Abstract
Background
Smoking, alcohol consumption, poor diet and low levels of physical activity significantly contribute to the burden of illness in developed countries. Whilst the links between specific and multiple risk behaviours and individual chronic conditions are well documented, the impact of these behaviours in mid-life across a range of later life outcomes has yet to be comprehensively assessed. This review aimed to provide an overview of behavioural risk factors in mid-life that are associated with successful ageing and the primary prevention or delay of disability, dementia, frailty and non-communicable chronic conditions.
Methods
A literature search was conducted to identify cohort studies published in English since 2000 up to Dec 2014. Multivariate analyses and a minimum follow-up of five years were required for inclusion. Two reviewers screened titles, abstracts and papers independently. Studies were assessed for quality. Evidence was synthesised by mid-life behavioural risk for a range of late life outcomes.
Findings
This search located 10,338 individual references, of which 164 are included in this review. Follow-up data ranged from five years to 36 years. Outcomes include dementia, frailty, disability and cardiovascular disease. There is consistent evidence of beneficial associations between mid-life physical activity, healthy ageing and disease outcomes. Across all populations studied there is consistent evidence that mid-life smoking has a detrimental effect on health. Evidence specific to alcohol consumption was mixed. Limited, but supportive, evidence was available relating specifically to mid-life diet, leisure and social activities or health inequalities.
Conclusions
There is consistent evidence of associations between mid-life behaviours and a range of late life outcomes. The promotion of physical activity, healthy diet and smoking cessation in all mid-life populations should be encouraged for successful ageing and the prevention of disability and chronic disease.
Background
Non-communicable chronic conditions and disabilities that manifest in later life are heavily influenced by physical and social exposures, including behaviours, across the life course [1,2], leading to an accumulation of risks in older age. The four main behavioural risk factors (smoking, excessive consumption of alcohol, poor diet and low levels of physical activity) have been estimated to contribute to close to half of the burden of illness in developed countries [3,4] and are known to be unequally distributed in the population particularly affecting the most vulnerable in society [5]. Importantly there is a contingent and temporal nature of these behavioural exposures in mid-life [6–8] and epidemiological data suggests that it ought to be possible to prevent or delay morbidity and mortality [9] whilst promoting successful aging and quality of life [10–12], if adoption of healthy behaviours is encouraged across life course and society.
Although many systematic reviews have looked at the links between individual behavioural risk factors and health outcomes [13–19], a comprehensive overview across behavioural risk factors and outcomes has yet to be undertaken. There remain uncertainties regarding associations between the relative contributions of behavioural and lifestyle factors, in mid-life specifically, to prevalence, risk and outcomes of non-communicable chronic conditions (for example, dementia, cancer and cardiovascular diseases), disabilities and frailty (including quality of life, and mortality). That is particularly true for the relationship between behavioural risk factors and frailty, where the operational definition of this complex syndrome is still controversial [20,21]; and for dementia where the aetiology and natural history of disease is still uncertain [22,23].
This systematic review was one of a series of reviews conducted to inform the development of UK national public health guidance on mid-life approaches to prevent dementia, disability and frailty in later life [24]. The aim of the review was to assess the behavioural risk factors in mid-life that are associated with successful ageing and the primary prevention or delay of disability, dementia, frailty (DDF) and non-communicable chronic conditions. The goal was not to summarise the whole of the epidemiological evidence on behavioural risk in adult life. Rather, we aimed to identify associations specifically derived from people in mid-life to inform the development of well-targeted interventions that will minimise the impact of ill health in later life. With a similar focus, the other two reviews in the series looked at the effectiveness of mid-life interventions on behavioural risks and late life outcomes, and at key issues for people in mid-life that prevent or limit, or which help or motivate them to take up and maintain healthy behaviours.
Methods
The review was conducted as a rapid systematic review to provide best available evidence within limited timescales. The scope of the review was defined by the funders (National Institute for Health and Care Excellence—NICE), after open consultation with stakeholders and the protocol (S1 Text) was agreed prior to the start of work. Established systematic review methods of NICE (NICE 2015) were broadly followed, except as described below.
Searches
The following electronic sources were searched for peer-reviewed studies published in the English language since the year 2000: MEDLINE, EMBASE, PsycINFO, CINAHL, Health Management Information Consortium, Social Science Citation Index, the HTA database, Cochrane Database of Systematic Reviews, and Cochrane Database of Abstracts of Reviews of Effectiveness as well as relevant websites (S2 Text).
Primary longitudinal cohort studies were identified using an observational study search filter [25]. To enable a manageable number of search hits, the searches were limited to studies in which terms related to mid-life/middle-aged were indexed in the title/abstract or MeSH term. Time constraints precluded hand searches or contact of authors for additional data.
Inclusion and exclusion criteria
Populations
The populations covered by this review included 1) mid-life adults (aged 40–64 years), 2) adults aged 39 and younger in populations at higher risk of health inequalities, which refer to people from disadvantaged and minority groups. For this review ‘disadvantaged and minority groups’ includes (but is not limited to) people of low socioeconomic status; ethnic minority groups; LGBT community groups; travellers; and other groups with protected characteristics under the equality and diversity legislation.
Studies in populations with mid-life dementia or pre-existing cognitive impairment, and non-communicable chronic conditions were excluded, as were studies of specific disabilities associated with modifiable behavioural risk factors
Exposure
Behavioural risk factors in the populations described above include (but are not limited to): physical activity; diet and nutrition; smoking; alcohol consumption; and social activity. Studies that reported mid-life weight change/cycling (as a health behaviour) were included whereas studies focusing on mid-life obesity (as a risk factor) were excluded.
Outcomes
Outcomes included (but were not limited to): dementia, disability (i.e. activities of daily living (ADL), instrumental activities of daily living (IADL), independence, mobility), frailty, quality of life, cardiovascular diseases and stroke, renal disease, cancer, chronic obstructive pulmonary disease, type 2 diabetes, osteoporosis and bone health, mental health.
Study design
Primary longitudinal cohort studies that reported multivariate analyses and provided information on the association between behavioural risk factors at mid-life and health or ageing outcomes in later life (>/ = 5 years follow-up) as listed above were eligible for inclusion. Cross-sectional and univariate analyses were excluded. Abstracts, letters and editorials were also excluded.
Identification of relevant studies
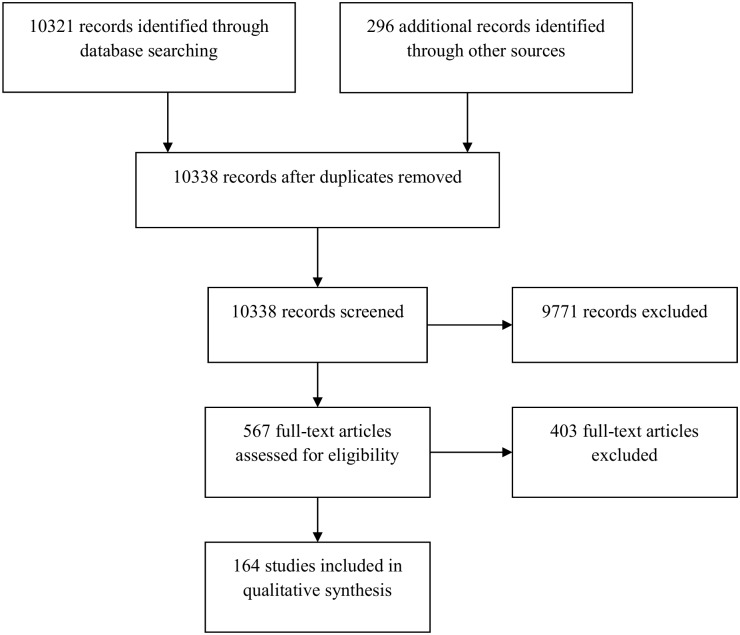
Titles, abstracts and papers were screened for inclusion by two reviewers. Differences between reviewers’ results were resolved by discussion and when necessary in consultation with a third reviewer. Fig 1 illustrates the flow chart for the study selection process. Studies excluded at the full paper screening stage are listed in S1 Table along with the reason for exclusion.
Fig 1. Search Results for Primary Studies.
Quality Assessment
Quality appraisal of cohort studies was done using a validated quality appraisal checklist [24]. Each full paper was assessed by one reviewer and checked for accuracy by another. A minimum of 10% of the studies was assessed twice and discrepancies resolved by discussion. No studies were excluded on the basis of quality.
Data extraction and evidence synthesis
Data was extracted on study detail, population and setting, study design, outcomes and method of analysis, and results. To ensure accurate reporting, the data extraction pro-forma was piloted against a selection of papers. A full summary of studies, exposures, outcomes and details of effect sizes are reported in Table 1.
Table 1. Overview of Included Studies.
| Study | Country | n | Age at baseline | Length of follow-up | Quality |
|---|---|---|---|---|---|
| Physical Activity (PA) | |||||
| Andel 2008 (Case control study) | Sweden | 264 dementia 2870 controls (90 twin pairs) | Mean 48.1 (SD 4.9) | 31.4 years | + |
| Britton 2008 | UK (England) | 5823 | 35–55 | 17 years | + |
| Carlson 2008 | US | 147 twin pairs | 45 (SD 3) | 20–40 years | ++ |
| Chang 2010 | Iceland | 4761 | 51 | 26 years | - |
| Chang 2013 (Same study as Chang 2010, different outcomes) | Iceland | 4753 | 51 (SD 7) | 25 years | - |
| Debette 2011 | USA | 1352 | 61±9 | 10yrs | + |
| Ekelund 2005 | UK (England) | 605 | 53 (mean) | 5.6 years | + |
| Elwood 2013 (Caerphilly cohort study) | UK (Wales) | 2235 | 45–59 | 30 years | ++ |
| Englund 2011 (Case-control study) | Sweden | 81 cases/ 156 controls | 57 (SD 5) | 11 years | - |
| Englund 2013 (Case-control study) | Sweden | 376 cases/402 controls | 54 (SD 6) | 11 years | - |
| Friedland 2001 (Case-control study) | US | 193 cases/358 controls (for total study, not reported for 40–59 year olds) | 40–59 | >12 (not fully reported) | - |
| Harmsen 2006 | Sweden | 6193 | 47–55 | 28 years | + |
| Hamer 2013 | UK (England) | 3454 | 63.7 (SD 8.9) | 8 years | ++ |
| Holtermann 2009 | Denmark | 4952 | 40–59 | 30 years | + |
| Holme 2007 (Oslo study) | Norway | 6382 | 40–49 | 28 years | - |
| Hu 2003 | Finland | 13290 | 35–64 | 12 years | + |
| Hu 2004 | Finland | 18892 | 25–74 (mean age 42–48) | 9.8 years | + |
| Hu 2005 | Finland | 47212 | 25–64 (mean age 41–46) | 17.7 years | + |
| Hu 2007 | Finland | 25–64 (mean age 42–49) | 18.9 years | + | |
| Knopman 2001 | USA | 10,963 | 47–70 | 6yrs | + |
| Lahti 2010 | Finland | 5437 women, 1257 men | 49–51 | 5–7 years | + |
| Lang 2007 | UK [England (ELSA study), and US] | 8702 (US)& 1507 (UK) (from 2 studies | 50–69 (mean 60.2 & 58) | 6 years | + |
| Malmberg 2006 | Finland | 1791 | 40–64 | 16 years | + |
| Meisinger 2007 | Germany | 3501 men, 3475 women | 45–74 | 8.6 years | + |
| Menotti 2006 | Italy | 1712 | 40–49 | 5 years | + |
| Morgan 2012 (Caerphilly cohort study) | UK (Wales) | 1005 | 45–59 | 16 years | + |
| Nokes 2012 | US | 244 | 35–45 | 6 years | + |
| Ostbye 2002 | US | 7845 (HRS study) 5037 | 51–61 | 5–6 yrs | - |
| Patel 2006 | Italy | 1001 | 40–60 | 7 years | + |
| Pitsavos 2004 | Greece (Corfu) | 529 | 49 ±6 | 40 years | + |
| Riserus 2007 | Sweden | 770 | 50 | 20 yrs (70–73 to 91–95) | + |
| Rovio 2005 | Sweden | 2000 | 50 | 21 years | + |
| Rovio 2007 (Same study as Rovio 2005) | Sweden | 1449 | 50 | 21 years | + |
| Sabia 2009 | England | 5123 | 44 (mean) | 17 years (85–88 to 02–04) | + |
| Stevens 2009 | England and Scotland | 1.29 million women | 50–64 (mean 56) | 96–01 to 05–07 Mean yrs of follow-up: 7.2 for cancer incidence; 8.9 for mortality | + |
| Sun 2010 | US | 13535 | 60 (mean age) | 14 years | ++ |
| Szoeke 2006 | US | 224 | 50 (mean) | 11 years | + |
