Abstract
BACKGROUND/OBJECTIVES
This is the first study to identify common genetic factors associated with the basal metabolic rate (BMR) and body mass index (BMI) in obese Korean women including overweight. This will be a basic study for future research of obese gene-BMR interaction.
SUBJECTS/METHODS
The experimental design was 2 by 2 with variables of BMR and BMI. A genome-wide association study (GWAS) of single nucleotide polymorphisms (SNPs) was conducted in the overweight and obesity (BMI > 23 kg/m2) compared to the normality, and in women with low BMR (< 1426.3 kcal/day) compared to high BMR. A total of 140 SNPs reached formal genome-wide statistical significance in this study (P < 1 × 10-4). Surveys to estimate energy intake using 24-h recall method for three days and questionnaires for family history, a medical examination, and physical activities were conducted.
RESULTS
We found that two NRG3 gene SNPs in the 10q23.1 chromosomal region were highly associated with BMR (rs10786764; P = 8.0 × 10-7, rs1040675; 2.3 × 10-6) and BMI (rs10786764; P = 2.5 × 10-5, rs10786764; 6.57 × 10-5). The other genes related to BMI (HSD52, TMA16, MARCH1, NRG1, NRXN3, and STK4) yielded P <10 × 10-4. Five new loci associated with BMR and BMI, including NRG3, OR8U8, BCL2L2-PABPN1, PABPN1, and SLC22A17 were identified in obese Korean women (P < 1 × 10-4). In the questionnaire investigation, significant differences were found in the number of starvation periods per week, family history of stomach cancer, coffee intake, and trial of weight control in each group.
CONCLUSION
We discovered several common BMR- and BMI-related genes using GWAS. Although most of these newly established loci were not previously associated with obesity, they may provide new insights into body weight regulation. Our findings of five common genes associated with BMR and BMI in Koreans will serve as a reference for replication and validation of future studies on the metabolic rate.
Keywords: GWAS, BMR, BMI, obesity
INTRODUCTION
The prevalence of overweight and obesity, a risk factor associated with the morbidity and mortality of several diseases, is increasing with epidemic proportions worldwide. Obesity, diagnosed on the basis of body mass index (BMI) or waist circumference (WC), derives from an imbalance between energy intake and energy expenditure; however, its underlying mechanisms are generally unclear [1,2]. Environmental factors play a crucial role in development of obesity, however, multiple twin and family studies have shown that genetic factors also have an important contribution to the etiology of obesity. Many genetic loci associated with obesity have been demonstrated; however, these loci can explain only a narrow portion of the genetic variances for obesity [2]. Genetic variants associated with "common polygenic obesity" have been widely studied through candidate gene approaches and genome-wide linkage studies. However, the candidate gene approach has failed for several reasons, including too small study samples, lack of adjustment for multiple testing, and insufficient replication. On the other hand, with high-throughput genotyping and the Human Genome Project, genome-wide linkage studies have established several methods for discovery of obesity susceptibility genes. In genome-wide linkage studies, the whole genome of individuals is scanned to investigate the linkage of chromosomal regions with obesity and associated traits [3].
Genome-wide association study (GWAS) recently demonstrated several single nucleotide polymorphisms (SNPs) associated with both BMI and obesity susceptibility loci, located in or near genes such as fat mass and obesity-associated protein (FTO), melanocortin receptor 4 (MC4R), transmembrane protein 18 (TMEM18), glucosamine-6-phosphate deaminase 2(GNPDA2), brain-derived neurotrophic factor(BDNF), neuronal growth regulator 1(NEGR1), SH2B adapter protein 1 (SH2B1), mitochondrial carrier homolog 2(MTCH2), and potassium channel tetramerization domain containing 15 (KCTD15) in Europeans as well as in different ethnic populations [2,4,5,6,7,8,9,10,11]. Many of these genes are involved in many biological pathways and expressed in numerous tissues, including the central nervous system [2]. Some of the new obesity genes (FTO, MC4R, TMEM18, GNPDA2, SH2B1, KCTD15, and BDNF) are expressed in the hypothalamus and are involved in a crucial center for energy balance and regulation of appetite [10]. In addition, meta-analyses of GWAS for obesity-related traits, including BMI and waist-hip ratio (WHR), have identified many regions of the genome associated with obesity [1]. Few studies to confirm the associations of BMI-associated loci with central obesity have been conducted. Because the crucial role of central obesity, as assessed by WC, is more strongly associated with the risk of hypertension, type 2 diabetes, cardiovascular diseases (CVD), and cancer than with obesity, it is necessary to determine whether BMI-associated loci can significantly predict central obesity [4]. In addition, as the accumulation of abdominal fat is a risk factor for CVD and type 2 diabetes, understanding mechanisms involved in regulation of fat accumulation and visceral fat mass is important in understanding obesity [3]. Obesity associated with fat mass is usually measured using BMI or WC. WC and WHR are also related to intraabdominal fat content and central obesity, rather than BMI [1].
Imbalance between energy expenditure and energy intake causes excessive fat accumulation, leading to an increase in the number and/or size of fat cells [12]. Basal metabolic rate (BMR) is the minimum energy required to retain the physiological functions of the body while awake. BMR accounts for approximately 45% to 70% of total energy expenditure in most healthy adults and is directly affected by age, sex, body surface area, body composition, genetic composition, pregnancy, and hormonal status [13,14]. Thus, estimating BMR is important for establishing strategies for obesity prevention programs [2]. For several years, numerous predictive equations for BMR have been developed in different populations. Many studies have reported on the influence of ethnicity on BMR, however, their results have been inconsistent [13,14]. Several GWAS on BMI, WC, WHR, extreme obesity phenotypes, and BMI-adjusted WHR in ethnic groups have been published; however, GWAS of BMR and BMI has not yet been confirmed in Korean women. To investigate the differences of the eating behavior and obesity-related life pattern between women with obesity and healthy control subjects, we conducted a survey using the 24-h recall method and a questionnaire.
