Abstract
Background and Purpose
Monocyte‐derived macrophages are critical in the development of atherosclerosis and can adopt a wide range of functional phenotypes depending on their surrounding milieu. High‐density lipoproteins (HDLs) have many cardio‐protective properties including potent anti‐inflammatory effects. We investigated the effects of HDL on human macrophage phenotype and the mechanisms by which these occur.
Experimental Approach
Human blood monocytes were differentiated into macrophages in the presence or absence of HDL and were then induced to either an inflammatory macrophage (M1) or anti‐inflammatory macrophage (M2) phenotype using LPS and IFN‐γ or IL‐4, respectively.
Key Results
HDL inhibited the induction of macrophages to an M1‐phenotype, as evidenced by a decrease in the expression of M1‐specific cell surface markers CD192 and CD64, as well as M1‐associated inflammatory genes TNF‐α, IL‐6 and MCP‐1 (CCL2). HDL also inhibited M1 function by reducing the production of ROS. In contrast, HDL had no effect on macrophage induction to the M2‐phenotype. Similarly, methyl‐β‐cyclodextrin, a non‐specific cholesterol acceptor also suppressed the induction of M1 suggesting that cholesterol efflux is important in this process. Furthermore, HDL decreased membrane caveolin‐1 in M1 macrophages. We confirmed that caveolin‐1 is required for HDL to inhibit M1 induction as bone marrow‐derived macrophages from caveolin‐1 knockout mice continued to polarize into M1‐phenotype despite the presence of HDL. Moreover, HDL decreased ERK1/2 and STAT3 phosphorylation in M1 macrophages.
Conclusions and Implications
We concluded that HDL reduces the induction of macrophages to the inflammatory M1‐phenotype via redistribution of caveolin‐1, preventing the activation of ERK1/2 and STAT3.
Linked Articles
This article is part of a themed section on Inflammation: maladies, models, mechanisms and molecules. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2016.173.issue-4
Abbreviations
- BMDMs
bone marrow‐derived macrophages
- Cav‐1−/−
caveolin‐1 knockout
- HDL
high‐density lipoprotein
- MβCD
methyl‐beta‐cyclodextrin
- M1
inflammatory macrophage
- M2
anti‐inflammatory macrophage
- WT
wild‐type
Tables of Links
| TARGETS | |
|---|---|
| Transporters a | Enzymes b |
| Na+/K+‐ATPase | ERK1 |
| ERK2 |
| LIGANDS | |
|---|---|
| Cholesterol | IL‐6 |
| H2O2 | LPS |
| IFN‐γ | MCP‐1 (CCL2) |
| IL‐4 | TNF‐α |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,2013b).
Introduction
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality globally (Greenland et al., 2010). It is well accepted that plasma cholesterol levels are a major determinant in the risk and severity of atherosclerosis (Hoogeveen et al., 2014) and that an inverse relationship exists between plasma high‐density lipoproteins (HDL) and CVD risk (Gordon et al., 1977), suggesting a protective role for HDL. One of the major functions of HDL is reverse cholesterol transport (RCT) from extra‐hepatic organs including the vascular wall to the liver for excretion into bile (Zhu et al., 2008; Hafiane and Genest, 2013; Barylski et al., 2014). However, HDL appears to exert a number of other anti‐atherosclerotic effects including reducing endothelial inflammation (Barter et al., 2004), reducing leukocyte activation (Rye and Barter, 2008; Murphy et al., 2011) and decreasing the production of myeloid cells (Murphy et al., 2013), which are intimately involved in the development and progression of the lesion. Increasing plasma levels of HDL also appears to directly impact macrophages in the atherosclerotic lesion and could contribute to atherosclerotic lesion regression (Feig et al., 2011; Feig et al., 2014).
Monocyte‐derived macrophages serve as critical players in the initiation and progression of atherosclerosis. Tissue macrophages can adopt a wide range of functional phenotypes depending on their surrounding milieu. Monocytes/macrophages surrounded by inflammatory stimuli such as LPS and cytokines are able to derive macrophages to a ‘classic’ inflammatory M1 phenotype, associated with tissue destruction, the production of ROS and secretion of inflammatory cytokines. On the other hand, cytokines such as IL‐4 and IL‐10 promote macrophages to a ‘non‐classic’ pro‐resolving M2 phenotype, which supports tissue repair, clearance of cell debris, angiogenesis and secretion of anti‐inflammatory cytokines (Tugal et al., 2013). Through the use of mouse models, it is largely thought that atherosclerotic lesions have an imbalance between these macrophage subsets such that lesions are largely comprised of M1 macrophages (Swirski et al., 2007; Tacke et al., 2007), which impair the resolution of this chronic inflammatory disease (Tacke et al., 2007). Dampening the M1 response or promoting M2 macrophages is a potential mechanism to promote the regression of atherosclerotic CVD.
Key findings from the Fisher laboratory have revealed that raising HDL levels regresses the atherosclerotic lesion in mouse models. Feig et al. (2011) discovered that increasing HDL levels via overexpression of human ApoA‐I not only reduces the size of the lesion and the lipid content but also alters the genetic signature of lesional macrophages from an inflammatory (M1) to a pro‐fibrotic (M2) phenotype. Increasing HDL levels may systemically have a number of effects that could result in lesional macrophages displaying an M2‐like phenotype. As monocytes circulate in plasma containing increased HDL levels, the influence on the type of macrophage could begin at this point. In the current study, we determined whether HDL influences the maturation and phenotype of monocytes to macrophages and the mechanisms by which it does this.
Methods
Isolation of HDL
Human HDL was isolated and purified using sequential ultracentrifugation with potassium bromide for density adjustment as previously described (McPherson et al., 2007). Plasma of healthy donors was obtained through the Red Cross Blood Bank of Australia. Plasma density was adjusted from 1.01 to 1.21 g·L−1 to isolate pure HDL. SDS‐PAGE gel was used to determine the purity of HDL using protein apoA‐1 as a positive control.
