tet-on mice. B. Western blot of MDA-7/IL-24 expression in mammary glands isolated from two MMTV-rtTA:IL24tet-on double transgenic mice fed regular or doxycycline-containing chow.
To test for mda-7/IL-24 inducibility, we fed MMTV-rtTA:IL24tet-on double compound transgenic females regular or doxycycline-containing chow. Mammary glands were harvested and subjected to western blot analysis (Figure 1B). In contrast to control mice that expressed very low levels of mda-7/IL-24, doxycycline induced robust mda-7/IL-24 expression (53-fold increase over background).
Transgenic mda-7/IL-24 expression suppresses Her2/Neu tumor formation
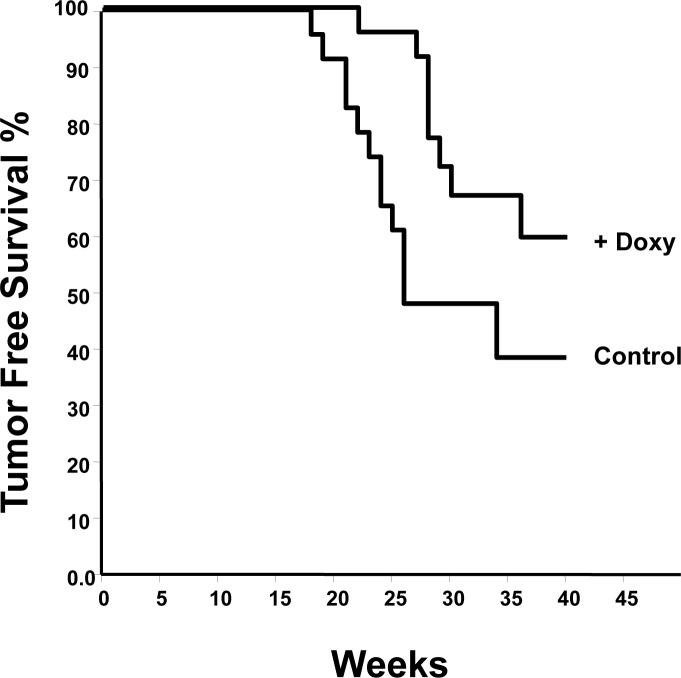
To determine the effect of mda-7/IL-24 on tumor development, we next crossed MMTV-rtTA:IL24tet-on mice with MMTV-Her2/neu transgenic mice [53]. A group of 23 MMTV-rtTA:IL24tet-on:MMTV-Her2/neu triple compound transgenics was fed chow-containing doxycycline, while a similar size group was fed regular chow. Mice were monitored weekly for signs of tumor formation. Kaplan-Meier tumor-free survival curves for the two groups were generated (Figure 2). It is clear that induction of mda-7/IL-24 significantly reduced tumor progression (P = 0.005) with hazard ratio of 2.7.
Figure 2. Kaplan-Meier analysis of tumor development in MMTV-rtTA:IL24tet-on:MMTV-Her2/neu triple compound transgenic mice fed regular chow (controls) or chow containing doxycycline.
Hazard ratio 2.1 (P = 0.005).
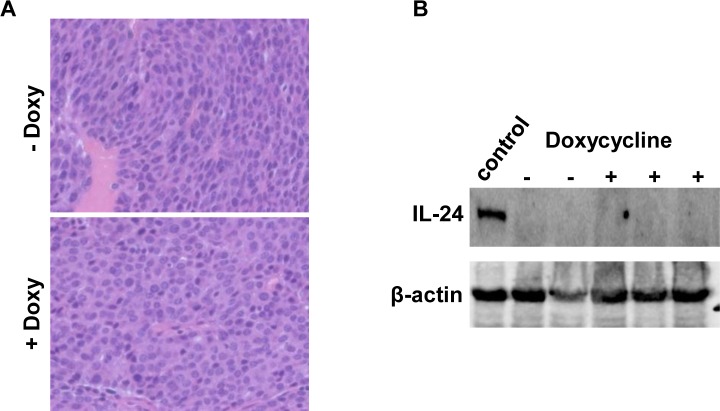
Tumors from both groups were fixed for histological analysis. Those from control mice not administered doxycycline were typical, poorly differentiated adenocarcinomas as previously characterized in these animals (Figure 3A). Tumors from doxycycline-treated mice were indistinguishable from the control group. Western analysis showed no MDA-7/IL-24 expression in tumors from either doxycycline-treated mice or untreated mice (Figure 3B). This result was confirmed in Western blots performed with a three-fold increase (150 μg) in the amount of protein loaded per lane (data not shown).
Figure 3.
A. H&E staining of tumors from MMTV-rtTA:IL24tet-on:MMTV-Her2/neu triple transgenic mice fed regular chow or chow containing doxycycline. Original magnification, 400X. B. Western blot demonstrating lack of expression of MDA-7/IL-24 in tumors that arose in either doxycycline-treated or untreated mice. Each lane contained 50μg protein; the positive control lane contained protein isolated from the mammary glands of double transgenic mice fed doxycyline as shown in Figure 1A.
