Abstract
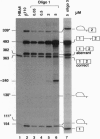
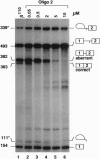
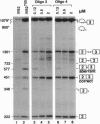
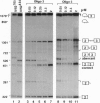
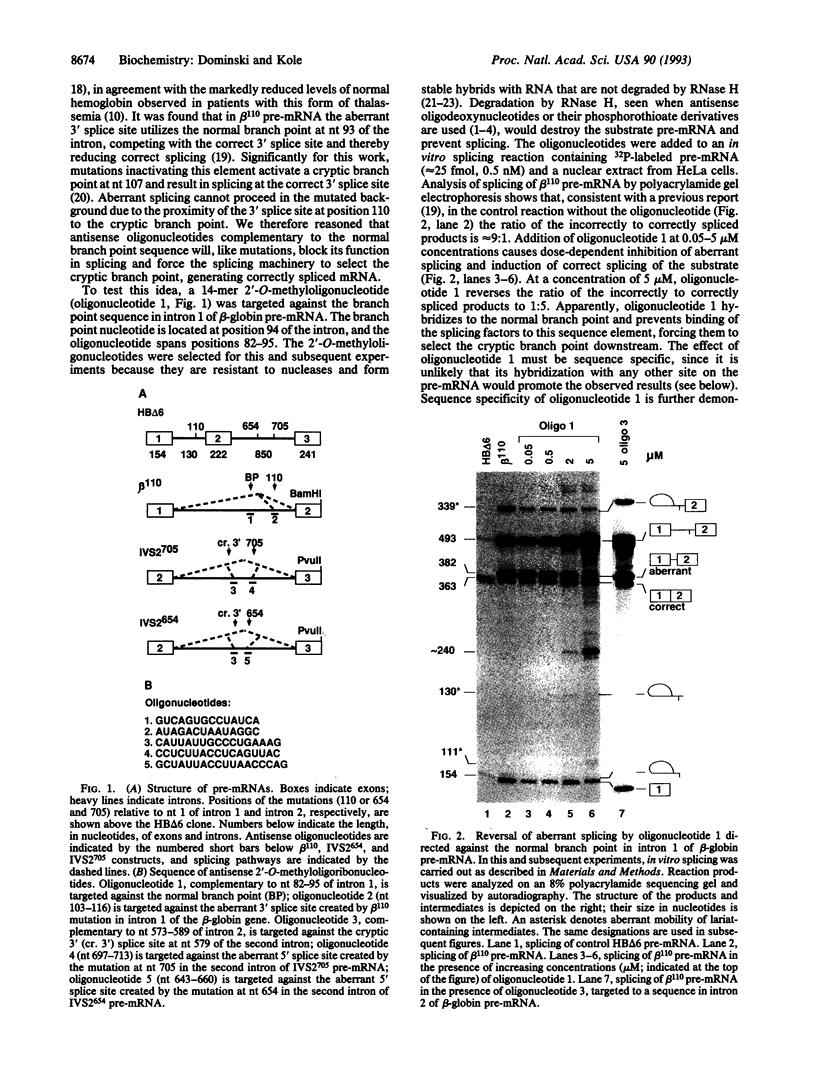
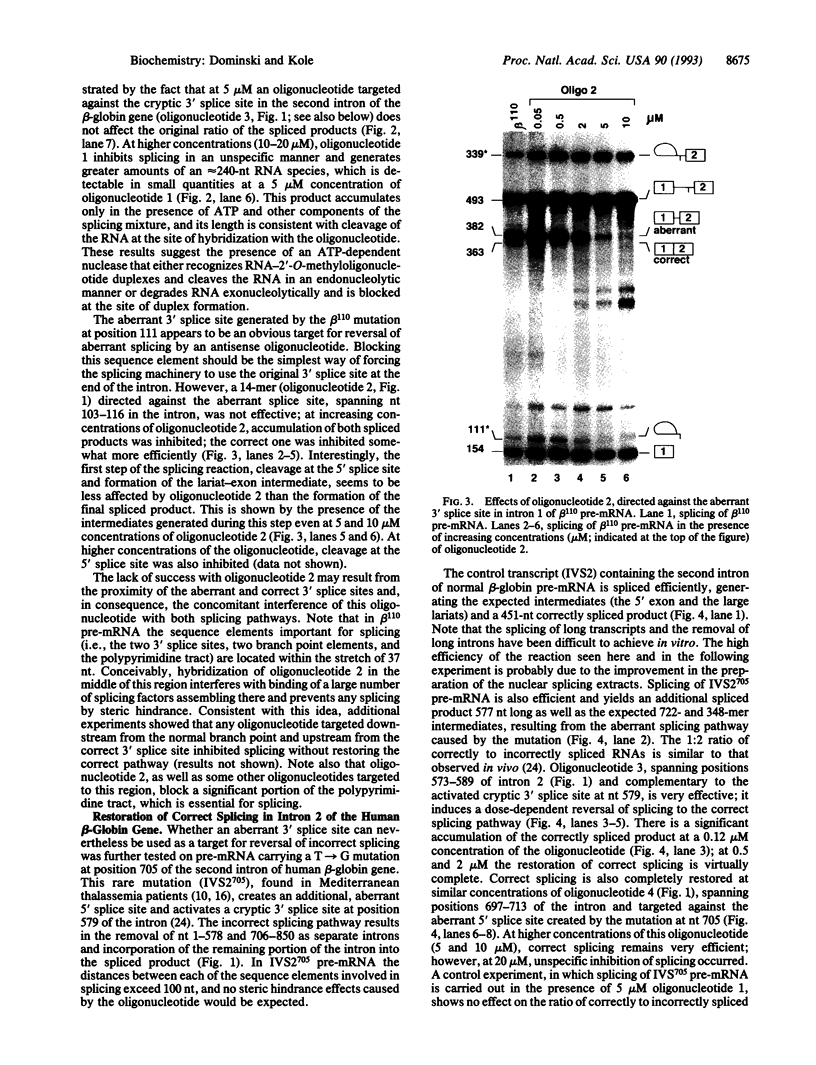
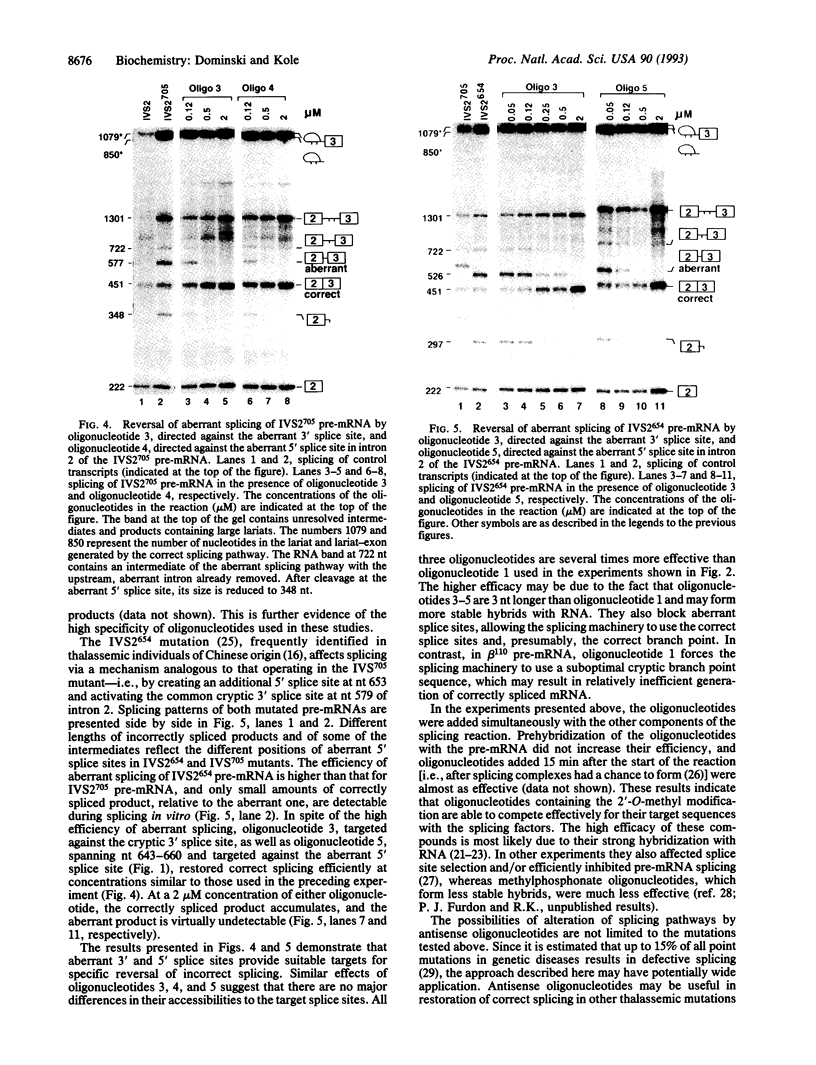
Antisense 2'-O-methylribooligonucleotides were targeted against specific sequence elements in mutated human beta-globin pre-mRNAs to restore correct splicing of these RNAs in vitro. The following mutations of the beta-globin gene, A-->G at nt 110 of the first intron (beta 110), T-->G at nt 705 and C-->T at nt 654 of the second intron (IVS2(705) and IVS2(654), respectively), which led to aberrant splicing of the corresponding pre-mRNAs, were previously identified as the underlying causes of beta-thalassemia. Aberrant splicing of beta 110 pre-mRNA was efficiently reversed by an oligonucleotide targeted against the branch point sequence in the first intron of the pre-mRNA but not by an oligonucleotide targeted against the aberrant 3' splice site. In both IVS2(705) and IVS2(654) pre-mRNAs, correct splicing was restored by oligonucleotides targeted against the aberrant 5' splice sites created by the mutations in the second intron or against a cryptic 3' splice site located upstream and activated in the mutated background. These experiments represent an approach in which antisense oligonucleotides are used to restore the function of a defective gene and not, as usual, to down-regulate the expression of an undesirable gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S. Antisense oligonucleotides as antiviral agents. Trends Biotechnol. 1992 May;10(5):152–158. doi: 10.1016/0167-7799(92)90203-8. [DOI] [PubMed] [Google Scholar]