Isolation of human monocytes
Human monocytes were isolated from buffy coats (containing white blood cells, red blood cells, platelets and plasma) of healthy donors through the Red Cross Blood Bank of Australia. Peripheral blood mononuclear cells were isolated using Ficoll‐paque density gradient centrifugation. Human primary monocytes were then purified using MACS Human Pan Monocyte Negative Selection Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of isolated monocytes, assessed using CD14+/CD16+ expression via flow cytometry, was greater than 85%.
Monocyte to macrophage differentiation and polarization
Freshly isolated monocytes (1 × 106 cells·mL−1) were re‐suspended in culture medium (RPMI 1640 supplemented with 10% FBS, 1% Non‐essential amino acids (NEAA), 100 IU·mL−1 penicillin and 100 g·mL−1 streptomycin). These cells were incubated for 7 days with or without 400 µg·mL−1 human HDL, and refreshed every 2 days. To stimulate the differentiation of monocytes to macrophages, 100 ng·mL−1 of human M‐CSF were also added. On day 7, culture medium, without either HDL or M‐CSF, was refreshed. LPS (100 ng·mL−1) plus IFN‐γ (20 ng·mL−1) or IL‐4 (20 ng·mL−1) were then added to induce differentiated primary macrophages (M0) into M1 or M2 phenotypes respectively.
Bone marrow‐derived macrophage isolation, differentiation and polarization
Murine bone marrow‐derived macrophages (BMDMs) were obtained from both femur and tibia of WT (wild‐type) C57Bl/6 and caveolin‐1 knockout (Cav‐1 −/−) mice and cultured using a standard protocol similar to human monocytes to macrophage differentiation. Isolated cells were suspended with RPMI 1640 Medium (containing 20% L‐cell‐conditioned medium, 10% FBS, 50 IU·mL−1 penicillin G and 50 µg·mL−1 streptomycin). Cells were then seeded into cell culture plates for 7 days with or without human HDL (400 µg·mL−1), refreshed every 2 days. On day 7, medium (without HDL) was refreshed. LPS (100 ng·mL−1) plus IFN‐γ (20 ng·mL−1) were then added to induce macrophages to the M1 phenotype.
Surface marker expression
Collected macrophages were incubated in either APC‐conjugated mouse anti‐human CD192 or FITC‐conjugated mouse anti‐human CD64 to detect M1 macrophage surface markers, or Allophycocyanin (APC)‐conjugated mouse anti‐human CD206 and FITC‐conjugated mouse anti‐human CD200R to detect M2 macrophage surface markers for 30 min at 4°C. Cells were washed using centrifugation to get rid of any non‐binding antibodies and were then analysed by flow cytometry using BD FACS Calibur (BD Biosciences, San Diego, CA, USA). Ten thousand cells were counted per sample, and the read‐out was analysed using Cell Quest Pro software (BD Biosciences). All antibodies mentioned were purchased from BD Biosciences.
ROS assay
Carboxy‐H2DCFDA (Invitrogen, Carlsbad, USA) that detects intracellular hydrogen peroxide (H2O2) was added 1 h before the cells were harvested to measure ROS production of macrophages. Fluorescent cells were determined by flow cytometry using BD Calibur flow cytometer (BD Biosciences). Again, 10 000 cells were counted per sample, and the read‐out was analysed using Cell Quest Pro software (BD Biosciences).
Real‐time PCR
Total RNA was extracted from macrophages using TRIzol (Invitrogen, Carlsbad, CA, USA) and treated with RNase‐Free DNase (Promega, Madison, WI, USA) before reverse‐transcribing 2 µg of total RNA into cDNA using Moloney‐Murine Leukemia Virus (M‐MLV) reverse transcriptase (Promega). Then 100 ng cDNA was used to perform real‐time PCR using FastStart Universal SYBR Green Master (Roches Applied Science, Indianapolis, IN, USA) on an ABI Prism 7500 Sequence Detection System (Applied Biosystems) to determine mRNA levels of target genes. Gene expressions were all normalized to 18S expression and assessed using the ΔΔCT calculation method. Primers used were designed by Roche's ProbeFinder software and synthesized by GeneWorks. Primer sequences are available upon request.
Cellular cholesterol labelling
BODIPY‐Cholesterol (Avanti Polar Lipids, Alabaster, Alabama, USA) that detects cellular cholesterol as previously described (Murphy et al., 2013), was added 1 h before the cells were harvested to measure cellular cholesterol content of macrophages. Flow cytometry was then used to analyse the MFI of fluorescent cells in the 10 000 cells per sample, with the read‐out being analysed using Cell Quest Pro software (BD Biosciences).
Extraction of protein from whole cell and membrane fraction
Whole cells were lysed using whole cell lysis buffer [10 mM of Tris (pH = 7.4), 0.1 M of NaCl, 1 mM of EDTA, 1 mM of EGTA, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X100, 10% glycerol and protease inhibitors (Roche Applied Science)]. Cell lysates were sonicated for 3 s at 14% intensity and incubated on ice for 30 min before centrifugation at 12 000 g in 4°C for 20 min to obtain supernatants of whole cell protein samples for further analysis. Membrane fractions were prepared via ultracentrifugation. Briefly, cells were first incubated with Buffer A [5 mM Tris‐HCl (pH = 7.5) containing protease inhibitors (Roche Applied Science)] at 4°C for 30 min before being placed in −80°C for 15 min. Cells were then thawed on ice and passed through a 25 G needle before centrifugation at 250 g to get rid of the postnuclear supernatant fraction. Supernatants were collected and then spun at 150 000 g for 60 min in 4°C using Beckman Optima MAX‐TL ultracentrifuge (Beckman Coulter, Brea, California, USA). Cell pellets were then resuspended using Buffer B [50 mM Tris‐HCl (pH = 7.5) containing 2 mM mercaptoethanol, 1% triton X‐100 and protease inhibitors (Roche Applied Science)]. All protein concentrations were then measured using Dc Protein Assay (Bio‐Rad Laboratories, Hercules, CA, USA).