Effect of mda-7/IL-24 expression on Her2/Neu tumor growth
We next asked whether induction of mda-7/IL-24 would reduce growth of pre-existing tumors. To this end, Her2/Neu tumors were allowed to form in triple compound transgenic mice fed normal chow. Once tumors were detected, half the mice were shifted to a doxycycline-containing diet. We found that tumors in these mice continued to develop over a 6-week period at the same rate as those in control mice (data not shown). These results are consistent with the observation above (Figure 3B) that once tumors are formed in triple compound transgenic mice, they shut down mda-7/IL-24 expression and no longer respond to doxycycline.
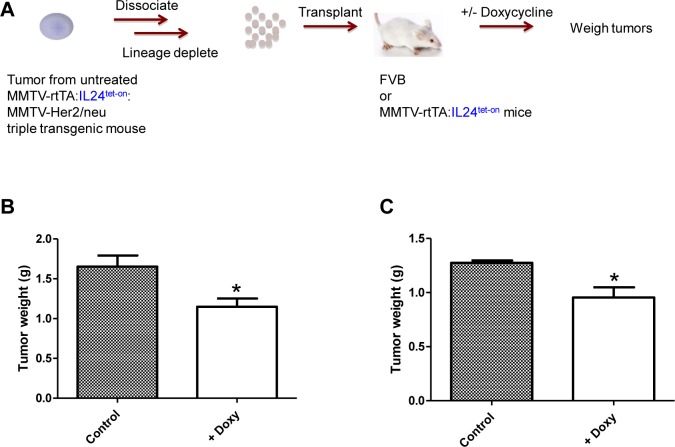
Next we determined whether MDA-7/IL-24 expression could suppress growth of pre-existing tumor cells in vivo. To this end, lineage-depleted tumor cells were isolated from MMTV-rtTA:IL24tet-on:MMTV-Her2/neu triple compound transgenic mice that had not been treated with doxycycline, then injected into the mammary fat pads of FVB mice (Figure 4A). Treatment of recipient mice with doxycycline to induce mda-7/IL-24 expression significantly inhibited tumor growth compared with untreated mice (P < 0.03) (Figure 4B). We obtained a similar result when the recipient mice were MMTV-rtTA:IL24tet-on double compound transgenics (P < 0.02) (Figure 4C).
Figure 4.
A. Schematic of experimental design. B. Effect of mda-7/IL-24 expression on the growth of tumor cells from MMTV-rtTA:IL24tet-on:MMTV-Her2/neu triple transgenic mice fed regular chow, which were dissociated into single cells and transplanted into FVB mice. The FVB mice were then fed regular chow or chow containing doxycycline for 12 weeks. Tumors were dissected and weighed (n = 8, P = 0.0275; unpaired Student t-test). C. Effect of MDA-7/IL-24 expression on growth of tumor cells from MMTV-rtTA:IL24tet-on:MMTV-Her2/neu triple transgenic mice fed regular chow, which were dissociated into single cells and transplanted into MMTV-rtTA:IL24tet-on double transgenic mice. The double transgenic mice were then fed regular chow or chow containing doxycycline for 12 weeks before MMTV-rtTA:IL24tet-on:MMTV-Her2/neu tumors were dissected and weighed (n = 8, P = 0.0152; unpaired Student t-test).
mda-7/IL-24 regulates p53 apoptosis effector related to PMP-22 (PERP) in breast cancer cells
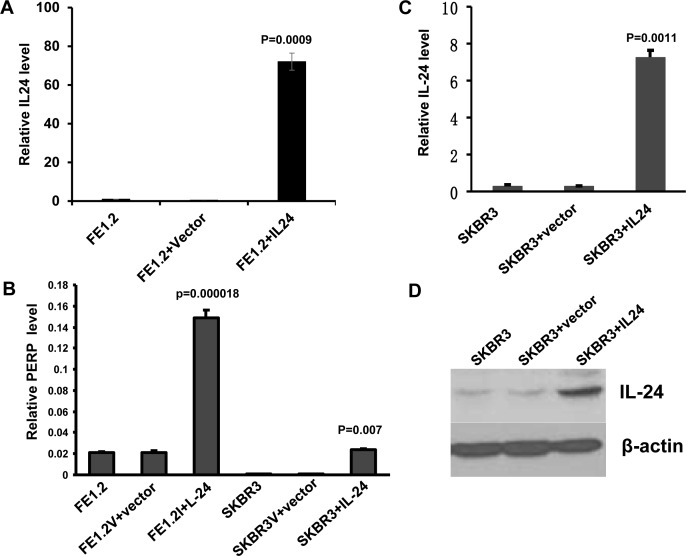
In a recent study, we identified gas3/pmp-22 as a downstream target of mda-7/IL-24 that mediates some of the growth inhibitory effects of this cytokine [44]. In the microarray analysis used to identify GAS3 [44], we also observed increased expression of another member of the GAS-3/PMP-22 family - p53 apoptosis effector related to PMP-22, PERP [54]. Here we show that the level of perp transcripts as determined by Q-rt-PCR was significantly higher in FE1.3 cells, expressing high levels of endogenous mda-7/IL-24, or in FE1.2 cells over-expressing mda-7/IL-24 (FE1.2+IL-24), than in control FE1.2+vector cells (Figure 5A and 5B). FE1.2 and FE1.3 are both breast cancer cell lines previously isolated from MNU-treated rats [55]. Over-expression of mda-7/IL-24 in the HER2+ breast cancer cell line SKBR3 (Figure 5C and 5D) also induced perp mRNA levels (Figure 5B).
