Abstract
Background
Immunosuppressive therapy may impact cancer risk in inflammatory bowel disease (IBD). Cancer specific data regarding risk and outcome are scarce and data for renal cell carcinoma (RCC) are lacking. We aimed(1) to identify risk factors for RCC development in IBD patients (2) to compare RCC characteristics, outcome and survival between IBD patients and the general population.
Methods
A PALGA (Dutch Pathology Registry) search was performed to establish a case group consisting of all IBD patients with incident RCC in The Netherlands (1991–2013). Cases were compared with two separate control groups: (A) with a population-based IBD cohort for identification of risk factors (B) with a RCC cohort from the general population to compare RCC characteristics and outcomes.
Results
180 IBD patients with RCC were identified. Pancolitis (OR 1.8–2.5), penetrating Crohn's disease (OR 2.8), IBD related surgery (OR 3.7–4.5), male gender (OR 3.2–5.0) and older age at IBD onset (OR 1.0–1.1) were identified as independent risk factors for RCC development. IBD patients had a significantly lower age at RCC diagnosis (p < 0.001), lower N-stage (p = 0.025), lower M-stage (p = 0.020) and underwent more frequently surgical treatment for RCC (p < 0.001) compared to the general population. This translated into a better survival (p = 0.026; HR 0.7) independent of immunosuppression.
Conclusions
IBD patients with a complex phenotype are at increased risk to develop RCC. They are diagnosed with RCC at a younger age and at an earlier disease stage compared to the general population. This translates into a better survival independent of immunosuppressive or anti-TNFα therapy.
Keywords: inflammatory bowel disease, renal cell carcinoma, immunosuppressive therapy
INTRODUCTION
Inflammatory bowel disease (IBD), including ulcerative colitis (UC), Crohn's disease (CD) and indeterminate colitis is a chronic inflammatory disorder of the gastrointestinal tract. Patients with this disease have an increased risk for both intestinal and various extra-intestinal malignancies [1, 2]. This risk is mainly attributed to two drivers: chronic inflammation and drug-induced immunosuppression [3]. Particularly immunosuppressive medication such as thiopurines and methotrexate may play a role in the development of extra-intestinal malignancies by impairing immunosurveillance of tumor cells or inducing DNA damage [4–7]. The potential associated cancer risk is an important growing concern given the need for prolonged immunosuppressive therapy in IBD, especially in view of the aging IBD population.
Various extra-intestinal malignancies, such as lymphoproliferative disorders and non-melanoma skin cancers occur more frequently in IBD patients compared to the general population, mainly in those using immunosuppression [2, 6, 8–12]. Although only limited evidence is available, it has been suggested that immunosuppression in IBD patients may increase the risk for a variety of solid malignancies, such as renal cell carcinoma (RCC). Indeed, RCC occurs more frequently in post-transplantation patients exposed to immunosuppressive medication [13]. In addition, the risk for urinary tract cancers in IBD patients on thiopurines seems to be elevated [3].
It is unknown whether and how IBD therapy impacts risk of cancer recurrence, outcome and survival. Aggregate data failed to demonstrate an effect of immunosuppression and anti-TNFα agents on recurrence of any cancer in IBD patients [5]. By contrast, cancer specific data on recurrence and outcome are scarce. For example, only eight case reports of IBD patients with RCC have been described and led to speculation on a putative association with immunosuppressive therapy [14–19]. As such, more data are needed to estimate RCC risk and to guide the subsequent individual IBD therapy.
To this end we established a nationwide cohort of IBD patients with incident RCC. We had a dual aim: (1) to identify risk factors for RCC development, and in particular to investigate the impact of IBD therapy on RCC development (2) to compare RCC characteristics, outcome and survival between IBD patients and the general population, including the impact of immunosuppression and anti-TNFα agents.
RESULTS
Patient identification
We identified 180 IBD patients who developed RCC in 69 of 87 hospital organizations in the Netherlands (Figure 1) [20]. Twenty-seven potential cases could not be verified and were excluded. To identify risk factors for RCC development we established a control group of 1800 IBD patients randomly selected from the IBD South Limburg Cohort (IBDSL; Case control study A). For the comparison of RCC characteristics and outcomes we identified a second control group using the Eindoven Cancer Registry (ECR). This search yielded 4388 patients with RCC in the general population (Case control study B).
Figure 1. Patient inclusion flowchart.
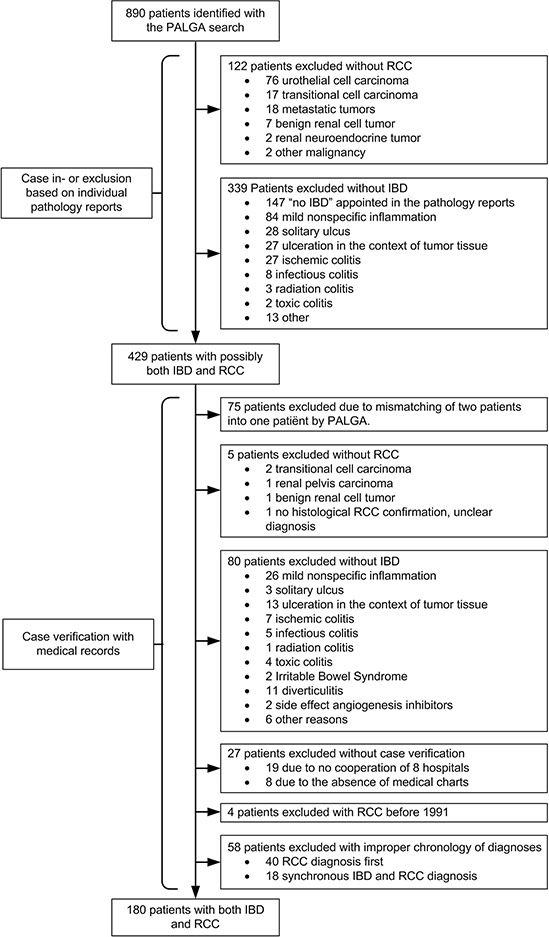
IBD, inflammatory bowel disease; RCC, renal cell carcinoma
Risk factors for RCC development - Case control study A
Potential clinical and demographic risk factors for RCC development were compared univariable between IBD cases who developed RCC and IBDSL control patients (Table 1). Male gender, Montreal E3 pancolitis, perianal disease activity, a stricturing and/or penetrating CD phenotype, and IBD related surgery occurred statistically significantly more frequent in the case group (p < 0.001 for all comparisons). Furthermore, cases had a statistically significantly longer duration of follow up since IBD diagnosis (p < 0.001), but used less thiopurines (p = 0.047) and anti-TNFα agents (p = 0.006) during follow up. We considered differences in inclusion period (IBD diagnosis since 1950 (cases) versus IBD diagnosis since 1991 (controls)) as a reason for these differences, since widespread use of thiopurines and the introduction of anti-TNFα therapy occurred in the last decade of inclusion. Using similar inclusion periods of IBD diagnosis for both cases and controls (since 1991) almost abolished treatment differences (5-aminosalicylic acids (5-ASA), 89.6% (cases) versus 89.8% (controls), p = 0.954; thiopurines, 35.6% versus 40.2%, p = 0.432; methotrexate, 0.0% versus 5.3%, p = 0.049; cyclosporine, 4.1% versus 1.5%, p = 0.102; anti-TNFα therapy, 15.1% versus 19.7%, p = 0.326).