Western blot
Protein samples were separated by 10–12% SDS‐PAGE gel and transferred onto a PVDF membrane. The membrane was then blocked using 5% fat‐free milk and incubated with various primary antibodies (1:1000) overnight in 4°C including β‐actin, Sodium Potassium ATPase (Abcam, Cambridge, UK), pSTAT1, pERK1/2, CAV‐1, pSTAT3 and STAT3 (Cell Signalling Technology, Danvers, MA, USA). The membrane was then incubated with secondary antibodies (1:4000), including Anti‐rabbit IgG (Cell Signalling Technology) or Goat anti‐mouse IgG (H + L) – HRP Conjugate Antibody (BioRad Laboratories), and the protein bands were visualized using enhanced luminal reagents (PerkinElmer, Waltham, MA, USA) and quantified using NIH FIJI software.
Animal experiments
Animals were bred and housed under standard conditions at the AMREP Animal Service Centre. Experimental procedures were approved by the Institutional Animal Ethics Committee in accordance with the Australian NHMRC guidelines. Male/female WT mice (on C57BL6 background) and Cav‐1 −/− mice of 10–12 weeks old were used. The Cav‐1 −/− mice were kindly provided by Robert Parton (University of Queensland) and were housed and bred at AMREP Animal Service Centre. A total of 39 mice were used in the current study. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Statistical analyses
Results are presented as mean ± SEM of n experiments. Each experiment was performed in triplicate to reduce error. If one of the triplicate values was anomalous, it was excluded. Triplicates were averaged to obtain one single value per experiment (n). Each value (n = 1) was then brought forward for statistical analysis. Statistical analyses used in these experiments were either one‐way ANOVA followed by a Tukey post hoc test, or two‐way ANOVA followed by a Tukey post hoc test. Values of P < 0.05 were deemed to be significant. Tukey post hoc tests were carried out only when F was significant (P < 0.05).
Results
HDL suppresses the induction of M1 pro‐inflammatory phenotype
It is well established that human macrophages can be induced in an in vitro setting to express an M1 pro‐inflammatory or M2 pro‐resolving phenotype using LPS + IFN‐γ or IL‐4 respectively (Murray et al., 2014). To study the influence of HDL in this process, human monocytes were incubated with HDL during the differentiation and induction protocol. Incubating macrophages with LPS + IFN‐γ for 24 h significantly increased M1‐related cell surface markers CD192 and CD64, while incubation with HDL significantly reduced both markers (Figure 1A,B). Consistent with this, LPS + IFN‐γ‐induced macrophages demonstrated substantially increased gene expression of the M1‐related cytokines, TNF‐α, MCP‐1(CCL2) and IL‐6. Likewise, incubation with HDL markedly suppressed these M1‐related genes (Figure 1C–E). When macrophages were induced with IL‐4, the M2‐related surface markers CD206 and CD200R significantly increased. However, incubation with HDL had no effect on either surface markers (Figure 1F,G) or the expression of M2‐related genes CD206 and SR‐B1 (Figure 1H,I).
Figure 1.
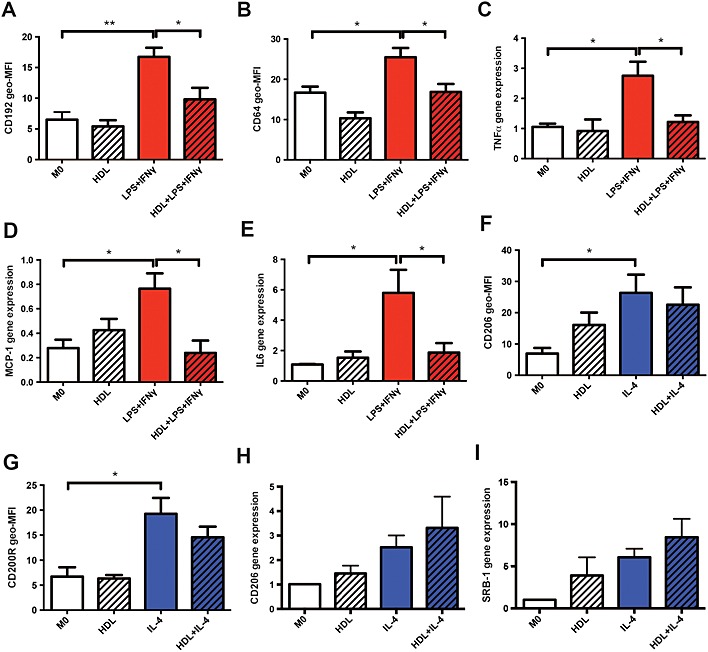
HDL reduces M1 macrophage polarization. Isolated monocytes were differentiated for 7 days to macrophages with or without HDL (400 µg·mL−1). They were then either polarized into an M1 or M2 phenotype using LPS + IFN‐γ or IL‐4 respectively. Cell surface markers were determined using flow cytometry (A, B, F and G) and mRNA expressions were measured using RT‐PCR (C, D, E, H and I). Data are presented as mean ± SEM of 3–6 individual donors, each performed in triplicate and analysed using one‐way ANOVA followed by Tukey's multiple comparisons test (*P < 0.05, **P < 0.01).