SUBJECTS AND METHODS
Study population
The study population consisted of 77 Korean female participants aged 18-34 years who were recruited between May and July 2012. This study was approved by the ethics committee of Sungshin Women's University (SSWU IRB 2012-003). The study included completion of anthropometrics, questionnaires (family history, a medical examination, and physical activities), and dietary intakes. Written informed consent was obtained from all participants. Subjects with BMI > 25 kg/m2 and WC > 85 cm2 were included. Subjects with vascular disease (e.g., myocardial infarction or stroke), disturbances of lipid metabolism, acute or chronic renal failure, and impaired hepatic function were excluded. Those taking diabetes medication or diet pills were also excluded. A total of 77 participants who fulfilled all requirements were divided into four groups including high vs. low BMR according to median value of BMR, 1426.3 kcal/day, and low BMI (< 23 kg/m2) vs. high BMI (> 23 kg/m2).
Measurement of anthropometric parameters and BMR
Anthropometry was performed for weight, height, BMI, BMR, WC, hip, lean body mass, fat mass, percent of fat mass, and WHR. WC was measured midway between the lowest rib and the superior border of the iliac crest at the end of normal expiration using an inelastic measuring tape to the nearest 0.1 cm. BMR measurement was performed using a MedGem® metabolic analyzer (Microlife USA, Dunedin, FL), a handheld indirect calorimeter used for its accuracy and portability in clinical settings, which is becoming popular in research studies as a screening tool and a criterion measure of RMR.
This device is used for calculation of RMR using florescent quenching of ruthenium in the presence of oxygen and the modified Weir equation with an assumed RQ of 0.85 for estimation of carbon dioxide production [15].
Questionnaires
Six divisions of general questionnaires were conducted; general question, family history, nutritive conditions with eating habits, weight-control history, exercise and diet intakes with eating pattern. Questions about age, smoking status, and drinking status were included as general questions, and questions on hypertension, diabetes, myocardial infarction, angina, stroke, and cancer (stomach, lung, breast, etc.) were inspected for the family history. Assessments of habitual diet intake, including the number of starvation periods per week, intake of fast-food, carbonated drink, coffee, snack, and dietary (food, health, nutritional) supplement, and number of times eating-out were performed. Environmental data, including weight control methods, degree of physical activity such as hard, normal, and light exercise were acquired through the questionnaires. Dietary intakes with three days recall methods were collected and analyzed using Can Pro 4.0 software.
GWA Genotyping
Samples were analyzed on Affymetrix Genome-Wide Human SNP array 6.0 in the DNALink (Songpa-gu, Seoul, Korea). Affymetrix Genome-Wide Human SNP array 6.0 comprises 906,600 genome-wide SNPs and 946,000 copy number variations. Approximately 500ng of genomic DNA was digested with two restriction enzymes, NSP I and Sty I, and processed according to the Affymetrix protocol. The digested segments were ligated to enzyme specific adaptors which incorporate a universal PCR priming sequence. PCR amplification using universal primers was performed in a reaction optimized for amplification of fragments between 200-1,100 base pairs. A fragmentation step then reduced the PCR product to segments of approximately 25-50 bp, which were then end-labeled using biotinylated nucleotides. The labeled product was then hybridized to a chip, washed, and detected. Images were analyzed using GeneChip Operating System software (Affymetrix, Santa Clara, CA, USA). For the data obtained from the chip, internal quality control measures were used: the QC call rate (Dynamic Model algorithm) always exceeded 86% and heterozygosity on the X chromosome correctly identified the gender of the individual. Genotype calling was performed using the Birdseed v2 algorithm.
Statistical analysis
For the analysis of GWAS data, the subjects were divided into two groups: high BMR and low BMI (control group) vs. low BMR and high BMI (case group). Continuous variables were expressed as mean ± SD, and differences between groups were assessed using Student's t-test. Categorical variables were represented as percentages and tested using the χ2 test. Hardy-Weinberg equilibrium (HWE) was assessed using the χ2 test. A P value > 0.05 was considered significant. In addition, all datasets were filtered to exclude samples or SNPs with > 5% missing values, variants with < 5% minor allele frequency, and samples deviating from the HWE, using PLINK, whole genome data analysis tool set. Quantitative trait association analyses for BMR and BMI in the 77 overweight women (BMR < 1426.3 kcal/day and BMI > 23 kg/m2) and normal-weight control subjects (BMRI > 1426.3 kcal/day and BMI < 23 kg/m2) were performed. BMI and BMR were analyzed as continuous traits, separately in obese and normal-weight subjects, with linear regression in PLINK, using dominant, codominant, and recessive models. Genotype and allele frequencies were compared between groups using the chi-square test, the Cochran Armitage trend test, or the Jonckheere-Terpstra test as appropriate. Data analysis was performed using SAS software version 9.1.3 (SAS Inc., Cary, NC, USA) and PLINK. [16].