Table 1. Univariable comparison of potential risk factors and confounders between cases (IBD patients who developed RCC) and controls (randomly selected IBD patients from IBDSL) for the identification of risk factors to develop RCC (case control study A).
| Variable | IBD and RCC cases (n = 180) | IBDSL (n = 1800) | Missing values (cases/IBDSL) | P-value |
|---|---|---|---|---|
| Male gender, n (%) | 114 (63.3) | 837 (46.5) | 0 | <0.001 |
| Ever smokeda, n (%) | 38 (62.3) | 421 (62.5) | 11/122 | 0.979 |
| Age at IBD diagnosis(y), median | 43 | 39 | 3/1 | 0.106 |
|
IBD typeb Ulcerative colitis, n (%) Crohn's disease, n (%) |
93 (56.4) 72 (43.6) |
1004 (55.8) 796 (44.2) |
0 |
0.885 |
|
Ulcerative colitis Extend Proctitis (E1), n (%) Left-sided colitis (E2), n (%) Pancolitis (E3), n (%) |
14 (17.5) 24 (30.0) 42 (52.5) |
243 (24.4) 472 (47.5) 279 (28.1) |
10/13 |
<0.001 |
|
Crohn's disease Extend Ileum (L1), n (%) Colon (L2), n (%) Ileocolonic (L3), n (%) Upper digestive (L4), n (%) Perianal disease activity, n (%) Phenotype Non stricturing/penetrating (B1), n (%) Stricturing (B2), n (%) Penetrating (B3), n (%) Stricturing and penetrating, n (%) |
27 (38.6) 19 (27.1) 24 (34.3) 2 (2.8) 21 (30.0) 19 (27.9) 16 (23.5) 15 (22.1) 18 (26.5) |
223 (28.1) 183 (23.0) 389 (48.9) 65 (8.2) 119 (14.9) 437 (54.9) 171 (21.5) 96 (12.1) 92 (11.6) |
2/1 1/0 2/0 4/0 |
0.054 0.106 0.001 <0.001 |
|
Medical therapy prior to RCC diagnosis 5ASA, n (%) Thiopurines, n (%) Methotrexate, n (%) Cyclosporine, n (%) Anti-TNFα therapy, n (%) |
145 (94.2) 49 (32.0) 3 (2.0) 5 (3.3) 16 (10.5) |
1605 (89.8) 717 (40.2) 95 (5.3) 26 (1.1) 350 (19.7) |
26/13 27/17 29/10 27/10 28/25 |
0.083 0.047 0.074 0.091 0.006 |
| IBD related surgery, n (%) | 86 (48.0) | 508 (28.3) | 1/8 | <0.001 |
| Calendar year of IBD diagnosis, median | 1989 | 2003 | 3/1 | <0.001 |
| Duration of follow up since IBD diagnosis (y), median | 19 | 7 | 3/30 | <0.001 |
IBD, Inflammatory bowel disease; IBDSL, IBD South Limburg cohort; RCC, renal cell carcinoma.
Smoking data were only available for patients with Crohn's Disease
Indeterminate colitis was not considered in this comparison since these patients were excluded from IBDSL
A multivariable logistic regression model that took the duration of follow up since IBD diagnosis into account was made separately for UC and CD patients to identify independent risk factors for RCC development. Included variables were: gender, age at IBD diagnosis, extend of UC and CD, perianal disease activity, CD phenotype and IBD related surgery. As prescribed medical therapy might be different and/or not reliable in early years of inclusion, we did not include these variables in this model. Therefore, we performed a sensitivity analysis including patients with an IBD diagnosis since 1991 in both the case and control group. Medical therapy was included in this logistic regression model.
Table 2 shows the final logistic regression models after backward elimination of the non-significant variables for both UC and CD patients. Patients with a more complex phenotype including Montreal E3 UC (OR 1.8–2.5, 95% CI 1.0–5.3), penetrating CD (OR 2.8, 95% CI 1.3–5.8) and/or IBD related surgery (OR 3.7–4.5, 95% CI 1.6–8.2) were at increased risk for RCC development. Furthermore, male gender (OR 3.2–5.0, 95% CI 1.7–13.2) and older age at IBD diagnosis but not age by itself (OR 1.0–1.1, 95% CI 1.0–1.1) were identified as independent risk factors. Use of 5-ASA (OR 0.2, 95% CI 0.0–0.7) protected against RCC development.