Preventing cell membrane lipid accumulation inhibits M1 polarization
Next, we explored the mechanism by which HDL suppresses the induction of macrophages to the M1 phenotype. Because the key function of HDL is its ability to remove lipids from cells (Tall et al., 2012), we examined whether lipid removal may be important. We used methyl‐beta‐cyclodextrin (MβCD) as a non‐specific cholesterol acceptor to explore this pathway. Incubating macrophages with MβCD significantly decreased the expression levels of CD192, similar to levels seen with HDL (Figure 2A). To confirm that HDL and MβCD were altering cholesterol content within these macrophages, we used a flow cytometry‐based method to assess cellular cholesterol (Murphy et al., 2013). We found that macrophages polarizing to an M1 phenotype had increased cellular cholesterol, while pre‐treating cells with HDL and MβCD could significantly prevent this increase to an equal degree, potentially through a decrease in cellular membrane cholesterol (Figure 2B). This suggests that preventing cell membrane lipid accumulation plays a significant role in the inhibitory action of HDL in M1 polarization.
Figure 2.
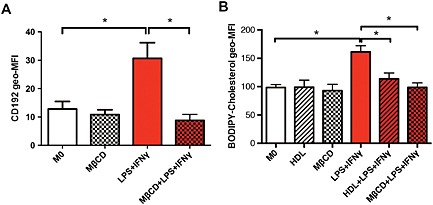
Preventing cell membrane lipid accumulation inhibits M1 polarization. Following a 1 h pretreatment with MβCD (5 mM), 7 day‐differentiated macrophages were then stimulated towards an M1 phenotype using LPS + IFN‐γ for 24 h. The CD192 inflammatory cell surface marker (A) and BODIPY‐cholesterol (B) were measured using flow cytometry. Data are presented as mean ± SEM of 5–9 individual donors, each performed in triplicate and analysed using one‐way ANOVA followed by Tukey's multiple comparisons test (*P < 0.05).
HDL prevents re‐distribution of caveolin‐1 to the membrane fractions of macrophages
Caveolae are specialized lipid raft domains that form flask‐shaped invaginations of plasma membrane by coupling cholesterol with caveolin‐1 in the membrane (Schlegel and Lisanti, 2001). Because HDL was demonstrated to decrease M1 induction via decrease in cellular cholesterol content, potentially from the membrane, we explored the role of caveolin‐1 in this context. Membrane fractions of macrophages were separated, and abundance of caveolin‐1 was assessed using western blot. Consistent with the notion that caveolae are formed when membrane cholesterol/lipid rafts are increased, we also saw an increased abundance of the major caveolae protein caveolin‐1 in the membrane fraction of M1 macrophages compared with control (M0) (Figure 3A). Again, consistent with our cholesterol data (Figure 2B), HDL markedly reduced the abundance of membrane caveolin‐1 in M1‐induced macrophages (Figure 3A). To determine if the reduction in caveolin‐1 in the membrane was due to an overall decrease in caveolin‐1, we examined total whole cell lysates and found no change amongst the different treatments (Figure 3B), suggesting that caveolin‐1 is retained within the cell and not shed. These data indicate that polarization of macrophages to an M1 phenotype favours the formation of caveolae by re‐distributing caveolin‐1 to the cell membrane and that incubation of these cells with HDL prevents this. Thus, it appears that reduced cellular cholesterol from macrophages inhibits cellular caveolin‐1 from localizing to the membrane and in turn prevents the polarization of M1 macrophages.
Figure 3.
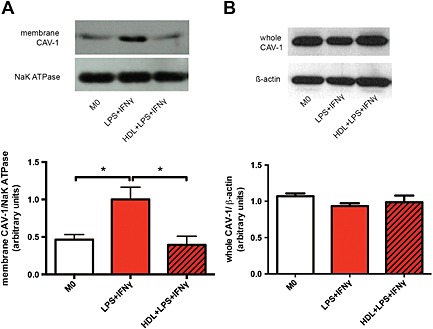
HDL prevents re‐distribution of caveolin‐1 to the membrane fractions of macrophages. Macrophages were pretreated with or without HDL (400 µg·mL−1) and induced into an M1 phenotype for 24 h. Protein of membrane (A) and whole cell (B) fractions were isolated from cells, run on a 12% SDS‐PAGE gel and analysed using western blots. Na/K ATPase was used as a membrane loading control. Data are presented as a mean ± SEM of four individual donors, each performed in triplicate, and were analysed using one‐way ANOVA followed by Tukey's multiple comparisons test (*P < 0.05).
Caveolin‐1 is required for HDL to inhibit M1 macrophage polarization
To determine if HDL requires caveolin‐1 to prevent the polarization of macrophages to an M1 phenotype, we cultured BMDMs from WT and Caveolin‐1 −/− (Cav‐1 −/−) mice. Consistent with our human macrophage data, WT macrophages incubated with HDL showed significant decrease in the expression of M1‐related cell surface marker CD64 (Figure 4A) and M1‐related genes, Tnf‐α and Il‐6 (Figure 4B,C). Interestingly, BMDMs from Cav‐1 −/− mice failed to respond to HDL, differentiating into M1 macrophages. This along with the data in Figure 3 suggests that caveolin‐1 is required for HDL to prevent the polarization of M1 macrophages and to inhibit the expression of inflammatory genes.
Figure 4.
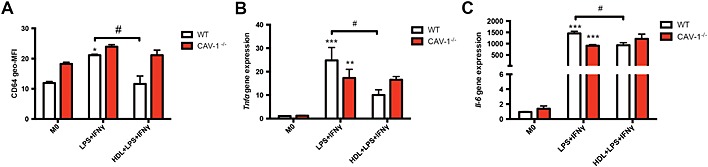
Caveolin‐1 is required to reduce M1 macrophage polarization. WT and Cav‐1 −/− mice were killed when they were 10–12 weeks‐old, and BMDMs were isolated. BMDMs were then cultured for 7 days with or without 400 µg·mL−1 of HDL before they were stimulated towards an M1 phenotype. The inflammatory M1‐related cell surface marker CD64 (A) was measured using flow cytometry. Gene expressions of Tnf‐α (B) and Il‐6 (C) were determined using RT‐PCR. Data are presented as mean ± SEM of 5–6 independent experiments, each performed in triplicate (two‐way ANOVA followed by Tukey's multiple comparisons test, *P < 0.05, **P < 0.01, ***P < 0.001 compared with M0 control group, # P < 0.05).