| Wannamethee 2001 | UK (England) | 7630 | 40–59 | 18.8 years | + |
| Wiles 2007 (Caerphilly cohort study) | UK (Wales) | 2512 | 45–59 | 10 years | ++ |
| Xu 2010 | South East Queensland, Australia | 564 | 45–60 yrs (mean 55) | 2001–06 | - |
| Yu 2003 (Caerphilly cohort study) | UK (Wales) | 1975 | 45–59 | 10.5 years | + |
| Physical Inactivity | |||||
| Christensen 2006 | Denmark | 376 | 50, 60, 70 | 25 years | - |
| Haapanen-Niemi 2000 | Finland | 2212 (295 PA) | 35–63 | 16 years | + |
| Diet | |||||
| Akbaraly 2013 | UK | 8815 | 35–55 years | 18 years | + |
| Britton 2008 | UK | 5823 | 35–55 years (mean: 44) | 20 years | + |
| Elwood 2013 | UK (Caerphilly) | 2235 | 45–59 years | 30 years | + |
| Eskelinen 2008 | Finland | 1449 | SD: 50.2 | 21 years | + |
| Eskelinen 2009 (CAIDE study) | Finland | 1409 | SD: 50.4 | 21 years | + |
| He 2004 | United States | 74063 | 38–63 years | 12 years | + |
| Hodge 2013 | Australia | 8660 | 50–69 years | 12 years | + |
| Hughes 2010 | Sweden | 3779 (3424 non-demented, 355 dementia cases) | Mean age 48 | 30 years | + |
| Hu 2007 | Finland | 29335 | 25–64 years | 18.9 years | + |
| Kesse-Guyot 2012 | France | 3054 | SD: 52.1 | 13.4 years | - |
| Laitala 2009 | Finland | 2606 | Mean age 46–52 | 28 years | ++ |
| Laitinen 2006 | Finland | 1449 | SD: 50.4 | 21 years | ++ |
| Laurin 2004 | US (Hawaii) | 2459 | 45–68 years (Mean: 51.2) | 30.2 years | + |
| Lehto 2013 | Finland | 2600 | 42–61 years | 20.1 years | + |
| Liu 2003 | United States | 74091 | 38–63 years | 12 years | + |
| Masaki 2003 | Japan | 5644 | 40–69 | 10 years | + |
| Miura 2004 | United States | 1710 | 40–55 years (Mean 48.5) | 39 years | + |
| Nakamura 2009 | Japan | 2316 | 47–60 years | 19 years | + |
| Nooyens 2011 (Doetinchem Cohort Study) | Netherlands | 2613 | 43–70 years | 10 years | + |
| Osler 2003 | Denmark | 7540 | 30–70 years | 36 years | + |
| Ross 2000 | US Hawaii | 8004 | 45–68 years | 30 years | + |
| Ruder 2011 | United States | 292797 | 40–61 years | 10 years | ++ |
| Ruusanen 2010 | Finland | 2232 | 42–60 years | 17.5 years | + |
| Sabia 2009 | UK | 5123 | 35–55 years | 17 years | + |
| Samieri 2013 | United States | 10670 | Upper 50s, lower 60s (SD: 59) | 15.2 years | + |
| Seccareccia 2003 | Italy | 1536 | 45–65 years | 30 years | + |
| Song 2006 | United States | 28349 | 45+ years | 9.8 years | + |
| Strandhagen 2000 | Sweden | 792 | Age 54 | 26 years | ++ |
| Tsugane 2004 | Japan | 39065 | 40–59 years | 11 years | + |
| Walda 2002 | Finland, Italy and The Netherlands | 2917 | 50–69 years | 20 years | + |
| Wang 2009 | United States | 38408 | 45+ years | 11.5 years | + |
| Wang 2012 | United States | 28082 | 39+ | 12.9 years | + |
| Wang 2008 | United States | 28766 | 45+ years (SD 53.8) | 10 years | + |
| Xu 2010 | Australia | 564 | 45–60 years | 5 years | - |
| Smoking | |||||
| Agahi 2013 | Sweden | 1060 | 30–50 | Up to 34yrs | - |
| Alonso 2009 | USA | 11,151 | 45–64 | Up to 10yrs | + |
| Baba 2006 | Japan | 41,307 | 40-59yrs | 11yrs | + |
| Blanco-Cedres 2002 | USA | 8,816 | 40-59yrs | 25yrs | + |
| Boudik 2006 | Prague | 926 men | Mean 46.1 (Middle aged men) | 21yrs | - |
| Britton 2008 | England | 5823 (civil servant) | 35–55 | 17yrs | + |
| Dubas 2007 | Sweden | 7388 | 47–55 | 28yrs (‘70-‘98) | ++ |
| Englund 2013 | Sweden | 778 | 54±5.9 | 11.2±2.6 | + |
| Fogelholm 2000 | Finland | 1143 | 36–88 | 10 yrs (‘85-‘95) | + |
| Gerber 2012 | Israel | 4633 | 50.1±6.5 | Median 26yrs (quartiles 1–3: 16–35) | ++ |
| Halperin 2008 | USA | 13,529 | 52.4±8.9 | Med 14.5yrs Max 20.5yrs | + |
| Hara 2002 | Japan | 41,484 | 40–59 | Never: 64,986 PA Former: 42,798 PA Current: 103,537 PA | + |
| Harmsen 2006 | Sweden | 7457 | Middle-age men | 28yrs | + |
| Holme 2007 | Norway | 6382(M) | 40–49 | 28yrs | + |
| Holmberg 2006 | Sweden | 22444 (M) 10902(W) | Men: 27–61 yrs. Women: 28–58 yrs | 19yrs (M) 15yrs (W) | + |
| Humphries 2001 | UK | 3052 men | 55.7±3.2 | 11yrs | + |
| Inoue 2004 | Japan | 92,792 | 40–69 (mean 53) | 10 yrs | + |
| Janzon 2004 | Sweden | 10619 | 49 yrs (28.3–57.6) | 14.0±4.5yrs (range 0.5–21.9 years) | + |
| Khalili 2002 | Sweden | 22 444 –(not clear) | Mean 42.2 | 17yrs | + |
| Kimm 2011 | Korea | 3252 | Men 51.9 ±8.7 Women 53.6 ±9.9 | 14yrs | + |
| Lim 2013 | Singapore | 48,251 | 45–74 | 93–98 to 2009 | - |
| Mannami 2004 | Japan | 19,782 men and 21,500 women | 40–59 | 90–92 to 01 (total of 461,761 person-year follow-up) | ++ |
| Moayyeri 2009 | UK | 25,311 | W: 64.7 (8.4) M: 61.9 (9.7) (40-75yrs) | 11.3yrs (SD = 1.5; range 9.2–14.1) | + |
| Nakayama 2000 | Japan | 998 | 40-64yrs | 20yrs | + |
| Noborisaka 2013 | Japan | 6998 | Men 84.3% bt 30-59yrs Women 86.3% bt 30-59yrs | 6 yrs | + |
| Nooyens 2008 | Netherlands | 1964 | 56.0 (7.0) | 5yrs | + |
| Nafziger 2007 | Sweden | 82927 | 30–60 | 10 years | ++ |
| Östenson 2012 | Sweden | 2382 | 47.2 (46.9–47.4) | 10 years | + |
| Ostbye 2002 | US | 7,845 | 51–61 years | HRS: 6yrs | - |
| Otani 2003 | Japan | 19,862 (cohort 1). 10,212 (cohort 2) | 48.9 (6.0) (cohort 1). 53.4 (8.2) (cohort 2) | 10yrs (cohort 1). 7yrs (cohort 2) | + |
| Patja 2005 | Finland | 41 372 | 25–64 | Mean follow-up 21 years | - |
| Pelkonen 2000 | Finland | 1582 | Not reported | 30yrs | ++ |
| Qiao 2000 | Finland | 1673 | Not reported | 35yrs | + |
| Qiu 2003 | China | 50,069 | 55.3±11.8 | 6yrs | + |
| Räikkönen 2001 | USA | 541 | 48.0±1.5 | 9.2yrs; SD, 3.4 years | + |
| Riserus 2007 | Sweden | 770 | 50 | 20 yrs (70–73 to 91–95) | + |
| Rusanen 2011 | USA | 21,123 | 50–60 | 17yrs | + |
| Sabia 2008 | England | 5388 | 35–55 | 85–88 to 97–99 | + |
| Sabia 2009 | England | 5123 | Mean 56yrs | 5yrs | + |
| Satoh 2006 | Japan | 2,764 | 35–44 | 10yrs | + |
| Sairenchi 2004 | Japan | 39,528 men and 88,613 women | 40–79 (sub group: 40–59) | 93–02 | + |
| Shaper 2003 | Britain | 7735 | 40–59 | 22yrs (‘78-‘00) | ++ |
| Sobue 2002 | Japan | 91,738 | 40–69 | 9yrs | ++ |
| Stevens 2009 | England Scotland | 1.3 million | 50–60 (women) | 5-9yrs | ++ |
| Strand 2013 | Norway | 48,793 | 35–50 | 35yrs | ++ |
| Strandberg 2008 | Finland | 1658 | 40–55 | 26yrs | + |
| Szoeke 2006 | Australia | 438 | 46–52 | 11 years | + |
| Tyas 2003 | Hawaii | 3734 | (mid-life) | (‘65–‘71) and (‘91–’96) | + |
| Whitmer 2005 | USA | 8,845 | 40–44 | 27yrs (‘64-‘03) | + |
| Wilcox 2006 | USA | 5820 Japanese American men | Mean 54yrs (45–68) | Up to 40yrs | ++ |
| Wannamethe 2001 | UK | 7735 | 40–59 | 16.8 yrs | + |
| Alcohol | |||||
| Anttila 2004 | Finland | 632 women and 386 men | Mean age 48.3 yrs | 1972–77 to 1998 | + |
| Beulens 2007 | Netherlands | 1,417 | 49–70 yrs | 1993–97 to 2005 | ++ |
| Elwood 2013 | Caerphilly, UK | 1,320 men | 45–59 | 1979–04 | + |
| Emberson 2005 | England,Wales, and Scotland | 7,735 | 40–59 | 1978/1980 to 1998/2000 | ++ |
| Englund 2013 | Sweden | 778 | 49–61 | 85–08 | + |
| Flood 2008 | USA | 49238 | Older than 50 y | 1995–1996 to 2000 | ++ |
| Iso 2004 | Japan | 19 544 | 40–59 | 1990–2000 | + |
| Lin 2005 | Japan | 110,792 | 40 to 79 years | 1988–1990 to 1999 | ++ |
| Moayyeri 2009 | UK | 25311 | 40–75 years | 1993–1997 to 2007 | + |
| Ostbye 2002 | US | HRS study: 7,845 people | HRS study: ages 51–61 yrs | HRS: 92–98 | - |
| Otani 2003 | Japan | 90004 | 40–59 Cohort 1. 40–69 Cohort 2 | Cohort I. After January 1, 1990–1999. Cohort II. January 1, 1993–1994–1999 | + |
| Qiu 2003 | China | 50069 | 40–80+ | 1994/1996-2000 | + |
| Sabia 2009 | England | 5,123 | Mean age 56 yrs | 97–99 to 02–04 | + |
| Sabia 2011 | France | 4073 men | Ages 40–50 for men and 35–50 for women | 10 yrs (1992 to 2002–04) | + |
| Stevens 2009 | England and Scotland | 1.29 million women | 96–01 to 05–07 Mean yrs of follow-up: 7.2 for cancer incidence; 8.9 for mortality | + | |
| Sun 2011 | US | 13,894 | 70+ | 84–00 | ++ |
| Tabak 2001 | Finland, Italy, Netherlands | 2,953 men (Finland: 1,186 men; Italy: 1,183; Netherlands: 667) | 40–59 | 20 yrs (1965–70 to 1990) | - |
| Virta 2010 | Finland | 1,486 | Mean age in 1981: 51.7 yrs (SD: 6.1) | 1975–81 to 1999–07 (mean follow-up: 22.8 yrs.) | ++ |
| Wannamethee 2002 | England,Wales, and Scotland | 7157 | 40-59yrs | (‘78–’80)-(2000) | + |
| Waki 2005 | Japan | 28,893 | 40–59 yrs | 10 yrs (baseline: 1990) | + |
| Wannamethee 2003 | England, Wales, Scotland | 7,608 men | 40–59 yrs | 1978–80 to 1983–85 | + |
| Wang 2010 | US | 19,220 | 38–89 yrs | 12.9 yrs. follow-up (baseline: 1992–95) | + |
| Willcox 2006 | Island of Oahu | 5820 | 45-68yrs | Not reported | ++ |
| Xu 2010 | South East Queensland, Australia | 564 | 45–60 yrs | 2001–06 | - |
| Weight Change/Weight Cycling | |||||
| Field 2009 | United States | 44842 | 30–55 | 16 years | + |
| Langlois 2001 | United States | 2180 | 50–74 | 22 years | ++ |
| Ravona-Springer 2013 | Israel | 10000 | 40–70 | 36 years | + |
| Waring 2010 | United States | 1577 | 40–50 | 11 years | ++ |
| Agrigoroaei 2011 | United States | 4995 | 33–84 | 9–10 years | ++ |
| Elwood 2013 | Caerphilly, UK | 1,320 men | 45–59 | 1979–04 | + |
| King 2007 | United States | 15708 | 45–64 | 11–13 years | ++ |
| Leisure/Cognitive Activity/Social Networks | |||||
| Bielak 2012 | Australia | 7152 | 20–24: 2404, 40–44: 2530, 60:64: 2551 | 7 years | ++ |
| Britton 2008 | UK (England) | 5823 | 35–55 | 17 years | + |
| Friedland 2001 | United States | 193 cases/358 controls (for total study, not reported for 40–59 year olds) | 40–59 | >12 (not fully reported) | - |
| Holtzman 2004 | United States | 354 | 50+ | 12.4 years | ++ |
| Kareholt 2011 | Sweden | 1643 | 57.4 | 20+ years | ++ |
| Raikkonen 2001 | United States | 541 | 42–50 | 9.2 years | ++ |
Guide: i. Data is from multivariate models. Where multiple models have been reported data from the most adjusted (or most relevant) model has been used. ii. Results: + = significant positive association, – = significant inverse association, 0 = no significant association; √ = study that shows improved outcomes; X = study that shows poorer outcomes. iii. Quality of study [++/+/-]. ++ = high quality; + = moderate quality; - = low quality.
Note 1: A positive association (+) with PA is the better outcome
Note: 2 Physical inactivity where multiple outcomes have reported the most adjusted data in this table; sig = p</ = 0.05; ns = not significant (p>0.05)
Note 3: A positive association (+) with smoking is a worst outcome
Note 4: A positive association (+) with alcohol is the worst outcome
Longitudinal associations have been tabulated and synthesised narratively. Data specific to health inequalities has been summarised separately. Due to the methodological and statistical heterogeneity it was not appropriate to conduct a meta-analysis.
Results
Search Results
The searches for primary studies and the grey literature located 10,338 articles after removing duplicates, 567 of which had relevant titles and abstracts. In total, 164 observational longitudinal cohort studies were included in the review (Fig 1). The behavioural risk factors for which we found published data in mid-life with relevant outcomes in later life were: physical activity and inactivity; diet; tobacco consumption; alcohol; weight change or weight cycling; leisure, cognitive activity or social networks; and combinations of the above. Studies of behaviour related to hearing or vision were sought, but none met the inclusion criteria.
Quality
Overall, the quality of studies is good (most studies were rated as high or moderate quality). Summary quality scores can be found in Table 1. Full quality assessments can be found in S2 Table.
Characteristics of included studies
Summary characteristics for studies by health behaviours are listed in Table 1. Forty-five papers were found relating to mid-life physical activity (PA) or inactivity, 48 for diet, 57 for smoking and 24 for alcohol. Four studies were included that reported an association between weight change patterns in mid-life and later outcomes. Three studies reported data for combinations of lifestyle behaviours. Four studies were found that examined the relationship between mid-life leisure/social activities and DDF outcomes. The data relating studies found and health behaviour is reported in Table 2.