RESULTS
Clinical characteristics of the study population
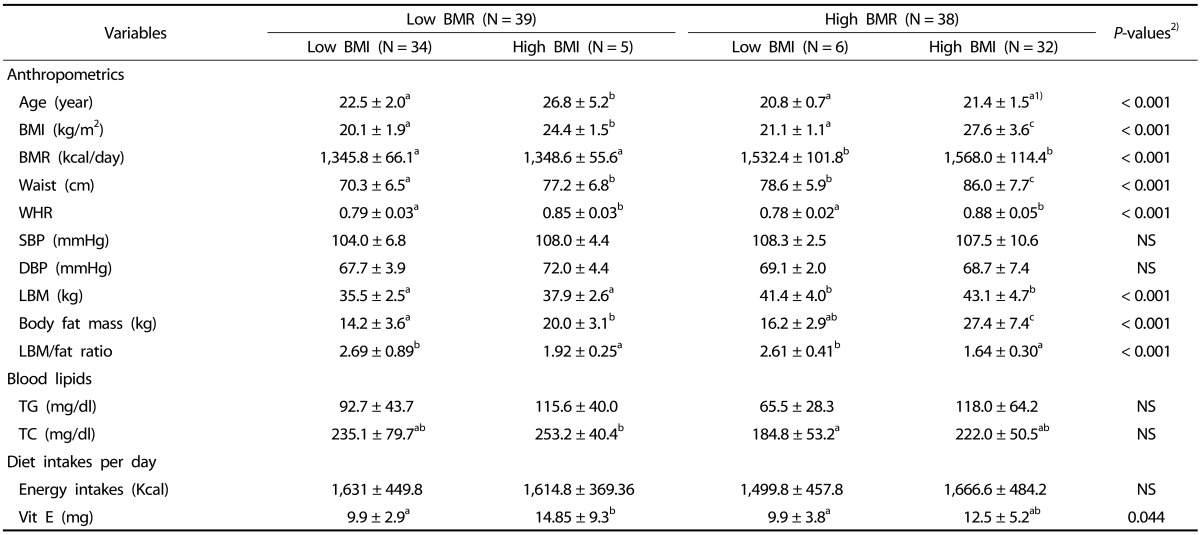
A total of 77 subjects were enrolled in the current study. Table 1 shows the clinical characteristics of the study population. The subjects were divided into several groups: normal vs. overweight according to BMI and low vs. high BMR. The mean BMR value was 1452.9 ± 141.4 kcal/day and the mean BMI was 23.6 ± 4.5 kcal/day in all subjects. In the BMR groups, subjects with a high BMI had significantly higher BMR than those with a low BMI (P < 0.001). Significant differences in BMI, BMR, waist measurement, hip measurement, lean body mass, and body fat mass were observed in each group according to BMR or BMI (Table 1). Blood TG and TC did not differ among the four groups.
Table 1. Demographic characteristics of the study subjects according to BMR and BMI.
1) Mean ± SD
2) P-values; statistical differences among 4 groups. NS; non-significant differences BMI: body mass index; BMR: basal metabolic rate; WHR: waist-hip ratio; SBP: systolic blood pressure; DBP: diastolic blood pressure; LBM: lean body mass; TG: triglyceride; TC, total cholesterol.
Genome-wide association study
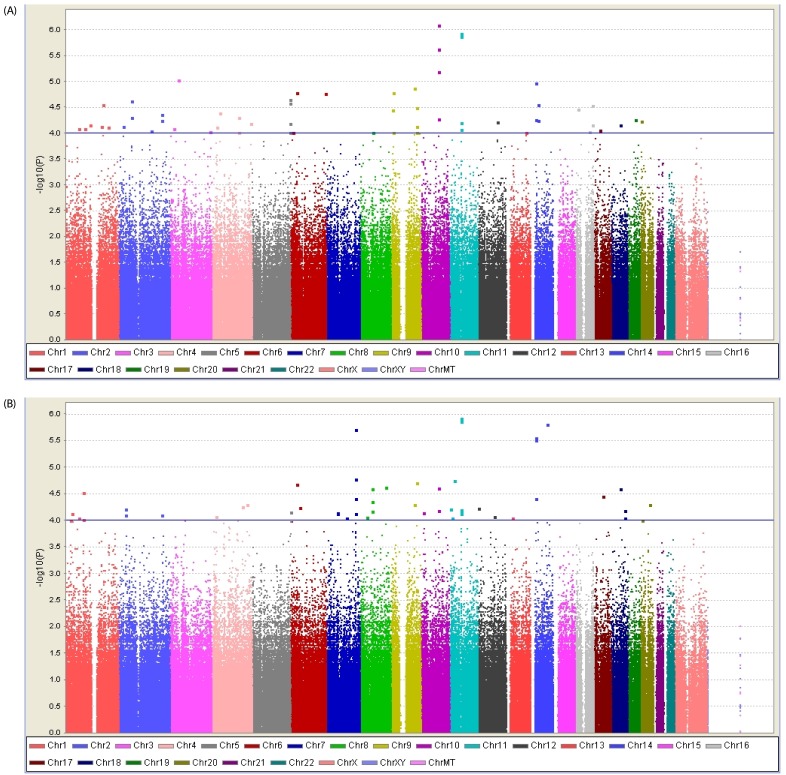
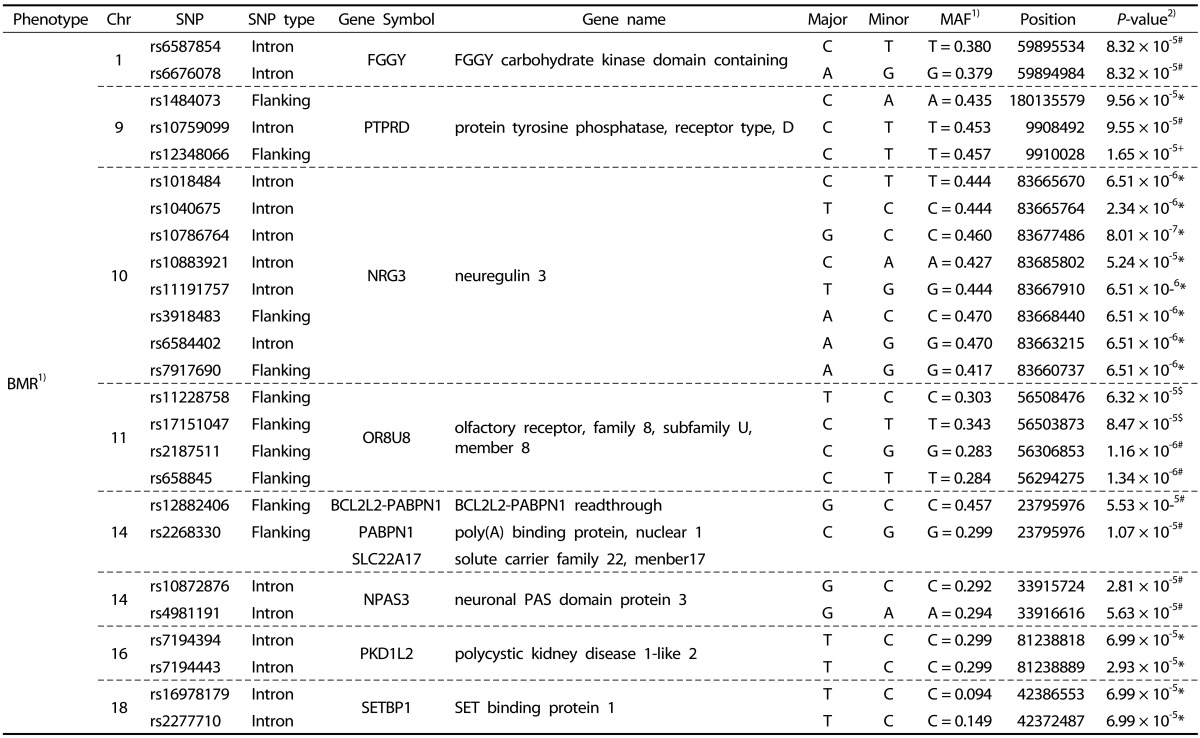
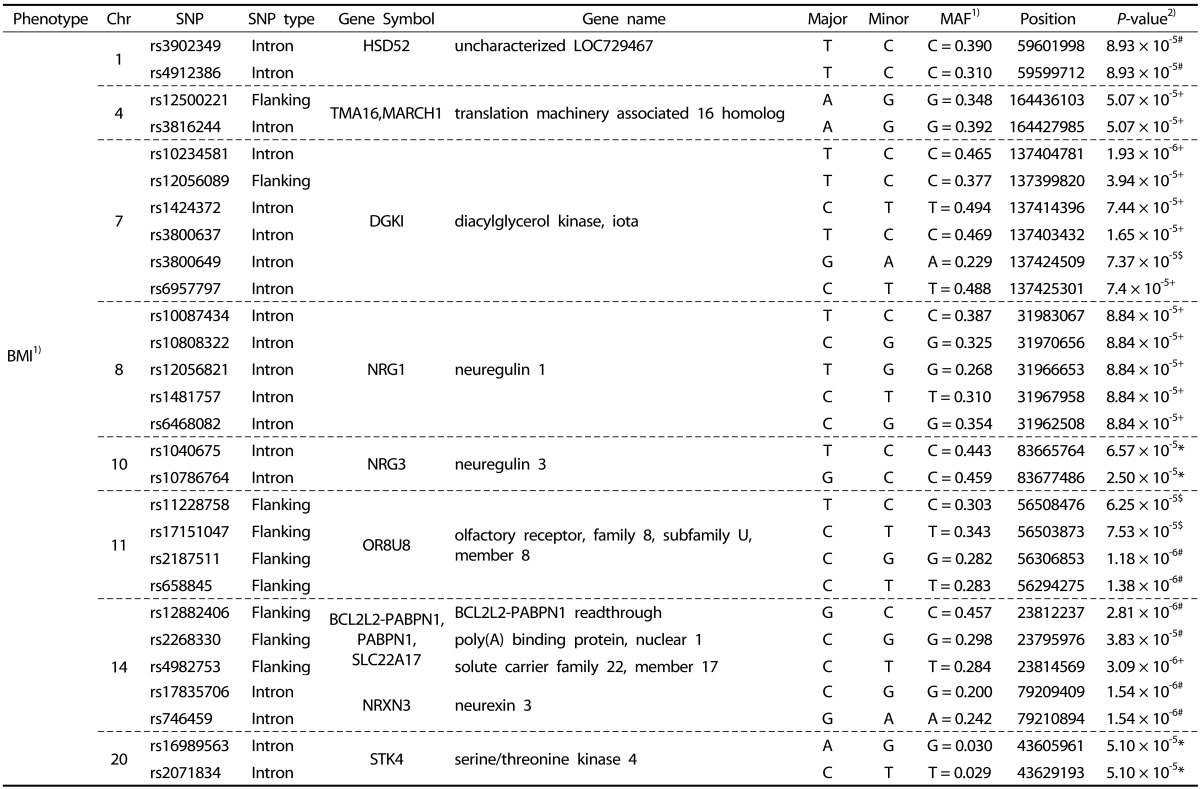
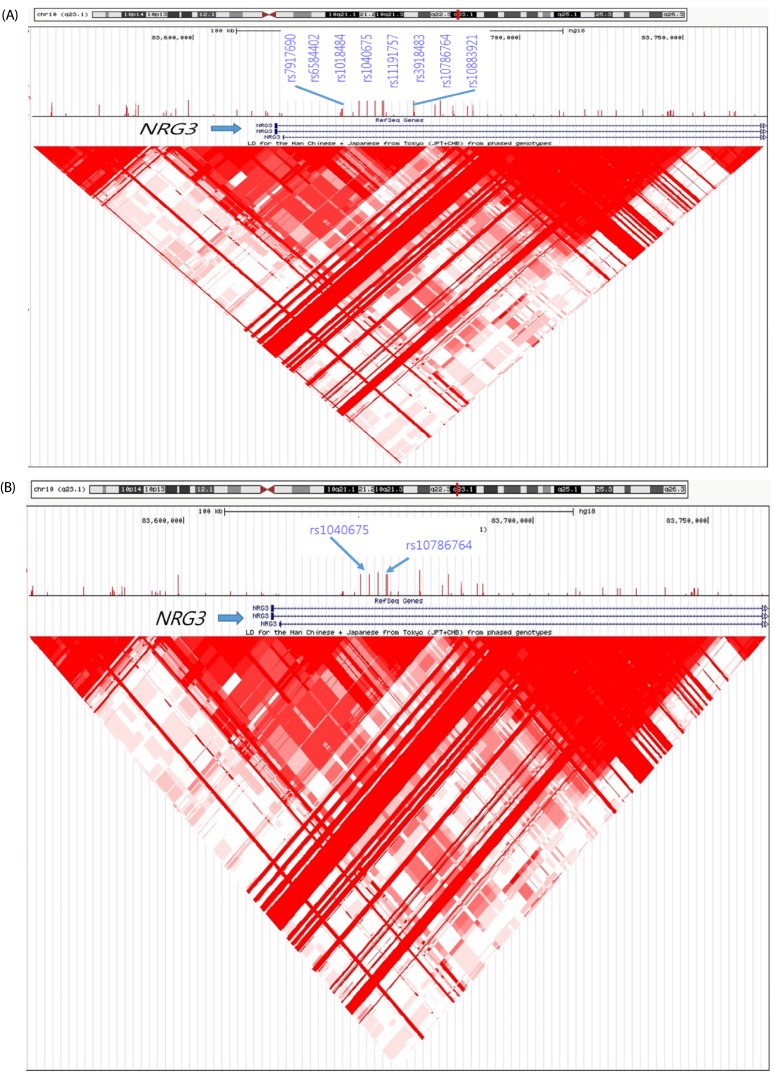
We performed systematic quality control steps on the raw genotype data and obtained 904,085 SNPs; SNPs with a minor allele frequency of < 1%, a call rate of < 95%, and a significant deviation from Hardy-Weinberg equilibrium in controls (P < 1 × 10-7) were excluded. SNPs likely to be false-positive associations due to wrong clustering were also excluded. After quality control, 710,315 and 710,679 SNPs were included in BMR and BMI study. The Manhattan plot of -log10 (P-value) from the GWAS and imputation analysis against the chromosome position showed that no genetic loci reached genome-wide significance with the threshold P-value of 1 × 10-7 (Fig. 1). For identification of susceptible loci associated with BMR, a two-step discovery