Table 2. Final multivariable regression model for the identification of independent risk factors to develop RCC.
| Model | Variable | Coefficient β | Odds ratio (95% confidence interval) | P-value |
|---|---|---|---|---|
| Ulcerative colitis (all cases, n = 1061) | Male gender IBD related surgery Age at IBD diagnosis Montreal E3 colitisa |
1.169 1.499 0.023 0.598 |
3.218 (1.715–6.040) 4.477 (2.433–8.238) 1.023 (1.006–1.042) 1.818 (1.045–3.163) |
<0.001 <0.001 0.009 0.034 |
| Ulcerative colitis (sensitivity analysis, n = 1015) | Male gender IBD related surgery Age at IBD diagnosis Montreal E3 colitisa 5-ASA |
1.609 1.306 0.028 0.922 –1.746 |
4.999 (1.889–13.226) 3.692 (1.578–8.641) 1.029 (1.006–1.051) 2.513 (1.2005.262) 0.174 (0.044–0.687) |
0.001 0.003 0.011 0.015 0.013 |
| Crohn's disease (all cases, n = 845) | Age at IBD diagnosis Penetrating disease |
0.035 1.021 |
1.035 (1.014–1.057) 2.776 (1.320–5.836) |
0.001 0.007 |
| Crohn's disease (sensitivity analysis, n = 811) | Age at IBD diagnosis | 0.049 | 1.051 (1.028–1.074) | <0.001 |
Similar inclusion periods of IBD diagnosis (since 1991) for cases and controls were used in the sensitivity analysis (case control study A). IBD, inflammatory bowel disease; 5-ASA, 5-aminosalicylic acids.
Reference category is patients with Montreal E1 or E2 colitis
RCC characteristics and survival - Case control study B
Univariable comparisons of RCC characteristics between IBD cases and the general population are shown in Table 3. IBD patients had a statistically significantly lower age at RCC diagnosis (p < 0.001), lower N-stage (p = 0.025), lower M-stage (p = 0.020) and underwent more frequently surgical treatment for RCC (p < 0.001). This may be attributed to a high percentage of incidentally diagnosed cancers in the case group (n = 80/180, 51.3%).
Table 3. Univariable comparison of RCC characteristics between cases (IBD patients who developed RCC) and controls (RCC patients in the general population derived from ECR) (case control study B).
| Variable | IBD and RCC cases (n = 180) | ECR (n = 4388) | Missing values(cases/ECR) | P-value |
|---|---|---|---|---|
| Male gender, n (%) | 114 (63.3) | 2659 (60.6) | 0 | 0.461 |
| Age at RCC diagnosis (y), median | 62 (27–83) | 66 | 0 | <0.001 |
|
Location Left-sided, n (%) Right-sided, n (%) |
89 (50.6) 87 (49.4) |
2119 (48.7) 2230 (51.3) |
4/39 |
0.631 |
|
Grade 1–2, n (%) 3–4, n (%) |
88 (72.7) 33 (27.3) |
1214 (69.3) 539 (30.7) |
59/2635 |
0.442 |
|
T stage T1–T2, n (%) T3–T4, n (%) |
130 (76.9) 39 (23.1) |
2509 (70.9) 1032 (29.1) |
11/847 |
0.089 |
|
N stage N0, n (%) N+, n (%) |
160 (94.1) 10 (5.9) |
3281 (88.6) 423 (11.4) |
10/684 |
0.025 |
|
M stage M0, n (%) M1, n (%) |
155 (87.1) 23 (12.9) |
2962 (80.0) 742 (20.0) |
2/684 |
0.020 |
| Surgery, n (%) | 168 (93.9) | 3318 (75.6) | 1/0 | <0.001 |
| Calendar year of RCC diagnosis, median | 2003 | 2007 | 0 | <0.001 |
RCC, renal cell carcinoma; IBD, inflammatory bowel disease; ECR, Eindhoven cancer registry.
Figure 2 displays the overall survival curves of the case and control group. IBD patients had a statistically significant better overall survival compared to the general population (p < 0.001). However, age at RCC diagnosis, T-stage, M-stage, surgical treatment and calendar year of RCC diagnosis emerged as confounders in a Cox model. Adjusted for these confounders, a protective effect of IBD on overall survival was still present (p = 0.026; HR 0.7; 95% CI 0.5–1.0).
Figure 2. Overall survival curves of the general and IBD population following RCC diagnosis.
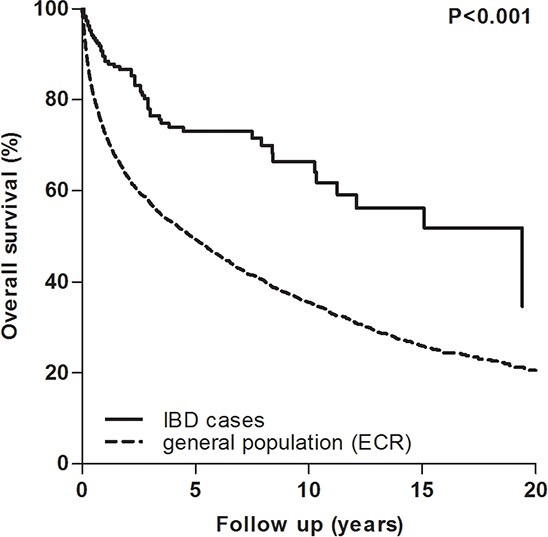
IBD, inflammatory bowel disease; ECR, Eindhoven cancer registry
RCC survival related to medical IBD therapy
Based on received IBD medication, we performed subgroup survival analysis for IBD cases with RCC. Patients who used immunosuppression (including thiopurines, methotrexate and cyclosporine) and/or anti-TNFα therapy before or after RCC diagnosis had a statistically significantly better disease free survival following RCC diagnosis compared to those who did not (Figure 3). Especially patients who were treated with immunosuppression and/or anti-TNFα agents after RCC diagnosis, showed a better disease free survival. However, a multivariable Cox analyses adjusted for the confounders TNM stage and age at RCC diagnosis, abolished this protective effect of immunosuppressive and anti-TNFα therapy (immunosuppression before RCC diagnosis, p = 0.946; immunosuppression after RCC diagnosis, p = 0.386; anti-TNFα therapy before RCC diagnosis, p = 0.673; anti-TNFα therapy after RCC diagnosis, p = 0.502). Similar survival curves were found for the effect of IBD therapy on overall survival (data not shown).