Table 2. Exposure and Outcomes.
| Physical activity and physical inactivity | Diet | Smoking | Alcohol | Weight change and cycling | Combined | Leisure/cognitive activity/social networks | |
|---|---|---|---|---|---|---|---|
| Healthy Ageing / Quality of Life / Well-being | [30]; [39]; [46]; [59]; [31]; [38]; [42]; [37]; [32] | [71]; [30]; [115]; [112]; [116]; [72] | [120]; [30]; [163]; [121]; [147]; [122]; [148]; [207]; [209]; [37]; [118] | [46]; [32]; [173]; [174] | No evidence was identified | No evidence was identified | [30] |
| Disability / Frailty | [34]; [41]; [40]; [49]; [33]; [35]; [37]; [36]; [43] | [60]; [73]; [117] | [120]; [40]; [59]; [43] | [46]; [40]; [122]; [37]; | [177] | [46] | [181] |
| Dementia & Cognition | [44]; [48]; [47]; [133]; [46]; [131]; [50]; [45]; [51]; [52] | [46]; [74]; [80]; [76]; [79]; [78]; [81]; [52] | [26]; [126]; [134]; [125]; [129]; [52]; [132]; [127]; [124] | [165]; [46]; [52]; [166]; [164]; | [178] | [184]; [46] | [181]; [49]; [182]; [183] |
| Overall mortality | [46]; [57]; [54]; [53]; [55]; [161]; [56] | [71]; [46]; [82]; [75]; [85]; [84]; [83] | [140]; [137]; [152]; [139]; [134]; [132]; [118]; [119] | [46]; [167]; [168]; [119]; | [179] | [46] | No evidence was identified |
| Cardiovascular (CVD) Outcomes | [46]; [57]; [62]; [53]; [60]; [28]; [61]; [56] | [71]; [97]; [98]; [96]; [111]; [100]; | [27]; [141]; [142]; [153]; [140]; [150]; [137]; [154]; [149]; [152]; [139]; [147]; [138]; [135]; [89]; [151]; [146]; | [93]; [46]; [167]; [171]; [89]; [169]; [170]; | No evidence was identified | [46]; [186] | [151] |
| Diabetes / Metabolic Syndrome | [65]; [63]; [64]; [66] | [46]; [106]; [66]; [104]; [105]; [102]; | [63]; [149]; [158]; [155]; [66]; [156]; [68] | [46]; [172] | [180] | [46] | No evidence was identified |
| Cancer | [46]; [68] | [46]; [107]; [108]; [83]; [109] | [162]; [159]; [160]; [161]; | [46]; [176]; [159]; [161]; | No evidence was identified | [46] | No evidence was identified |
| Mental health | [69]; [70]; | [114]; [113]; [70] | [120] | [70] | No evidence was identified | No evidence was identified | No evidence was identified |
Exposures were measured a number of different ways in the included studies. For physical activity all studies used self-reports of activity except one, which used an accelerometer. Dietary data was also self-reported through interview and food questionnaires. For smoking, many studies used self-reports with questionnaires and interviews with a number of studies using biochemical testing to confirm smoking status. All studies assessing the impact of alcohol, weight change/weight cycling and leisure and social activities used self-administered lifestyle questionnaires and interviews. The outcome and exposure measures are comprehensively reported in S4 Table.
Only four studies explicitly examining health inequalities were identified: three looking at gender or ethnicity [26–28] and one at lower socioeconomic status groups [29].
Summary of health behaviours and outcomes
A full summary of studies, exposures and outcomes are reported in S3 Table. An overview of reported outcomes in relation to health behaviours is shown in Tables 3 and 4. Due to the large volume of data found only the main findings are reported below.
Table 3. Overall Summary of Studies.
| Results: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Successful ageing | Disability and frailty | Dementia | Total mortality | CVD outcomes (events and mortality) | Diabetes (MetS) | Cancer (and cancer mortality) | Other chronic diseases | Mental health |
| Physical Activity | ||||||||
| √√√ | √√√√√ 0 | √√√√√√ 00 | √√√√√ | √√√√√√√√√ | √√√√√ | √√ X 000 | √ 0 | |
| Physical Inactivity | ||||||||
| 0 | 0 | X0 | ||||||
| Tobacco | ||||||||
| XXX | XX (mobility) 0X0X (fract) | XXXXXX (dementia) 00XXX (cognition) | X(XXX)XXX √√√√√√ (Ex-smokers) | XXXXXX0 (mortality) XXXXXXXXXXX0 (CVD) | XXX0 (Dia) X0 (MetS) | XXXXXX (lung, pancreatic,colorectal,cancer) | X (kidney disease) 0 (ex smoker) | No studies |
| Smokeless Tobacco | ||||||||
| √ (insulin, weight) | ||||||||
| Alcohol | ||||||||
| √X | X (ADL)0X (fract) | X0 (dementia APOE4) 0 (cognition) XX (Abstainers (compared to mod or infreq) XXX Heavy drinkers (APOE4 compared to non-drinkers) | X | 000 XX (Heavy cf occasional) √ (Reg cf occasional) | X (Diabetes—mod or high) X0√ (MetS) | 000X | 0 (COPD) | √ |
| Weight Change | ||||||||
| X (hip fracture) Weight loss of > 10% from max | X Weight variability | 0 Weight cycling | X Weight cycling when OW at midlife | |||||
| Leisure, Cognitive Activities and Social Network | ||||||||
| 0 | 0 | √ (dementia) √√√ (cognition) | ||||||
| Combined Lifestyle | ||||||||
| √0 (cog) | √ √ | √0 | 0 | 0 | ||||
Key: 0 = no significant association; √ = study that shows improved outcomes; X = study that shows poorer outcomes
Table 4. Overall Summary of Studies of Diet and Dietary Component.
| Results: | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diet or components of diet | Successful ageing | Disability & frailty | Dementia | Total mortality | CVD outcomes | Diabetes (MetS) | Cancer | Other chronic diseases | Mental health |
| Healthy dietary pattern | √√ | √ | √ | 0 | √ | ||||
| Mediterranean diet | √ | √√ | √ | ||||||
| Western diet | X | ||||||||
| Fruit and vegetables | √ 0 0 | √ 0 | √√ 0 | √ 0 | 0 | 0 0 | √ | ||
| Fat (saturated) | X | X | 0 | X | |||||
| Fat (polyunsaturated) | √ | 0 | |||||||
| Fat (monounsat) | 0 | ||||||||
| Fat (total) | 0 | X | |||||||
| Fish | X | √ 0 | √ women 0 men | √ | |||||
| Meat | √ (>1 per 2 d) | √ (1-2/wk) | |||||||
| Red and processed meat | X | X | X X | ||||||
| Coffee | 0 | √ 0 | X (heavy) | √√ | √√ | 0 | |||
| Tea | √ | 0 | |||||||
| Caffeine | √ | 0 | |||||||
| Milk | √ | ||||||||
| Salt | X | ||||||||
| Glycaemic index/GL | √0 | ||||||||
| Protein | 0 | 0 | |||||||
| Chocolate | √ (1–3 times/month only) | ||||||||
| Fibre | 0 | ||||||||
| Micronutrients | 0 | √ | 0 | ||||||
| Flavonoids | 0 | √ | 0 | ||||||
Key: 0 = no significant association; √ = study that shows improved outcomes; X = study that shows poorer outcomes
The impact of physical activity (PA) and physical inactivity (PI)
Summary data for physical activity, physical inactivity and sedentary behaviour studies, including study location, duration, period of follow-up, effect sizes, and quality scores are reported in Table 3. Note that we found no studies specifically looking at the consequences of physical inactivity or sedentary behaviour for dementia and cognition outcomes, diabetes/metabolic syndrome, or mental health.
Healthy ageing and well-being
There is consistent evidence [30–32] that PA in mid-life is positively associated with healthy and successful ageing outcomes. Healthy ageing or successful survival was defined in the three included studies as having no history of major chronic diseases and no cognitive impairment, physical impairment, or mental health limitations. No studies specifically aimed to assess physical inactivity; most studies reported physical activity in quintiles so reported lower levels of PA indirectly. No studies we found assessed quality of life.
Disability/frailty
There is consistent evidence that PA in mid-life is related to more positive outcomes in terms of disability and frailty in later life. Five of the six studies reported beneficial associations between mid-life PA and physical mobility [33] or physical functioning [34] or disability [35–37]. One study [38] reported no significant association with disability. Another study [39] found no association between inactivity in leisure time PA in mid-life and disability at age 75.
Three studies reported on the association between mid-life PA and bone fractures or bone health. One study reported less risk of hip or wrist fractures [40,41]. One reported improved bone mineral density in those who took part in PA in mid-life [42], and another reported no significant association with the risk of osteoarthritis [43].
Dementia & cognition
There is consistent evidence that PA in mid-life is associated with lower risk of dementia or better cognitive function in later life. Of the six prospective studies reporting on dementia or Alzheimer’s disease, four studies reported a significant beneficial association [44–47] and four studies non-significant associations [48–51]. Two studies found a significant inverse association between light and regular PA, but not for heavy PA [47,49]. Two studies found a positive association between mid-life PA and improved cognitive function in later life [34,52].
Overall mortality
There is consistent evidence [46,53–56] that regular, moderate and high intensity PA in mid-life is related to lower mortality in later life. One study [46,56] reports lower all-cause mortality but another [57] found no significant relationship between leisure time inactivity in mid-life and all-cause mortality.
Cardiovascular outcomes
There is strong evidence [28,46,53,54,58–62] of the beneficial effect of mid-life PA on CVD events or mortality. Six papers reported a significant association between mid-life PA and stroke [59], CVD risk [56,62], coronary heart disease (CHD) events [54,56,60], myocardial infarction [28], ischaemic heart disease [54] and three papers reported lower CVD-related mortality from stroke [61], CHD [56] and CVD [53] related to PA in mid-life.
Another [57] found a significant positive relationship between a single item measure of leisure time inactivity in mid-life and CVD mortality. However, no significant association was found when an index measure of leisure time physical activity was used.
Diabetes/metabolic syndrome
There is some consistent evidence that PA in mid-life is related to lower incidence of diabetes in later life [46,60,63,64]. In addition, three studies reported a beneficial association between mid-life PA and diabetes preconditions, two reported metabolic syndrome [63,65] and one reported insulin sensitivity [66].
Impact on cancer
The evidence relating to the associations between PA in mid-life and cancer is mixed. Four studies [46,53,66,67] reported longitudinal associations between mid-life physical activity and cancer or cancer mortality; however no significant relationship between mid-life PA and incident and fatal pancreatic cancer [67], lung, stomach, colorectal, lymphatic/hematopoietic cancers [68], or cancer mortality [53,68] was observed. One study [68] found lower rates of total cancer, upper digestive tract cancers (oral, oesophagus, stomach cancer) in those who participated in moderate or vigorous PA at mid-life, and increased risk of bladder cancer in those who participated in vigorous exercise compared to those who did not. Conversely, total cancer mortality was lower in those who took part in moderate or high levels of PA [46].
Impact on mental health
The evidence for an association between mid-life PA and mental health is inconclusive. One prospective cohort study [69] reported less risk of anxiety and/or depression for heavy PA at five-year follow-up but not at 10 years. Another study [70] found no significant association between mental well-being, including anxiety and depression and mid-life PA.
The impact of overall diet and dietary patterns
Summary data, including study location, duration, period of follow-up, effect sizes, and quality scores for overall diet and dietary patterns is reported in Table 4.
Healthy ageing/quality-of-life/well-being
There is consistent evidence [30,71,72] that a ‘healthy’ diet in mid-life is related to healthy and successful ageing. A Mediterranean type diet is associated with more successful ageing [72]. A Western dietary pattern (characterised by high intakes of fried and sweet food, processed food and red meat, refined grains, and high-fat dairy products) was associated with less successful ageing. In these studies healthy ageing is defined as no major chronic diseases or major impairments in cognitive or physical function or mental health. No studies we found assessed quality of life or wellbeing.
Disability/frailty
There is limited evidence that ‘healthy’ diet or dietary patterns in mid-life is related to better functioning. One study reported associations between mid-life diet and ADL [73], in which men who ate meat at least once every two days or more were less likely to have impairment in ADL.
Dementia & cognition
There is limited evidence that ‘healthy’ diet or dietary patterns in mid-life is related to dementia and cognitive functioning in later life. Two studies reported beneficial associations between fruit and vegetable intake in mid-life and dementia [46,74]. Two studies examined relationships between coffee consumption in mid-life and dementia [75–77]. One study reported that moderate coffee consumption was associated with lower risk of dementia, but not tea drinking [75,77]. Another found no significant association with dementia [76]. One reported a non-significant relationship between mid-life dietary antioxidant intake, flavonoids and dementia [78]. A greater risk of dementia was also reported in those consuming moderate compared to low amounts of saturated fat compared to those consuming moderate compared to low amounts of polyunsaturated fat [79].
One study reported better cognitive outcomes for those consuming a ‘healthy pattern’ diet [80], characterised as consumption of fruit, whole grains, vegetables, and negatively correlated with meat and poultry, refined grains, animal fat, and processed meat. More than two portions of fruit and vegetables a day was associated with better cognitive performance [52]; however, two studies reported no significant association with cognitive function [46,81]. Higher levels of total or saturated fat were associated with greater cognitive impairment in later life [82].
Overall mortality
The evidence on the impact of diet/dietary patterns on mortality is mixed. Three studies reported associations between fruit and/or vegetable intake and total mortality. One reported significantly lower risk of death in people consuming higher levels of fruit and vegetables at mid-life [83], another reported significantly lower overall mortality for each increase of 20g/day in vegetable intake [84]. Associations between >3 portions fruit and vegetables/day were not significant in one study [46]. There is limited evidence for increased mortality with greater fish consumption in those at high risk of CHD [85].
Cardiovascular outcomes
Evidence suggests a healthy diet has a positive impact on cardiovascular outcomes. Two studies reported beneficial effects of a Mediterranean diet pattern with lower risk of CHD events and mortality [86,87]. One reported fruit and vegetable intake was associated with lower CVD mortality [83]; however another [46] reported non-significant associations. Findings from one study [88] suggest that high intakes of flavonoids may be associated with decreased risk of ischaemic stroke and possibly with reduced CVD mortality.
One study [89] reported lower risk of cerebrovascular disease in those consuming meat one-two times a month compared to those consuming no meat or those who ate meat more than once a week. A lower risk of CHD events and mortality was found in women when meat was replaced with fish [90]. Another study reported no significant association between fatty fish consumption and heart failure but lower risk of heart failure in those consuming marine omega 3 fatty acids once a week [91]. Higher intakes of marine omega 3 fatty acids were not significantly associated with heart failure.
Heavier coffee drinkers showed a higher risk of CHD events and mortality compared to moderate coffee drinkers [92]. Associations were not significant for light or no coffee drinking compared to moderate intake.
A higher risk of CVD was reported for those consuming diets with the highest compared to the lowest dietary glycaemic index and glycaemic load [93]. In another study associations between glycaemic index and glycaemic load, and CVD events were not significant [91]. One study found no significant associations between mid-life protein intake and ischemic heart disease [94].