design was applied in the Korean female subjects. In an initial discovery step based on the sample of 77 female subjects, we selected all loci with a P value of < 10-4 using continuous variables; 239 loci associated with BMR satisfied this criterion. In a second analysis, we attempted to replicate the strongest SNP association in the groups divided according to BMR and BMI. Using continuous variables, 118 SNPs in the whole chromosome including the NRG3 and PTPRD regions reached our current threshold for genome-wide significance (data not shown). The subjects were divided into two groups: high-BMR and low-BMI (control group) vs. low-BMR and high-BMI (case group). In the second analysis, 49 additional loci including rs1018484 in NRG3 and a locus (rs11228758) at chromosome 11 in the intron of olfactory receptor, family 8, subfamily U, member 8 (OR8U8; GenBank NW_003871073) reached genome-wide significance (Tables 2 and 3). A total of 140 SNPs reached formal genome-wide statistical significance in this study (P < 1 × 10-4). First, NRG3 gene SNP (rs10786764) in the 10q23.1 chromosomal region was highly associated with both BMR (P = 8.0 × 10-7) and BMI (P = 8.0 × 10-7), and NRG3 gene SNP (rs1040675), also a gene commonly associated with BMR and BMI even though it was not as strong as rs10786764 in the linkage disequilibrium (LD) plot (Fig. 2). The FGGY gene SNP rs6676078 also reached genome-wide significance (P = 8.3 × 10-5). The TNR, B3GNT2, FZD7, OR2Y1, MGAT1, NPAS3, PKD1L2, and SETBP1 genes showed weaker associations (P < 1 × 10-5) with BMR. Seven other genes associated with BMI (HSD52, TMA16, MARCH1, DGKI, NRG1, NRXN3, and STK4) yielded P < 10 × 10-4. The five common genes associated with both BMR and BMI were NRG3, OR8U8, BCL2L2-PABPN1, PABPN1, and SLC22A17 (P < 1 × 10-4).
Fig. 1. The Manhattan plot for the genome-wide association study (GWAS) of obesity in the Korean female subjects.
This plot is based on -log10 (P-value) from GWAS and imputation analysis against chromosome position; each color represents a different chromosome. Blue horizontal line indicates the suggestive association threshold, P = 1 × 10-4. (A) BMR and (B) BMI in these study subjects.