HDL reduces phosphorylation of ERK1/2 via caveolin‐1 in macrophages
Next, we examined how caveolin‐1 could be involved in regulating the expression of inflammatory genes. There is an accumulation of evidence to suggest that caveolin‐1 is important in mediating anti‐inflammatory effects in murine macrophages through signalling pathways such as ERK1/2 (Medina et al., 2006; Wang et al., 2006). Therefore, to determine if HDL mediates its anti‐inflammatory effects via ERK1/2 and if this requires caveolin‐1, we examined ERK1/2 phosphorylation in WT and Cav‐1 −/− BMDMs. Here, we discovered that HDL inhibited the phosphorylation of ERK1/2 in WT macrophages, consistent with inhibition of inflammatory surface marker and gene expression. However, incubation of Cav1 −/− macrophages with HDL failed to perturb the phosphorylation of ERK1/2 in response to LPS + IFN‐γ (Figure 5A). Evidence also indicates a role for caveolin‐1 to interact or interfere with STAT3 to dampen the expression of key inflammatory genes (Shah et al., 2002; Yuan et al., 2011). However, we found that LPS + IFN‐γ did not influence the phosphorylation of STAT3 in WT or Cav‐1 −/− BMDMs, nor did HDL affect STAT3 phosphorylation in WT BMDMs (Figure 5B).
Figure 5.
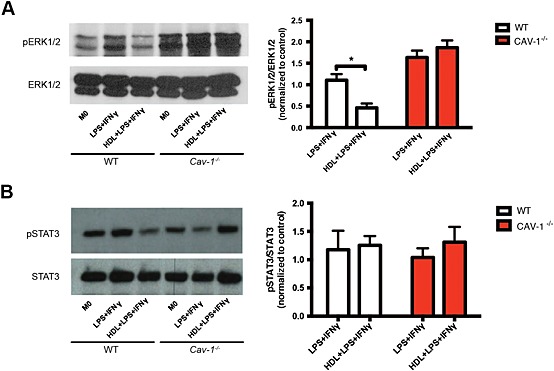
HDL mediated anti‐inflammatory effects on ERK1/2, but not STAT3, via caveolin‐1. WT and Cav‐1 −/− mice were killed when they were 10–12 weeks‐old, and BMDMs were isolated. BMDMs were then cultured for 7 days with or without 400 µg·mL−1 of HDL before they were stimulated for 10 min with LPS + IFN‐γ; they were then harvested for western blot analysis to detect the phosphorylation of ERK1/2 (A) and STAT3 (B). Data are presented as mean ± SEM of 6–8 experiments, each performed in triplicate and normalized to M0 control values (one‐way ANOVA followed by Tukey's multiple comparisons test, * P < 0.05).
HDL reduces phosphorylation of ERK1/2 and STAT3 in human macrophages
Our data in mice (Figure 5A) clearly shows a role for HDL in suppressing ERK1/2 signalling in macrophages. Thus, we aimed to determine if this was also true in human macrophages. As expected, when M0 human macrophages were polarized with LPS + IFN‐γ, we observed a robust increase in ERK1/2 phosphorylation. HDL incubation significantly decreased ERK1/2 phosphorylation recapitulating our results in mice (Figure 6A). We did also find a statistically significant increase in STAT3 phosphorylation upon LPS + IFN‐γ induction, albeit to a lesser degree than pERK1/2, and this phosphorylation of STAT3 was prevented by HDL pre‐treatment (Figure 6B). Thus, it is possible that STAT3 may be more important in human macrophage polarization and also suggests that pERK1/2 is likely the major pathway that HDL is inhibiting. We also examined the effect of STAT1, which can also play a role in the IFN‐γ induction of macrophages to an M1 phenotype (Ramana et al., 2002). While we observed an increase in STAT1 phosphorylation at Y701 and S727, HDL failed to inhibit this event, suggesting that HDL does not inhibit M1 polarization via the STAT1 pathway (Figure 6C).
Figure 6.
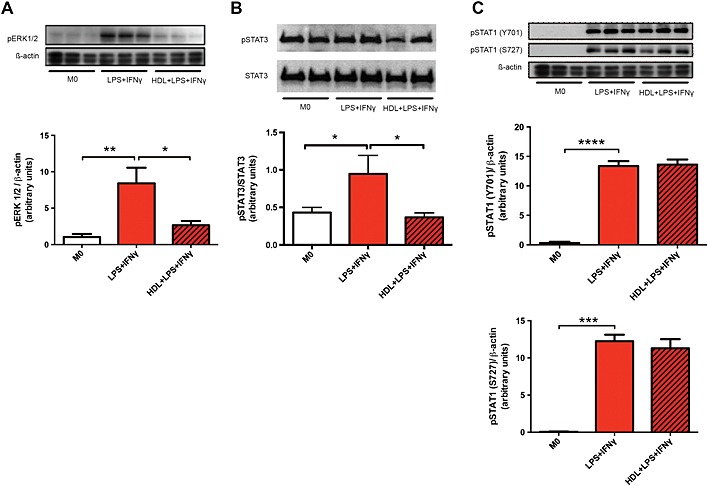
HDL reduces the phosphorylation of ERK1/2 and STAT3, but not STAT1 of M1‐induced macrophages. Seven day differentiated macrophages were pretreated with or without HDL before a 5 min (A) or 10 min (B and C) stimulation with LPS + IFN‐γ. pERK1/2, pSTAT3 and pSTAT1 were then measured using western blot analysis. Data are presented as mean ± SEM of 3–4 individual donors, each performed in triplicate (one‐way ANOVA followed by Tukey's multiple comparisons test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
HDL reduces ROS production of M1 macrophages
To determine if the decrease in M1 polarization as measured by cell surface and gene expression changes its function, we used carboxy‐H2CDFDA to detect ROS production via flow cytometry as macrophages produce ROS in order to neutralize pathogens (Mokgobu et al., 2015). Stimulating macrophages towards an M1 phenotype with LPS + IFN‐γ demonstrated a greater capacity to produce ROS compared with basal levels observed in M0 macrophages. When pre‐treated with HDL, ROS production of M1 macrophages was significantly attenuated (Figure 7).