One study reported no significant associations between total, saturated, monounsaturated or polyunsaturated fat and fatal or non-fatal cardiovascular events [95].
Fruit and possibly vitamin E intake may also have protective effects against COPD [96]. In men, diets higher in fruits and vegetables and lower in meats (except fish) may reduce the risk of developing high blood pressure [97]. In women results of one study [98] suggest that higher intake of dietary magnesium may have a modest effect on the development of hypertension. Intakes of low-fat dairy products, calcium, and vitamin D were each inversely associated with risk of hypertension in middle-aged and older women [99]. While higher intake of all fruits (but not all vegetables) was significantly associated with reduced risk of hypertension in women [100]; higher intake of saturated fats, monounsaturated fats, and trans fats were each associated with increased risk of hypertension among middle-aged and older women [101].
Diabetes/metabolic syndrome
The evidence for the impact of diet on diabetes and metabolic syndrome is mixed. One study reported that a dietary pattern low in staples and high in milk was associated with lower risk of diabetes [102]. Another reported no statistically significant association between fruit and vegetables and diabetes [46]. Higher saturated fat intake at mid-life was associated with lower insulin sensitivity [66]. One study [103] reported lower risk of diabetes in women eating moderate and high amounts of fish and shellfish with a significant trend with greater fish and shellfish intake. One study found increased risk of diabetes in those consuming higher levels of red and processed meat with a significant trend from lower to higher intake [104].
Two studies reported lower risk of diabetes with coffee intake. One conducted on men and women found a significant trend towards lower risk for diabetes with increasing coffee consumption [105]. The other [106] reported a significant inverse relationship for women consuming three or more cups of coffee a day with a significant trend. In men only one-two cups/day was significantly associated with lower risk of diabetes but there was also a significant inverse trend between coffee consumption and diabetes.
Impact on cancer
There is inconsistent evidence regarding the impact of diet/dietary patterns on cancers. One study [107] found no clear associations between four dietary patterns and cancer. Two studies reported no significant associations between fruit and vegetables and cancer incidence or mortality [46,83]. One study reported lower risk of colorectal cancer with consumption of fruit, dietary calcium, vitamin A and vitamin C; but higher risk of colorectal cancer with consumption of red and processed meat [108]. The study reported lower incidence of colorectal and rectal cancer in those consuming high volumes of milk but found no significant associations between protein and fibre intake with colorectal cancer. Another study [109] found a significant association between high salt intake and higher risk of gastric cancer in men but not women. One [110] reported lower risk of heart failure when chocolate was consumed 1–3 times month compared to no chocolate consumption. There is no significant relationship reported between flavonoids and total cancer or site-specific cancers [111].
Impact on mental health
There is limited evidence regarding the impact on mental health. One study [112] reported less psychological distress in those with the highest compared to lowest adherence to the Mediterranean diet. Light or heavy coffee consumption was also associated with lower risk of severe depression [113]. However, one [70] reported no significant association between coffee drinking and anxiety, depression or psychological symptoms, but reported lower scores on the mental health scale on the SF-36 general health questionnaire. There was no association with tea or caffeine intake. One study [114] found no association between dietary zinc intake and depression.
Impact on other conditions
There is limited evidence regarding the impact of diet on other outcomes. One study reported intake of fruits and vegetables may reduce long-term risk of weight gain in middle-aged women [115]. Another study in women reported that weight gain was inversely associated with the intake of high-fibre, wholegrain foods but positively related to the intake of refined-grain foods [116]. Higher coffee and caffeine intake was also associated with a significantly lower incidence of Parkinson’s Disease [117].
The impact of tobacco
Summary data, including study location, duration, period of follow-up, effect sizes, and quality scores for tobacco is reported in Table 3.
Healthy ageing/quality-of-life/well-being
There is consistent evidence demonstrating a detrimental association between smoking and healthy ageing, quality of life or well-being outcomes. One study [30] showed that not smoking was related to a favourable older life; another [118] found that never-smokers lived longer than heavy smokers, and their extra years were of better quality. Health-related quality of life deteriorated with an increase in daily cigarettes smoked in a dose-dependent manner. The third [119] suggests that ‘ever smoking’ is associated with overall survival and a borderline association with exceptional survival (i.e. free of a set of major diseases and impairments).
Disability/frailty
There is consistent evidence demonstrating the dose-response relationship between smoking and impaired mobility. One study [120] suggests that a history of smoking, especially heavy smoking, with or without quitting, is associated with an earlier onset, and more rapid development, of mobility problems during the transition from middle age to old age. Another [37] showed a consistent adverse dose-response relationship between smoking and ill-health (i.e. disability, impaired mobility, health care utilisation, self-reported health).
There is inconsistent evidence demonstrating associations between smoking and low energy fractures. One [121] showed that among women, smokers had a higher risk for vertebral fractures than non-smokers. Among men, smokers had a greater risk for low energy fractures, vertebral fractures, proximal humerus fractures, and hip fractures. Other papers showed no association between smoking and wrist fractures [40] or osteoporotic fractures [122]. One study [43,123] found a positive association between smoking and risk of osteoarthritis. No studies we found assessed frailty.
Dementia & cognition
There is strong evidence demonstrating the association between smoking in mid-life and dementia, or cognitive decline, in later life. The association between smoking and specific types of dementia is less clear. In most studies smoking was strongly associated with dementia [26,124–127], subsequent risk of hospitalisation with dementia [26], and with being diagnosed with dementia [128]. Two studies [52,129] showed an association between smoking and cognition. One [51] showed that smoking in middle age is associated with memory deficit and decline in reasoning abilities; long-term ex-smokers (compared to current smokers and recent ex-smokers) are less likely to have cognitive deficits in memory, vocabulary, and verbal fluency. Another [130] reported greater decline in memory function, cognitive flexibility, and cognitive function among smokers. The declines in all cognitive domains were larger with increasing number of pack-years smoked. Only one study [131] found that smoking at baseline was not associated with change in cognitive decline whilst another [132] looking at dementia death failed to demonstrate an association with smoking. In another study [133], mid-life smoking was associated with an increased rate of progression of vascular brain injury, global and hippocampal atrophy.
Overall mortality
There is strong evidence demonstrating a dose-response relationship between smoking in mid-life and total mortality. Compared to never smokers, smokers are at increased risk of mortality. Five studies [118,134–137] showed that current smokers showed the highest risks of total mortality with a dose-response relationship with increasing number of cigarettes smoked. One [135] showed that men smoking persistently were most at risk, while those who persisted in quitting had no increased risk of death compared with non-smokers. Another [138] concluded that smokers across the entire range of pulmonary function may increase their expectation of lifespan by giving up smoking. Finally, one study [139] showed that compared to current smokers, never smokers, long-term quitters and new quitters had a decreased risk of all-cause mortality; the same association was observed for never smokers and long-term quitters for other non-lung cancer mortality. Compared to those who maintained their smoking habit, individuals that reduced or quit smoking had a decreased risk of all-cause mortality [140].
Cardiovascular outcomes
Smoking or having smoked is consistently associated with increased risk of cardiovascular mortality and CVD. Six studies provide evidence of a strong association between smoking and cardiovascular mortality [137,139–143], with current smokers being more likely to die from cardiovascular events compared to non-smokers. Only one study didn’t support this association [144]. Other studies provide evidence of a strong association between smoking and cardiovascular events and outcomes [59,134,145–153]. The highest risks for both CHD events and stroke were seen in heavy current smokers [134]. Smoking also increases the risk of CHD in men of all APOE genotypes but particularly in men carrying the e4 allele [145,154]. Only one showed no association between smoking and CHD [146]; all other studies have shown an association between smoking and stroke [59,146,147] and myocardial infarction [148].
Diabetes/metabolic syndrome
Cigarette smoking is an independent and modifiable risk factor for type 2 diabetes; however, the evidence for insulin sensitivity and metabolic syndrome is not sufficient to conclude. Three studies [68,155,156], demonstrated cigarette smoking is an independent and modifiable risk factor for type 2 diabetes and one did not [157]. One [68] reported the risk of diabetes in those who switched from smoking cigarettes to pipe or cigars remained equal to the risk in continuing cigarette smokers. Other studies showed that smoking is a risk factor for type 2 diabetes independently of BMI and physical activity [155]; however another [157] found that smoking was associated with the metabolic syndrome but not diabetes. Finally, one study [66] found no significant association with insulin sensitivity and smoking in men.
There is some evidence to suggest that the use of smokeless tobacco in mid-life is related to type 2 diabetes [158]. The use of smokeless tobacco was associated with low insulin response but not low insulin sensitivity.
Impact on cancer
Evidence showed a consistent association between smoking and cancer with a dose-response effect. The dose-response and exposure association seems to depend on the type of cancer considered [134,159,160]; current cigarette smokers showed the highest risk of total cancer with a strong dose-response effect. In a study [161] looking specifically at pancreatic cancer in women, pancreatic cancer incidence was greater in current smokers, with the risk increasing with the number of cigarettes smoked. One [159] showed that smoking was significantly associated with colorectal cancer in men and not significantly in women. Current cigarette smoking is associated with an elevated lung cancer risk approximately 10- to 20-fold higher for squamous cell and small cell carcinoma and 2- to 3-fold higher for adenocarcinoma in both men and women [160]. Another [162] confirmed the strong association between smoking and cancer, and cancer-related mortality. Compared to current smokers, never smokers, long-term quitters and new quitters also had a decreased risk of lung cancer mortality [139].
Impact on other conditions
One study reported that being a smoker was significantly associated with weight loss [163].
No studies were identified which assessed the impact on mental health.
The impact of alcohol
Summary data, including study location, duration, period of follow-up, effect sizes, and quality scores for alcohol is reported in Table 3.
Disability/frailty
Two studies reported higher odds for ill-health and osteoporotic fractures among alcohol drinkers compared to non-drinkers, while one study found no link between alcohol intake and wrist fractures [40]. Conversely, the risk for any incident osteoporotic fracture was reported to be higher among male alcohol users compared to male teetotallers [122]. A large study [37] showed that respondents with a past drinking problem had the highest odds for ill-health in terms of ADL dependence, difficulty climbing stairs, difficulty walking, poor health, and hospitalization. No studies we found assessed frailty.
Dementia & cognition
There is consistent evidence demonstrating an association between alcohol abstinence and/or heavy drinking and cognitive impairment [52,164–166]. Compared to moderate alcohol intake, alcohol abstinence was associated with a higher risk of poor executive function and poor memory [52]. One study reported no association with impairment cognition or dementia [46].
Overall mortality
Evidence was mixed for associations between alcohol consumption and all-cause mortality. One study [119] reported excessive alcohol consumption was associated with overall and exceptional survival at age 85 years. One [167] showed that alcohol intake was associated with a higher risk for all-cause mortality. Another [168] showed that excessive mortality was significantly associated with heavy drinking among men diagnosed with cancer and cardiovascular disease in this time period. One reported no association between ethanol consumption and chronic obstructive pulmonary disease mortality [169].
Cardiovascular outcomes
The evidence regarding alcohol use and cardiovascular outcomes is inconsistent. Three studies [167,170,171] showed a significant association between alcohol intake and cardiovascular outcomes. Regular drinkers had a significantly lower risk of major CHD events, CHD deaths, and CVD deaths in comparison with occasional drinkers after full adjustment for lifestyle characteristics and pre-existing disease [170]. Heavy drinkers had a higher risk of both major CHD and stroke compared to occasional drinkers [167,171]. No significant associations were observed between alcohol intake and cardiovascular outcomes in three studies (e.g., disease development, death) [46,93,144].
Diabetes/metabolic syndrome
Findings were consistent with respect to the influence of alcohol intake on diabetes/metabolic syndrome. Three studies [172–174] found significant positive associations between alcohol intake levels and diabetes/metabolic syndrome, while one study [46] did not find an association.
Impact on cancer
There is mixed evidence demonstrating the absence of an association between alcohol intake and cancer. Three studies [46,175,176] did not find significant associations between alcohol intake and incident and fatal pancreatic cancer; cancer in general; and colorectal cancer, respectively. In contrast, another study [159] showed a significantly higher risk for colorectal cancer among men who drink alcohol compared to non-drinkers. That study [159] reported that approximately half of the reported colorectal cancer cases may be preventable by tobacco and alcohol controls in middle-aged Japanese men; however the portion attributable to alcohol is not known.
Impact on mental health
Evidence on the impact on mental health is limited. One study [70] of women indicated that past alcohol drinkers had lower anxiety than non-drinkers.
No studies were identified which assessed the impact on healthy ageing, quality of life, well-being.
The impact of weight change/weight cycling
Summary data, including study location, duration, period of follow-up, effect sizes, and quality scores for weight change/weight cycling is reported in Table 3.
Disability/frailty
There is limited evidence that weight loss of more than 10% of max body weight in mid-life is related to hip fractures [177]. The impact was statistically significant for those aged 50–64 and 65–74 years who were weight cycling (as defined by study authors) in mid-life.
Dementia and cognition
There is limited evidence to suggest that weight change in mid-life is related to dementia [178]. Those in the highest two quartiles of weight change had a significantly higher risk of dementia, independent of the direction of weight change.
Overall mortality
One study reported no association between weight cycling in mid-life and mortality [179].
Diabetes/metabolic syndrome
One study [180] reported longitudinal associations between weight change/weight cycling and diabetes. The impact was not significant for weight change or weight cycling but overall weight status was more important in those who were overweight or obese at increased risk of diabetes.
No studies were identified which assessed the impact on healthy ageing, quality of life, well-being, cardiovascular outcomes, cancer or mental health.
The impact of leisure, cognitive activities and social network
Summary data, including study location, duration, period of follow-up, effect sizes, and quality scores from leisure, cognitive activities and social network studies is reported in Table 3.
Healthy ageing/quality-of-life/well-being
There is little evidence to determine if leisure and cognitive activities, and an individual’s social network in mid-life are related to successful aging. One study [30] reported longitudinal associations between leisure, cognitive activities, social network and successful aging in both men and women. While there was a beneficial association these were non-significant. No studies we found assessed quality of life or well-being.
Dementia and cognition
There is some evidence that leisure and cognitive activities, and an individual’s social network in mid-life is related to a lower risk of cognitive decline in later life. One study [49] reported an association between diversity and intensity of participation in intellectual, physical and passive activities and lower risk of AD. Three studies [181–183] reported longitudinal associations between leisure, cognitive activities, social network and dementia or cognitive impairment. All studies reported a beneficial association between mid-life factors and dementia or cognitive decline in later life; however in one study the associations were non-significant for some activities including social, organisational and physical activities [183]. There is limited evidence for a prospective association between mid-life mental distress and dementia [184].