Table 2. Summary of BMR-associated genes SNPs showing a P-value of < 1 × 10-4 in Korean female subjects.
1) BMR: basal metabolic rate; MAF: minor allele frequency.
2) P-values; Statistical differences among 4 groups. * Dominant, + Recessive, # Allele, $ Co-dominant
Table 3. Summary of BMI-associated genes SNPs showing a P-value of < 1 × 10-4 in Korean female subjects.
1) BMI: body mass index; MAF: minor allele frequency
2) P-values; Statistical differences among 4 groups. * Dominant, + Recessive, # Allele, $ Co-dominant
Fig. 2. Linkage Disequilibrium Plot figure of NRG3 gene SNPs (rs10786764 and rs1040675), black arrow on chromosome 10, in obese Korean women including overweight.
The darker red in each diamond shape, stronger LD in association with BMR (A) and BMI (B).
Dietary intake and physical activity assessments using a questionnaire
We conducted a questionnaire investigation, including items on dietary intake and physical activity in all subjects. We found that total energy intakes (Kcal/day) including carbohydrate, protein, lipid, vitamin, and minerals did not differ among the 4 groups except vit E intakes (Table 1). Higher Vit E intakes were observed in high BMI than in low BMI, with no significant difference between low BMR and high BMR. However, significant differences in habitual eating behavior, trial of weight control, number of starvation periods per week, and family history were observed between the low BMR and high BMR groups. Sixteen women (41.0%) with low BMR skipped a meal four or five times per week; however, nine subjects (23.6%) with high BMR often went hungry (P = 0.014). In the low BMR group, 33.3% of the women had been working on their weight, while only 3.3% in the high BMR group were making a desperate effort to watch their weight. (P = 0.005). From questions about the family history of gastric cancer, it was found that women in the high BMR have higher family history rates than subjects in the low BMR group (P = 0.013).
