Abstract
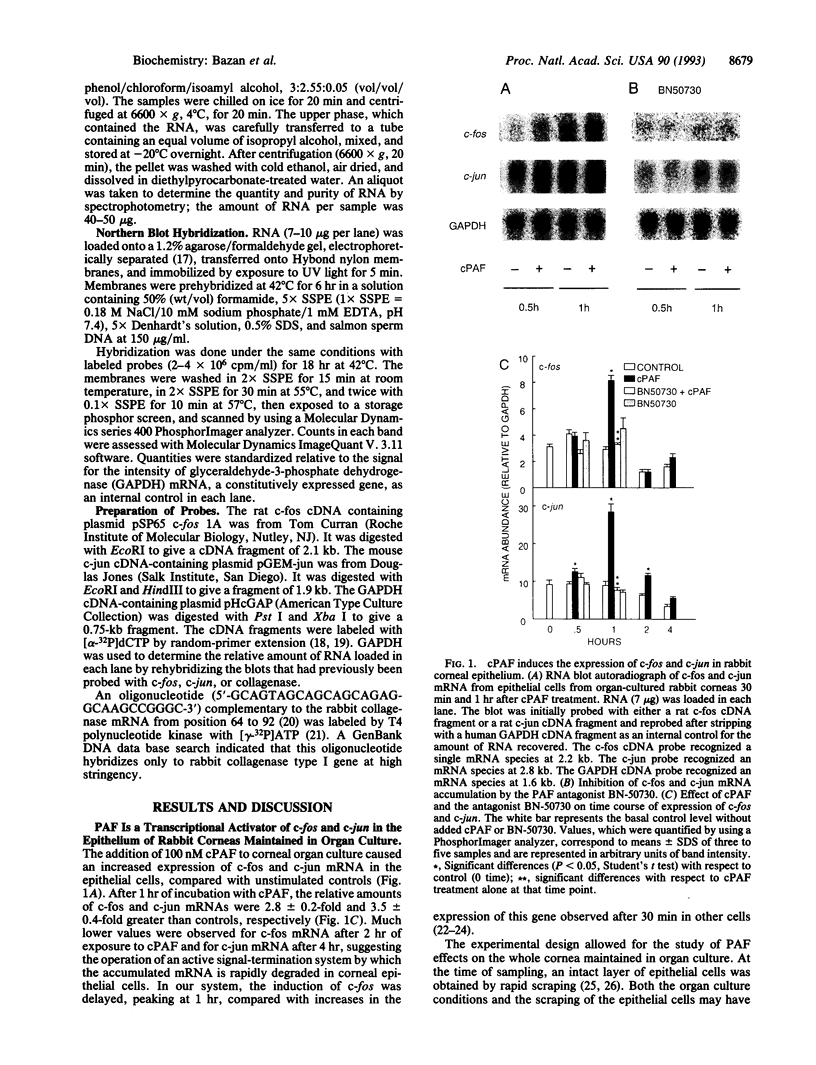
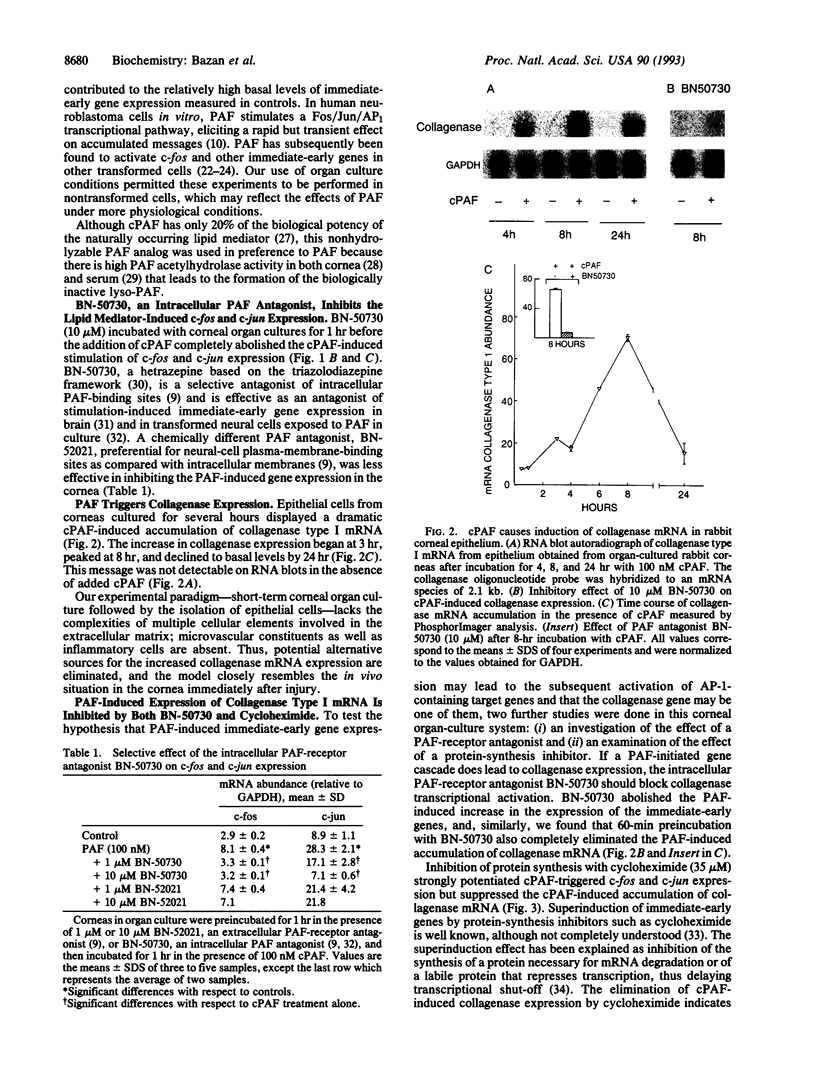
Platelet-activating factor (PAF), a potent lipid mediator involved in inflammatory and immune responses, accumulates rapidly in response to injury in a variety of tissues, including the corneal epithelium. However, the precise role of this compound in the cascade of events following insult has not been defined. Here we examined the effect of PAF on gene expression in the epithelial cells of rabbit corneas in organ culture. We found that incubation with 100 nM methylcarbamoyl PAF, a nonhydrolyzable analog of PAF, produced rapid transient 2.8- and 3.5-fold increases in the expression of c-fos and c-jun, respectively, at 1 hr, followed by increased expression of the collagenase type I gene beginning at 3 hr and peaking at 14-fold by 8 hr. Addition of the protein-synthesis-inhibitor cycloheximide superinduced c-fos and c-jun, strongly potentiating the PAF effect, but inhibited the induction of collagenase type I expression, suggesting the existence of a transcriptional factor linking the two events. BN-50730, a selective antagonist of intracellular PAF-binding sites, blocked the expression of the immediate-early genes as well as the increase in collagenase type I mRNA. Our results suggest that one of the functions of PAF may be to enhance the breakdown of the extracellular matrix as a part of the remodeling process during corneal wound healing after injury. Pathologically, a PAF-induced overproduction of collagenase may be a factor in the development of corneal ulcers, as well as other pathophysiological conditions such as cartilage destruction in arthritis. If so, inhibitors of this signal-transduction pathway may be useful as tools for further investigation and, eventually, as therapeutic agents to treat such disorders.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G., Hecquet F., Etienne A., Braquet P., Mencia-Huerta J. M. Role of platelet-activating factor (PAF) in the ovoimplantation in the rat: effect of the specific PAF-acether antagonist, BN 52021. Prostaglandins. 1988 Feb;35(2):233–241. doi: 10.1016/0090-6980(88)90090-1. [DOI] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan H. E., Birkle D. L., Beuerman R., Bazan N. G. Cryogenic lesion alters the metabolism of arachidonic acid in rabbit cornea layers. Invest Ophthalmol Vis Sci. 1985 Apr;26(4):474–480. [PubMed] [Google Scholar]