Cardiovascular outcomes
We found no evidence on the association between leisure activity, cognitive activity or social networks in mid-life and late life cardiovascular outcomes apart from one study which found no correlation with reduced hypertension in women [151].
No studies were identified which assessed the impact of these behaviours on disability, frailty, overall mortality, diabetes, cancer or mental health.
Combined behavioural risks
Summary data, including study location, duration, period of follow-up, effect size, and quality scores from combined lifestyle studies are reported in Table 3.
Dementia & cognition
There is limited evidence to suggest that combined healthy behaviours in mid-life is related to less risk of dementia in later life. Three studies [46,185,186] reported longitudinal associations between healthy behaviours and dementia or cognitive impairment; one study [185] reported that modifiable psychosocial and behavioural factors have protective effects on cognition and another [164] reported that higher levels of consumption was associated with higher risk of cognitive impairment, in particular that binge drinking was found to be an independent risk factor for cognitive impairment; however in one study the associations were non-significant[46].
Overall mortality
There is evidence to suggest that reducing unhealthy behaviours or adopting a healthier lifestyle in mid-life is related to reduced death. Two cohort studies [46,187] reported a significant negative association between number of unhealthy behaviours and mortality.
Cardiovascular outcomes
There is inconsistent evidence that reducing unhealthy behaviours or adopting healthier behaviours in mid-life is related to reduced CVD. One study reported a significant negative association between number of unhealthy behaviours and CVD [187] while the other [46] reported no association. Importantly the combined behaviours in these two studies vary.
Diabetes/metabolic syndrome
There is limited evidence that association exists between reducing unhealthy combined lifestyle behaviours in mid-life are related to diabetes. One study examined the impact of combined lifestyle on diabetes; while there was a negative correlation this was non-significant [46].
Cancer
There is no evidence that association exists between reducing unhealthy combined lifestyle behaviours and cancer. One study examined the impact of combined lifestyle on cancer and there was no correlation [46].
No studies were identified which assessed the impact on healthy ageing, quality of life, well-being, disability, frailty or mental health.
Disadvantaged and minority groups
For this review ‘disadvantaged and minority groups’ includes (but is not limited to) people of low socioeconomic status; ethnic minority groups; LGBT community groups; travellers; and other groups with protected characteristics under the equality and diversity legislation. One study examined associations between mid-life smoking and reported no difference in the risk of developing dementia by gender or ethnicity [26] or the risk of cardiovascular disease, total CHD or myocardial infarction by gender [143]. One [28] reported less risk of myocardial infarction in women participating in leisure time sports, however this result was based on a small sample size. High alcohol intake in lower SES groups is related to poorer cognitive performance[29]. No association between alcohol consumption and cognitive performance was found in those in intermediate or high socioeconomic groups. There is limited evidence and where evidence is available this is generally weak. Most of these groups also have substantially lower life expectancy than the general population, with fewer surviving into age groups of the highest risk.
Discussion
This review provides an overview of the available evidence from high quality observational studies reporting on the association between specific behavioural risk factors, in mid-life, and successful ageing and the primary prevention or delay of DDF and non-communicable chronic conditions. The conclusions of individual cohort studies are consistent with existing knowledge and best practice for a range of specific non communicable disorders (for example, reducing alcohol and tobacco consumption, and increasing physical activity and intake of fruit and vegetables).
The findings that consistently emerge as being of societal importance in their range of potential impact are physical activity, which is beneficial, and smoking is consistently found to be associated with DDF and non-communicable chronic conditions. Other factors for which the evidence base is not as strong are diet, alcohol, weight change and social/leisure activities.
For Physical Activity there was consistent evidence within the 45 studies identified that mid-life PA is associated with better late life DDF and NCC outcomes. Only two studies specifically focused on physical inactivity. This finding is in alignment with previous research that suggests regular PA can reduce the risk of chronic disease and improve one’s health and well-being whereas a lack of physical activity leads to poorer health [188–190].
The review found a wealth of evidence from longitudinal cohort studies relating to the association between mid-life smoking and poorer DDF and NCC outcomes. The impact of tobacco is well known as previous research demonstrates that tobacco consumption is an important risk factor for CVD, cancer and hypertension, and is a major cause of premature mortality [191,192]. Whilst smoking cessation is associated with weight gain and a subsequent increase in risk of diabetes, the long-term benefits of giving up smoking outweigh the adverse effects of early weight gain.
There is some consistent evidence from 48 studies that healthy diets have beneficial effects on DDF and NCC outcomes and higher consumption of saturated fat or processed and red meat is associated with poorer ageing, DDF and NCC outcomes. Previous research has also demonstrated a strong relationship between diet, dietary patterns or nutrient intakes, and prevention and management of chronic diseases [193,194] including diabetes [195] and CVD [196].
Evidence from 24 studies specific to mid-life alcohol consumption was mixed and inconsistent with smaller effect sizes than for smoking and physical activity. Some studies reported negative outcomes (e.g. for dementia, mortality and cancer) and some more positive outcomes (e.g. for ageing and mental health). Previous research suggests that alcohol is a significant cause of mortality, morbidity and social problems, both in developed and in developing countries [197], causing an estimated 4% of the global disease burden including chronic diseases and certain cancers, as well as poor health in general [198–200].
Four studies were included that reported an association between weight change patterns in mid-life and later outcomes, including increased risk of hip fracture, mortality, diabetes and dementia. For issues specific to weight change or weight cycling it is known that being overweight increases the risks for many chronic medical conditions, including diabetes, heart disease, hypertension and stroke [201]. Modest reductions in weight can lead to important health benefits [67,202], while fatigue and unintentional weight loss can affect an individual’s physical, psychological, social and cognitive functioning [203,204]. Combinations of lifestyle behaviours were also protective factors in relation to cognitive function, diabetes, vascular disease, cancer, dementia and mortality.
There is little evidence on the impact of leisure, cognitive activities and social network and combined lifestyles. Studies that focused on disadvantaged groups have tended to be few in number and of lower quality indicating that this is a neglected research area.
Limitations of the Evidence, Gaps
Limited evidence was found specifically relating to mid-life behaviours for leisure activities including cognitive activities and social networks, weight change and weight cycling and smokeless tobacco. While many diet-related studies were found they covered a broad range of diets and dietary components. There were some diets or dietary components for which studies specifically pertaining to mid-life was not found.
For the following exposures only one study was identified:
Evidence of the impact of PA on stroke [61]; CHD [56,205]; CVD [53]; bone mineral density [42]; insulin sensitivity [66]; and risk of anxiety and/or depression [69].
Evidence of the impact of diet, consumption of dietary calcium, vitamin A, vitamin C, zinc and flavonoids on CVD mortality [83], overall mortality [84], and psychological distress [206].
Evidence on the impact of tobacco on total cancer [134]; pancreatic cancer [175]; colorectal cancer [159]; risks of lung cancer [207] and lung cancer mortality [139]; cancer related mortality [162]; quality of life [118]; psychological distress [37]; impaired mobility [120]; risk of chronic kidney condition [208]; and smokeless tobacco on type 2 diabetes [209]; weight and weight maintenance [210].
Evidence on the impact of alcohol on major coronary heart disease and stroke [167]; ADL dependence, difficulty climbing stairs, difficulty walking, poor health, and hospitalization [37]; wrist fractures [40]; dementia [46]; COPD mortality [169]; incident and fatal pancreatic cancer [175,211]; cancer in general[46]; and colorectal cancer [176].
No studies were found which reported: physical inactivity and healthy ageing/quality of life/well-being, dementia, diabetes/metabolic syndrome, cancer or mental health; the relationship between alcohol consumption and healthy ageing/quality of life/well-being; weight change/weight cycling with healthy ageing/quality of life/well-being, CVD outcomes, cancer or mental health; impacts on disability/frailty, diabetes/metabolic syndrome, cancer, overall mortality, mental health or other chronic diseases; or the impact of combined lifestyle behaviours and healthy ageing/quality of life/well-being, disability/frailty, mental health or other chronic diseases; studies of behaviour related to hearing or vision. The challenge in assessing the impact of these exposures is a pronounced lack of data.
While no included studies reported relationship between mid-life physical activity and dementia, a recent meta-analysis [212] of prospective studies was published subsequently to the completion of the review and reported a fixed effects risk ratio of 0.61 (95% CI 0.52–0.73) indicating an overall reduction in risk of Alzheimer’s disease in physically active older adults.
There is also substantial heterogeneity in the measurement of exposures and outcomes included in this review making precise comparisons between studies problematic. While the review only includes longitudinal observational studies, which can show an association between mid-life risk factors and later life outcomes, associations from this type of study are not necessarily causal.
There was a paucity of data relating to disadvantaged groups covered by the equality and diversity legislation. While we found evidence assessing the risks of developing: dementia by gender or ethnicity [26]; CVD, total CHD or MI by gender [143], and in women [28] only, also alcohol intake in lower SES groups [29]. No longitudinal data was found relevant to other groups covered by the equality and diversity legislation such as LGBT groups or travellers. The absence of large-scale, high-quality research in these communities is problematic as there is a need for a more detailed and accurate picture of the disparities these communities face. Disadvantaged groups are significantly more likely to experience pervasive discrimination [213] and without clear evidence, the challenges faced are exacerbated. Whilst there have been some gains for visibility and equality for these communities, many disparities remain, particularly for women and the poorest (most vulnerable) in our society. Poorer health and well-being will persist, if high rates of discrimination are allowed to continue resulting in the experience of deeper inequality. Solutions to the disparities addressed above must focus both on reducing discrimination in general and improving conditions of living. Given that discrimination has a cumulative negative effect on health, there are plausible reasons for anticipating differences for disadvantaged groups or settings. These are the important considerations that should be made to ensure that inequities are reduced. There is therefore very little information about how mid-life behaviours in disadvantaged communities’ impact on population-level health outcomes in later life. More data is needed to understand how the behavioural risks are differentially modulated in these disadvantaged groups; with the aim of designing interventions that are fit for purpose.
Limitations of the Review
The remit of this rapid review was specifically to identify mid-life behavioural risk factors for DDF outcomes and common NCCs in later life. Determinants of DDF over the whole life course were not included. Pragmatically, due to the wide scope of the review, the large amount of literature covering behavioural risk factors and the outcomes of interest, and the timescales for the review, the searches were focused on studies with mid-life-related terms in the title, abstract or related MeSH indexing to identify those studies that specifically aimed to report on mid-life exposure. The implication is that cohort studies that have followed individuals from mid- to late life and reported associations of interest without specifying mid-life terms in the title or abstract were not identified by the searches. This might explain some of the gaps in evidence and further work is on-going to address this limitation.
Although we conducted a rapid systematic review it is unlikely that our overall conclusions would vary greatly had a systematic review been conducted over a longer period of time [199]. Another strength is that unlike other rapid reviews a quality assessment of the included studies was undertaken reducing the limitations associated with the evidence synthesis process and results [200].
Also, only OECD countries are included, which may impact on the implications of findings within the broader global health context. However, few studies from non-OECD countries were found in the searches.
Conclusion
The long-term impact of beneficial behavioural factors in middle to older age adults was greater chance of successful ageing and the primary prevention or delay of disability, dementia, frailty, and non-communicable chronic conditions. The exposures and health behaviours in mid-life identified in this review are important considerations both in identifying possible differential mechanisms for action (in order to reduce ill-health in the population) and the design of interventions to improve healthy behaviours.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
(DOCX)
(DOCX)
Acknowledgments
We wish to thank Frances Cater (Secretary/Administrator) for her administrative support. Written permission has been obtained for her contributions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the National Institute for Health and Care Excellence (NICE), invitation to tender reference DDER 42013, and supported by the National Institute for Health Research School for Public Health Research. The scope of the work was defined by NICE and the protocol was agreed with NICE prior to the start of work. The funders had no role in data analysis, preparation of the manuscript or decision to publish.