Figure 7.
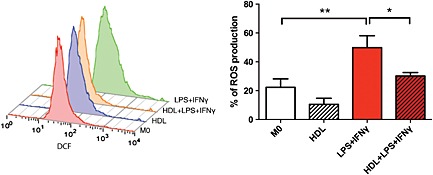
HDL reduces ROS production in M1 macrophages. After monocytes were differentiated to macrophages for 7 days in the presence or absence of HDL (400 µg·mL−1), macrophages were stimulated towards an M1 phenotype for 24 h. ROS production was measured using flow cytometry. Data presented as mean ± SEM of seven individual donors, each performed in triplicate and analysed using one‐way ANOVA followed by Tukey's multiple comparisons test (*P < 0.05, **P < 0.01).
Discussion and conclusions
In the present study, we demonstrated using flow cytometry, RT‐PCR and functional assay of ROS production that HDL prevents human monocytes from differentiating into an M1 pro‐inflammatory phenotype while having no effect on the M2 phenotype. We also show that caveolin‐1 is critical for this effect of HDL and that this effect appears to be mediated through cell membrane lipid removal preventing the abundance of caveolin‐1 at the cell membrane. This event is linked to a marked reduction in the phosphorylation of inflammatory ERK1/2 and STAT3 signalling pathways in M1‐induced macrophages.
An inverse relationship between HDL‐C and the risk of CVD events has long been well established (Castelli et al., 1977; Gordon et al., 1977). HDL exerts many atheroprotective effects ranging from inhibiting adhesion molecule expression on the endothelium to inhibiting the oxidation of LDL in the subendothelial space, as well as transporting cholesterol from cholesterol‐laden peripheral tissues such as lipidated macrophages back into the liver for excretion (Tsompanidi et al., 2010). Our findings identify yet another anti‐inflammatory role of HDL, that is, that of suppressing inflammatory macrophage phenotypes. This is consistent with the recent publication by De Nardo et al (2014) uncovering an anti‐inflammatory role of HDL to down‐regulate toll‐like receptor‐induced inflammatory cytokines in macrophages via activation of the ATF3 transcriptional regulator. In addition to the study, Feig et al (2011) and Sanson et al (2013) also demonstrate the ability of HDL to inhibit M1‐related inflammatory genes in murine macrophages, however, both studies differ in that an up‐regulation in M2‐related genes observed in IL‐4‐induced murine macrophages was not observed in our human macrophages. As well, our data align with the recent study by Colin et al. (2014) where again, contrary to murine macrophages, HDL was not observed to influence human M2 macrophage polarization (Colin et al., 2014).
The ability of HDL to prevent macrophages from being induced into an M1 phenotype provides further insight into the potential anti‐atherosclerotic role of HDL in retarding plaque progression. Using immunological techniques, an abundance of M1 macrophages has been located in plaque lipid core, whereas M2 macrophages are predominantly situated in the stable cell‐enriched regions of the atherosclerotic plaque away from the lipid core (Chinetti‐Gbaguidi et al., 2011). Furthermore, studies looking at plaque size and composition have found that administering native HDL significantly increases smooth muscle cell proliferation as well as decreasing MMP‐9 expression, suggesting that the capacity of HDL to inhibit M1 macrophages from releasing inflammatory cytokines may result in a more stable plaque by attenuating fibrous cap thinning (Nicholls et al., 2005).
Monocyte‐derived macrophages to foam cell formation is a critical step in the formation of atherosclerotic lesions (Dushkin, 2012). Studies looking at cholesterol content within macrophages in lesions have noted a greater accumulation of lipids and bigger lipid droplets within M1 macrophages than M2 macrophages, further contributing to the burden of foam cells (Chinetti‐Gbaguidi et al., 2011) and highlighting the importance of reducing cholesterol level within M1 macrophages as well as macrophage number. Interestingly, M1 macrophages appear to have better cholesterol transport handling than M2 macrophages due to an up‐regulation of the cholesterol transporter genes ABCA1 and ABCG1 (Chinetti‐Gbaguidi et al., 2011). It is thus not unexpected that the removal of cellular cholesterol, potentially by modulating cell membrane levels using MβCD or HDL in the current experiment effectively reduces the induction of macrophages to the M1 phenotype.
Interestingly, our data revealed an important and previously uncharacterized role for caveolin‐1 in mediating the effects of HDL. Caveolin‐1 is a structural protein that binds to membrane cholesterol, forming structural invaginations of the membrane known as caveolae (Simons and Toomre, 2000). Caveolin‐1 is involved in many cellular processes such as lipid regulation, pathway signalling and also recently being reported to play a part in the development of atherosclerosis and inflammation (Gargalovic and Dory, 2003). Our data showed that membrane caveolin‐1 was reduced by HDL but remained inside the cell, suggesting re‐distribution within the cell. Many studies have suggested both pro‐atherosclerotic and anti‐atherosclerotic effects of cavoelin‐1, depending on their cell type and disease state (Pavlides et al., 2012). We have previously shown the critical role of caveolin‐1 in the differentiation of monocytes to macrophages (Fu et al., 2012). We now show that knocking out caveolin‐1 diminished the effect of HDL on M1 induction, a finding consistent with reports by Wang et al. (2006) and Yuan et al. (2011) pointing towards an anti‐inflammatory effect of caveolin‐1 in macrophages (Wang et al., 2006; Yuan et al., 2011).