- Akli S., Chelly J., Mezard C., Gandy S., Kahn A., Poenaru L. A "G" to "A" mutation at position -1 of a 5' splice site in a late infantile form of Tay-Sachs disease. J Biol Chem. 1990 May 5;265(13):7324–7330. [PubMed] [Google Scholar]
- Bennett C. F., Chiang M. Y., Chan H., Shoemaker J. E., Mirabelli C. K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1992 Jun;41(6):1023–1033. [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Cheng T. C., Orkin S. H., Antonarakis S. E., Potter M. J., Sexton J. P., Markham A. F., Giardina P. J., Li A., Kazazian H. H., Jr beta-Thalassemia in Chinese: use of in vivo RNA analysis and oligonucleotide hybridization in systematic characterization of molecular defects. Proc Natl Acad Sci U S A. 1984 May;81(9):2821–2825. doi: 10.1073/pnas.81.9.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G., Leonetti J. P., Milhaud P., Mechti N., Lebleu B. Antisense inhibitors of HIV: problems and perspectives. Antiviral Res. 1992 Apr;17(4):279–287. doi: 10.1016/0166-3542(92)90023-x. [DOI] [PubMed] [Google Scholar]
- Dobkin C., Bank A. Reversibility of IVS 2 missplicing in a mutant human beta-globin gene. J Biol Chem. 1985 Dec 25;260(30):16332–16337. [PubMed] [Google Scholar]
- Dominski Z., Kole R. Cooperation of pre-mRNA sequence elements in splice site selection. Mol Cell Biol. 1992 May;12(5):2108–2114. doi: 10.1128/mcb.12.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworniczak B., Aulehla-Scholz C., Kalaydjieva L., Bartholomé K., Grudda K., Horst J. Aberrant splicing of phenylalanine hydroxylase mRNA: the major cause for phenylketonuria in parts of southern Europe. Genomics. 1991 Oct;11(2):242–246. doi: 10.1016/0888-7543(91)90129-3. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- Ghosh M. K., Cohen J. S. Oligodeoxynucleotides as antisense inhibitors of gene expression. Prog Nucleic Acid Res Mol Biol. 1992;42:79–126. doi: 10.1016/s0079-6603(08)60574-7. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Antisense antivirals. Antisense Res Dev. 1991 Winter;1(4):361–364. doi: 10.1089/ard.1991.1.361. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Sohn R. L., Buckler A. J., Pelletier J., Call K. M., Housman D. E. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanvey J. C., Peffer N. J., Bisi J. E., Thomson S. A., Cadilla R., Josey J. A., Ricca D. J., Hassman C. F., Bonham M. A., Au K. G. Antisense and antigene properties of peptide nucleic acids. Science. 1992 Nov 27;258(5087):1481–1485. doi: 10.1126/science.1279811. [DOI] [PubMed] [Google Scholar]
- Huisman T. H. Frequencies of common beta-thalassaemia alleles among different populations: variability in clinical severity. Br J Haematol. 1990 Aug;75(4):454–457. doi: 10.1111/j.1365-2141.1990.tb07781.x. [DOI] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Iwai S., Ohtsuka E. Sequence-dependent hydrolysis of RNA using modified oligonucleotide splints and RNase H. FEBS Lett. 1987 May 11;215(2):327–330. doi: 10.1016/0014-5793(87)80171-0. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Kulka M., Smith C. C., Aurelian L., Fishelevich R., Meade K., Miller P., Ts'o P. O. Site specificity of the inhibitory effects of oligo(nucleoside methylphosphonate)s complementary to the acceptor splice junction of herpes simplex virus type 1 immediate early mRNA 4. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6868–6872. doi: 10.1073/pnas.86.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J., Sun D., Klotman M., Agrawal S., Zamecnik P., Gallo R. Specific inhibition of human immunodeficiency virus type 1 replication by antisense oligonucleotides: an in vitro model for treatment. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11209–11213. doi: 10.1073/pnas.89.23.11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Oligonucleotide-directed DNA triple-helix formation: an approach to artificial repressors? Antisense Res Dev. 1991 Fall;1(3):277–281. [PubMed] [Google Scholar]