References
- 1.Kuh D, New Dynamics of Ageing (NDA) Preparatory Network (2007) A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci 62: 717–721. [DOI] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381: 752–762. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (2002) The World Health Report 2002: Reducing risks, promoting healthy life. World Health Organization. [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. (2013) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blane D. The life course, the social gradient and health In Marmot M, Wilkinson R, editors. Social Determinants of Health. Oxford University Press; 2006. pp. 54–77. [Google Scholar]
- 6.Newman AB, Glynn NW, Taylor CA, Sebastiani P, Perls TT, Mayeux R, et al. (2011) Health and function of participants in the Long Life Family Study: A comparison with other cohorts. Aging 3: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimäki M, Dugravot A (2011) Does cognitive reserve shape cognitive decline? Ann Neurol 70: 296–304. 10.1002/ana.22391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wills AK, Lawlor DA, Marthews FE, Aihie Sayer A, Bakra E, Ben-Shlomo Y, et al. (2011) Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med 8: e1000440 10.1371/journal.pmed.1000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K (2001) A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch Intern Med 161: 1703–1708. [DOI] [PubMed] [Google Scholar]
- 10.Khaw K-T, Wareham N, Bingham S, Welch A, Luben R, Day N (2008) Combined impact of health behaviours and mortality in men and women: The EPIC-Norfolk Prospective Population Study. PLoS Med 5: e12 10.1371/journal.pmed.0050012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myint PK, Smith RD, Luben RN, Surtees PG, Wainwright NW, Wareham NJ, et al. (2011) Lifestyle behaviours and quality-adjusted life years in middle and older age. Age Ageing 40: 589–595. 10.1093/ageing/afr058 [DOI] [PubMed] [Google Scholar]
- 12.Sabia S, Singh-Manoux A, Hagger-Johnson G, Cambois E, Brunner E, Kivimaki M (2012) Influence of individual and combined healthy behaviours on successful aging. CMAJ 18: 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisinger C, Godtfredsen NS (2007) Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res 9: 631–646. [DOI] [PubMed] [Google Scholar]
- 14.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S (2010) Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 153: 182–193. 10.7326/0003-4819-153-3-201008030-00258 [DOI] [PubMed] [Google Scholar]
- 15.Echouffo-Tcheugui JB, Batty GD, Kivimäki M, Kengne AP (2013) Risk models to predict hypertension: a systematic review. PLoS One 8: e67370 10.1371/journal.pone.0067370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison SL, Ding J, Tang EY, Siervo M, Robinson L, Jagger C, et al. (2014) Cardiovascular disease risk models and longitudinal changes in cognition: a systematic review. PLoS One 9: e114431 10.1371/journal.pone.0114431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt-Lunstad J, Smith TB, Layton JB (2010) Social relationships and mortality risk: a meta-analytic review. PLoS Med 7: e1000316 10.1371/journal.pmed.1000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Micha R, Wallace S (2010) Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 7: e1000252 10.1371/journal.pmed.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Xiong X, Liu W (2013) Yoga for essential hypertension: a systematic review. PLoS One 8: e76357 10.1371/journal.pone.0076357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed N, Mandel R, Fain MJ (2007) Frailty: an emerging geriatric syndrome. Am J Med 120: 748–753. [DOI] [PubMed] [Google Scholar]
- 21.Andrew MK, Mitnitski AB (2008) Different ways to think about frailty? Am J Med 121: e21. [DOI] [PubMed] [Google Scholar]
- 22.Brayne C, Gao L, Dewey M, Matthews FE (2006) Dementia before death in ageing societies—the promise of prevention and the reality. PLoS Med 3: e397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews F, Brayne C (2005) Correction: The incidence of dementia in England and Wales: findings from the five identical sites of the MRC CFA Study. PLoS Med 2: e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Clinical Excellence. Methods for the development of NICE public health guidance. NICE. 2012. Available: http://publications.nice.org.uk/methods-for-the-development-of-nice-public-health-guidance-third-edition-pmg4. [PubMed]
- 25.ISSG Search Filters Resource. Available: https://sites.google.com/a/york.ac.uk/issg-search-filters-resource/home
- 26.Alonso A, Mosley TH, Gottesman RF, Catellier D, Sharrett AR, Coresh J (2009) Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 80: 1194–1201. 10.1136/jnnp.2009.176818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baba S, Iso H, Mannami T, Sasaki S, Okada K, Konishi M, et al. (2006) Cigarette smoking and risk of coronary heart disease incidence among middle-aged Japanese men and women: The JPHC Study Cohort I. Eur J Cardiovasc Prev Rehabil 13: 207–213. [DOI] [PubMed] [Google Scholar]
- 28.Meisinger C, Löwel H, Heier M, Kandler U, Döring A (2007) Association of sports activities in leisure time and incident myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Eur J Cardiovasc Prev Rehabil 14: 788–792. [DOI] [PubMed] [Google Scholar]
- 29.Sabia S, Guéguen A, Berr C, Berkman L, Ankri J, Goldberg M, et al. (2011) High alcohol consumption in middle-aged adults is associated with poorer cognitive performance only in the low socio-economic group. Results from the GAZEL cohort study. Addiction 106: 93–101. 10.1111/j.1360-0443.2010.03106.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Britton A, Shipley M, Singh-Manoux A, Marmot MG (2008) Successful aging: the contribution of early-life and midlife risk factors. J Am Geriatr Soc 56: 1098–1105. 10.1111/j.1532-5415.2008.01740.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamer M, Lavoie KL, Bacon SL (2014) Taking up physical activity in later life and healthy ageing: the English longitudinal study of ageing. Br J Sports Med 48: 239–243. 10.1136/bjsports-2013-092993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Q, Townsend MK, Okereke OI, Franco OH, Hu FB, Grodstein F (2010) Physical activity at midlife in relation to successful survival in women at age 70 years or older. Arch Intern Med 170: 194–201. 10.1001/archinternmed.2009.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang IA, Guralnik JM, Melzer D (2007) Physical activity in middle-aged adults reduces risks of functional impairment independent of its effect on weight. J Am Geriatr Soc 55: 1836–1841. [DOI] [PubMed] [Google Scholar]
- 34.Chang M, Saczynski JS, Snaedal J, Bjornsson S, Einarsson B, Garcia M, et al. (2013) Midlife physical activity preserves lower extremity function in older adults: Age gene/environment susceptibility-Reykjavik study. J Am Geriatr Soc 61: 237–242. 10.1111/jgs.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmberg JJ, Miilunpalo SI, Pasanen ME, Vuori IM, Oja P (2006) Associations of leisure-time physical activity with mobility difficulties among middle-aged and older adults. J Aging Phys Act 14: 133–153. [DOI] [PubMed] [Google Scholar]
- 36.Patel KV, Coppin AK, Manini TM, Lauretani F, Bandinelli S, Ferrucci L, et al. (2006) Midlife physical activity and mobility in older age: The InCHIANTI Study. Am J Prev Med 31: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostbye T, Taylor DH, Jung SH (2002) A longitudinal study of the effects of tobacco smoking and other modifiable risk factors on ill health in middle-aged and old Americans: results from the Health and Retirement Study and Asset and Health Dynamics among the Oldest Old survey. Prev Med 34: 334–345. [DOI] [PubMed] [Google Scholar]
- 38.Lahti J, Laaksonen M, Lahelma E, Rahkonen O (2010) The impact of physical activity on physical health functioning—a prospective study among middle-aged employees. Prev Med 50: 246–250. 10.1016/j.ypmed.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 39.Christensen U, Støvring N, Schultz-Larsen K, Schroll M, Avlund K (2006) Functional ability at age 75: Is there an impact of physical inactivity from middle age to early old age? Scand J Med Sci Sports 16: 245–251. [DOI] [PubMed] [Google Scholar]
- 40.Englund U, Nordström P, Nilsson J, Hallmans G, Svensson O, Bergström U, et al. (2013) Active commuting reduces the risk of wrist fractures in middle-aged women—The UFO study. Osteoporos Int 24: 533–540. 10.1007/s00198-012-1988-8 [DOI] [PubMed] [Google Scholar]
- 41.Englund U, Nordström P, Nilsson J, Bucht G, Björnstig U, Hallmans G, et al. (2011) Physical activity in middle-aged women and hip fracture risk: the UFO study. Osteoporos Int 22: 499–505. 10.1007/s00198-010-1234-1 [DOI] [PubMed] [Google Scholar]
- 42.Nokes NR, Tucker LA (2012) Changes in hip bone mineral density and objectively measured physical activity in middle-aged women: a 6-year prospective study. Am J Health Promot 26: 341–347. 10.4278/ajhp.100622-QUAN-208 [DOI] [PubMed] [Google Scholar]
- 43.Szoeke CEI, Cicuttini FM, Guthrie JR, Clark MS, Dennerstein L (2006). Factors affecting the prevalence of osteoarthritis in healthy middle-aged women: Data from the longitudinal Melbourne Women's Midlife Health Project. Bone 39: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 44.Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M (2008) Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci 63: 62–66. [DOI] [PubMed] [Google Scholar]
- 45.Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, et al. (2005) Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol 4: 705–711. [DOI] [PubMed] [Google Scholar]
- 46.Elwood P, Galante J, Pickering J, Palmer S, Bayer A, Ben-Shlomo Y, et al. (2013) Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly Cohort Study. PLoS One 8: e81877 10.1371/journal.pone.0081877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, et al. (2010) The effect of midlife physical activity on cognitive function among older adults: AGES-Reykjavik Study. J Gerontol A Biol Sci Med Sci 65: 1369–1374. 10.1093/gerona/glq152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL (2008) Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement 4: 324–331. 10.1016/j.jalz.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen C, et al. (2001) Patients with Alzheimer's disease have reduced activities in midlife compared with healthy control-group members. Proc Natl Acad Sci U S A: 98: 3440–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan GS, Gallacher J, Bayer A, Fish M, Ebrahim S, Ben-Shlomo Y (2012) Physical activity in middle-age and dementia in later life: Findings from a prospective cohort of men in caerphilly, South Wales and a meta-analysis. J Alzheimers Dis 31: 569–580. 10.3233/JAD-2012-112171 [DOI] [PubMed] [Google Scholar]
- 51.Rovio S, Kåreholt I, Viitanen M, Winblad B, Tuomilehto J, Soininen H, et al. (2007) Work-related physical activity and the risk of dementia and Alzheimer's disease. Int J Geriatr Psychiatry 22: 874–882. [DOI] [PubMed] [Google Scholar]
- 52.Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A (2009) Health behaviors from early to late midlife as predictors of cognitive function: The Whitehall II study. Am J Epidemiol 170: 428–437. 10.1093/aje/kwp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu G, Tuomilehto J, Silventoinen K, Barengo NC, Peltonen M, Jousilahti P (2005) The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47 212 middle-aged Finnish men and women. Int J Obes 29: 894–902. [DOI] [PubMed] [Google Scholar]
- 54.Holtermann A, Mortensen OS, Burr H, Søgaard K, Gyntelberg F, Suadicani P (2009) The interplay between physical activity at work and during leisure time—risk of ischemic heart disease and all-cause mortality in middle-aged Caucasian men. Scand J Work Environ Health 35: 466–474. [DOI] [PubMed] [Google Scholar]
- 55.Menotti A, Lanti M, Maiani G, Kromhout D (2006) Determinants of longevity and all-cause mortality among middle-aged men. Role of 48 personal characteristics in a 40-year follow-up of Italian Rural Areas in the Seven Countries Study. Aging Clin Exp Res 18: 394–406. [DOI] [PubMed] [Google Scholar]
- 56.Yu S, Huang YS (2003) Knowledge of, attitudes toward, and activity to prevent osteoporosis among middle-aged and elderly women. J Nurs Res 11: 65–72. [DOI] [PubMed] [Google Scholar]
- 57.Haapanen-Niemi N, Miilunpalo S, Pasanen M, Vuori I, Oja P, Malmberg J (2000) Body mass index, physical inactivity and low level of physical fitness as determinants of all-cause and cardiovascular disease mortality—16 y follow-up of middle-aged and elderly men and women. Int J Obes Relat Metab Disord 24: 1465–1474. [DOI] [PubMed] [Google Scholar]
- 58.Meisinger C, Löwel H, Heier M, Kandler U, Döring A (2007) Association of sports activities in leisure time and incident myocardial infarction in middle-aged men and women from the general population: The MONICA/KORA Augsburg cohort study. Eur J Cardiovasc Prev Rehabil 14: 788–792. [DOI] [PubMed] [Google Scholar]
- 59.Harmsen P, Lappas G, Rosengren A, Wilhelmsen L (2006) Long-term risk factors for stroke: twenty-eight years of follow-up of 7457 middle-aged men in Göteborg, Sweden. Stroke 37: 1663–1667. [DOI] [PubMed] [Google Scholar]
- 60.Hu G, Jousilahti P, Borodulin K, Barengo NC, Lakka TA, Nissinen A, et al. (2007) Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis 194: 490–497. [DOI] [PubMed] [Google Scholar]
- 61.Pitsavos C, Panagiotakos DB, Chrysohoou C, Kokkinos P, Menotti A, Singh S, et al. (2004) Physical activity decreases the risk of stroke in middle-age men with left ventricular hypertrophy: 40-year follow-up (1961–2001) of the Seven Countries Study (The Corfu cohort). J Hum Hypertens 18: 495–501. [DOI] [PubMed] [Google Scholar]
- 62.Hu G, Tuomilehto J, Silventoinen K, Barengo N, Jousilahti P (2004) Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J 25: 2212–2219. [DOI] [PubMed] [Google Scholar]
- 63.Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim LL (2007) Leisure time physical activity in middle age predicts the metabolic syndrome in old age: results of a 28-year follow-up of men in the Oslo study. BMC Public Health 7: 154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu G, Qiao Q, Silventoinen K, Eriksson JG, Jousilahti P, Lindström J, et al. (2003) Occupational, commuting, and leisure-time physical activity in relation to risk for Type 2 diabetes in middle-aged Finnish men and women. Diabetologia 46(3): 322–329. [DOI] [PubMed] [Google Scholar]
- 65.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ (2005) Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy caucasians: The Medical Research Council Ely Study. Diabetes Care 28: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 66.Risérus U, Arnlöv J, Berglund L (2007) Long-term predictors of insulin resistance: role of lifestyle and metabolic factors in middle-aged men. Diabetes Care 30: 2928–2933. [DOI] [PubMed] [Google Scholar]
- 67.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, West DS, et al. (2001) Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 134: 1–11. [DOI] [PubMed] [Google Scholar]
- 68.Wannamethee SG, Shaper AG, Walker M (2001) Physical activity and risk of cancer in middle-aged men. Br J Cancer 85: 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiles NJ, Haase AM, Gallacher J, Lawlor DA, Lewis G (2007) Physical activity and common mental disorder: results from the Caerphilly study. Am J Epidemiol 165: 946–954. [DOI] [PubMed] [Google Scholar]
- 70.Xu Q, Anderson D, Courtney M (2010) A longitudinal study of the relationship between lifestyle and mental health among midlife and older women in Australia: findings from the Healthy Aging of Women Study. Health Care Women Int 31: 1082–1096. 10.1080/07399332.2010.486096 [DOI] [PubMed] [Google Scholar]