Figure 5. MDA-7/IL-24 directly induces PERP transcription.
A. Expression of mda-7/IL-24 by Q-rtPCR in FE1.3 cells alone and in FE1.2 cells transfected with vector (FE1.2+vector) or MSCV-IL-24 (FE1.2+IL-24), as previously described (44). B. Expression of PERP by Q-rtPCR in FE1.2 or SKBR3 cells transfected with vector or mda-7/IL-24 retroviruses. C. Expression of MDA-7/IL-24 by Q-rtPCR in SKBR3 cells transfected with vector or mda-7/IL-24 retroviruses. D. Expression of MDA-7/IL-24 by Western blotting in SKBR3 cells transfected with empty vector or mda-7/IL-24 retroviruses.
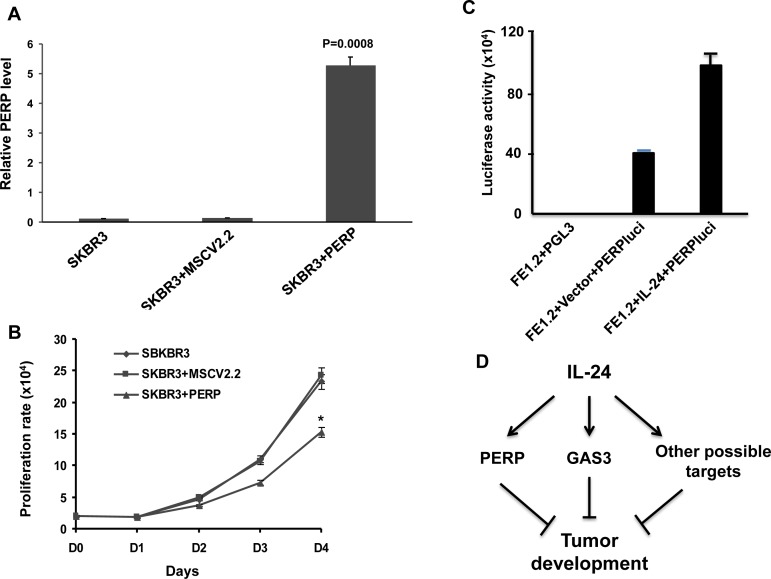
To determine the effect of over-expressing PREP on growth of SKBR3 cells, we used a retroviral expression vector (Figure 6A). Prep overexpression significantly inhibited proliferation compared to untransfected or vector alone (Figure 6B). To further investigate perp regulation by MDA-7/IL-24, a luciferase vector driven by the perp promoter (PERPluci) was transiently transfected into FE1.2+IL-24 and FE1.2+vector cells. This led to a significant up-regulation of luciferase activity in PERPluci-transfected cells in response to MDA-7/IL-24 (Figure 6C). These results demonstrate that members of the PMP-22 family of tumor suppressor genes play an important role in mda-7/IL-24-mediated growth inhibition of HER2+ breast cancer cells (Figure 6D).
Figure 6. MDA-7/IL-24 regulates PERP expression.
A. Expression of PERP by Q-rtPCR in SKBR3 cells transfected with vector or MSCV-perp retroviruses. B. Growth rate of SKBR3, SKBR3 cells transfected with vector (SKBR3-vector) or perp cDNA (SKBR3-prep). * denotes P = 0.0074 at day 4 comparing SKBR3-vector with SKBR3-prep. C. Luciferase activity in FE1.2, FE1.2+vector and FE1.2+MSCV-IL-24 after transfection with a luciferase vector driven by the PERP promoter (PERPluci) as previously described (75). D. A model for mda-7/IL-24 suggesting that it induces PERP (this study), GAS3 (44) and likely other factors that collectively suppress tumor development.
DISCUSSION
We report that mammary tumor development was significantly inhibited in MMTV-rtTA:IL24tet-on:MMTV-Her2/neu triple compound transgenic mice in which mda-7/IL-24 was over-expressed by means of a doxycycline inducible promoter. Moreover, transplantation of tumors induced in the triple compound transgenic mice that were not treated with doxycycline into FVB or MMTV-rtTA:IL24tet-on double compound transgenic mice resulted in tumors that grew significantly slower after treatment with the mda-7/IL-24 inducer. These results provide direct evidence for tumor inhibitory activity of MDA-7/IL-24 in Her2/Neu-induced breast cancer.
