Abstract
Background
Risk of high-grade dysplasia and invasive carcinoma in intraductal papillary mucinous neoplasms (IPMN) of the pancreas is increased in main-duct compared to branch-duct lesions. We hypothesized that isolated uncinate duct dilation may also be a radiographic indicator of high-risk disease as the primary drainage of this portion of the gland originates from a distinct embryologic precursor.
Methods
All patients with available preoperative imaging who underwent resection for IPMN between 1994 and 2010 were included (n=184). Imaging studies were reviewed by an experienced radiologist who was blinded to the pathologic results, and studies were categorized as main-duct, branch- duct, or combined-duct. The presence of uncinate duct dilation was assessed as a risk factor for tumors which proved to have high-grade dysplasia (HGD) or invasive carcinoma (IC) on pathologic assessment.
Results
IPMN with HGD or IC were identified in 82 of 184 cases (45%). Without considering uncinate duct dilation, IPMN with HGD or IC were present in 84% of patients with main-duct IPMN (n= 31/37), 58% with combined-duct IPMN (n= 23/40), and 26% with branch-duct IPMN (n=28/107). Dilation of the uncinate duct was observed in 47 patients, with or without main duct dilation, and 30 of these (64%) contained HGD or IC on pathology. Isolated uncinate duct dilation without main duct dilation was observed in 17 patients, and 11 (65%) had HGD. On multivariate analysis of IPMN without associated main-duct dilation, uncinate duct dilation was independently associated with IPMN with HGD or IC (p=0.002).
Conclusion
Uncinate duct dilation on preoperative radiologic imaging appears to be an additional risk factor for IPMN-associated high-grade dysplasia or adenocarcinoma.
INTRODUCTION
Intraductal mucinous papillary neoplasms (IPMN) are mucinous cystic tumors of the pancreas with the ability to progress to invasive cancer. IPMN comprise a spectrum of precursor lesions ranging from low-grade dysplasia to moderate dysplasia to high-grade dysplasia (carcinoma in-situ) to invasive carcinoma. These lesions originate from the cells of the pancreatic ductal system and involve the main pancreatic duct or ductal side branches. IPMN are being discovered with increasing frequency, and management remains controversial.
Several radiographic and clinical features are associated with high-risk IPMN (high-grade dysplasia or invasive disease). When these features are present, resection is generally indicated. These features include the presence of symptoms, the identification of carcinoma on cytologic analysis of cyst contents, or any of the following imaging findings: main duct dilation > 6mm, cyst size ≥ 3cm, and solid component or mural nodularity.1 Patients with branch duct IPMN with none of the above indications for resection are typically followed with surveillance cross-sectional imaging, and resection is offered if concerning radiographic features arise.
Embryologically, the pancreas forms from rotation of the ventral and dorsal buds. The ventral bud forms the posterior and inferior portion of the head and the uncinate process. The dorsal bud forms the anterior head, body, and tail. The fusion of the ventral duct with the distal dorsal duct forms the main pancreatic duct, and the accessory duct persists from the proximal dorsal bud.2 Recent studies have confirmed the persistence of one or two dominant ducts within the uncinate process, reportedly developing from components of both the dorsal and ventral buds. 3, 4
Some patients with IPMN present with dilation of the primary drainage of the uncinate process. It is unknown whether development of IPMN within these structures may behave in a similar fashion as main duct IPMN. In addition to the known predictors of IPMN malignancy, we hypothesized that dilation of the uncinate duct is a radiographic indicator of high-risk disease, IPMN associated with high-grade dysplasia (HGD) or invasive carcinoma (IC). In the present study, we performed a retrospective review to define the radiographic features associated with IPMN with HGD or IC, and in specific, the association of the involvement of the uncinate drainage.