- Mayeda A., Hayase Y., Inoue H., Ohtsuka E., Ohshima Y. Surveying cis-acting sequences of pre-mRNA by adding antisense 2'-O-methyl oligoribonucleotides to a splicing reaction. J Biochem. 1990 Sep;108(3):399–405. doi: 10.1093/oxfordjournals.jbchem.a123213. [DOI] [PubMed] [Google Scholar]
- Neckers L., Whitesell L., Rosolen A., Geselowitz D. A. Antisense inhibition of oncogene expression. Crit Rev Oncog. 1992;3(1-2):175–231. [PubMed] [Google Scholar]
- Rapaport E., Misiura K., Agrawal S., Zamecnik P. Antimalarial activities of oligodeoxynucleotide phosphorothioates in chloroquine-resistant Plasmodium falciparum. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8577–8580. doi: 10.1073/pnas.89.18.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Sameshima Y., Akiyama T., Mori N., Mizoguchi H., Toyoshima K., Sugimura T., Terada M., Yokota J. Point mutation of the p53 gene resulting in splicing inhibition in small cell lung carcinoma. Biochem Biophys Res Commun. 1990 Dec 14;173(2):697–703. doi: 10.1016/s0006-291x(05)80091-9. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Lamond A. I., Beijer B., Neuner P., Ryder U. Highly efficient chemical synthesis of 2'-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989 May 11;17(9):3373–3386. doi: 10.1093/nar/17.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui L. C. The spectrum of cystic fibrosis mutations. Trends Genet. 1992 Nov;8(11):392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in human U2 snRNA can suppress a branch site mutation. Genes Dev. 1989 Oct;3(10):1545–1552. doi: 10.1101/gad.3.10.1545. [DOI] [PubMed] [Google Scholar]