- 71.Akbaraly T, Sabia S, Hagger-Johnson G, Tabak AG, Shipley MJ, Jokela M, et al. (2013) Does overall diet in midlife predict future aging phenotypes? A cohort study. Am J Med 126: 411–419. 10.1016/j.amjmed.2012.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samieri C, Sun Q, Townsend MK, Chiuve SE, Okereke OI, Willett WC, et al. (2013) The association between dietary patterns at midlife and health in aging an observational study. Ann Intern Med 159: 584–591. 10.7326/0003-4819-159-9-201311050-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura Y, Hozawa A, Turin TC, Takashima N, Okamura T, Hayakawa T, et al. (2009) Dietary habits in middle age and future changes in activities of daily living—NIPPON DATA80. Gerontology 55: 707–713. 10.1159/000235906 [DOI] [PubMed] [Google Scholar]
- 74.Hughes TF, Andel R, Small BJ, Borenstein AR, Mortimer JA, Wolk A, et al. (2010) Midlife fruit and vegetable consumption and risk of dementia in later life in Swedish Twins. Am J Geriatr Psychiatry 18: 413–420. 10.1097/JGP.0b013e3181c65250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eskelinen MH, Tuomilehto J, Soininen H, Kivipelto M (2009) Healthy diet at midlife and the risk of late-life dementia and Alzheimer's disease: A population-based CAIDE study. Alzheimers Dement 5: 284. [DOI] [PubMed] [Google Scholar]
- 76.Laitala VS, Kaprio J, Koskenvuo M, Räihä I, Rinne JO, Silventoinen K (2009) Coffee drinking in middle age is not associated with cognitive performance in old age. Am J Clin Nutr 90: 640–646. 10.3945/ajcn.2009.27660 [DOI] [PubMed] [Google Scholar]
- 77.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M (2009) Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis 16: 85–91. [DOI] [PubMed] [Google Scholar]
- 78.Laurin D, Masaki KH, Foley DJ, White LR, Launer LJ (2004) Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu-Asia Aging Study. Am J Epidemiol 159: 959–67. [DOI] [PubMed] [Google Scholar]
- 79.Laitinen MH, Ngandu T, Rovio S, Helkala EL, Uusitalo U, Viitanen M, et al. (2006) Fat intake at midlife and risk of dementia and Alzheimer’s Disease: a population-based study. Dement Geriatr Cogn Disord 22: 99–107. [DOI] [PubMed] [Google Scholar]
- 80.Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Hercberg S, Galan P (2012) A healthy dietary pattern at midlife is associated with subsequent cognitive performance. J Nutr 142: 909–915. 10.3945/jn.111.156257 [DOI] [PubMed] [Google Scholar]
- 81.Nooyens AC, Bueno-de-Mesquita HB, van Boxtel MP, van Gelder BM, Verschuren WM (2011) Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Br J Nutr 106: 752–761. 10.1017/S0007114511001024 [DOI] [PubMed] [Google Scholar]
- 82.Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, et al. (2008) Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry 23: 741–747. 10.1002/gps.1969 [DOI] [PubMed] [Google Scholar]
- 83.Strandhagen E, Hansson PO, Bosaeus I, Isaksson B, Eriksson H (2000) High fruit intake may reduce mortality among middle-aged and elderly men. The study of men born in 1913. Eur J Clin Nutr 54: 337–341. [DOI] [PubMed] [Google Scholar]
- 84.Seccareccia F, Alberti-Fidanza A, Fidanza F, Farchi G, Freeman KM, Mariotti S, et al. (2003) Vegetable intake and long-term survival among middle-aged men in Italy. Ann Epidemiol 13: 424–430. [DOI] [PubMed] [Google Scholar]
- 85.Osler M, Andreasen AH, Hoidrup S (2003) No inverse association between fish consumption and risk of death from all-causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol 56: 274–279. [DOI] [PubMed] [Google Scholar]
- 86.Guallar-Castillon P, Rodríguez-Artalejo F, Tormo MJ, Sánchez MJ, Rodríguez L, Quirós JR, et al. (2012) Major dietary patterns and risk of coronary heart disease in middle-aged persons from a Mediterranean country: The EPIC-Spain cohort study. Nutr Metab Cardiovasc Dis 22: 192–199. 10.1016/j.numecd.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 87.Menotti A, Alberti-Fidanza A, Fidanza F (2012) The association of the Mediterranean Adequacy Index with fatal coronary events in an Italian middle-aged male population followed for 40 years. Nutr Metab Cardiovasc Dis 22: 369–375. 10.1016/j.numecd.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 88.Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen J (2008) Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr 100: 890–895. 10.1017/S0007114508945694 [DOI] [PubMed] [Google Scholar]
- 89.Qiu D, Mei J, Tanihata T, Kawaminami K, Minowa M (2003) A cohort Study an Cerebrovascular Disease in middle-aged and elderly population in rural areas in Jiangxi Province, China. J Epidemiology 13: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lajous M, Willett WC, Robins J, Young JG, Rimm E, Mozaffarian D, et al. (2013) Changes in fish consumption in midlife and the risk of coronary heart disease in men and women. Am J Epidemiol 178: 382–391. 10.1093/aje/kws478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levitan EB, Wolk A, Mittleman MA (2009) Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: a population-based prospective study of middle-aged and elderly men. Eur Heart J 30: 1495–1500. 10.1093/eurheartj/ehp111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Happonen P, Voutilainen S, Salonen JT (2004) Coffee drinking is dose-dependently related to the risk of acute coronary events in middle-aged men. J Nutr 134: 2381–2386. [DOI] [PubMed] [Google Scholar]
- 93.Beulens JW, de Bruijne LM, Stolk RP, Peeters P, Bots ML, Grobbee DE, et al. (2007) High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol 50: 14–21. [DOI] [PubMed] [Google Scholar]
- 94.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB (2010) Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr 92: 1265–1272. 10.3945/ajcn.2010.29626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leosdottir M, Nilsson PM, Nilsson JÅ, Berglund G (2007) Cardiovascular event risk in relation to dietary fat intake in middle-aged individuals: Data from the Malmo Diet and Cancer Study. Eur J Cardiovasc Prev Rehabil 14: 701–706. [DOI] [PubMed] [Google Scholar]
- 96.Walda IC, Tabak C, Smit HA, Räsänen L, Fidanza F, Menotti A, et al. (2002) Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countries. Eur J Clin Nutr 56: 638–643. [DOI] [PubMed] [Google Scholar]
- 97.Miura K, Greenland P, Stamler J, Liu K, Daviglus ML, Nakagawa H (2004) Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men: the Chicago Western Electric Study. Am J Epidemiol 159: 572–580. [DOI] [PubMed] [Google Scholar]
- 98.Song Y, Sesso HD, Manson JE, Cook NR, Buring J, Liu S (2006) Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am J Cardiol 98: 1616–1621. [DOI] [PubMed] [Google Scholar]
- 99.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD (2008) Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 100.Wang L, Manson JE, Gaziano JM, Buring JE, Sesso HD (2012) Fruit and vegetable intake and the risk of hypertension in middle-aged and older women. Am J Hypertension 25: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang L, Manson JE, Forman JP, Gaziano JM, Buring JE, Sesso HD (2010) Dietary fatty acids and the risk of hypertension in middle-aged and older women. Hypertension 56: 598–604. 10.1161/HYPERTENSIONAHA.110.154187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Villegas R, Yang G, Gao YT, Cai H, Li H, Zheng W, et al. (2010) Dietary patterns are associated with lower incidence of type 2 diabetes in middle-aged women: the Shanghai Women’s Health Study. Int J Epidemiol 39: 889–899. 10.1093/ije/dyq008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villegas R, Xiang YB, Elasy T, Li HL, Yang G, Cai H, et al. (2011) Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr: 543–551. 10.3945/ajcn.111.013193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song Y, Manson JE, Buring JE, Liu S (2004) A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The Women’s Health Study. Diabetes Care 27: 2108–2115. [DOI] [PubMed] [Google Scholar]
- 105.Tuomilehto J, Hu G, Bidel S, Lindström J, Jousilahti P (2004) Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. JAMA 291: 1213–1219. [DOI] [PubMed] [Google Scholar]
- 106.Kato M, Noda M, Inoue M, Kadowaki T, Tsugane S (2009) Psychological factors, coffee and risk of diabetes mellitus among middle-aged Japanese: A population-based prospective study in the JPHC study cohort. Endocrine J 56: 459–468. [DOI] [PubMed] [Google Scholar]
- 107.Masaki M, Sugimori H, Nakamura KI, Tadera M (2003) Dietary patterns and stomach cancer among middle-aged male workers in Tokyo. Asian Pac J Cancer Prev 4: 61–66. [PubMed] [Google Scholar]
- 108.Ruder EH, Thiébaut AC, Thompson FE, Potischman N, Subar AF, Park Y, et al. (2011) Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr 94:1607–1619. 10.3945/ajcn.111.020701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsugane S, Sasazuki S, Kobayashi M, Sasaki S (2004) Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer 90: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mostofsky E, Levitan EB, Wolk A, Mittleman MA (2010) Chocolate intake and incidence of heart failure: a population-based prospective study of middle-aged and elderly women. Circ Heart Fail 3: 612–616. 10.1161/CIRCHEARTFAILURE.110.944025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD (2009) Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutrition 89: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hodge A, Almeida OP, English DR, Giles GG, Flicker L (2013) Patterns of dietary intake and psychological distress in older Australians: benefits not just from a Mediterranean diet. Int Psychogeriatr 25: 456–66. 10.1017/S1041610212001986 [DOI] [PubMed] [Google Scholar]
- 113.Ruusunen A, Lehto SM, Tolmunen T, Mursu J, Kaplan GA, Voutilainen S (2010) Coffee, tea and caffeine intake and the risk of severe depression in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Public Health Nutr 13: 1215–1220. 10.1017/S1368980010000509 [DOI] [PubMed] [Google Scholar]
- 114.Lehto SM, Ruusunen A, Tolmunen T, Voutilainen S, Tuomainen TP, Kauhanen J (2013) Dietary zinc intake and the risk of depression in middle-aged men: a 20-year prospective follow-up study. J Affect Disord 150: 682–685. 10.1016/j.jad.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 115.He K, Hu FB, Colditz GA, Manson JE, Willett WC, Liu S (2004) Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes 28: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 116.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G (2003) Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr 78: 920–927. [DOI] [PubMed] [Google Scholar]
- 117.Ross G, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, et al. (2000) Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283: 2674–2679. [DOI] [PubMed] [Google Scholar]
- 118.Strandberg AY, Strandberg TE, Pitkälä K, Salomaa VV, Tilvis RS, Miettinen TA (2008) The effect of smoking in midlife on health-related quality of life in old age: A 26-year prospective study. Arch Intern Med 168: 1968–1974. 10.1001/archinte.168.18.1968 [DOI] [PubMed] [Google Scholar]
- 119.Willcox BJ, He Q, Chen R, Yano K, Masaki KH, Grove JS, et al. (2006) Midlife risk factors and healthy survival in men. JAMA 296: 2343–2350. [DOI] [PubMed] [Google Scholar]
- 120.Agahi N, Shaw BA (2013) Smoking trajectories from midlife to old age and the development of non-life-threatening health problems: a 34-year prospective cohort study. Prev Med 57: 107–112. 10.1016/j.ypmed.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Åkesson K (2006) Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int 17: 1065–1077. [DOI] [PubMed] [Google Scholar]
- 122.Moayyeri A, Kaptoge S, Luben RN, Wareham NJ, Bingham S, Reeve J, et al. (2009) Estimation of absolute fracture risk among middle-aged and older men and women: The EPIC-Norfolk population cohort study. Eur J Epidemiol 24: 259–266. 10.1007/s10654-009-9337-8 [DOI] [PubMed] [Google Scholar]
- 123.Szoeke CEI, Cicuttini FM, Guthrie JR, Clark MS, Dennerstein L (2006) Factors affecting the prevalence of osteoarthritis in healthy middle-aged women: data from the longitudinal Melbourne Women's Midlife Health Project. Bone 39: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 124.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64: 277–281. [DOI] [PubMed] [Google Scholar]
- 125.Rusanen M, Kivipelto M, Quesenberry CP, Zhou J, Whitmer RA (2011) Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med 171: 333–339. 10.1001/archinternmed.2010.393 [DOI] [PubMed] [Google Scholar]
- 126.Kimm H, Lee PH, Shin YJ, Park KS, Jo J, Lee Y, et al. (2011) Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch Gerontol Geriatr 52: e117–e122. 10.1016/j.archger.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 127.Tyas SL, White LR, Petrovitch H, Ross GW, Foley DJ, Heimovitz HK, et al. (2003) Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging 24: 589–596. [DOI] [PubMed] [Google Scholar]
- 128.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K (2005) Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 330: 1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sabia S, Marmot M, Dufouil C, Singh-Manoux A (2008) Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med 168: 1165–1173. 10.1001/archinte.168.11.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nooyens AC, van Gelder BM, Verschuren WM (2008) Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am J Public Health 98: 2244–2250. 10.2105/AJPH.2007.130294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. (2001) Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 56: 42–48. [DOI] [PubMed] [Google Scholar]
- 132.Strand BH, Langballe EM, Hjellvik V, Handal M, Næss Ø, Knudsen GP, et al. (2013) Midlife vascular risk factors and their association with dementia deaths: results from a Norwegian prospective study followed up for 35 years. J Neurol Sci 324(1–2): 124–130. 10.1016/j.jns.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 133.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77: 461–468. 10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shaper AG, Wannamethee SG, Walker M (2003) Pipe and cigar smoking and major cardiovascular events, cancer incidence and all-cause mortality in middle-aged British men. Int J Epidemiol 32: 802–808. [DOI] [PubMed] [Google Scholar]
- 135.Qiao Q, Tervahauta M, Nissinen A, Tuomilehto J (2000) Mortality from all causes and from coronary heart disease related to smoking and changes in smoking during a 35-year follow-up of middle-aged Finnish men. EurHeart J 21: 1621–1626. [DOI] [PubMed] [Google Scholar]
- 136.Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H (2006) Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest 130: 1129–1137. [DOI] [PubMed] [Google Scholar]
- 137.Hara M, Sobue T, Sasaki S, Tsugane S (2002) Smoking and risk of premature death among middle-aged Japanese: Ten-year follow-up of the Japan public health center-based prospective study on cancer and cardiovascular diseases (JPHC study) cohort I. Jpn J Cancer Res 93: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pelkonen M, Tukiainen H, Tervahauta M, Notkola IL, Kivelä SL, Salorinne Y, et al. (2000) Pulmonary function, smoking cessation and 30 year mortality in middle aged Finnish men. Thorax 55: 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lim SH, Tai BC, Yuan JM, Mimi CY, Koh WP (2011) Smoking cessation and mortality among middle-aged and elderly Chinese in Singapore: the Singapore Chinese Health Study. Tob Control 22: 235–240. 10.1136/tobaccocontrol-2011-050106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gerber Y, Myers V, Goldbourt U (2012) Smoking reduction at midlife and lifetime mortality risk in men: A prospective cohort study. Am J Epidemiol 175: 1006–1012. 10.1093/aje/kwr466 [DOI] [PubMed] [Google Scholar]