MATERIALS AND METHODS
Review of our prospectively maintained pancreatic surgery database identified 184 patients who had available preoperative imaging and who underwent resection for IPMN between June 1994 and March 2010. Operative specimens were identified pathologically as IPMN if they met the following criteria: grossly identifiable cystic structures measuring at least 1 cm in greatest diameter, connection to the remainder of the ductal system, and lined by flat, papillary mucinous, or oncocytic epithelium exhibiting varying degrees of cytoarchitectural atypia. Tumors were classified using the World Health Organization classification as either IPMN with low-grade dysplasia, IPMN with moderate-grade dysplasia, IPMN with high-grade dysplasia, or IPMN-associated adenocarcinoma.5
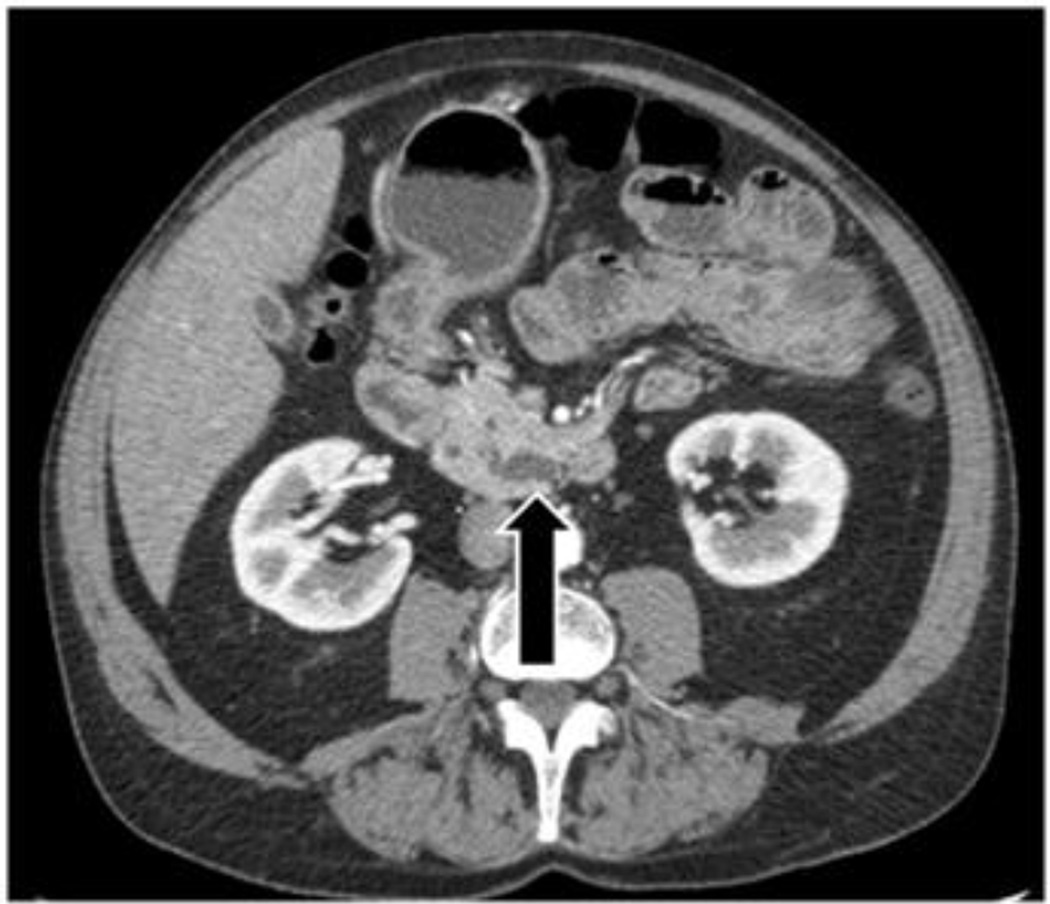
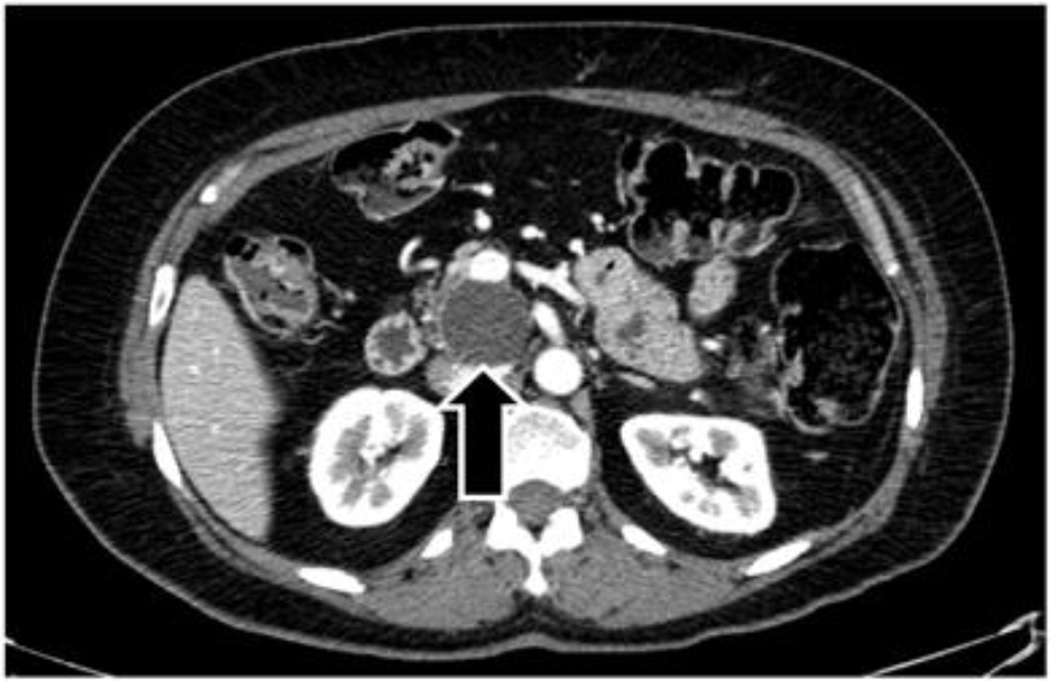
Imaging studies were reviewed by an experienced hepatopancreatobiliary radiologist (RD) blinded to the pathologic diagnosis. The following radiographic features were recorded on available CT or MR examinations on a Picture Archiving and Communication System (PACS): size, presence of mural nodularity or mass, and dilation of the main duct, branch ducts and/or uncinate duct. The size of the largest cystic lesion, when present, was measured in millimeters by electronic calipers on a PACS workstation. IPMN were classified based on imaging characteristics as main-duct, branch-duct or combined-duct. Main-duct IPMN had dilation of main pancreatic duct > 6mm, branch-duct IPMN had cystic dilation of branch ducts, and combined-duct IPMN had both main and branch duct dilation. For purposes of classification, the uncinate duct was considered a branch duct. The classification of uncinate disease was separated into two categories and when main duct dilation was absent both categories were defined as branch duct for the initial analysis. Uncinate duct dilatation was defined as a dilated tubular structure of fluid density (on CT) or signal intensity (on MRI) measuring ≥ 4mm, extending to the papilla (Figure 1a). Alternatively, an uncinate process cystic lesion was identified when no communication to the major papilla was present (Figure 1b).
Figure 1.
a) Arrow points to the dilated uncinate duct. b) Arrow points to uncinate process cystic lesion
The association between radiologic findings and pathology was assessed using t-test for continuous variables and χ2 analysis for categorical variables. We used multivariable logistic regression analyses to assess the independent association of radiologic findings with IPMN with HGD or IC on surgical pathology. P values <0.05 were considered statistically significant.
RESULTS
A total of 213 patients underwent surgical resection of IPMN during the study period. Imaging was available for review in 184 of these patients and these patients comprise the study population. Patient and tumor characteristics are shown in Table 1.
TABLE 1.