Figure 3. Disease free survival curves in IBD subgroups with RCC based on IBD medication received.
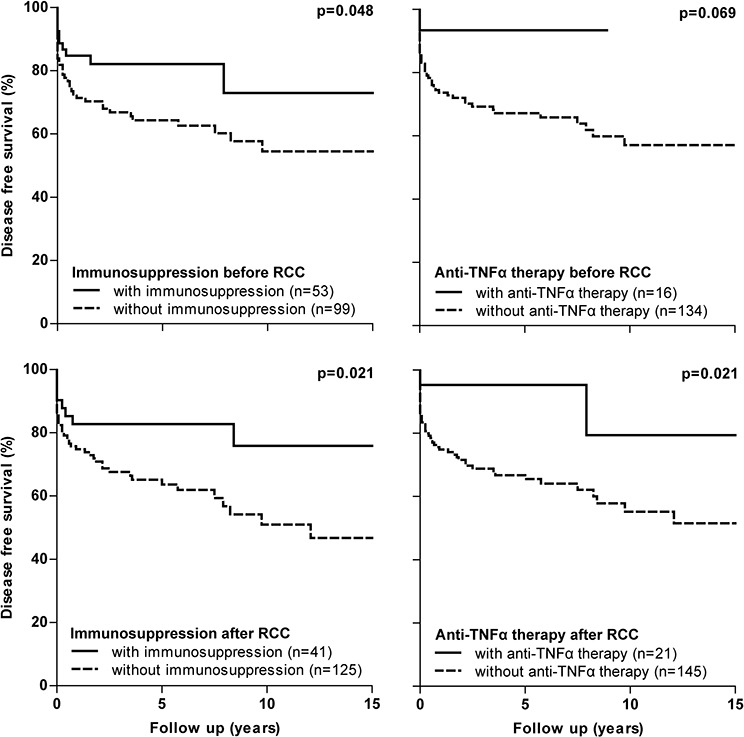
RCC, renal cell carcinoma
Following a similar strategy as for the identification of risk factors we performed a sensitivity analysis focusing on patients who carried an IBD diagnosis since 1991. We determined the effect of medical therapy on disease free and overall survival. All survival analyses and Cox models showed similar results as shown above (data not shown).
DISCUSSION
One of the key findings of our study is that IBD patients with a complex phenotype (including Montreal E3 UC, penetrating CD and/or IBD related surgery) are at increased risk to develop RCC. They are younger at diagnosis and carry a lower RCC stage compared to the general population. This translates into a better disease free and overall survival. The second key finding of our study is that immunosuppressive and anti-TNFα therapy does not adversely affect disease free and overall survival in IBD patients following RCC diagnosis.
A better survival in our IBD cohort with RCC may be caused by frequent abdominal imaging in these patients, which leads to incidental findings such as RCC. Due to the widespread use of imaging techniques, the incidental detection of RCC in the general population significantly increased in recent decades to approximately 40% [21–23]. This compares to 51% for incidentally detected RCC in our IBD cohort. Previous studies have shown that patients with these incidentally detected RCC are diagnosed at an earlier stage, which is translated into a better survival after correction for confounders (TNM stage, age at RCC diagnosis, calendar year) [22, 24]. This is in line with our study in which IBD patients (including a high proportion of incidentally diagnosed cancers) received earlier RCC diagnosis and had a better survival.
We found that patients with a more complex IBD phenotype are at increased risk to develop RCC. A more frequent and intensive use of the health care system, including abdominal imaging, may be associated with this phenomenon. Indeed, another study found that IBD patients exposed to anti-TNFα agents (generally prescribed for patients with a more complex IBD phenotype) developed RCC at a younger age and received earlier RCC surgery compared to IBD patients unexposed to anti-TNFα therapy or patients having rheumatoid arthritis [25]. Other risk factors for RCC development were male gender and older age at IBD onset (not age by itself), but not the use of medical therapy. One could hypothesize that with increasing age, potential cancer risk factors accumulate until IBD onset with subsequently accelerated carcinogenesis. As such, patients who develop IBD later in life are at increased risk to develop early colorectal cancer (<8 y) and more widespread colorectal neoplasia [26, 27]. The role of immunosuppression and/or anti-TNFα agents in relation to cancer development remains to be clarified as the literature reports contradictory results [3, 5, 28].
Results of our study demonstrated no adverse effect of immunosuppression and/or anti-TNFα therapy on both disease free and overall survival following RCC. These therapies were mainly (re)started or continued after RCC diagnosis in patients with low stage RCC and as a corollary these patients showed a better disease free and overall survival (Figure 3). For example, 32 out of 41 patients (82.1%) who used immunosuppressive therapy and 17 out of 21 patients (81.0%) with anti-TNFα therapy after RCC diagnosis had a T1 RCC. Adjustment for TNM stage abolished the protective effect of immunosuppressive and anti-TNFα therapy and no differences on survival were subsequently found. These findings are in line with the only available, prospective study in IBD patients, which showed no negative impact of immunosuppressive agents on recurrent cancer of any type [5]. Other data originates from observational studies including patients with rheumatoid arthritis or solid organ transplants. No difference in any new or recurrent malignancy incidence was observed in rheumatoid arthritis patients exposed or unexposed to anti-TNFα agents [7, 29]. A study in post-transplant setting demonstrated a recurrence rate of 0% for incidentally diagnosed RCC and of 30% for symptomatic RCC, although a formal comparison to a control group was lacking [30].
Despite concerns regarding a cancer inducing effect of anti-TNFα therapy, TNFα blockers have been previously considered as a therapeutic strategy for RCC [31, 32]. Previous studies showed that TNFα can act as an autocrine tumor growth factor and that its presence is associated with poor prognosis. Indeed, phase I/II trials in RCC demonstrated an anti-tumor effect of anti-TNFα treatment [32]. However, the most recent phase II trial in 2010 showed no beneficial effect of anti-TNFα therapy in metastatic RCC [31]. Similarly, results of our study did not show a better survival of metastatic RCC in patients treated with anti-TNFα agents (data not shown).