Further downstream, HDL was observed to prevent M1 macrophage polarization via ERK1/2 and STAT3, but not STAT1, signalling pathways. We found that ERK1/2 phosphorylation can be activated upon M1‐induction and inhibited by HDL in both human and murine macrophages. Critically, ERK1/2 has been shown to be associated with caveolin‐1 (Cohen et al., 2003; Medina et al., 2006). Therefore, our data suggest that HDL‐induced, cholesterol efflux‐associated, re‐distribution of caveolin‐1 prevents ERK1/2 phosphorylation and thus inhibits the expression of inflammatory M1‐macrophage‐associated genes. STAT3 has been previously shown to be induced upon inflammatory stimulation of macrophages with inflammatory mediators (Guo et al., 2014; Vasamsetti et al., 2015). Although in our hands, while this was true in human macrophages, we failed to see a robust increase of phosphorylation of STAT3 in murine BMDMs. This may reflect differences between human and mouse; however, this discrepancy between species also revealed a common pathway through ERK 1/2 that was suppressed in both mouse and human macrophages by HDL. Furthermore, previous studies show that IFN‐γ stimulation induces M1 polarization via STAT1 phosphorylation (Tugal et al., 2013). Our data, while consistent with this, also clearly show that this is not the mechanism by which HDL acts.
Asides from examining M1 phenotype using cell surface markers and gene expression of M1‐associated cytokines, the effect of HDL on the functional capacity of LPS + IFN‐γ‐induced M1 macrophages was examined. Our data confirms an increase in ROS generation in M1 macrophages (Mokgobu et al., 2015) where it is likely to trigger activation of the NFκB pathway, and in turn increase inflammatory cytokines expression (Fenyo and Gafencu, 2013). Furthermore, in the setting of atherosclerosis, oxidative modification of LDLs induced by ROS results in an increase in foam cell formation (Yla‐Herttuala, 1991). The anti‐oxidative properties of HDL in the current study are thus consistent with its potential anti‐atherosclerotic role.
In summary, we demonstrated that HDL prevents the induction of human macrophages into an M1 phenotype by preventing the accumulation of caveolin‐1 to the cell membrane. These results provide further insights into the cardio‐protective role of HDL, which may aid in atherosclerotic lesion regression/resolution and suggest that therapies targeted to redistribute membrane caveolin‐1 in immune cells may be a novel therapeutic angle for dampening inflammation.
Author contributions
M. K. S. L. designed and performed the experiments, analysed the data and wrote the manuscript. X. L. M. designed the experiments and analysed in part the data in the study. Y. F. designed and performed in part the experiments and analysed in part the data in the study. A. A‐S. performed in part experiments in the study. D. D. helped perform in part the experiments in the study. M. A. F‐R. created this line of C57/Black 6‐backcrossed caveolin‐1 knockout mice. R. P. provided intellectual input. D. S. provided intellectual input. A. J. M. helped design the experiments and wrote in part and finalized the manuscript. J. P. F. C‐D. conceptualized the study and finalized the manuscript.
Conflict of interest
Authors declare that they have no conflict of interest.
Acknowledgements
M. K. S. L. and A. A‐S. were supported by the Bright Sparks Program from Baker IDI Heart & Diabetes Institute Australia. A. J. M. was supported by a Career Development fellowship from the National Health and Medical Research Council (NHMRC), a future leader fellowship from the National Heart Foundation, and a Viertel award from Diabetes Australia Research Trust Australia. This work was also supported by an NHMRC programme grant (APP10363652) and by the Victorian Government's Operational Infrastructure Support (OIS) programme.
Lee, M. K. S. , Moore, X.‐L. , Fu, Y. , Al‐Sharea, A. , Dragoljevic, D. , Fernandez‐Rojo, M. A. , Parton, R. , Sviridov, D. , Murphy, A. J. , and Chin‐Dusting, J. P. F. (2016) High‐density lipoprotein inhibits human M1 macrophage polarization through redistribution of caveolin‐1. British Journal of Pharmacology, 173: 741–751. doi: 10.1111/bph.13319.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013a). The concise guide to PHARMACOLOGY 2013/14: transporters. Br J Pharmacol 170: 1706–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013b). The concise guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM (2004). Antiinflammatory properties of HDL. Circ. Res. 95: 764–772. [DOI] [PubMed] [Google Scholar]
- Barylski M, Toth PP, Nikolic D, Banach M, Rizzo M, Montalto G (2014). Emerging therapies for raising high‐density lipoprotein cholesterol (HDL‐C) and augmenting HDL particle functionality. Best Pract. Res. Clin. Endocrinol. Metab. 28: 453–461. [DOI] [PubMed] [Google Scholar]
- Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB et al. (1977). HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 55: 767–772. [DOI] [PubMed] [Google Scholar]
- Chinetti‐Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y et al. (2011). Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 108: 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J et al. (2003). Caveolin‐1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 284: C457–474. [DOI] [PubMed] [Google Scholar]
- Colin S, Fanchon M, Belloy L, Bochem AE, Copin C, Derudas B et al. (2014). HDL does not influence the polarization of human monocytes toward an alternative phenotype. Int. J. Cardiol. 172: 179–184. [DOI] [PubMed] [Google Scholar]
- De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M et al. (2014). High‐density lipoprotein mediates anti‐inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 15: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushkin MI (2012). Macrophage/foam cell is an attribute of inflammation: Mechanisms of formation and functional role. Biochemistry (Mosc.) 77: 327–338. [DOI] [PubMed] [Google Scholar]
- Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA (2014). High‐density lipoprotein and atherosclerosis regression: Evidence from preclinical and clinical studies. Circ. Res. 114: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J et al. (2011). HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte‐derived cells. Proc. Natl. Acad. Sci. U. S. A. 108: 7166–7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyo IM, Gafencu AV (2013). The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology 218: 1376–1384. [DOI] [PubMed] [Google Scholar]
- Fu Y, Moore XL, Lee MK, Fernandez‐Rojo MA, Parat MO, Parton RG et al. (2012). Caveolin‐1 plays a critical role in the differentiation of monocytes into macrophages. Arterioscler. Thromb. Vasc. Biol. 32: e117–125. [DOI] [PubMed] [Google Scholar]
- Gargalovic P, Dory L (2003). Caveolins and macrophage lipid metabolism. J. Lipid Res. 44: 11–21. [DOI] [PubMed] [Google Scholar]
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977). High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA et al. (2010). American College of Cardiology F, American Heart A. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 56: e50–103. [DOI] [PubMed] [Google Scholar]
- Guo H, Jin D, Chen X (2014). Lipocalin 2 is a regulator of macrophage polarization and NF‐κB/STAT3 pathway activation. Mol. Endocrinol. 28: 1616–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafiane A, Genest J (2013). HDL, atherosclerosis, and emerging therapies. Cholesterol 2013: 891403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J et al. (2014). Small dense low‐density lipoprotein‐cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler. Thromb. Vasc. Biol. 34: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PA, Young IS, McKibben B, McEneny J (2007). High density lipoprotein subfractions: Isolation, composition, and their duplicitous role in oxidation. J. Lipid Res. 48: 86–95. [DOI] [PubMed] [Google Scholar]
- Medina FA, de Almeida CJ, Dew E, Li J, Bonuccelli G, Williams TM et al. (2006). Caveolin‐1‐deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 74: 6665–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokgobu MI, Cholo MC, Anderson R, Steel HC, Motheo MP, Hlatshwayo TN et al. (2015). Oxidative induction of pro‐inflammatory cytokine formation by human monocyte‐derived macrophages following exposure to manganese in vitro . J. Immunotoxicol. 12: 98–103. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D et al. (2011). Neutrophil activation is attenuated by high‐density lipoprotein and apolipoprotein AI in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 31: 1333–1341. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Bijl N, Yvan‐Charvet L, Welch CB, Bhagwat N, Reheman A et al. (2013). Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat. Med. 19: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al. (2014). Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls SJ, Cutri B, Worthley SG, Kee P, Rye KA, Bao S et al. (2005). Impact of short‐term administration of high‐density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 25: 2416–2421. [DOI] [PubMed] [Google Scholar]
- Pavlides S, Gutierrez‐Pajares JL, Danilo C, Lisanti MP, Frank PG (2012). Atherosclerosis, caveolae and caveolin‐1. Adv. Exp. Med. Biol. 729: 127–144. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana CV, Gil MP, Schreiber RD, Stark GR (2002). Stat1‐dependent and ‐independent pathways in IFN‐γ‐dependent signaling. Trends Immunol. 23: 96–101. [DOI] [PubMed] [Google Scholar]
- Rye KA, Barter PJ (2008). Antiinflammatory actions of HDL: A new insight. Arterioscler. Thromb. Vasc. Biol. 28: 1890–1891. [DOI] [PubMed] [Google Scholar]
- Sanson M, Distel E, Fisher EA (2013). HDL induces the expression of the M2 macrophage markers arginase 1 and Fizz‐1 in a STAT6‐dependent process. PLoS One 8: e74676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A, Lisanti MP (2001). The caveolin triad: Caveolae biogenesis, cholesterol trafficking, and signal transduction. Cytokine Growth Factor Rev. 12: 41–51. [DOI] [PubMed] [Google Scholar]
- Shah M, Patel K, Fried VA, Sehgal PB (2002). Interactions of STAT3 with caveolin‐1 and heat shock protein 90 in plasma membrane raft and cytosolic complexes. Preservation of cytokine signaling during fever. J. Biol. Chem. 277: 45662–45669. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R et al. (2007). Ly‐6Chi monocytes dominate hypercholesterolemia‐associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J et al. (2007). Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall AR, Yvan‐Charvet L, Westerterp M, Murphy AJ (2012). Cholesterol efflux: A novel regulator of myelopoiesis and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 32: 2547–2552. [DOI] [PubMed] [Google Scholar]
- Tsompanidi EM, Brinkmeier MS, Fotiadou EH, Giakoumi SM, Kypreos KE (2010). HDL biogenesis and functions: Role of HDL quality and quantity in atherosclerosis. Atherosclerosis 208: 3–9. [DOI] [PubMed] [Google Scholar]
- Tugal D, Liao X, Jain MK (2013). Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 33: 1135–1144. [DOI] [PubMed] [Google Scholar]
- Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S (2015). Metformin inhibits monocyte‐to‐macrophage differentiation via AMPK‐mediated inhibition of STAT3 activation: Potential role in atherosclerosis. Diabetes 64: 2028–2041. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Song R, Choi AM (2006). Caveolin‐1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am. J. Respir. Cell Mol. Biol. 34: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yla‐Herttuala S (1991). Macrophages and oxidized low density lipoproteins in the pathogenesis of atherosclerosis. Ann. Med. 23: 561–567. [DOI] [PubMed] [Google Scholar]
- Yuan K, Huang C, Fox J, Gaid M, Weaver A, Li G et al. (2011). Elevated inflammatory response in caveolin‐1‐deficient mice with Pseudomonas aeruginosa infection is mediated by STAT3 protein and nuclear factor κB (NF‐κB). J. Biol. Chem. 286: 21814–21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A et al. (2008). Increased cellular free cholesterol in macrophage‐specific Abca1 knock‐out mice enhances pro‐inflammatory response of macrophages. J. Biol. Chem. 283: 22930–22941. [DOI] [PMC free article] [PubMed] [Google Scholar]