- Bazan H. E., Reddy S. T., Lin N. Platelet-activating factor (PAF) accumulation correlates with injury in the cornea. Exp Eye Res. 1991 Apr;52(4):481–491. doi: 10.1016/0014-4835(91)90046-h. [DOI] [PubMed] [Google Scholar]
- Bazan H. E., Reddy S. T., Woodland J. M., Bazan N. G. The accumulation of platelet activating factor in the injured cornea may be interrelated with the synthesis of lipoxygenase products. Biochem Biophys Res Commun. 1987 Dec 31;149(3):915–920. doi: 10.1016/0006-291x(87)90495-5. [DOI] [PubMed] [Google Scholar]
- Bazan N. G., Squinto S. P., Braquet P., Panetta T., Marcheselli V. L. Platelet-activating factor and polyunsaturated fatty acids in cerebral ischemia or convulsions: intracellular PAF-binding sites and activation of a fos/jun/AP-1 transcriptional signaling system. Lipids. 1991 Dec;26(12):1236–1242. doi: 10.1007/BF02536539. [DOI] [PubMed] [Google Scholar]
- Behrendtsen O., Alexander C. M., Werb Z. Metalloproteinases mediate extracellular matrix degradation by cells from mouse blastocyst outgrowths. Development. 1992 Feb;114(2):447–456. doi: 10.1242/dev.114.2.447. [DOI] [PubMed] [Google Scholar]
- Berman M. The pathogenesis of corneal epithelial defects. Acta Ophthalmol Suppl. 1989;192:55–64. doi: 10.1111/j.1755-3768.1989.tb07095.x. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Hall M. N., Cress E. A., Snyder F. Inactivation of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine by a plasma acetylhydrolase: higher activities in hypertensive rats. Biochem Biophys Res Commun. 1983 Jun 15;113(2):666–671. doi: 10.1016/0006-291x(83)91778-3. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Brenner C. A., Adler R. R., Rappolee D. A., Pedersen R. A., Werb Z. Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev. 1989 Jun;3(6):848–859. doi: 10.1101/gad.3.6.848. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung J. H. Healing of rabbit corneal alkali wounds in vitro. Cornea. 1990 Jan;9(1):36–40. [PubMed] [Google Scholar]
- Cobianchi F., Wilson S. H. Enzymes for modifying and labeling DNA and RNA. Methods Enzymol. 1987;152:94–110. doi: 10.1016/0076-6879(87)52013-4. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Plucinska I. M., Mayer A. S., Gross R. H., Brinckerhoff C. E. A gene for rabbit synovial cell collagenase: member of a family of metalloproteinases that degrade the connective tissue matrix. Biochemistry. 1987 Sep 22;26(19):6156–6165. doi: 10.1021/bi00393a032. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Z., Nakamura M., Miki I., Minami M., Watanabe T., Seyama Y., Okado H., Toh H., Ito K., Miyamoto T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991 Jan 24;349(6307):342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Hurst J. S., Balazy M., Bazan H. E., Bazan N. G. The epithelium, endothelium, and stroma of the rabbit cornea generate (12S)-hydroxyeicosatetraenoic acid as the main lipoxygenase metabolite in response to injury. J Biol Chem. 1991 Apr 15;266(11):6726–6730. [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Koltai M., Hosford D., Guinot P., Esanu A., Braquet P. Platelet activating factor (PAF). A review of its effects, antagonists and possible future clinical implications (Part I). Drugs. 1991 Jul;42(1):9–29. doi: 10.2165/00003495-199142010-00002. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lynch J. M., Henson P. M. The intracellular retention of newly synthesized platelet-activating factor. J Immunol. 1986 Oct 15;137(8):2653–2661. [PubMed] [Google Scholar]
- Marcheselli V. L., Rossowska M. J., Domingo M. T., Braquet P., Bazan N. G. Distinct platelet-activating factor binding sites in synaptic endings and in intracellular membranes of rat cerebral cortex. J Biol Chem. 1990 Jun 5;265(16):9140–9145. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Mazer B., Domenico J., Sawami H., Gelfand E. W. Platelet-activating factor induces an increase in intracellular calcium and expression of regulatory genes in human B lymphoblastoid cells. J Immunol. 1991 Mar 15;146(6):1914–1920. [PubMed] [Google Scholar]
- Mitchell T. I., Coon C. I., Brinckerhoff C. E. Serum amyloid A (SAA3) produced by rabbit synovial fibroblasts treated with phorbol esters or interleukin 1 induces synthesis of collagenase and is neutralized with specific antiserum. J Clin Invest. 1991 Apr;87(4):1177–1185. doi: 10.1172/JCI115116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Honda Z., Izumi T., Sakanaka C., Mutoh H., Minami M., Bito H., Seyama Y., Matsumoto T., Noma M. Molecular cloning and expression of platelet-activating factor receptor from human leukocytes. J Biol Chem. 1991 Oct 25;266(30):20400–20405. [PubMed] [Google Scholar]
- O'Flaherty J. T., Redman J. F., Jr, Schmitt J. D., Ellis J. M., Surles J. R., Marx M. H., Piantadosi C., Wykle R. L. 1-O-alkyl-2-N-methylcarbamyl-glycerophosphocholine: a biologically potent, non-metabolizable analog of platelet-activating factor. Biochem Biophys Res Commun. 1987 Aug 31;147(1):18–24. doi: 10.1016/s0006-291x(87)80081-5. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Platelet-activating factor. J Biol Chem. 1990 Oct 15;265(29):17381–17384. [PubMed] [Google Scholar]