While our results demonstrated a robust tumor inhibition in triple compound transgenic mice after transgene induction, the expression of mda-7/IL-24 was found to be negligible in tumors harvested prior to euthanasia. This observation suggests that mda-7/IL-24 expression subsides during tumor development. This conclusion is supported by our findings that tumor growth was not inhibited in triple compound transgenic mice that had not been treated with doxycycline but were then treated with doxycycline for 6 weeks (not shown). Thus, mda-7/IL-24 expression appears to be shut down during tumor evolution, explaining the delayed, rather than complete, suppression of tumor formation in the doxycycline-treated mice, and the robust growth of these tumors once formed. Since mda-7/IL-24 acts as tumor suppressor, loss of transgene expression, perhaps as a result of limited delivery of doxycycline to the tumor mass or epigenetic silencing, may promote selection of faster growing cancer cells that eventually dominate. This result raises the possibility that future cancer therapy may require a delivery mechanism that ensures persistent expression/delivery of this cytokine to the tumor microenvironment. Alternatively, combining mda-7/IL-24 with a second therapeutic agent that may directly target the tumor, activate the immune system and/or affect the tumor microenvironment might promote a more sustained therapeutic response [7, 15-17, 24-26, 48, 56]. A recent study suggests that phosphorylation of MDA-7/IL-24 is required for its anti-cancer activity in a single lung cancer cell line, H1299 [57]. The authors suggest that phosphomimetic MDA-7/IL-24 or pharmacological induction of phosphorylation of endogenous or exogenous MDA-7/IL-24 may further promote its anti-tumor effects. An alternate strategy could employ high throughput screening and combinatorial chemistry approaches, to identify small molecules that can either stabilize mda-7/IL-24 mRNA, enhance translation of mda-7/IL-24 mRNA into protein or promote IL-20R1/IL-20R2 or IL-22R1/IL-20R2 receptor activation to induce endogenous mda-7/IL-24 mRNA production and protein in target cells [58-68].
Transplantation of lineage-depleted tumor cells from triple compound transgenic mice fed regular chow into FVB or MMTV-rtTA:IL24tet-on double compound transgenic mice treated with doxycycline inhibited subsequent tumor growth. These results are consistent with the notion that high local MDA-7/IL-24 is growth suppressive, but that once tumor cells grow, MDA-7/IL-24 expression in specific contexts may be shut down through mechanisms that are currently not fully understood.
In the past decade, various downstream target genes were suggested as potential mediators of growth suppression by mda-7/IL-24 [rev. in 7, 8, 15, 16]. We recently discovered the GAS-3/PMP-22 tumor suppressor gene as a novel target for this cytokine [44]. Our identification here of the MDA-7/IL-24 target PERP, another member of the GAS-3/PMP-22 family with strong tumor inhibitory activity, further highlights the importance of this gene family in malignant transformation. Since PERP is also a direct target of p53, our data suggest that MDA-7/IL-24 can induce growth arrest independently of p53, as supported by previous work [69-71], and may, therefore, be a useful inhibitor of tumors harboring TP53 mutations or deletions.
The anti-tumor effects of mda-7/IL-24 are likely mediated not only by autonomous cellular mechanisms through its downstream targets (Figure 6, model), but also through induction of an immune response. This is exemplified in the transplantation experiment shown in Figure 4 in which IL-24-transgenic tumor cells were injected into immuno-competent FvB mice, but an immune response may also play a role in the transgenic mouse experiment (Figure 3). In an accompanying paper by Menezes et al. [72] three distinct transgenic mouse models of breast cancer were utilized to determine the role of MDA-7/IL-24 in mammary tumorigenesis. The findings in that paper provide further proof of the relevance of MDA-7/IL-24 in tumor suppression in mouse models with an intact immune system. The findings further demonstrate the role of MDA-7/IL-24 in eliciting an antitumor immune response and show that adenovirus mediated mda-7/IL-24 delivery induces infiltrating CD8+ T cells expressing high levels of levels of IFN-γ expression [72]. Thus, induction of exogenous mda-7/IL-24 may not only suppress cell proliferation directly but may also elicit an immune response in immune-competent animals, further attenuating tumor growth.
In summary, our data provides strong support for the role of mda-7/IL-24 as a suppressor of Her2/Neu-induced breast cancer and should encourage development of a therapy based on continuous mda-7/IL-24 delivery into tumor cells, and based on a preponderance of data in the literature in combination with other therapeutic agents. Although direct injection of recombinant IL-24 or viral-based deliveries are possible, an appealing direction is to identify novel drugs that can upregulate or stabilize endogenous levels of this cytokine tumor suppressor.
MATERIALS AND METHODS
Generation of Tg mice
mda-7/IL-24 mRNA, isolated from our rat mammary tumor cell line FE1.3 [55] was used to derive cDNA that was cloned into the EcoRI site of plasmid PtetOS-4264 [51] kindly supplied by Dr. D. Dumont, Sunnybrook Health Sciences Centre, Toronto, Canada. This construct was microinjected into pronuclei of FVB mouse oocytes, which were then implanted into pseudopregnant recipients by the Toronto Centre for Phenogenomics (www.phenogenomics.ca). Pups were screened for the presence of mda-7/IL-24 at 2-3 weeks of age using the forward primer ATGGTTCTCCAAGCCTTCCT and reverse primer CCCTGAGGACAACAGGGATA. In order to express mda-7/IL-24 specifically in the mammary gland, transgenic founders were crossed with MMTV-rtTA mice in an FVB background [52] kindly supplied to us by Dr. L. A. Chodosh, University of Pennsylvania School of Medicine. The double compound transgenics were identified using the forward primer ATCCGCACCCTTGATGACTCCG and the reverse primer GGCTATCAACCAACACACTGCCAC. Finally, FVB mice carrying the MMTV- neu transgene [53] (FVB/N-Tg(MMTVneu)202Mul/J) were obtained from Jackson Labs (Bar Harbor, MN). Presence of the neu transgene was verified using the forward primer TTTCCTGCAGCAGCCTACGC and the reverse primer CGGAACCCACATCAGGCC. The double compound transgenic mice were then crossed with the MMTV-neu transgenic mice to obtain triple compound transgenics, screened using the three primer sets detailed above. Females were used for mammary tumor analysis.