Our study has important clinical implications for the evidence-based management of IBD therapy in patients with a history of RCC. As no adverse effect of IBD therapy on disease free and overall survival was observed, our data suggest that these agents could be considered following RCC. Cancer specific data are lacking to date, although case-by-case management is encouraged based on the characteristics and expected evolution of the cancer, the probable impact of IBD therapy on cancer evolution, and IBD severity [4, 7]. The impact of dose, duration and time interval following RCC remains to be assessed in larger prospectively followed cohorts. In addition, more cancer specific data are needed for other types of cancer to develop individualized evidence-based management strategies in IBD patients with cancer.
The present study has several limitations. First, the retrospective nature of data collection could have influenced the completeness and accuracy of the extracted data. For example, medication use was difficult to ascertain from older medical records. To address this issue, we performed sensitivity analyses including patients with similar calendar years of IBD diagnosis or RCC diagnosis in the case and control group, disseminating missing values and errors across groups. Second, the use of multiple databases and registries resulted in different ways of data collection and the absence of some variables. For example, potential risk factors and confounders, such as smoking behavior, hypertension, body mass index and incidental detection of RCC, were not available in IBDSL or the ECR. Given this limitation some of our results need to be interpreted with caution. However, it was inevitable to use multiple databases to address our research questions. Finally, selection bias may have been introduced as we used different registries and databases. For example, cases were identified nationwide whereas controls with RCC and controls with IBD were ascertained from two different registries in the south of The Netherlands. However, previous studies confirmed that these population-based registries are representative of the total Dutch population [33].
In conclusion, we identified a complex IBD phenotype as a risk factor to develop RCC. IBD patients were diagnosed with RCC at a younger age and at an earlier disease stage compared to the general population, which translated into a better disease free and overall survival following RCC. Immunosuppressive and anti-TNFα therapy did not adversely affect this better survival. This observation may aid physicians in guiding IBD therapy following RCC diagnosis and treatment.
MATERIALS AND METHODS
Study design and data sources
In order to study risk factors and the clinical course of RCC in IBD patients, we performed two retrospective nationwide case control studies. We established a case group consisting of all IBD patients who developed RCC in The Netherlands assembled over 22 years, using PALGA (Dutch nationwide network and registry of histo- and cytopathology) [34]. Subsequently, these cases were included in the following two case control studies:
The first case control study aimed for the identification of risk factors to develop RCC. Controls were randomly sampled from IBDSL, a population-based IBD registry [35].
The second case control study was performed to compare RCC characteristics and outcomes between IBD patients and the general population. Controls were identified from the ECR and included patients from the general population who developed RCC [36].
The study was approved by the Privacy Commission and Scientific Council of PALGA and by the Medical Ethics Review Committee region Arnhem - Nijmegen (Registration number 2013/419).
Case identification
PALGA was searched in order to identify all IBD patients with concomitant RCC in The Netherlands from January 1991 until May 2013. The PALGA registry contains pathology reports generated in the Netherlands since 1971 and has complete national coverage since 1991 encompassing all pathology laboratories from all academic and non-academic hospitals in the Netherlands [34]. We performed a PALGA search with the following search terms: “ulcerative colitis”, “Crohn's disease”, “indeterminate colitis”, or “chronic idiopathic inflammatory bowel disease” combined with all “primary carcinomas of the kidney” or “metastasis of kidney cancer”. Cases were further confirmed or excluded after careful evaluation of the individual pathology reports and/or medical records (Figure 1).
All patients with UC, CD or indeterminate colitis who developed a histologically confirmed RCC following IBD diagnoses were included. The diagnosis of IBD was based on a combination of clinical, endoscopic, histological and radiographic criteria [37]. The following exclusion criteria were used: no diagnosis of IBD, no diagnosis of RCC, RCC diagnosis before IBD diagnosis and RCC diagnosis before 1991.
Controls (A)–IBD South limburg cohort
Controls for the identification of risk factors to develop RCC were randomly selected from IBDSL. IBDSL is a prospectively followed, population-based IBD registry in an area in the southeast of The Netherlands between Germany and Belgium, called South-Limburg. This area has a population of approximately 645.000 inhabitants with a low migration rate and covers one academic and two general district hospitals [38]. Adult patients in this area with a diagnosis of UC or CD based on a combination of endoscopic, radiologic and histological evidence are included in this cohort since 1991 [38]. It includes 2807 IBD patients (40.9% CD, 59.0% UC), which represents 93% of the regional IBD population [35]. We randomly included patients with an IBD diagnosis between 1991 and 2011. An unmatched study design was chosen given the relatively large number of cases, allowing adjustment for possible confounders as well as to avoid missing potential risk factors [39].
Controls (B)–Eindhoven cancer registry
Controls to compare RCC characteristics and outcomes were identified from the ECR, maintained by the Netherlands Comprehensive Cancer Organization. The registry prospectively collects data on all newly diagnosed cancers in the southern part of The Netherlands since 1955 [36]. This area includes 10 general hospitals and 6 regional pathology laboratories, comprising approximately 2.3 million inhabitants (15% of the Dutch population) [40]. Tumor characteristics and patient characteristics are routinely extracted from medical records by specially trained administrators of the cancer registry. By means of an independent case ascertainment method, the completeness of the registration is estimated to exceed 95%. [41] We included all patients with a histologically confirmed RCC between 1991 and 2010 from the ECR as controls.
Data extraction
Two authors (L.D and L.N) extracted demographic and clinical variables from anonymized medical records for patients included in the case group. Extracted data included gender, date of birth, smoking history (ever/never), primary sclerosing cholangitis, IBD characteristics and RCC characteristics. We collected the following IBD characteristics: type of IBD, date of diagnosis, phenotype according to the Montreal Classification, and medical and surgical treatment. Exposure to 5-ASA, thiopurines, anti-TNFα agents, cyclosporine or methotrexate was defined as “used” or “not used” since dosage/duration could not be reliably retrieved for all cases. RCC characteristics included: date of diagnosis, location, tumor stage according to the TNM classification (7th edition), differentiation grade according to Fuhrman [42], whether the tumor was incidentally detected or not, treatment, outcome and survival.