Demographics
| Patient/tumor characteristic (n=184) |
|
|---|---|
| Median age - yrs (range) | 68 (34–88) |
| Imaging study | |
| Pancreatic protocol CT scan | 73 (40%) |
| Abdominal CT scan | 49 (27%) |
| MRI | 56 (30%) |
| MRI and Abdominal CT scan | 6 (3%) |
| Median radiologic diameter - cm (range) | 2.9 (1–13) |
| Solid component/mural nodule – n (%) | 40 (22%) |
| ≥ 3 cm – n (%) | 83 (45%) |
| Symptomatic – n (%) | 101 (55%) |
| Operation | |
| Pancreatoduodenectomy – n (%) | 122 (66%) |
| Distal pancreatectomy – n (%) | 46 (25%) |
| Total pancreatectomy – n (%) | 4 (2%) |
| Central pancreatectomy – n (%) | 5 (3%) |
| Enucleation – n (%) | 7 (4%) |
| Pathology | |
| Low-grade dysplasia/unspecified – n (%) | 31 (17%) |
| Moderate-grade dysplasia – n (%) | 71 (39%) |
| High-grade dysplasia – n (%) | 53 (29%) |
| Invasive adenocarcinoma – n (%) | 29 (16%) |
| Type | |
| Main-duct – n (%) | 37 (20%) |
| Combined-duct – n (%) | 40 (22%) |
| Branch-duct – n (%) | 107 (58%) |
| Dilation of uncinate duct – n (%) | 47 (26%) |
| Uncinate process cystic lesion – n (%) | 50 (27%) |
IPMN with HGD or IC were identified in 82 of 184 cases (45%). Table 2 shows the characteristics of pathologic high-risk (HGD or IC) and low-risk lesions (moderate and low-grade dysplasia). Using the standard classification without regard to uncinate duct dilation, IPMN with HGD or IC were present in 84% of patients with main-duct IPMN (n=31/37), 58% with combined-duct IPMN (n= 23/40), and 26% with branch-duct IPMN (n=28/107). When compared to branch-duct IPMN, IPMN with main duct dilation (including both main-duct and combined-duct IPMN) were more likely to harbor high-risk lesions (26% vs. 70%, respectively: p<0.0001). Size ≥ 3.0 cm and the radiographic evidence of mural nodularity or solid component were associated with IPMN with HGD or IC, while the presence of symptoms was not.
TABLE 2.
Univariate analysis – malignant vs non-malignant IPMN
| Malignant IPMN n=82 |
Non-malignant IPMN n=102 |
P | |
|---|---|---|---|
| Median age - years (range) | 68 (37–85) | 68 (34–88) | 0.553 |
| Median radiologic size - cm (range) | 3.2 (1–13) | 2.7 (1–7) | 0.006 |
| Solid component/mural nodule – n (%) | 32 (39%) | 8 (8%) | <0.001 |
| ≥ 3 cm – n (%) | 45 (61%) | 38 (38%) | 0.004 |
| Symptomatic – n (%) | 49 (60%) | 52 (51%) | 0.297 |
| Uncinate process cystic lesion – n (%) | 22 (28%) | 28 (27%) | 1 |
| Type | |||
| Main-duct – n (%) | 31 (38%) | 6 (6%) | <0.001 |
| Combined-duct – n (%) | 23 (28%) | 17 (17%) | 0.073 |
| Branch-duct – n (%) | 28 (34%) | 79 (77%) | <0.001 |
| Ductal dilation | |||
| Main duct – n | 54 | 23 | <0.001 |
| Branch duct – n | 27 | 33 | 1 |
| Uncinate duct – n | 30 | 17 | 0.004 |
“Malignant IPMN” = IPMN with HGD or IC; “Non-malignant IPMN” = IPMN with moderate of low-grade dysplasia
Uncinate process cystic lesions were seen in 50 patients, and dilation of the uncinate duct was observed in 47 patients. As seen in Table 2, uncinate process cystic lesion was not associated with IPMN with HGD or IC. However, uncinate duct dilation was associated with pathologic IPMN with HGD or IC, which were identified in 30 of the 47 patients (64%) with a dilated uncinate duct. Isolated uncinate duct dilation without main duct dilation was seen in 17 patients. The indication for surgery in these patients was size ≥ 3cm in 14 patients, 2 of whom also had solid component. Three patients with lesions <3cm with no solid component underwent surgery due to symptoms of abdominal and/or back pain. 11 (65%) of these 17 patients with isolated uncinate duct dilation without main duct dilation had HGD, while 6 had moderate-grade dysplasia. A solid component was present in 2 of the 11 patients (18%) with IPMN with HGD or IC, and in none of the 6 patients with low-risk disease (p = 0.5417). Lesion diameter ≥ 3cm was present in 9 of the 11 patients (82%) with IPMN with HGD or IC and 5 of the 6 patients (83%) with low-risk disease (p = 1). Symptoms were present in 10 of the 11 patients (91%) with IPMN with HGD or IC and 3 of the 6 patients (50%) with low-risk disease (p = 0.0987). Of the 3 patients with isolated uncinate duct dilation and no other radiologic risk factors, 2 patients had HGD. IPMN with HGD or IC was present in 65% of patients with a dilated uncinate duct and normal caliber main duct compared to 18% of patients with branch duct dilation and normal caliber main and uncinate ducts (p = 0.0002).