Tumor analysis
Mice were bred to yield 46 triple compound transgenic females. Twenty-three of these were administered doxycycline in their feed beginning at 21 days of age as described above, while 23 received no doxycycline and acted as controls. All mice were weighed and palpated weekly for the presence of mammary tumors. Kaplan-Meier tumor-free survival analysis was performed using the PAST program (P.D. Ryan and Ø. Hammer, University of Oslo) comparing the treatment group with controls (23 mice per group for more than 25 weeks of treatment). P-value for the Kaplan-Meier curve was calculated using the Wilcoxon method. Differences were considered statistically significant at p < 0.05. Tumors were weighed then cut in half. One half was placed in formalin prior to histological analysis, the other half was snap frozen in liquid nitrogen and stored at −80. Significance of tumor weight differences between treated and untreated animals was determined using an unpaired Student t-test.
Effect of mda-7/IL-24 expression on growth of transplanted tumor cells
Lineage-depleted tumor cells were isolated from triple compound transgenic mice that had not been treated with doxycycline according to an established procedure [73, 74]. Eight six week-old FVB female mice were then injected with 10,000 cells into the No 4 mammary gland fat pads under isofluorane anaesthesia. Four of the mice were administered doxycycline in their feed as described above, and 4 mice were given feed with no doxycycline as controls. The mice were euthanized 12 weeks after transplantation and the tumors were dissected and weighed. A similar experiment was performed using the double compound transgenic mice as recipients of the tumor cells.
Effect of mda-7/IL-24 expression on the growth of tumors in Tg mice
Ten triple compound transgenic mice not treated with doxycycline, in which small mammary tumors (<0.25 cm) had been detected, were randomized into two groups. Five mice received doxycycline in their feed as described above and 5 received the diet with no doxycycline. After 6 weeks, the mice were euthanized and tumors collected for analysis.
Animal care
Animal protocols were approved by the University of Toronto in accordance with the guidelines of the Canadian Council of Animal Care.
Western blotting
Western blotting was conducted as described [44]. Polyclonal rabbit anti-rat IL-24 antibodies were obtained from GenHunter (dilution of 1:1,000); β-actin from Sigma (dilution of 1:50,000).
Q-rtPCR amplification
RNA levels were quantified by the real-time rtPCR (q-rtPCR) in a StepOne Plus thermal cycler (Applied Biosystems) using specific primers and TaqMan probes (Genomed), as described [44]. The β-actin gene was used as control; SuperReal PreMix Plus SYBR®Green (TianGen, Beijing) was used for detection. The primers for PERP amplification were: sense-TCTTCCTTTAGTGGCGGTGT, anti-sense-ACGTCTGGATGTGGTTGCTA-3.
Cloning and transduction
Transduction of mda-7/IL-24 or vector alone into SKBR3 cells was previously described [43, 44]. Expression of mda-7/Il-24 in these cells was confirmed by Q-rt-PCR [43, 44]. The human perp gene was isolated from human peripheral blood mononuclear cells (PBMC) by PCR using forward: ATCTCGAGCAGGCCACTCTCTGCTGTC and Reverse: CTCCCACATTCATTCCCAAGT primers. The cDNA was first cloned into pCR2.1 plasmid using TA cloning kit (Invitrogen) and then subcloned into XhoI and EcoRI sites of the retroviral vector MSCV2.2 [43]. MSCV-perp and vector was then transfected into SKBR3 cells, as described [43]. The expression of prep in the transduced cells was determined using Q-rt-PCR using forward:ATAACTGGGCCTACGGCTTT and Reverse:CTCCCACATTCATTCCCAAGT primers.
Luciferase assay
pPERPluc1 was purchased from addgene and deposited by Dr. Tyler Jacks, as described [75]. This plasmid (2μg) was transfected into FE1.2+IL-24 and FE1.2+vector cells, using lipofectamine 2000 (Life technology, Beijing, China). After 48 h of transfection, luciferase assays were performed in triplicate as described [75, 76].