Incidentally diagnosed cancers were considered to be tumors discovered during investigations performed for reasons other than for RCC related symptoms including palpable tumor, haematuria (both macroscopic and microscopic), flank pain and signs of cachexia related to the disease [21]. RCC outcome included disease free survival (duration since RCC diagnosis until recurrence or death) and overall survival (duration since RCC diagnosis until death). Histopathological subtype could not be obtained reliably due to the great variability of morphology reporting standards since 1991.
Similar variables with corresponding definitions were extracted from registries (IBDSL and ECR) for patients included in the control groups.
Statistics
For both case control studies we compared potential risk factors, RCC characteristics and/or outcomes between cases and controls with a univariable analysis. X2 test or Fisher exact test (if expected cell counts were < 5) were used for categorical data and independent Student t test (if normally distributed) or Mann-Whitney U test (if not normally distributed) were used for continuous data. Variables with a P-value of < 0.1 in univariable analyses were included in a multivariable model. As the control group in a case control study should reflect the entire source population that gave rise to the cases, we did not exclude IBD patients who developed RCC from the control groups [43]. These patients were in both models analyzed as cases.
For case control study A, identifying independent risk factors to develop RCC, we performed a multivariable logistic regression model with backward sampling. This model was adjusted for the duration of follow up (fixed variable), which was defined as the time since IBD onset until the date of RCC diagnosis (cases) or the end of follow up or death (controls). For case control study B, comparing RCC outcome between IBD cases and the general population, we made Kaplan Meier survival curves and performed log rank analysis. Subsequently confounder correction was performed with Cox regression model with forward sampling. A covariate was considered as a confounder when the beta coefficient of the variable of interest (IBD yes/no) changed by 10% or more [44].
A P-value of < 0.05 (2 sided) was considered to be statistically significant. All missing values were considered to be at random and were excluded from analyses. All statistical analyses were performed with IBM SPSS statistics version 20.0 (SPSS Inc, Chicago, IL).
ACKNOWLEDGMENTS AND FUNDING
The authors would like to thank Prof. Dr. L.A.L.M. Kiemeneij for his epidemiological and statistical advice.
PALGA group: PM Kluin (University Medical Centre Groningen, Groningen), M Hogenes (Ziekenhuisgroep Twente, Almelo), AF Hamel (Ommelander Hospital Group, Winschoten), R Natté (Haga Hospital, Den Haag), CM van Dijk (Groene Hart Hospital, Gouda), HVN Kusters-Vandevelde (Canisius-Wilhelmina Hospital, Nijmegen), SH Sastrowijoto (Orbis Medical Centre, Sittard), AP Willig (Sint Jans Gasthuis, Weert)
IBD/RCC group: G Dijkstra (University Medical Centre Groningen, Groningen), AE van der Meulen-de Jong (Leiden University Medical Centre, Leiden), MK Vu (Rijnland Hospital, Leiderdorp), A Cats (Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam), JBAG Haanen (Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam), CJ van der Woude (Erasmus Medical Centre, Rotterdam), MGVM Russel (Medical Spectrum Twente, Enschede), B Oldenburg (University Medical Centre Utrecht, Utrecht), JJ Meeuse (Hospital Rivierenland, Tiel), S Corporaal (Medical Centre Leeuwarden, Leeuwarden), AM Zonneveld (Antonius Hospital, Sneek), PJ Wahab (Rijnstate Hospital, Arnhem), SJ van den Hazel (Slingeland Hospital, Doetinchem), WGN Mares (Hospital Gelderse Vallei, Ede), RJ Lieverse (Hospital Bethesda, Hoogeveen), MAC Meijssen (Diaconessenhuis Meppel, Meppel), K Thuernau (Röpcke-Zweers Hospital, Hardenberg), D Janik (Ommelander Hospital Group, Delfzijl), H van der Heide (Martini Hospital, Groningen), IJ Klompmaker (Wilhelmina Hospital Assen, Assen), CJM Bolwerk (Reinier de Graaf Groep, Delft), R Stuyt (Haga Hospital, Den Haag), AA van Bodegraven (Vrije University Medical Centre, Amsterdam), CY Ponsioen (Academic Medical Centre, Amsterdam), RL West (Sint Franciscus Gasthuis, Rotterdam), RNM Zeijen (Vlietland Hospital, Schiedam), TJ Tang (IJsselland Hospital, Capelle aan den IJssel), PJ Wismans (Havenziekenhuis, Rotterdam), P Dewint (Van Weel-Bethesda Hospital, Dirksland; Maasstad Hospital, Rotterdam), JYL Lai (Groene Hart Hospital, Gouda; Langeland Hospital, Zoetermeer), ACITL Tan (Canisius-Wilhelmina Hospital, Nijmegen), ACTM Depla (Slotervaart Hospital, Amsterdam), ETP Keulen (Orbis Medical Centre, Sittard), LE Oostenbrug (Atrium Medical Centre, Heerlen), ME Bartelink (Deventer Hospital, Deventer), GW Erkelens (Gelre Hospital, Apeldoorn), J Vecht (Isala, Zwolle), JWM Tjhie-Wensing (Elkerliek Hospital, Helmond), TEH Römkens (Jeroen Bosch Hospital, Den Bosch), WA de Boer (Bernhoven, Uden), RK Linskens (Sint Anna Hospital, Geldrop), ML Verhulst (Máxima Medical Centre, Eindhoven), AHJ Naber (Tergooi Hospitals, Hilversum), GC Noomen (Medical Centre Alkmaar, Alkmaar), PEP Dekkers (Zaans Medical Centre, Zaandam), PP Viergever (Gemini Hospital, Den Helder), JR Vermeijden (Meander Medical Centre, Amersfoort), M Willems (Hospital Sint jansdal, Harderwijk), HGT Lam (Diakonessenhuis, Utrecht), N Mahmmod (Sint Antonius, Nieuwegein), BCAJ Loffeld (Zuwe Hofpoort Hospital, Woerden), JM Jansen (Onze Lieve Vrouwe Gasthuis, Amsterdam; BovenIJ Hospital, Amsterdam), PCF Stokkers (Sint Lucas Andreas Hospital, Amsterdam), JP Kuijvenhoven (Kennemer Gasthuis, Haarlem) MJ Wagtmans (Rode Kruis Hospital, Beverwijk), CHM Clemens (Diaconessenhuis Leiden, Leiden), LT Vlasveld (Hospital Bronovo, Den Haag), PJ Bus (Laurentius Hospital, Roermond), RPM Dahlmans (Sint Jans Gasthuis, Weert), R Beukers (Albert Schweitzer Hospital, Dordrecht), PCJ ter Borg (Ikazia Hospital, Rotterdam), PPJ van der Veek (Medical Centre Haaglanden, Den Haag), JT Sarneel (Admiraal De Ruyter Hospital, Goes), S Vandebosch (ZorgSaam Zeeuws-Vlaanderen, Terneuzen), E Halet (Franciscus Hospital Roosendaal, Roosendaal), MCM Rijk (Amphia Hospital, Breda), MJAL Grubben (Sint Elisabeth Hospital, Tilburg), PJM Kil (Sint Elisabeth Hospital, Tilburg), LPL Gilissen (Catharina Hospital, Eindhoven), FL Wolters (VieCuri, Venlo), M Uiterwaal (Spaarne Hospital, Hoofddorp).
