Abstract
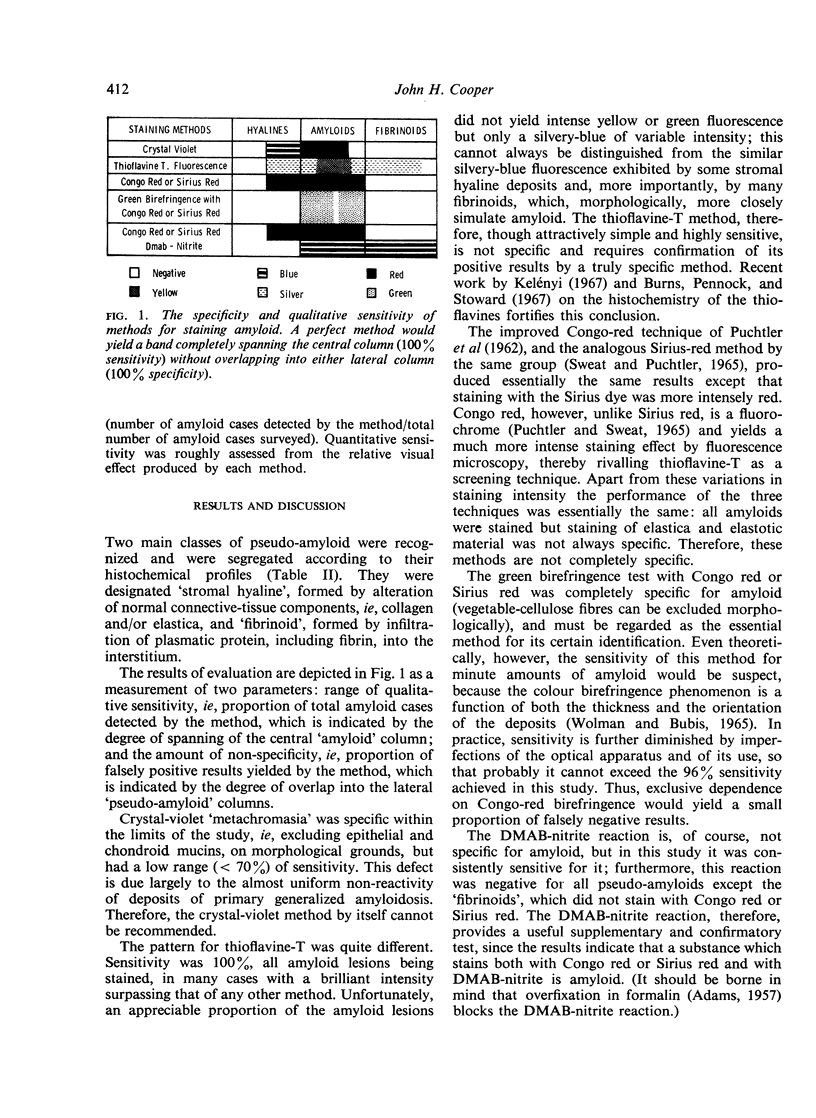
Six current histological methods for demonstrating amyloid (crystal violet, thioflavine-T fluorescence, Congo-red staining and fluorescence, Sirius-red staining, and Congo- or Sirius-red birefringence) were applied in 25 cases of amyloidosis of various types and 47 pseudo-amyloid lesions. The results were compared and were correlated with those of ancillary histochemical tests and clinico-pathological data and each method's sensitivity and specificity for amyloid was evaluated. Thioflavine-T and, to a lesser degree, Congo-red fluorescence and Sirius-red staining proved very sensitive but not specific. Green birefringence with Congo or Sirius red was specific but not completely sensitive. The coexistence of Congo-red (and Sirius-red) staining and a positive DMAB-nitrite reaction occurred in all amyloid specimens and appeared specific for amyloid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRNETT R. J., SELIGMAN A. M. Demonstration of protein-bound sulfhydryl and disulfide groups by two new histochemical methods. J Natl Cancer Inst. 1952 Aug;13(1):215–216. [PubMed] [Google Scholar]
- Burns J., Pennock C. A., Stoward P. J. The specificity of the staining of amyloid deposits with thioflavine T. J Pathol Bacteriol. 1967 Oct;94(2):337–344. doi: 10.1002/path.1700940211. [DOI] [PubMed] [Google Scholar]
- Cohen A. S. The constitution and genesis of amyloid. Int Rev Exp Pathol. 1965;4:159–243. [PubMed] [Google Scholar]
- GLENNER G. G., LILLIE R. D. Observations on the diazotization-coupling reaction for the histochemical demonstration of tyrosine: metal chelation and formazan variants. J Histochem Cytochem. 1959 Nov;7:416–422. doi: 10.1177/7.6.416. [DOI] [PubMed] [Google Scholar]
- KRAMER H., WINDRUM G. M. The metachromatic staining reaction. J Histochem Cytochem. 1955 May;3(3):227–237. doi: 10.1177/3.3.227. [DOI] [PubMed] [Google Scholar]
- MISSMAHL H. P. Polarisationsopticher Beitrag zur Kongorotfärbung des Amyloid. Z Wiss Mikrosk. 1957 Jan;63(3):133–139. [PubMed] [Google Scholar]
- Nebut M., Hartmann L. Identification histolgique de la substance amyloïde par la thioflavine T en lumbière U.V. Ann Biol Clin (Paris) 1966 May-Jun;24(5):703–708. [PubMed] [Google Scholar]
- Puchtler H., Sweat F. Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem. 1965 Nov-Dec;13(8):693–694. doi: 10.1177/13.8.693. [DOI] [PubMed] [Google Scholar]
- ROGERS D. R. SCREENING FOR AMYLOID WITH THE THIOFLAVIN-T FLUORESCENT METHOD. Am J Clin Pathol. 1965 Jul;44:59–61. doi: 10.1093/ajcp/44.1.59. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ P. SENILE CEREBRAL, PANCREATIC INSULAR AND CARDIAC AMYLOIDOSIS. Trans N Y Acad Sci. 1965 Feb;27:393–413. doi: 10.1111/j.2164-0947.1965.tb02977.x. [DOI] [PubMed] [Google Scholar]
- Saeed S. M., Fine G. Thioflavin-T for amyloid detection. Am J Clin Pathol. 1967 May;47(5):588–593. doi: 10.1093/ajcp/47.5.588. [DOI] [PubMed] [Google Scholar]
- Sweat F., Puchtler H. Demonstration of amyloid with direct cotton dyes. Experiences with a new method for the selective staining of amyloid by sirius red F3BA and sirius supra scarlet GG-CF. Arch Pathol. 1965 Dec;80(6):613–620. [PubMed] [Google Scholar]
- VASSAR P. S., CULLING C. F. Fluorescent stains, with special reference to amyloid and connective tissues. Arch Pathol. 1959 Nov;68:487–498. [PubMed] [Google Scholar]
- Wolman M., Bubis J. J. The cause of the green polarization color of amyloid stained with Congo red. Histochemie. 1965 Jan 12;4(5):351–356. doi: 10.1007/BF00306246. [DOI] [PubMed] [Google Scholar]