In order to examine factors associated with IPMN with HGD or IC in branch-duct IPMN, a multivariate logistic regression analysis was performed. Only patients without main duct dilation were included in this analysis (n = 107). Three factors were used in the model: any solid component, size ≥ 3.0 cm, and uncinate duct dilation. The results of this analysis are demonstrated in Table 3. Uncinate duct dilation was independently associated with IPMN with HGD or IC (p=0.002).
TABLE 3.
Multivariate analysis – Factors associated with high-risk branch-duct IPMN (n=107)
| Variable | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Solid component/mural nodule | 2.133 | 0.576 to 7.896 | 0.257 |
| ≥ 3 cm | 1.287 | 0.471 to 3.522 | 0.623 |
| Uncinate duct dilation | 7.177 | 2.121 to 24.284 | 0.002 |
DISCUSSION
In the current study, preoperative imaging findings were correlated to post-resection pathology in patients with IPMN. In addition to the commonly cited risk factors for high-risk tumors such as size, main-duct dilation, and solid component, uncinate duct dilation on preoperative imaging was also found to be associated with high-risk disease.6–11 This is the first report examining uncinate duct dilation as a potential risk factor for high risk IPMN.
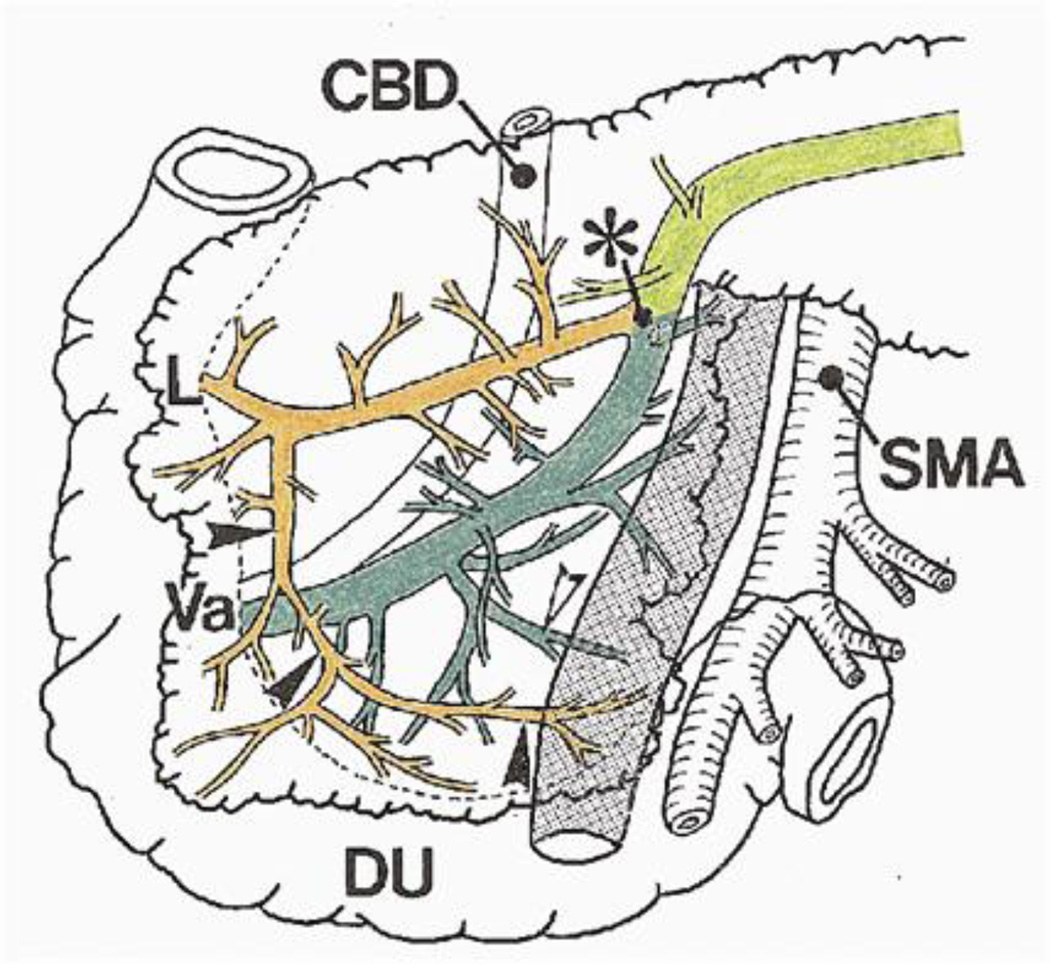
The anatomy of uncinate ductal drainage is variable. Embryologically, the pancreas forms from the foregut. The ventral and dorsal pancreatic buds rotate and fuse to form the fully developed pancreas. Classically, the proximal portion of the main pancreas duct and the uncinate ductal branches were thought to arise from the ventral pancreatic bud.12 More recently, there has been evidence that remnants of both the ventral and dorsal pancreatic buds comprise the uncinate process. In one series of 15 cadaveric specimens, double drainage of the uncinate into both the main and accessory ducts was noted in all specimens.4 A similar study of 17 specimens showed more variation with single drainage to the main duct in 59%, single drainage to the accessory duct in 18%, and double drainage in 24%.3 The accessory duct receives a branch which runs obliquely to drain the anteroinferior uncinate process, and the main duct receives a shorter branch draining the posterior aspect of the uncinate process (Figure 2).4 Regardless of the exact anatomical footprint, these studies demonstrate that the uncinate process has a dominant ductal system that may be embryologically similar to the drainage of the body and tail of the pancreas. These ducts may be analogous to the main pancreatic duct.