This work was not supported by any company or grants.
Footnotes
CONFLICTS OF INTEREST
None of the authors report conflicts of interest that are relevant to the submitted manuscript.
REFERENCES
- 1.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. The American journal of gastroenterology. 2010;105:1480–1487. doi: 10.1038/ajg.2009.760. [DOI] [PubMed] [Google Scholar]
- 3.Beaugerie L. Inflammatory bowel disease therapies and cancer risk: where are we and where are we going? Gut. 2012;61:476–483. doi: 10.1136/gutjnl-2011-301133. [DOI] [PubMed] [Google Scholar]
- 4.Bernheim O, Colombel JF, Ullman TA, Laharie D, Beaugerie L, Itzkowitz SH. The management of immunosuppression in patients with inflammatory bowel disease and cancer. Gut. 2013;62:1523–1528. doi: 10.1136/gutjnl-2013-305300. [DOI] [PubMed] [Google Scholar]
- 5.Beaugerie L, Carrat F, Colombel JF, Bouvier AM, Sokol H, Babouri A, Carbonnel F, Laharie D, Faucheron JL, Simon T, de Gramont A, Peyrin-Biroulet L, for the CSG Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2013 doi: 10.1136/gutjnl-2013-305763. [DOI] [PubMed] [Google Scholar]
- 6.Biancone L, Onali S, Petruzziello C, Calabrese E, Pallone F. Cancer and immunomodulators in inflammatory bowel diseases. Inflammatory bowel diseases. 2015;21:674–698. doi: 10.1097/MIB.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 7.Beaugerie L. Use of immunosuppressants and biologicals in patients with previous cancer. Digestive diseases. 2013;31:254–259. doi: 10.1159/000353382. [DOI] [PubMed] [Google Scholar]
- 8.Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lemann M, Cosnes J, Hebuterne X, Cortot A, Bouhnik Y, Gendre JP, Simon T, Maynadie M, Hermine O, Faivre J, Carrat F, Group CS. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, Carbonnel F, Colombel JF, Dupas JL, Godeberge P, Hugot JP, Lemann M, Nahon S, Sabate JM, Tucat G, Beaugerie L, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–1628. doi: 10.1053/j.gastro.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 10.Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sorensen HT, Baron JA. Risk of Cancer in Patients with Inflammatory Bowel Diseases: a Nationwide Population-Based Cohort Study with 30 Years of Follow Up. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013 doi: 10.1016/j.cgh.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jess T, Horvath-Puho E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. The American journal of gastroenterology. 2013;108:1869–1876. doi: 10.1038/ajg.2013.249. [DOI] [PubMed] [Google Scholar]
- 12.Jussila A, Virta LJ, Pukkala E, Farkkila MA. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scandinavian journal of gastroenterology. 2013;48:1405–1413. doi: 10.3109/00365521.2013.846402. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67:1167–1198. doi: 10.2165/00003495-200767080-00006. [DOI] [PubMed] [Google Scholar]
- 14.Boers-Sonderen MJ, Mulder SF, Nagtegaal ID, Jacobs JF, Wanten GJ, Hoentjen F, van Herpen CM. Severe exacerbation of Crohn's disease during sunitinib treatment. European journal of gastroenterology & hepatology. 2014;26:234–236. doi: 10.1097/MEG.0b013e328365ac54. [DOI] [PubMed] [Google Scholar]
- 15.Cameron C, Greenbaum L, Sato T, Trost B, Lundeen B, Kelly ME. Renal cell carcinoma in a patient with cystinosis and inflammatory bowel disease: a case report. Pediatric nephrology. 2008;23:1167–1170. doi: 10.1007/s00467-008-0773-6. [DOI] [PubMed] [Google Scholar]
- 16.Satsangi J, Marshall J, Roskell D, Jewell D. Ulcerative colitis complicated by renal cell carcinoma: a series of three patients. Gut. 1996;38:148–150. doi: 10.1136/gut.38.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plaisier PW. Ulcerative colitis and renal cell carcinoma. Gut. 1996;38:936. doi: 10.1136/gut.38.6.936-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostadinova-Kunovska S, Petrusevska G, Grcevska L, Banev S, Dzikova S, Bogoeva B, Polenakovic M. [The possible pathogenesis of AA type amyloidosis in a patient with ulcerative colitis and renal cell carcinoma] Acta medica Croatica: casopis Hravatske akademije medicinskih znanosti. 2006;60:251–254. [PubMed] [Google Scholar]
- 19.Keller AS, Bouldin MB, Drage LA, Hauser SC, Davis MD. Linear IgA bullous dermatosis: an association with ulcerative colitis versus renal cell carcinoma. Digestive diseases and sciences. 2003;48:783–789. doi: 10.1023/a:1022805329847. [DOI] [PubMed] [Google Scholar]
- 20. http.//www.zorgatlas.nl/zorg/ziekenhuiszorg/algemene-en-academische-ziekenhuizen/aanbod/locaties-algemene-en-academische-ziekenhuizen
- 21.Beisland C, Medby PC, Beisland HO. Renal cell carcinoma: gender difference in incidental detection and cancer-specific survival. Scandinavian journal of urology and nephrology. 2002;36:414–418. doi: 10.1080/003655902762467558. [DOI] [PubMed] [Google Scholar]