DISCUSSION
Twenty two genes/chromosome regions that reached genome-wide association significance (P < 1×10-4, 44 SNPs) in this GWAS were investigated. Five common genes (NRG3, OR8U8, BCL2L2-PABPN1, PABPN1, and SLC22A17) yielded P < 1 × 10-5 in the BMR and BMI groups. From the questionnaire investigation, differences in items including family history and food intake were observed between the low BMR and high BMR groups. These findings provide new insights into the genetic etiology of obesity in relation to BMR and BMI. Recently, common variations of fat mass and obesity-related genes, including FTO, MC4R, and TMEM18, have been consistently associated with obesity traits and BMI in GWAS [17,18]. GWAS have identified approximately 40 SNPs showing a significant association with BMI, a widely used measurement of adiposity. However, only eight of these associations have been verified by follow-up GWAS using more complicated evaluations of adiposity. Among these eight, an SNP close to the FTO gene has been studied for discovery of its function, while the remaining seven SNPs adjacent to, or within, the NEGR1, TMEM18, ETV5, FLJ35779, LINGO2, SH2B1, and GIPR genes are less well reported than FTO, particularly in connection with obesity. Some reports did not demonstrate the putative mechanism associating the FLJ35779 and LINGO2 genes with obesity. All of these genes are expressed in the brain, and SH2B1 and GIPR have been directly linked to appetite regulation. SH2B1 is an enhancer of intracellular signaling in the JAK-STAT pathway, and GIPR is the receptor for an appetite-linked hormone (GIP) produced by the alimentary tract. NEGR1, ETV5, and SH2B1 have suggested roles in neurite outgrowth; therefore, SNPs within these genes may influence the energy balance circuitry. These functions may contribute to their effects on the obese phenotype [19,20,21].
However, these genes and functions have shown association with BMI only, not BMR. Thus far, GWAS have confirmed at least 50 loci associated with BMI, WHR, body fat percentage, and extreme obesity. Some of these have been demonstrated to replicate in non-white populations and in children and adolescents. Despite the many new findings, the sizes of the established loci are small, and they can explain only a narrow portion of the inter-individual variations in BMI. In addition, most of these newly discovered loci were not previously linked to obesity [19]. From this study, we supposed that the new loci associated with BMR and BMI in obese Korean women might be associated with NRG3, OR8U8, BCL2L2-PABPN1, PABPN1, and SLC22A17 genes. However, these genes and their functions related to obesity, BMR, and BMI have not yet been reported. The neuregulin 3(NRG3) gene located at 10q22-q24 is involved in multiple psychiatric disorders including cognitive impairment [22]. As recently reported, genetic polymorphisms and haplotypes in the NRG3 gene may play a role in Alzheimer disease; however, NRG3 was not associated with BMR and BMI [23]. The current study shows a different result. OR8U8 is located in a cluster of seven olfactory receptor genes, previously associated with odor perception. Among these genes, for the first time, an SNP in the OR7G3 gene (rs10414255) was also found to be associated with adiposity and eating behaviors [22]. In the study by Choquette et al., OR8U8, an olfactory receptor gene, was found to be associated with obesity and/or BMI; this result should be considered as potential new speculation on the effects of polymorphisms in olfactory receptor genes on eating behaviors and obesity, and should be further studied in other populations. However, our study found a different result.
Recently, the overexpression of solute carrier family 22 member 17 (SLC22A17) was found to be an independent prognostic factor involved in the aggressive behavior of endometrial carcinoma cells [24]. The organic cation transporter (OCT, SLC22) family is a family of polyspecific transmembrane proteins involved in the uptake or excretion of many cationic drugs, toxins, and endogenous metabolites in many tissues. Two novel rat SLC22 genes, SLC22A17 (BOCT1) and SLC22A23 (BOCT2), were recently cloned and characterized. However, some reports showed that although these novel family members have conserved sequence elements like the other OCT members, these proteins were unable to transport typical substrates as the SLC22 family, and another possible function of SLC22A17 was suggested [25]. The SLC22 family has not yet been associated with obesity and/or BMI; however, further studies and experiments are needed in order to test this novel hypothesis.
In the current study we investigated the eating behavior and lifestyle of the low BMR and high BMR groups, and BMR- and BMI-related genes were identified. Subjects with a high BMR had a higher rate of family history of gastric cancer and higher coffee intake, as well high quantities of protein and vitamin E intake, compared to those with a low BMR. Interestingly, the number of starvation periods per week and the trial of weight control in the subjects with low BMR were higher than in those with a high BMR. Because they have a high BMI, women with a low BMR are more likely to be overweight, thus this population may need to watch their weight. Therefore, understanding the genetic causes of obesity susceptibility by investigating BMR- and BMI-related genes may reveal some of the underlying mechanisms leading to the prevention and possible treatments of obesity in the future. Despite the limitation of small number of subjects in this study because of economics, several common BMR- and BMI-related genes were discovered using GWAS. Although most of these newly established loci were not previously associated with obesity, they may provide new insights into body weight regulation. Further studies in gene discovery and function are needed in order to increase our understanding of obesity.
Footnotes
This work was supported by Korea Food Research Institute and NRF grant funded by MSIP (2014R1A2A1A11049611/1).