Acknowledgments
This work was supported by research grants from the Canadian Breast Cancer Foundation to MCA and YBD, the Science and Technology Department of Guizhou Province innovation and project grants (6012, 4001) and The Natural Science Foundation of China (grant 81472609) to YBD, and Canadian Breast Cancer Foundation (Ontario), Canadian Cancer Society Research Institute and Terry Fox Foundation to EZ. Support from the National Foundation for Cancer Research (NFCR) and the Samuel Waxman Cancer Research Foundation (SWCRF) to PBF is also acknowledged. PBF is a SWCRF Investigator and holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111:1888–1898. doi: 10.1038/bjc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto-Ibusuki M, Arnedos M, André F. Targeted therapies for ER+/HER2- metastatic breast cancer. BMC Med. 2015;13:137. doi: 10.1186/s12916-015-0369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- 5.Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, Pestka S, Fisher PB. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 6.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 7.Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, et al. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010;21:381–391. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitaker EL, Filippov VA, Duerksen-Hughes PJ. Interleukin 24: mechanisms and therapeutic potential of an anti-cancer gene. Cytokine Growth Factor Rev. 2012;23:323–331. doi: 10.1016/j.cytogfr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U S A. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci U S A. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ, Alexandre D, Chen Y, Vozhilla N, Mei MX, Christiansen KA, Sivo F, Goldstein NI, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 12.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S, Grimm EA. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 13.Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci U S A. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dash R, Bhoopathi P, Das SK, Sarkar S, Emdad L, Dasgupta S, Sarkar D, Fisher PB. Novel mechanism of MDA-7/IL-24 cancer-specific apoptosis through SARI induction. Cancer Res. 2014;74:563–574. doi: 10.1158/0008-5472.CAN-13-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, Dent P, Wang XY, Sarkar D, Fisher PB. MDA-7/IL-24: multifunctional cancer killing cytokine. Adv Exp Med Biol. 2014;818:127–153. doi: 10.1007/978-1-4471-6458-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WY, Cheng YT, Lei HY, Chang CP, Wang CW, Chang MS. IL-24 inhibits the growth of hepatoma cells in vivo. Genes Immun. 2005;6:493–499. doi: 10.1038/sj.gene.6364233. [DOI] [PubMed] [Google Scholar]
- 19.Yan S, Zhang H, Xie Y, Sheng W, Xiang J, Ye Z, Chen W, Yang J. Recombinant human interleukin-24 suppresses gastric carcinoma cell growth in vitro and in vivo. Cancer Invest. 2010;28:85–93. doi: 10.3109/07357900903095672. [DOI] [PubMed] [Google Scholar]
- 20.Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther. 2010;17:447–456. doi: 10.1038/cgt.2009.91. [DOI] [PubMed] [Google Scholar]
- 21.Wei N, Fan JK, Gu JF, Liu XY. Double-regulated oncolytic adenovirus-mediated interleukin-24 overexpression exhibits potent antitumor activity on gastric adenocarcinoma. Hum Gene Ther. 2010;21:855–864. doi: 10.1089/hum.2009.207. [DOI] [PubMed] [Google Scholar]
- 22.Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, Dmitriev IP, Lesniak MS, Shah K, Grant S, Curiel DT, Fisher PB, Dent P. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18:1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, Yacoub A, Curiel DT, Fisher PB, Dent P. Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol Ther. 2010;10:1290–1305. doi: 10.4161/cbt.10.12.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, Sarkar S, Wang XY, Hedvat M, Dmitriev IP, Curiel DT, Grant S, Dent P, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol. 2012;227:2145–2153. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar S, Quinn BA, Shen XN, Dash R, Das SK, Emdad L, Klibanov AL, Wang XY, Pellecchia M, Sarkar D, Fisher PB. Therapy of prostate cancer using a novel cancer terminator virus and a small molecule BH-3 mimetic. Oncotarget. 2015;6:10712–10727. doi: 10.18632/oncotarget.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, Rahmani M, Wei J, Hedvat M, Dent P, Dmitriev IP, Curiel DT, Grant S, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci U S A. 2011;108:8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–37. [PubMed] [Google Scholar]
- 28.Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P, Fisher PB. mda-7/IL-24: exploiting cancer's Achilles' heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, Senzer N, Nemunaitis J. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007;224:300–307. doi: 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther. 2008;8:1633–1643. doi: 10.1517/14712598.8.10.1633. [DOI] [PubMed] [Google Scholar]
- 33.Leng RX, Pan HF, Tao JH, Ye DQ. IL-19, IL-20 and IL-24: potential therapeutic targets for autoimmune diseases. Expert Opin Ther Targets. 2011;15:119–126. doi: 10.1517/14728222.2011.534461. [DOI] [PubMed] [Google Scholar]
- 34.Sauane M, Gupta P, Lebedeva IV, Su ZZ, Sarkar D, Randolph A, Valerie K, Gopalkrishnan RV, Fisher PB. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” antitumor activity. Cancer Res. 2006;66:11869–11877. doi: 10.1158/0008-5472.CAN-06-1887. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Kang X, Shi L, Li J, Xu W, Qian H, Wu M, Yin Z. mda-7/IL-24 induces apoptosis in human HepG2 hepatoma cells by endoplasmic reticulum stress. Oncol Rep. 2008;20:437–442. [PubMed] [Google Scholar]