- 141.Blanco-Cedres L, Daviglus ML, Garside DB, Liu K, Pirzada A, Stamler J, et al. (2002) Relation of cigarette smoking to 25-year mortality in middle-aged men with low baseline serum cholesterol: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol 155: 354–360. [DOI] [PubMed] [Google Scholar]
- 142.Boudik F, Reissigova J, Hrach K, Tomečková M, Bultas J, Anger Z, et al. (2006) Primary prevention of coronary artery disease among middle aged men in Prague: twenty-year follow-up results. Atherosclerosis 184: 86–93. [DOI] [PubMed] [Google Scholar]
- 143.Baba S, Iso H, Mannami T, Sasaki S, Okada K, Konishi M, et al. (2006) Cigarette smoking and risk of coronary heart disease incidence among middle-aged Japanese men and women: the JPHC Study Cohort I. Eur J Cardiovasc Prev Rehabil 13: 207–213. [DOI] [PubMed] [Google Scholar]
- 144.Qiu D, Mei J, Tanihata T, Kawaminami K, Minowa M (2003) A cohort study on cerebrovascular disease in middle-aged and elderly population in rural areas in Jiangxi Province, China. J Epidemiol 13: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Humphries SE, Talmud PJ, Hawe E, Bolla M, Day IN (2001) Apolipoprotein E4 and coronary heart disease in middle-aged men who smoke: a prospective study. Lancet 358: 115–119. [DOI] [PubMed] [Google Scholar]
- 146.Satoh H, Nishino T, Tomita K, Saijo Y, Kishi R, Tsutsui H (2006) Risk factors and the incidence of coronary artery disease in young middle-aged Japanese men: Results from a 10-year cohort study. Int Med 45: 235–239. [DOI] [PubMed] [Google Scholar]
- 147.Mannami T, Iso H, Baba S, Sasaki S, Okada K, Konishi M, et al. Cigarette smoking and risk of stroke and its subtypes among middle-aged Japanese men and women: the JPHC Study Cohort I. Stroke 35: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 148.Nakayama T, Yokoyama T, Yoshiike N, Zaman MM, Tanaka H, Detels R (2000) Population attributable fraction of stroke incidence in middle-aged and elderly people: contributions of hypertension, smoking and atrial fibrillation. Neuroepidemiol 19: 217–226. [DOI] [PubMed] [Google Scholar]
- 149.Janzon E, Hedblad B, Berglund G, Engström G (2004) Tobacco and myocardial infarction in middle-aged women: a study of factors modifying the risk. J Intern Med 256: 111–118. [DOI] [PubMed] [Google Scholar]
- 150.Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertension 21: 148–152. [DOI] [PubMed] [Google Scholar]
- 151.Raikkonen K, Matthews KA, Kuller LH (2001) Trajectory of psychological risk and incident hypertension in middle-aged women. Hypertension 38: 798–802. [PubMed] [Google Scholar]
- 152.Khalili P, Nilsson PM, Nilsson JÅ, Berglund G (2002) Smoking as a modifier of the systolic blood pressure-induced risk of cardiovascular events and mortality: a population-based prospective study of middle-aged men. J Hypertension 20: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 153.Dudas KA, Wilhelmsen L, Rosengren A (2007) Predictors of coronary bypass grafting in a population of middle-aged men. Eur J Cardiovasc Prev Rehabil 14: 122–127. [DOI] [PubMed] [Google Scholar]
- 154.Humphries SE, Talmud PJ, Hawe E, Bolla M, Day IN, Miller GJ (2001) Apolipoprotein E4 and coronary heart disease in middle-aged men who smoke: a prospective study. Lancet 358: 115–119. [DOI] [PubMed] [Google Scholar]
- 155.Patja K, Jousilahti P, Hu G, Valle T, Qiao Q, Tuomilehto J (2005) Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J Int Med 258: 356–362. [DOI] [PubMed] [Google Scholar]
- 156.Sairenchi T, Iso H, Nishimura A, Hosoda T, Irie F, Saito Y, et al. (2004) Cigarette smoking and risk of type 2 diabetes mellitus among middle-aged and elderly Japanese men and women. Am J Epidemiol 160: 158–162. [DOI] [PubMed] [Google Scholar]
- 157.Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim LL (2007) Leisure time physical activity in middle age predicts the metabolic syndrome in old age: Results of a 28-year follow-up of men in the Oslo study. BMC Public Health 7: 154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ostenson CG, Hilding A, Grill V, Efendic S (2012) High consumption of smokeless tobacco ("snus") predicts increased risk of type 2 diabetes in a 10-year prospective study of middle-aged Swedish men. Scand J Public Health 40: 730–737. 10.1177/1403494812459814 [DOI] [PubMed] [Google Scholar]
- 159.Otani T, Iwasaki M, Yamamoto S, Sobue T, Hanaoka T, Inoue M, et al. (2003) Alcohol consumption, smoking, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan Public Health Center-based prospective study. Cancer Epidemiol Biomarkers Prev 12: 1492–500. [PubMed] [Google Scholar]
- 160.Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, Tsugane S (2002) Cigarette smoking and subsequent risk of lung cancer by histologic type in middle-aged Japanese men and women: The JPHC study. Int J Cancer 99: 245–251. [DOI] [PubMed] [Google Scholar]
- 161.Stevens RJ, Roddam AW, Spencer EA, Pirie KL, Reeves GK, Green J, et al. (2009) Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int J Cancer 124: 2400–2405. 10.1002/ijc.24196 [DOI] [PubMed] [Google Scholar]
- 162.Inoue M, Hanaoka T, Sasazuki S, Sobue T, Tsugane S, JPHC Study Group (2004) Impact of tobacco smoking on subsequent cancer risk among middle-aged Japanese men and women: data from a large-scale population-based cohort study in Japan—the JPHC study. Prev Med 38: 516–522. [DOI] [PubMed] [Google Scholar]
- 163.Fogelholm M, Kujala U, Kaprio J, Sarna S (2000) Predictors of weight change in middle-aged and old men. Obes Res 8: 367–373. [DOI] [PubMed] [Google Scholar]
- 164.Virta JJ, Järvenpää T, Heikkilä K, Perola M, Koskenvuo M, Räihä I, et al. (2009) Midlife alcohol consumption and later risk of cognitive impairment: A twin follow-up study. J Alzheimers Dis 22: 939–948. [DOI] [PubMed] [Google Scholar]
- 165.Anttila T, Helkala EL, Viitanen M, Kåreholt I, Fratiglioni L, Winblad B, et al. (2004) Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ 329: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sabia S, Guéguen A, Berr C, Berkman L, Ankri J, Goldberg M, et al. (2011) High alcohol consumption in middle-aged adults is associated with poorer cognitive performance only in the low socio-economic group. Results from the GAZEL cohort study. Addiction 106: 93–101. 10.1111/j.1360-0443.2010.03106.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Emberson JR, Shaper AG, Wannamethee SG, Morris RW, Whincup PH (2005) Alcohol intake in middle age and risk of cardiovascular disease and mortality: accounting for intake variation over time. Am J Epidemiol 161: 856–863. [DOI] [PubMed] [Google Scholar]
- 168.Lin Y, Kikuchi S, Tamakoshi A, Wakai K, Kawamura T, Iso H, et al. (2005) Alcohol consumption and mortality among middle-aged and elderly Japanese men and women. Ann Epidemiol 15: 590–597. [DOI] [PubMed] [Google Scholar]
- 169.Tabak C, Smit HA, Räsänen L, Fidanza F, Menotti A, Nissinen A, et al. (2001) Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology 12: 239–245. [DOI] [PubMed] [Google Scholar]
- 170.Wannamethee SG, Shaper AG (2002) Taking up regular drinking in middle age: effect on major coronary heart disease events and mortality. Heart 87: 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Iso H, Baba S, Mannami T, Sasaki S, Okada K, Konishi M, et al. (2004) Alcohol consumption and risk of stroke among middle-aged men: the JPHC Study cohort I. Stroke 35: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 172.Waki K, Noda M, Sasaki S, Matsumura Y, Takahashi Y, Isogawa A, et al. (2005) Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: A population-based prospective study in the JPHC study cohort 1. Diabet Med 22: 323–331. [DOI] [PubMed] [Google Scholar]
- 173.Wannamethee SG, Shaper AG (2003) Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr 77: 1312–1317. [DOI] [PubMed] [Google Scholar]
- 174.Wang L, Lee IM, Manson JE, Buring JE, Sesso HD (2010) Alcohol consumption, weight gain, and risk of becoming overweight in middle-aged and older women. Arch Int Med 170: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stevens RJ, Roddam AW, Spencer EA, Pirie KL, Reeves GK, Green J, et al. (2009) Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int J Cancer 124: 2400–2405. 10.1002/ijc.24196 [DOI] [PubMed] [Google Scholar]
- 176.Flood A, Rastogi T, Wirfält E, Mitrou PN, Reedy J, Subar AF, et al. (2008) Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr 88: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Langlois JA, Mussolino ME, Visser M, Looker AC, Harris T, Madans J (2001) Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: The NHANES I epidemiologic follow-up study. Osteoporos Int 12: 763–768. [DOI] [PubMed] [Google Scholar]
- 178.Ravona-Springer R, Schnaider-Beeri M, Goldbourt U (2013) Body weight variability in midlife and risk for dementia in old age. Neurology 80(18): 1677–1683. 10.1212/WNL.0b013e3182904cee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Field AE, Malspeis S, Willett W (2009) Weight cycling and mortality among middle-aged or older women. Arch Int Med 169: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Waring ME, Eaton CB, Lasater TM, Lapane KL (2010) Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol 171: 550–556. 10.1093/aje/kwp433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bielak AA, Anstey KJ, Christensen H, Windsor TD (2012) Activity engagement is related to level, but not change in cognitive ability across adulthood. Psychol Aging 27: 219–228. 10.1037/a0024667 [DOI] [PubMed] [Google Scholar]
- 182.Holtzman RE, Rebok GW, Saczynski JS, Kouzis AC, Doyle KW, Eaton WW (2004) Social network characteristics and cognition in middle-aged and older adults. J Gerontol B Psychol Sci Soc Sci 59: 278–284. [DOI] [PubMed] [Google Scholar]
- 183.Kåreholt I, Lennartsson C, Gatz M, Parker MG (2011) Baseline leisure time activity and cognition more than two decades later. Int J Geriatr Psychiatry 26: 65–74. 10.1002/gps.2490 [DOI] [PubMed] [Google Scholar]
- 184.Skogen JC, Bergh S, Stewart R, Knudsen AK, Bjerkeset O (2015) Midlife mental distress and risk for dementia up to 27years later: the Nord-Trondelag Health Study (HUNT) in linkage with a dementia registry in Norway. BMC Geriatr 15: 23 10.1186/s12877-015-0020-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Agrigoroaei S, Lachman ME (2011) Cognitive functioning in midlife and old age: combined effects of psychosocial and behavioral factors. J Gerontol B Psychol Sci Soc Sci 66 Suppl 1: i130–i140. 10.1093/geronb/gbr017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Virta JJ, Heikkilä K, Perola M, Koskenvuo M, Räihä I, Rinne JO, et al. (2013) Midlife cardiovascular risk factors and late cognitive impairment. Eur J Epidemiol 28: 405–416. 10.1007/s10654-013-9794-y [DOI] [PubMed] [Google Scholar]
- 187.King DE, Mainous AG, Geesey ME (2007) Turning back the clock: adopting a healthy lifestyle in middle age. Am J Med 120: 598–603. [DOI] [PubMed] [Google Scholar]
- 188.Foster C, Hillsdon M, Thorogood M, Kaur A, Wedatilake T (2005) Interventions for promoting physical activity. Cochrane Database Syst Rev: CD003180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L (2008) Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev: CD005381. [DOI] [PubMed] [Google Scholar]
- 190.Baker PR, Francis DP, Soares J, Weightman AL, Foster C (2015) Community wide interventions for increasing physical activity. Cochrane Database Syst Rev: CD008366 10.1002/14651858.CD008366.pub3 [DOI] [PubMed] [Google Scholar]
- 191.Doll R, Peto R, Boreham J, Sutherland I (2004) Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 328: 1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Doll R (1996) Mortality from smoking worldwide. Br Med Bull 52: 12–21. [DOI] [PubMed] [Google Scholar]
- 193.Smart NA, Marshall BJ, Daley M, Boulos E, Windus J, Baker N, et al. (2011) Low-fat diets for acquired hypercholesterolaemia. Cochrane Database Syst Rev: CD007957 10.1002/14651858.CD007957.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S (2013) Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev: CD008722 10.1002/14651858.CD008722.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Champagne CM (2009) The usefulness of a Mediterranean-based diet in individuals with type 2 diabetes. Curr Diab Rep 9:389–395. [DOI] [PubMed] [Google Scholar]
- 196.Lavie CJ, Milani RV, Mehra MR, Ventura HO (2009) Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol 54: 585–594. 10.1016/j.jacc.2009.02.084 [DOI] [PubMed] [Google Scholar]
- 197.World Health Organization (1999) Global Status Report on Alcohol. World Health Organization. [Google Scholar]
- 198.Room R, Babor T, Rehm J (2005) Alcohol and public health. Lancet 365: 519–530. [DOI] [PubMed] [Google Scholar]
- 199.Rodgers A, Ezzati M, Hoorn SV, Lopez AD, Lin RB, Murray CJ (2004) Distribution of major health risks: findings from the Global Burden of Disease Study. PLoS Med 1: e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Brand DA, Saisana M, Rynn LA, Pennoni F, Lowenfels AB (2007) Comparative analysis of alcohol control policies in 30 countries. PLoS Med 4: e151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Panel, NHLBI Obesity Education Initiative Expert (2000) Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in Adults. Bethesda, Maryland: National Institutes of Health. [Google Scholar]
- 202.Pi-Sunyer FX (2007) How effective are lifestyle changes in the prevention of type 2 diabetes mellitus? Nutr Rev 65: 101–110. [DOI] [PubMed] [Google Scholar]
- 203.Benzein EG, Berg AC (2005) The level of and relation between hope, hopelessness and fatigue in patients and family members in palliative care. Palliat Med 19: 234–240. [DOI] [PubMed] [Google Scholar]
- 204.Ream E, Richardson A (1996) Fatigue: a concept analysis. Int J Nurs Stud 33: 519–529. [DOI] [PubMed] [Google Scholar]
- 205.Yu S, Huang YC (2003) Knowledge of, attitudes toward, and activity to prevent osteoporosis among middle-aged and elderly women. J Nurs Res 11: 65–72. [DOI] [PubMed] [Google Scholar]
- 206.Hodge A, O’Dea K, English DR, Giles GG, Flicker L (2014) Dietary patterns as predictors of successful ageing. J Nutr Health Aging 18: 221–227. 10.1007/s12603-013-0405-0 [DOI] [PubMed] [Google Scholar]
- 207.Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, Tsugane S (2002) Cigarette smoking and subsequent risk of lung cancer by histologic type in middle-aged Japanese men and women: The JPHC study. Int J Cancer 99: 245–251. [DOI] [PubMed] [Google Scholar]
- 208.Noborisaka Y, Ishizaki M, Yamada Y, Honda R, Yokoyama H, Miyao M, et al. (2013) The effects of continuing and discontinuing smoking on the development of chronic kidney disease (CKD) in the healthy middle-aged working population in Japan. Environ Health Prev Med 18: 24–32. 10.1007/s12199-012-0285-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Östenson CG, Hilding A, Grill V, Efendic S (2012) High consumption of smokeless tobacco (“snus”) predicts increased risk of type 2 diabetes in a 10-year prospective study of middle-aged Swedish men. Scand J Public Health 40: 730–737. 10.1177/1403494812459814 [DOI] [PubMed] [Google Scholar]
- 210.Nafziger AN, Lindvall K, Norberg M, Stenlund H, Wall S (2007) Who is maintaining weight in a middle-aged population in Sweden? A longitudinal analysis over 10 years. BMC Public Health 7: 108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stevens RJ, Roddam AW, Spencer EA, Pirie KL, Reeves GK, Green J, et al. (2009) Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int J Cancer 124: 2400–2405. 10.1002/ijc.24196 [DOI] [PubMed] [Google Scholar]
- 212.Beckett M, Ardern C, Rotondi M (2015) A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer's disease in older adults. BMC Geriatr 15: 9 10.1186/s12877-015-0007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.United Kingdom Equality Act. In: Elizabeth II. Chapter 15. London: Stationery Office: Parliament. Great Britain. 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.