Figure 2.
The uncinate duct runs obliquely draining the anteroinferior part of the uncinate process (black arrowheads). The uncinate process is also drained in part by small branches to the main pancreatic duct (white arrowheads). Asterisk, junction of main and accessory pancreatic ducts; CBD, common bile duct; L, lesser duodenal papilla; Va, ampulla of Vater; DU, duodenum. From: Takahashi S, et al. Surgery 1999;125:178–85. Reprinted with permission.
Many investigators have sought to determine predictors of invasive IPMN. Factors associated with invasive disease include symptoms, mural nodules or solid component, size, biliary dilation, and elevated serum CA19-9.10, 11, 13, 14 Preoperative cyst fluid cytology is inaccurate in determining invasive lesions.15, 16 The prevalence of invasive IPMN with main duct dilation ranges from 57% to 92%.1, 6, 8, 11, 17–22 Invasive IPMN is found in 6% to 46% of patients who undergo resection for branch-duct disease.1, 8, 11, 17–22 This is the first study to examine the risk of malignancy in resected IPMN with uncinate duct dilation. The majority of patients with uncinate duct dilation also had associated main duct dilation, which would predict a high rate of malignancy. In those patients with uncinate duct dilation in the absence of main duct dilation, the rate of IPMN with HGD or IC was 65%, a risk level more similar to main-duct IPMN than to branch-duct IPMN.
Resection is recommended for patients with IPMN at high risk for malignancy, as the risks of the lesion progressing to malignancy are felt to outweigh the risks of resection. Radiologic characteristics of the lesion often guide patient selection for resection. Observation with radiologic follow-up is generally recommended in asymptomatic patients with branch-duct IPMN measuring < 3cm without a solid component since the rate of malignancy is thought to be less than 3%.9, 23–28 At the authors’ institution, patients not meeting these criteria should be offered resection if they are appropriate surgical candidates. These patients are offered resection, but other factors may influence the decision to operate, such as family history of pancreatic cancer, age, and patient preference. This report suggests that uncinate duct dilation may be a factor to assist in selecting patients for resection.