References
- 1.Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol. 2014;382:740–757. doi: 10.1016/j.mce.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, van Vliet-Ostaptchouk JV, Wijmenga C, van der Schouw YT. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrientspecific food preference. Am J Clin Nutr. 2009;90:951–959. doi: 10.3945/ajcn.2009.27781. [DOI] [PubMed] [Google Scholar]
- 3.Xi B, Cheng H, Shen Y, Chandak GR, Zhao X, Hou D, Wu L, Wang X, Mi J. Study of 11 BMI-associated loci identified in GWAS for associations with central obesity in the Chinese children. PLoS One. 2013;8:e56472. doi: 10.1371/journal.pone.0056472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu CT, Monda KL, Taylor KC, Lange L, Demerath EW, Palmas W, Wojczynski MK, Ellis JC, Vitolins MZ, Liu S, Papanicolaou GJ, Irvin MR, Xue L, Griffin PJ, Nalls MA, Adeyemo A, Liu J, Li G, Ruiz-Narvaez EA, Chen WM, Chen F, Henderson BE, Millikan RC, Ambrosone CB, Strom SS, Guo X, Andrews JS, Sun YV, Mosley TH, Yanek LR, Shriner D, Haritunians T, Rotter JI, Speliotes EK, Smith M, Rosenberg L, Mychaleckyj J, Nayak U, Spruill I, Garvey WT, Pettaway C, Nyante S, Bandera EV, Britton AF, Zonderman AB, Rasmussen-Torvik LJ, Chen YD, Ding J, Lohman K, Kritchevsky SB, Zhao W, Peyser PA, Kardia SL, Kabagambe E, Broeckel U, Chen G, Zhou J, Wassertheil-Smoller S, Neuhouser ML, Rampersaud E, Psaty B, Kooperberg C, Manson JE, Kuller LH, Ochs-Balcom HM, Johnson KC, Sucheston L, Ordovas JM, Palmer JR, Haiman CA, McKnight B, Howard BV, Becker DM, Bielak LF, Liu Y, Allison MA, Grant SF, Burke GL, Patel SR, Schreiner PJ, Borecki IB, Evans MK, Taylor H, Sale MM, Howard V, Carlson CS, Rotimi CN, Cushman M, Harris TB, Reiner AP, Cupples LA, North KE, Fox CS. Genome-wide association of body fat distribution in African ancestry populations suggests new loci. PLoS Genet. 2013;9:e1003681. doi: 10.1371/journal.pgen.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi B, Zhao X, Chandak GR, Shen Y, Cheng H, Hou D, Wang X, Mi J. Influence of obesity on association between genetic variants identified by genome-wide association studies and hypertension risk in Chinese children. Am J Hypertens. 2013;26:990–996. doi: 10.1093/ajh/hpt046. [DOI] [PubMed] [Google Scholar]
- 6.Melén E, Granell R, Kogevinas M, Strachan D, Gonzalez JR, Wjst M, Jarvis D, Ege M, Braun-Fahrländer C, Genuneit J, Horak E, Bouzigon E, Demenais F, Kauffmann F, Siroux V, Michel S, von Berg A, Heinzmann A, Kabesch M, Probst-Hensch NM, Curjuric I, Imboden M, Rochat T, Henderson J, Sterne JA, McArdle WL, Hui J, James AL, William Musk A, Palmer LJ, Becker A, Kozyrskyj AL, Chan-Young M, Park JE, Leung A, Daley D, Freidin MB, Deev IA, Ogorodova LM, Puzyrev VP, Celedón JC, Brehm JM, Cloutier MM, Canino G, Acosta-Pérez E, Soto-Quiros M, Avila L, Bergström A, Magnusson J, Söderhäll C, Kull I, Scholtens S, Marike Boezen H, Koppelman GH, Wijga AH, Marenholz I, Esparza-Gordillo J, Lau S, Lee YA, Standl M, Tiesler CM, Flexeder C, Heinrich J, Myers RA, Ober C, Nicolae DL, Farrall M, Kumar A, Moffatt MF, Cookson WO, Lasky-Su J. Genome-wide association study of body mass index in 23 000 individuals with and without asthma. Clin Exp Allergy. 2013;43:463–474. doi: 10.1111/cea.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rask-Andersen M, Moschonis G, Chrousos GP, Marcus C, Dedoussis GV, Fredriksson R, Schiöth HB. The STK33-linked SNP rs4929949 is associated with obesity and BMI in two independent cohorts of Swedish and Greek children. PLoS One. 2013;8:e71353. doi: 10.1371/journal.pone.0071353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monda KL, Chen GK, Taylor KC, Palmer C, Edwards TL, Lange LA, Ng MC, Adeyemo AA, Allison MA, Bielak LF, Chen G, Graff M, Irvin MR, Rhie SK, Li G, Liu Y, Liu Y, Lu Y, Nalls MA, Sun YV, Wojczynski MK, Yanek LR, Aldrich MC, Ademola A, Amos CI, Bandera EV, Bock CH, Britton A, Broeckel U, Cai Q, Caporaso NE, Carlson CS, Carpten J, Casey G, Chen WM, Chen F, Chen YD, Chiang CW, Coetzee GA, Demerath E, Deming-Halverson SL, Driver RW, Dubbert P, Feitosa MF, Feng Y, Freedman BI, Gillanders EM, Gottesman O, Guo X, Haritunians T, Harris T, Harris CC, Hennis AJ, Hernandez DG, McNeill LH, Howard TD, Howard BV, Howard VJ, Johnson KC, Kang SJ, Keating BJ, Kolb S, Kuller LH, Kutlar A, Langefeld CD, Lettre G, Lohman K, Lotay V, Lyon H, Manson JE, Maixner W, Meng YA, Monroe KR, Morhason-Bello I, Murphy AB, Mychaleckyj JC, Nadukuru R, Nathanson KL, Nayak U, N'diaye A, Nemesure B, Wu SY, Leske MC, Neslund-Dudas C, Neuhouser M, Nyante S, Ochs-Balcom H, Ogunniyi A, Ogundiran TO, Ojengbede O, Olopade OI, Palmer JR, Ruiz-Narvaez EA, Palmer ND, Press MF, Rampersaud E, Rasmussen-Torvik LJ, Rodriguez-Gil JL, Salako B, Schadt EE, Schwartz AG, Shriner DA, Siscovick D, Smith SB, Wassertheil-Smoller S, Speliotes EK, Spitz MR, Sucheston L, Taylor H, Tayo BO, Tucker MA, Van Den Berg DJ, Edwards DR, Wang Z, Wiencke JK, Winkler TW, Witte JS, Wrensch M, Wu X, Yang JJ, Levin AM, Young TR, Zakai NA, Cushman M, Zanetti KA, Zhao JH, Zhao W, Zheng Y, Zhou J, Ziegler RG, Zmuda JM, Fernandes JK, Gilkeson GS, Kamen DL, Hunt KJ, Spruill IJ, Ambrosone CB, Ambs S, Arnett DK, Atwood L, Becker DM, Berndt SI, Bernstein L, Blot WJ, Borecki IB, Bottinger EP, Bowden DW, Burke G, Chanock SJ, Cooper RS, Ding J, Duggan D, Evans MK, Fox C, Garvey WT, Bradfield JP, Hakonarson H, Grant SF, Hsing A, Chu L, Hu JJ, Huo D, Ingles SA, John EM, Jordan JM, Kabagambe EK, Kardia SL, Kittles RA, Goodman PJ, Klein EA, Kolonel LN, Le Marchand L, Liu S, McKnight B, Millikan RC, Mosley TH, Padhukasahasram B, Williams LK, Patel SR, Peters U, Pettaway CA, Peyser PA, Psaty BM, Redline S, Rotimi CN, Rybicki BA, Sale MM, Schreiner PJ, Signorello LB, Singleton AB, Stanford JL, Strom SS, Thun MJ, Vitolins M, Zheng W, Moore JH, Williams SM, Ketkar S, Zhu X, Zonderman AB, Kooperberg C, Papanicolaou GJ, Henderson BE, Reiner AP, Hirschhorn JN, Loos RJ, North KE, Haiman CA NABEC Consortium; UKBEC Consortium; BioBank Japan Project; AGEN Consortium. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat Genet. 2013;45:690–696. doi: 10.1038/ng.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen W, Cho YS, Zheng W, Dorajoo R, Kato N, Qi L, Chen CH, Delahanty RJ, Okada Y, Tabara Y, Gu D, Zhu D, Haiman CA, Mo Z, Gao YT, Saw SM, Go MJ, Takeuchi F, Chang LC, Kokubo Y, Liang J, Hao M, Le Marchand L, Zhang Y, Hu Y, Wong TY, Long J, Han BG, Kubo M, Yamamoto K, Su MH, Miki T, Henderson BE, Song H, Tan A, He J, Ng DP, Cai Q, Tsunoda T, Tsai FJ, Iwai N, Chen GK, Shi J, Xu J, Sim X, Xiang YB, Maeda S, Ong RT, Li C, Nakamura Y, Aung T, Kamatani N, Liu JJ, Lu W, Yokota M, Seielstad M, Fann CS, Wu JY, Lee JY, Hu FB, Tanaka T, Tai ES, Shu XO Genetic Investigation of ANthropometric Traits (GIANT) Consortium. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet. 2012;44:307–311. doi: 10.1038/ng.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Mägi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segrè AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpeläinen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga JJ, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JR, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson JO, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MT, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AI, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proença C, Chen YD, Chen CM, Chines PS, Clarke R, Coin L, Connell J, Day IN, den Heijer M, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJ, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Grässler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen AL, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jørgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, König IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaløy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimäki T, Lettre G, Liu J, Lokki ML, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O'Donnell CJ, O'Rahilly S, Ong KK, Oostra B, Paré G, Parker AN, Perola M, Pichler I, Pietiläinen KH, Platou CG, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstråle M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo ML, Tardif JC, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tönjes A, Tuomi T, van Meurs JB, van Ommen GJ, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CI, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kähönen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Grönberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin MR, Kaprio J, Karpe F, Khaw KT, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PE, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, McCarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O'Connell JR, Peltonen L, Schlessinger D, Strachan DP, van Duijn CM, Wichmann HE, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, McCarthy MI, Hirschhorn JN, Ingelsson E, Loos RJ Procardis Consortium; MAGIC. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day FR, Loos RJ. Developments in obesity genetics in the era of genome-wide association studies. J Nutrigenet Nutrigenomics. 2011;4:222–238. doi: 10.1159/000332158. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Wang S. Role of kruppel-like transcription factors in adipogenesis. Dev Biol. 2013;373:235–243. doi: 10.1016/j.ydbio.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Sabounchi NS, Rahmandad H, Ammerman A. Best-fitting prediction equations for basal metabolic rate: informing obesity interventions in diverse populations. Int J Obes (Lond) 2013;37:1364–1370. doi: 10.1038/ijo.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JE, Poh BK, Nik Shanita S, Izham MM, Chan KQ, Tai MD, Ng WW, Ismail MN. Predicting basal metabolic rates in Malaysian adult elite athletes. Singapore Med J. 2012;53:744–749. [PubMed] [Google Scholar]
- 15.Compher C, Hise M, Sternberg A, Kinosian BP. Comparison between Medgem and Deltatrac resting metabolic rate measurements. Eur J Clin Nutr. 2005;59:1136–1141. doi: 10.1038/sj.ejcn.1602223. [DOI] [PubMed] [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Namjou B, Keddache M, Marsolo K, Wagner M, Lingren T, Cobb B, Perry C, Kennebeck S, Holm IA, Li R, Crimmins NA, Martin L, Solti I, Kohane IS, Harley JB. EMR-linked GWAS study: investigation of variation landscape of loci for body mass index in children. Front Genet. 2013;4:268. doi: 10.3389/fgene.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10:51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speakman JR. Functional analysis of seven genes linked to body mass index and adiposity by genome-wide association studies: a review. Hum Hered. 2013;75:57–79. doi: 10.1159/000353585. [DOI] [PubMed] [Google Scholar]
- 20.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang KS, Xu N, Wang L, Aragon L, Ciubuc R, Arana TB, Mao C, Petty L, Briones D, Su BB, Luo X, Camarillo C, Escamilla MA, Xu C. NRG3 gene is associated with the risk and age at onset of Alzheimer disease. J Neural Transm (Vienna) 2014;121:183–192. doi: 10.1007/s00702-013-1091-0. [DOI] [PubMed] [Google Scholar]
- 23.Choquette AC, Bouchard L, Drapeau V, Lemieux S, Tremblay A, Bouchard C, Vohl MC, Pérusse L. Association between olfactory receptor genes, eating behavior traits and adiposity: results from the Quebec Family Study. Physiol Behav. 2012;105:772–776. doi: 10.1016/j.physbeh.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto T, Asaka R, Suzuki A, Takatsu A, Kashima H, Shiozawa T. Immunohistochemical detection of a specific receptor for lipocalin2 (solute carrier family 22 member 17, SLC22A17) and its prognostic significance in endometrial carcinoma. Exp Mol Pathol. 2011;91:563–568. doi: 10.1016/j.yexmp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Bennett KM, Liu J, Hoelting C, Stoll J. Expression and analysis of two novel rat organic cation transporter homologs, SLC22A17 and SLC22A23. Mol Cell Biochem. 2011;352:143–154. doi: 10.1007/s11010-011-0748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]