- 36.Pataer A, Hu W, Xiaolin L, Chada S, Roth JA, Hunt KK, Swisher SG. Adenoviral endoplasmic reticulum-targeted mda-7/interleukin-24 vector enhances human cancer cell killing. Mol Cancer Ther. 2008;7:2528–2535. doi: 10.1158/1535-7163.MCT-08-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, Curiel DT, Grant S, Pellecchia M, et al. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010;70:5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahmani M, Mayo M, Dash R, Sokhi UK, Dmitriev IP, Sarkar D, Dent P, Curiel DT, Fisher PB, Grant S. Melanoma differentiation associated gene-7/interleukin-24 potently induces apoptosis in human myeloid leukemia cells through a process regulated by endoplasmic reticulum stress. Mol Pharmacol. 2010;78:1096–1104. doi: 10.1124/mol.110.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadife N, Nemos C, Frippiat JP, Hamade T, Perrot A, Dalloul A. Interleukin-24 mediates apoptosis in human B-cells through early activation of cell cycle arrest followed by late induction of the mitochondrial apoptosis pathway. Leuk Lymphoma. 2013;54:587–597. doi: 10.3109/10428194.2012.717079. [DOI] [PubMed] [Google Scholar]
- 40.Park MA, Hamed HA, Mitchell C, Cruickshanks N, Dash R, Allegood J, Dmitriev IP, Tye G, Ogretmen B, Spiegel S, Yacoub A, Grant S, Curiel DT, et al. A serotype 5/3 adenovirus expressing MDA-7/IL-24 infects renal carcinoma cells and promotes toxicity of agents that increase ROS and ceramide levels. Mol Pharmacol. 2011;79:368–380. doi: 10.1124/mol.110.069484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70:3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus WC, Grant S, Curiel DT, Fisher PB, et al. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010;70:1120–1129. doi: 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xuan W, Li YJ, Liu G, Ben-David Y, Archer MC. Interleukin-24 induces expression of beta4 integrin but suppresses anchorage-independent growth of rat mammary tumor cells by a mechanism that is independent of beta4. Mol Cancer Res. 2009;7:433–42. doi: 10.1158/1541-7786.MCR-08-0252. [DOI] [PubMed] [Google Scholar]
- 44.Li YJ, Liu G, Li Y, Vecchiarelli-Federico LM, Liu JC, Zacksenhaus E, Shan SW, Yang BB, Li Q, Dash R, Fisher PB, Archer MC, Ben-David Y. mda-7/IL-24 expression inhibits breast cancer through upregulation of growth arrest-specific gene 3 (gas3) and disruption of beta1 integrin function. Mol Cancer Res. 2013;11:593–603. doi: 10.1158/1541-7786.MCR-12-0496. [DOI] [PubMed] [Google Scholar]
- 45.Mhashilkar AM, Stewart AL, Sieger K, Yang HY, Khimani AH, Ito I, Saito Y, Hunt KK, Grimm EA, Roth JA, Meyn RE, Ramesh R, Chada S. MDA-7 negatively regulates the beta-catenin and PI3K signaling pathways in breast and lung tumor cells. Mol Ther. 2003;8:207–219. doi: 10.1016/s1525-0016(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 46.McKenzie T, Liu Y, Fanale M, Swisher SG, Chada S, Hunt KK. Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neu-overexpressing breast cancer cells. Surgery. 2004;136:437–442. doi: 10.1016/j.surg.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, Vorburger SA, Pataer A, Swisher SG, Ramesh R, Kawase K, Meyn RE, Hunt KK. mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: correlation with expression of bcl-2 family members. Cancer Gene Ther. 2006;13:490–502. doi: 10.1038/sj.cgt.7700915. [DOI] [PubMed] [Google Scholar]
- 48.Bocangel D, Zheng M, Mhashilkar A, Liu Y, Ramesh R, Hunt KK, Chada S. Combinatorial synergy induced by adenoviral-mediated mda-7 and Herceptin in Her-2+ breast cancer cells. Cancer Gene Ther. 2006;13:958–968. doi: 10.1038/sj.cgt.7700972. [DOI] [PubMed] [Google Scholar]
- 49.Bhutia SK, Das SK, Azab B, Menezes ME, Dent P, Wang XY, Sarkar D, Fisher PB. Targeting breast cancer-initiating/stem cells with melanoma differentiation-associated gene-7/interleukin-24. Int J Cancer. 2013;133:2726–2736. doi: 10.1002/ijc.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukumar S, Notario V, Martin-Zanca D, Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983;306:658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- 51.Shockett P, Schatz D. Inducible gene expression using an autoregulatory, tetracycline-controlled system. Curr Protoc Mol Biol. 2002;Chapter 16(Unit 16):4. doi: 10.1002/0471142727.mb1614s60. [DOI] [PubMed] [Google Scholar]
- 52.Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, Chodosh LA. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. Faseb J. 2002;16:283–292. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- 53.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, and Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, Attardi LD. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol. 2003;13:1985–1990. doi: 10.1016/j.cub.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 55.Li YJ, Song R, Korkola JE, Archer MC, Ben-David Y. Cyclin D1 is necessary but not sufficient for anchorage-independent growth of rat mammary tumor cells and is associated with resistance of the Copenhagen rat to mammary carcinogenesis. Oncogene. 2003;22:3452–3462. doi: 10.1038/sj.onc.1206411. [DOI] [PubMed] [Google Scholar]