There are some potential explanations for the high risk of malignancy in IPMN associated with uncinate duct dilation. Higher risk may be due to the embryologic formation of the main duct and uncinate duct from the ventral bud conferring a risk more closely resembling main-duct IPMN. It is also plausible that some of the patients with uncinate duct dilation had pancreas divisum and the duct of Wirsung was visualized as the uncinate duct. However, it is difficult to reliably assess pancreas divisum on cross-sectional imaging. It is also possible that we have overestimated the risk associated with uncinate duct dilation since all of the patients in this series were at high risk by virtue of being selected for resection.
Additional limitations to this study include the retrospective nature, and the fact that the imaging performed was not uniform among all patients with some studies, such as a dedicated pancreatic protocol CT scan, providing thinner slices through the pancreas. Furthermore, the studies were reviewed by a single experienced radiologist, but he was blinded to the pathologic diagnosis. Therefore, it would be presumed that the quality of imaging would be balanced between those with uncinate duct dilation and those without.
Despite these limitations, this is the first study to show that dilation of the uncinate duct is associated with IPMN with HGD or IC. Additional studies are necessary to confirm the validity of our findings.
CONCLUSION
Uncinate duct dilation on preoperative radiologic imaging appears to be an additional risk factor for IPMN-associated high-grade dysplasia or adenocarcinoma.
Acknowledgments
This study was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
REFERENCES
- 1.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 2.Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt F, Maignan A, Ploteau S, et al. New anatomical data on the drainage patterns of the uncinate process of the pancreas. Surg Radiol Anat. 2010;32:777–781. doi: 10.1007/s00276-010-0680-y. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Akita K, Goseki N, Sato T. Spatial arrangement of the pancreatic ducts in the head of the pancreas with special reference to the branches of the uncinate process. Surgery. 1999;125:178–185. [PubMed] [Google Scholar]
- 5.Longnecker DSAG, Hruban RH, Kloppel G. Intraductal papillary mucinous neoplasms. IARC Press; 2000. [Google Scholar]
- 6.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen PJ, D'Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2010 doi: 10.1007/s00423-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–1249. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 12.Carlson BM. Human embryology and developmental biology. Mosby: St Louis; 2004. [Google Scholar]
- 13.Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010;45:952–959. doi: 10.1007/s00535-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto S, Lawler LP, Horton KM, Eng J, Hruban RH, Fishman EK. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006;186:687–695. doi: 10.2214/AJR.04.1820. [DOI] [PubMed] [Google Scholar]
- 15.Pitman MB, Michaels PJ, Deshpande V, Brugge WR, Bounds BC. Cytological and cyst fluid analysis of small (> or =3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology. 2008;8:277–284. doi: 10.1159/000134276. [DOI] [PubMed] [Google Scholar]
- 16.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Choi BS, Kim TK, Kim AY, et al. Differential diagnosis of benign and malignant intraductal papillary mucinous tumors of the pancreas: MR cholangiopancreatography and MR angiography. Korean J Radiol. 2003;4:157–162. doi: 10.3348/kjr.2003.4.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doi R, Fujimoto K, Wada M, Imamura M. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery. 2002;132:80–85. doi: 10.1067/msy.2002.125386. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa Y, Unger TA, Taylor S, Kozarek RA, Traverso LW. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12–18. doi: 10.1016/S1091-255X(02)00152-X. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 20.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–265. doi: 10.1097/00004836-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas > or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234–242. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. quiz 309–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahani DV, Saokar A, Hahn PF, Brugge WR, Fernandez-Del Castillo C. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238:912–919. doi: 10.1148/radiol.2382041806. [DOI] [PubMed] [Google Scholar]
- 26.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–657. doi: 10.1097/01.sla.0000124299.57430.ce. discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–819. doi: 10.1016/j.cgh.2008.04.005. quiz 719. [DOI] [PubMed] [Google Scholar]
- 28.Walsh RM, Vogt DP, Henderson JM, et al. Natural history of indeterminate pancreatic cysts. Surgery. 2005;138:665–670. doi: 10.1016/j.surg.2005.07.019. discussion 70–1. [DOI] [PubMed] [Google Scholar]