- 22.Palsdottir HB, Hardarson S, Petursdottir V, Jonsson A, Jonsson E, Sigurdsson MI, Einarsson GV, Gudbjartsson T. Incidental detection of renal cell carcinoma is an independent prognostic marker: results of a long-term, whole population study. The Journal of urology. 2012;187:48–53. doi: 10.1016/j.juro.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Ficarra V, Prayer-Galetti T, Novella G, Bratti E, Maffei N, Dal Bianco M, Artibani W, Pagano F. Incidental detection beyond pathological factors as prognostic predictor of renal cell carcinoma. European urology. 2003;43:663–669. doi: 10.1016/s0302-2838(03)00142-8. [DOI] [PubMed] [Google Scholar]
- 24.Gudbjartsson T, Thoroddsen A, Petursdottir V, Hardarson S, Magnusson J, Einarsson GV. Effect of incidental detection for survival of patients with renal cell carcinoma: results of population-based study of 701 patients. Urology. 2005;66:1186–1191. doi: 10.1016/j.urology.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Wauters SJL, Verschueren P, van Assche G, Vermeire S, Ferrante M. P628. Anti-TNF treatment and renal cell carcinoma in patients with inflammatory bowel disease, rheumatoid arthritis and spondyloarthropathy: trigger or cure? Journal of Crohn's & colitis. 2015;1:S395–396. [Google Scholar]
- 26.Baars JE, Kuipers EJ, van Haastert M, Nicolai JJ, Poen AC, van der Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. Journal of gastroenterology. 2012;47:1308–1322. doi: 10.1007/s00535-012-0603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brackmann S, Andersen SN, Aamodt G, Roald B, Langmark F, Clausen OP, Aadland E, Fausa O, Rydning A, Vatn MH. Two distinct groups of colorectal cancer in inflammatory bowel disease. Inflammatory bowel diseases. 2009;15:9–16. doi: 10.1002/ibd.20542. [DOI] [PubMed] [Google Scholar]
- 28.Hudesman D, Lichtiger S, Sands B. Risk of extraintestinal solid cancer with anti-TNF therapy in adults with inflammatory bowel disease: review of the literature. Inflammatory bowel diseases. 2013;19:644–649. doi: 10.1097/MIB.0b013e318280ebbd. [DOI] [PubMed] [Google Scholar]
- 29.Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, Walsh C, Lawson R, Reynolds A, Emery P. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2011;70:1895–1904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 30.Penn I. The effect of immunosuppression on pre-existing cancers. Transplantation. 1993;55:742–747. doi: 10.1097/00007890-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Larkin JM, Ferguson TR, Pickering LM, Edmonds K, James MG, Thomas K, Banerji U, Berns B, de Boer C, Gore ME. A phase I/II trial of sorafenib and infliximab in advanced renal cell carcinoma. British journal of cancer. 2010;103:1149–1153. doi: 10.1038/sj.bjc.6605889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, Charles K, Ahern R, King DM, Eisen T, Corringham R, DeWitte M, et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4542–4549. doi: 10.1200/JCO.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- 33.Aben KK, Luth TK, Janssen-Heijnen ML, Mulders PF, Kiemeney LA, van Spronsen DJ. No improvement in renal cell carcinoma survival: a population-based study in the Netherlands. European journal of cancer. 2008;44:1701–1709. doi: 10.1016/j.ejca.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cellular oncology: the official journal of the International Society for Cellular Oncology. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Heuvel TR, Jonkers DM, Jeuring SF, Romberg-Camps MJ, Oostenbrug LE, Zeegers MP, Masclee AA, Pierik MJ. Cohort Profile: The Inflammatory Bowel Disease South Limburg Cohort (IBDSL) International journal of epidemiology. 2015 doi: 10.1093/ije/dyv088. [DOI] [PubMed] [Google Scholar]
- 36.Coebergh MLGJ-H JWW, Louwman WJ, Voogd AC. Cancer incidence, care and survival in the South of the Netherlands 1955–1999, a report from the Eindhoven Cancer Registry with cross-border implications. Comprehensive Cancer Centre South (ECR), Eindhoven. 2001 [Google Scholar]
- 37.Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. American journal of epidemiology. 1999;149:916–924. doi: 10.1093/oxfordjournals.aje.a009735. [DOI] [PubMed] [Google Scholar]
- 38.Romberg-Camps MJ, Bol Y, Dagnelie PC, Hesselink-van de Kruijs MA, Kester AD, Engels LG, van Deursen C, Hameeteman WH, Pierik M, Wolters F, Russel MG, Stockbrugger RW. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflammatory bowel diseases. 2010;16:2137–2147. doi: 10.1002/ibd.21285. [DOI] [PubMed] [Google Scholar]
- 39.Rose S, Laan MJ. Why match? Investigating matched case-control study designs with causal effect estimation. The international journal of biostatistics. 2009;5 doi: 10.2202/1557-4679.1127. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. http.//www.eindhovencancerregistry.nl
- 41.Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. International journal of epidemiology. 1993;22:369–376. doi: 10.1093/ije/22.3.369. [DOI] [PubMed] [Google Scholar]
- 42.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. The American journal of surgical pathology. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Rothman KJ. Oxford University Press; 2012. Epidemiology: an introduction. [Google Scholar]
- 44.Twisk J. Cambridge UK: Cambridge University Press; 2006. Applied Multilevel Analysis: A Practical Guide. [Google Scholar]