- 56.Sarkar D, Su ZZ, Vozhilla N, Park ES, Randolph A, Valerie K, Fisher PB. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- 57.Panneerselvam J, Shanker M, Jin J, Branch CD, Muralidharan R, Zhao YD, Chada S, Munshi A, Ramesh R. Phosphorylation of interleukin (IL)-24 is required for mediating its anti-cancer activity. Oncotarget. 2015;6:16271–86. doi: 10.18632/oncotarget.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001;98:10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebedeva IV, Su ZZ, Sarkar D, Gopalkrishnan RV, Waxman S, Yacoub A, Dent P, Fisher PB. Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene. 2005;24:585–596. doi: 10.1038/sj.onc.1208183. [DOI] [PubMed] [Google Scholar]
- 60.Lebedeva IV, Sarkar D, Su ZZ, Gopalkrishnan RV, Athar M, Randolph A, Valerie K, Dent P, Fisher PB. Molecular target-based therapy of pancreatic cancer. Cancer Res. 2006;66:2403–2413. doi: 10.1158/0008-5472.CAN-05-3510. [DOI] [PubMed] [Google Scholar]
- 61.Lebedeva IV, Washington I, Sarkar D, Clark JA, Fine RL, Dent P, Curiel DT, Turro NJ, Fisher PB. Strategy for reversing resistance to a single anticancer agent in human prostate and pancreatic carcinomas. Proc Natl Acad Sci U S A. 2007;104:3484–3489. doi: 10.1073/pnas.0700042104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB. Chemoprevention by perillyl alcohol coupled with viral gene therapy reduces pancreatic cancer pathogenesis. Mol Cancer Ther. 2008;7:2042–2050. doi: 10.1158/1535-7163.MCT-08-0245. [DOI] [PubMed] [Google Scholar]
- 63.Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB. Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/interleukin-24 and perillyl alcohol. Cancer Res. 2008;68:7439–7447. doi: 10.1158/0008-5472.CAN-08-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park MA, Zhang G, Mitchell C, Rahmani M, Hamed H, Hagan MP, Yacoub A, Curiel DT, Fisher PB, Grant S, Dent P. Mitogen-activated protein kinase kinase 1/2 inhibitors and 17-allylamino-17-demethoxygeldanamycin synergize to kill human gastrointestinal tumor cells in vitro via suppression of c-FLIP-s levels and activation of CD95. Mol Cancer Ther. 2008;7:2633–2648. doi: 10.1158/1535-7163.MCT-08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hedvat M, Emdad L, Das SK, Kim K, Dasgupta S, Thomas S, Hu B, Zhu S, Dash R, Quinn BA, Oyesanya RA, Kegelman TP, Sokhi UK, et al. Selected approaches for rational drug design and high throughput screening to identify anti-cancer molecules. Anticancer Agents Med Chem. 2012;12:1143–1155. doi: 10.2174/187152012803529709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarkar S, Azab B, Quinn BA, Shen X, Dent P, Klibanov AL, Emdad L, Das SK, Sarkar D, Fisher PB. Chemoprevention gene therapy (CGT) of pancreatic cancer using perillyl alcohol and a novel chimeric serotype cancer terminator virus. Curr Mol Med. 2014;14:125–140. doi: 10.2174/1566524013666131118110827. [DOI] [PubMed] [Google Scholar]
- 67.Sarkar S, Quinn BA, Shen X, Dent P, Das SK, Emdad L, Sarkar D, Fisher PB. Reversing translational suppression and induction of toxicity in pancreatic cancer cells using a chemoprevention gene therapy approach. Mol Pharmacol. 2015;87:286–295. doi: 10.1124/mol.114.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinn BA, Dash R, Sarkar S, Azab B, Bhoopathi P, Das SK, Emdad L, Wei J, Pellecchia M, Sarkar D, Fisher PB. Pancreatic cancer combination therapy using a BH3 mimetic and a synthetic tetracycline. Cancer Res. 2015;75:2305–2315. doi: 10.1158/0008-5472.CAN-14-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawabe S, Nishikawa T, Munshi A, Roth JA, Chada S, Meyn RE. Adenovirus-mediated mda-7 gene expression radiosensitizes non-small cell lung cancer cells via TP53-independent mechanisms. Mol Ther. 2002;6:637–644. [PubMed] [Google Scholar]
- 70.Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P, Fisher PB. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 71.Xue XB, Zheng JW, Wang CJ, Chen K, Hu HY, Hu H, Yu Y, Wu ZD. Adenovirus vector expressing MDA-7/IL-24 selectively induces growth arrests and apoptosis in human hepatocellular carcinoma cell lines independent of the state of p53 gene. Zhonghua Gan Zang Bing Za Zhi. 2006;14:670–675. [PubMed] [Google Scholar]
- 72.Menezes ME, Shen XN, Das SK, Emdad L, Guo C, Yuan F, Li YJ, Archer MC, Zacksenhaus E, Windle JJ, Subler MA, Ben-David Y, Sarkar D, Wang XY, Fisher PB. MDA-7/IL-24 functions as a tumor suppressor gene in vivo in transgenic mouse models of breast cancer. Oncotarget. 2015 Oct 12; doi: 10.18632/oncotarget.6047. doi: 10.18632[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu JC, Voisin V, Bader GD, Deng T, Pusztai L, Symmans WF, Esteva FJ, Egan SE, Zacksenhaus E. Seventeen-gene signature from enriched Her2/Neu mammary tumor-initiating cells predicts clinical outcome for human HER2+:ERalpha- breast cancer. Proc Natl Acad Sci U S A. 2012;109:5832–5837. doi: 10.1073/pnas.1201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res. 2007;67:8671–8681. doi: 10.1158/0008-5472.CAN-07-1486. [DOI] [PubMed] [Google Scholar]
- 75.Reczek EE, Flores ER, Tsay AS, Attardi LD, Jacks T. Multiple responses elements and differential p53 binding control Perp expression during apoptosis. Mol Cancer Res. 2003;1:1048–57. [PubMed] [Google Scholar]
- 76.Li YJ, Zhao X, Vecchiarelli-Federico LM, Li Y, Datti A, Cheng Y, Ben-David Y. Drug-mediated inhibition of Fli-1 for the treatment of leukemia. Blood cancer journal. 2012;2:e54. doi: 10.1038/bcj.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]