- Quinn C. O., Scott D. K., Brinckerhoff C. E., Matrisian L. M., Jeffrey J. J., Partridge N. C. Rat collagenase. Cloning, amino acid sequence comparison, and parathyroid hormone regulation in osteoblastic cells. J Biol Chem. 1990 Dec 25;265(36):22342–22347. [PubMed] [Google Scholar]
- Sakai J., Hung J., Zhu G., Katakami C., Boyce S., Kao W. W. Collagen metabolism during healing of lacerated rabbit corneas. Exp Eye Res. 1991 Mar;52(3):237–244. doi: 10.1016/0014-4835(91)90086-t. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Squinto S. P., Block A. L., Braquet P., Bazan N. G. Platelet-activating factor stimulates a fos/jun/AP-1 transcriptional signaling system in human neuroblastoma cells. J Neurosci Res. 1989 Dec;24(4):558–566. doi: 10.1002/jnr.490240414. [DOI] [PubMed] [Google Scholar]
- Squinto S. P., Braquet P., Block A. L., Bazan N. G. Platelet-activating factor activates HIV promoter in transfected SH-SY5Y neuroblastoma cells and MOLT-4 T lymphocytes. J Mol Neurosci. 1990;2(2):79–84. doi: 10.1007/BF02876914. [DOI] [PubMed] [Google Scholar]
- Thomson W., Pepper L., Payton A., Carthy D., Scott D., Ollier W., Silman A., Symmons D. Absence of an association between HLA-DRB1*04 and rheumatoid arthritis in newly diagnosed cases from the community. Ann Rheum Dis. 1993 Jul;52(7):539–541. doi: 10.1136/ard.52.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabandt A., Aicher W. K., Gay R. E., Sukhatme V. P., Fassbender H. G., Gay S. Spontaneous expression of immediately-early response genes c-fos and egr-1 in collagenase-producing rheumatoid synovial fibroblasts. Rheumatol Int. 1992;12(2):53–59. doi: 10.1007/BF00300977. [DOI] [PubMed] [Google Scholar]
- Tripathi Y. B., Kandala J. C., Guntaka R. V., Lim R. W., Shukla S. D. Platelet activating factor induces expression of early response genes c-fos and TIS-1 in human epidermoid carcinoma A-431 cells. Life Sci. 1991;49(23):1761–1767. doi: 10.1016/0024-3205(91)90319-7. [DOI] [PubMed] [Google Scholar]
- Wolf G. The molecular basis of the inhibition of collagenase by vitamin A. Nutr Rev. 1992 Oct;50(10):292–294. doi: 10.1111/j.1753-4887.1992.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Ye R. D., Prossnitz E. R., Zou A. H., Cochrane C. G. Characterization of a human cDNA that encodes a functional receptor for platelet activating factor. Biochem Biophys Res Commun. 1991 Oct 15;180(1):105–111. doi: 10.1016/s0006-291x(05)81261-6. [DOI] [PubMed] [Google Scholar]